Rheumatoid Arthritis and Osteoporosis as Prototypes of Immunosenescence in Osteoimmunology: Molecular Pathways of Inflammaging and Targeted Therapies
Abstract
1. Introduction
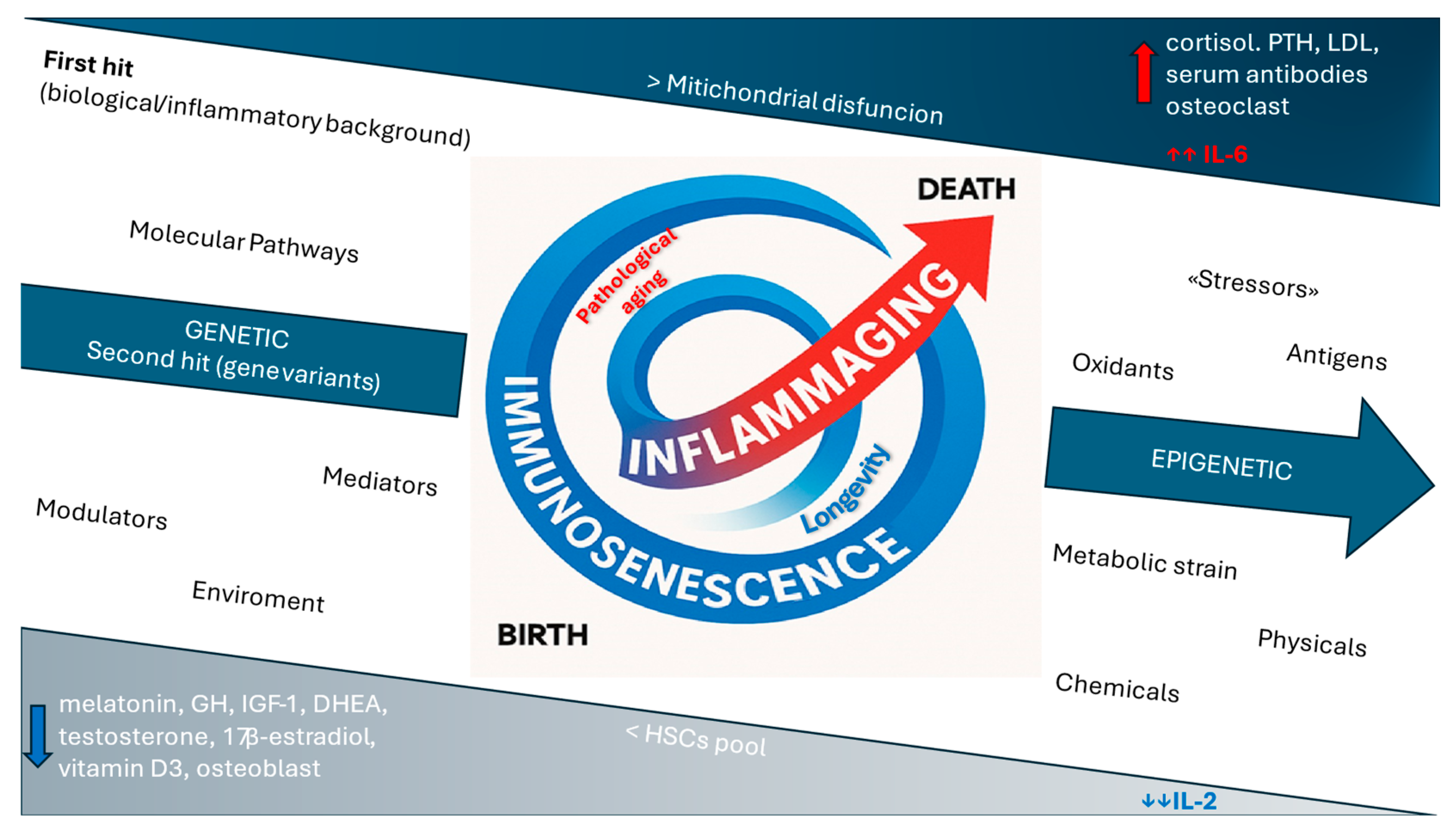
2. Immunosenescence and Inflammaging: The Contribution to Rheumatic Disorders
3. Rheumatoid Arthritis in the Elderly: From Molecular and Cellular Basis to Clinical Features
Conventional and Advanced Therapies in Rheumatoid Arthritis: Considerations for the Elderly
4. Osteoporosis: A Pathophysiological and Immunological Perspective of Inflammaging
Molecular Targeted Therapy of Osteoporosis
5. Gender Considerations
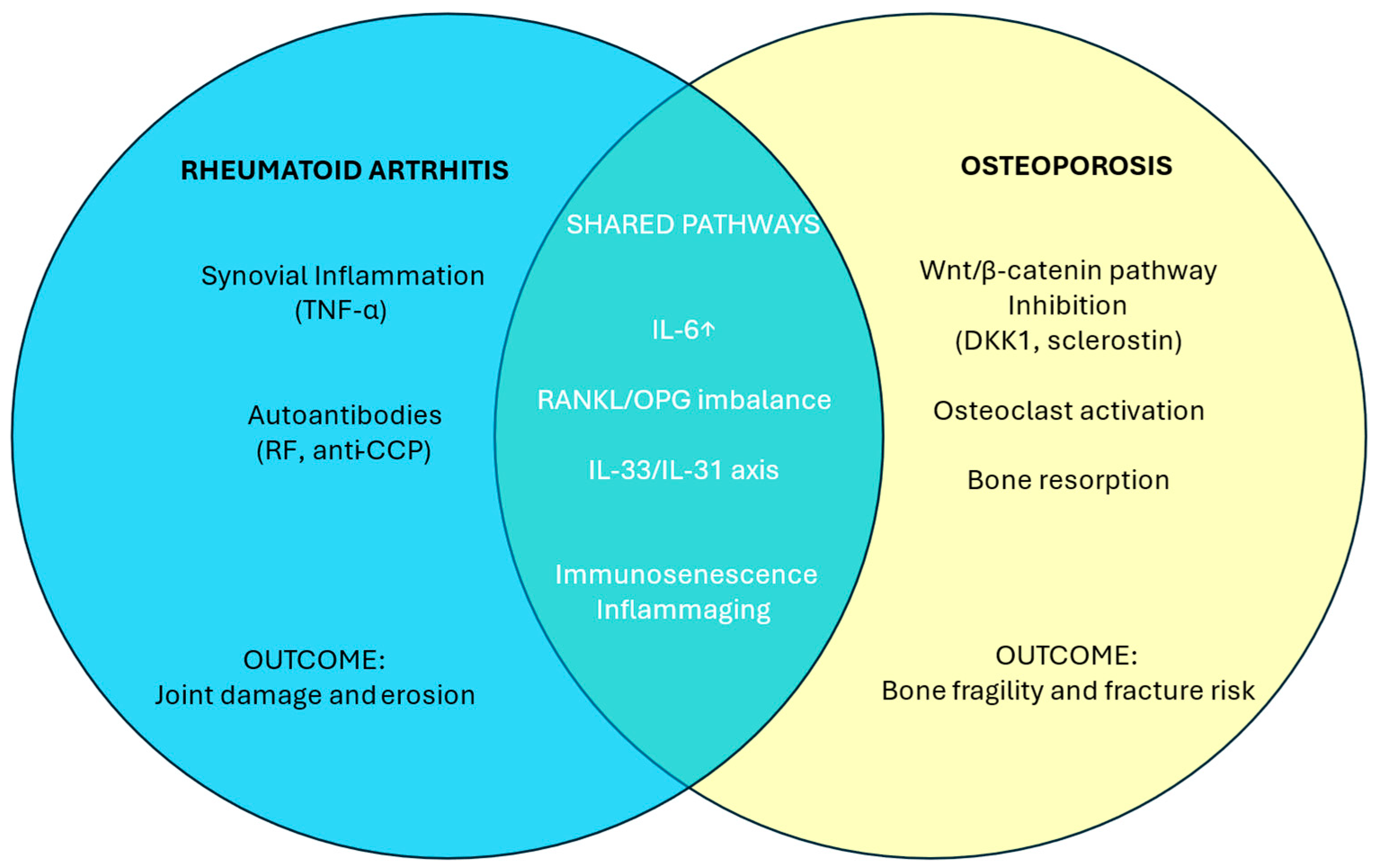
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, K.-A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Effros, R.B. Genetic Alterations in the Ageing Immune System: Impact on Infection and Cancer. Mech. Ageing Dev. 2003, 124, 71–77. [Google Scholar] [CrossRef]
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The Interplay between Immunosenescence and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, P.; Jiang, B.; Liu, K.; Zhang, L.; Wang, H.; Tian, Y.; Li, K.; Liu, G. Modulation of the Vitamin D/Vitamin D Receptor System in Osteoporosis Pathogenesis: Insights and Therapeutic Approaches. J. Orthop. Surg. Res. 2023, 18, 860. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Wu, C.-C.; Liao, M.-T.; Shyu, J.-F.; Hung, C.-F.; Yen, T.-H.; Lu, C.-L.; Lu, K.-C. Role of Nutritional Vitamin D in Osteoporosis Treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Komagamine, M.; Komatsu, N.; Ling, R.; Okamoto, K.; Tianshu, S.; Matsuda, K.; Takeuchi, T.; Kaneko, Y.; Takayanagi, H. Effect of JAK Inhibitors on the Three Forms of Bone Damage in Autoimmune Arthritis: Joint Erosion, Periarticular Osteopenia, and Systemic Bone Loss. Inflamm. Regen. 2023, 43, 44. [Google Scholar] [CrossRef]
- Straub, R.H.; Cutolo, M.; Zietz, B.; Schölmerich, J. The Process of Aging Changes the Interplay of the Immune, Endocrine and Nervous Systems. Mech. Ageing Dev. 2001, 122, 1591–1611. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular Mechanisms and Diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Gay, N.J.; Gangloff, M. Structure and Function of Toll Receptors and Their Ligands. Annu. Rev. Biochem. 2007, 76, 141–165. [Google Scholar] [CrossRef]
- Erceg, N.; Micic, M.; Forouzan, E.; Knezevic, N.N. The Role of Cortisol and Dehydroepiandrosterone in Obesity, Pain, and Aging. Diseases 2025, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Samaras, N.; Samaras, D.; Frangos, E.; Forster, A.; Philippe, J. A Review of Age-Related Dehydroepiandrosterone Decline and Its Association with Well-Known Geriatric Syndromes: Is Treatment Beneficial? Rejuvenation Res. 2013, 16, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Auchus, R.J.; El-Hajj Fuleihan, G.; Handelsman, D.J.; Kalyani, R.R.; McClung, M.; Stuenkel, C.A.; Thorner, M.O.; Verbalis, J.G. Hormones and Aging: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1835–1874. [Google Scholar] [CrossRef]
- Stamou, M.I.; Colling, C.; Dichtel, L.E. Adrenal Aging and Its Effects on the Stress Response and Immunosenescence. Maturitas 2023, 168, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, A.; Yalcinkaya, R.; Sardh, F.; Landegren, N. Immune Dynamics throughout Life in Relation to Sex Hormones and Perspectives Gained from Gender-Affirming Hormone Therapy. Front. Immunol. 2025, 15, 1501364. [Google Scholar] [CrossRef]
- Heffner, K.L. Neuroendocrine Effects of Stress on Immunity in the Elderly: Implications for Inflammatory Disease. Immunol. Allergy Clin. N. Am. 2011, 31, 95–108. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Di Silvestre, D.; Ginaldi, L. Sex and Gender Aspects for Patient Stratification in Allergy Prevention and Treatment. Int. J. Mol. Sci. 2020, 21, 1535. [Google Scholar] [CrossRef]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex Differences in Immune Responses: Hormonal Effects, Antagonistic Selection, and Evolutionary Consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems Analysis of Sex Differences Reveals an Immunosuppressive Role for Testosterone in the Response to Influenza Vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Zhai, Z.-M. IL-2 and Its Bidirectional Regulatory Effect on Immune Activation and Immune Tolerance. Chin. Pharmacol. Bull. 2013, 29, 319–322. [Google Scholar] [CrossRef]
- Takeuchi, T.; Yoshida, H.; Tanaka, S. Role of Interleukin-6 in Bone Destruction and Bone Repair in Rheumatoid Arthritis. Autoimmun. Rev. 2021, 20, 102884. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, Y.; Wang, K.; Zhu, L.; Dong, J.; Zhao, J.; Wang, Y.; Li, H.; Sun, X.; Lu, Y. Low Dose IL-2 Suppress Osteoclastogenesis in Collagen-induced Arthritis via JNK Dependent Pathway. Immun. Inflamm. Dis. 2020, 8, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, N.; Zhao, X.; Ding, T.; Xue, H.; Gao, C.; Li, X.; Wang, C. Low-Dose Interleukin-2: Biology and Therapeutic Prospects in Rheumatoid Arthritis. Autoimmun. Rev. 2020, 19, 102645. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Farrell, E.; Vis, M.; Colin, E.M.; Lubberts, E. Animal Models of Bone Loss in Inflammatory Arthritis: From Cytokines in the Bench to Novel Treatments for Bone Loss in the Bedside—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 27–47. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef]
- Piazza, J.R.; Dmitrieva, N.O.; Charles, S.T.; Almeida, D.M.; Orona, G.A. Diurnal Cortisol Profiles, Inflammation, and Functional Limitations in Aging: Findings from the MIDUS Study. Health Psychol. 2018, 37, 839–849. [Google Scholar] [CrossRef]
- Macfarlane, E.; Zhou, H.; Seibel, M.J. Endogenous Glucocorticoids during Skeletal Ageing. Explor. Endocr. Metab. Dis. 2024, 1, 191–212. [Google Scholar] [CrossRef]
- Wang, S.; Huo, T.; Lu, M.; Zhao, Y.; Zhang, J.; He, W.; Chen, H. Recent Advances in Aging and Immunosenescence: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; de Luca, M.; Ottaviani, E.; de Benedictis, G. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; El Aidy, S.; Hugenholtz, F.; van der Gaast–de Jongh, C.; Savelkoul, H.F.J.; De Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Ottaviani, E.; Franceschi, C. The Invertebrate Phagocytic Immunocyte: Clues to a Common Evolution of Immune and Neuroendocrine Systems. Immunol. Today 1997, 18, 169–174. [Google Scholar] [CrossRef]
- McGuire, P.J. Mitochondrial Dysfunction and the Aging Immune System. Biology 2019, 8, 26. [Google Scholar] [CrossRef]
- Geiger, H.; Denkinger, M.; Schirmbeck, R. Hematopoietic Stem Cell Aging. Curr. Opin. Immunol. 2014, 29, 86–92. [Google Scholar] [CrossRef]
- Pavlov-Dolijanovic, S.; Bogojevic, M.; Nozica-Radulovic, T.; Radunovic, G.; Mujovic, N. Elderly-Onset Rheumatoid Arthritis: Characteristics and Treatment Options. Medicina 2023, 59, 1878. [Google Scholar] [CrossRef]
- Wei, L.; Chen, X.; Liu, M. Global, Regional, and National Burden and Trends of Rheumatoid Arthritis among the Elderly Population: An Analysis Based on the 2021 Global Burden of Disease Study. Front. Immunol. 2025, 16, 1547763. [Google Scholar] [CrossRef]
- Yazici, Y.; Paget, S.A. ELDERLY-ONSET RHEUMATOID ARTHRITIS. Rheum. Dis. Clin. N. Am. 2000, 26, 517–526. [Google Scholar] [CrossRef] [PubMed]
- van Schaardenburg, D.; Breedveld, F.C. Elderly-Onset Rheumatoid Arthritis. Semin. Arthritis Rheum. 1994, 23, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.; de Gargiulo, M.L.Á.; Khoury, M.; Suárez, L.; de los Correa, M.Á.; Pera, M.; Saravia, N.; Gómez, G. Elderly-Onset Rheumatoid Arthritis Receives Less Aggressive Therapies than Young-Onset Rheumatoid Arthritis in an Argentinian Cohort. Reumatol. Clín. (Engl. Ed.) 2024, 20, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E. Accelerated Immunosenescence in Rheumatoid Arthritis: Impact on Clinical Progression. Immun. Ageing 2020, 17, 6. [Google Scholar] [CrossRef]
- Weyand, C.M.; Yang, Z.; Goronzy, J.J. T-Cell Aging in Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2014, 26, 93–100. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Hajeer, A.H.; Dababneh, A.; Makki, R.; Garcia-Porrua, C.; Thomson, W.; Ollier, W. Seronegative Rheumatoid Arthritis in Elderly and Polymyalgia Rheumatica Have Similar Patterns of HLA Association. J. Rheumatol. 2001, 28, 122–125. [Google Scholar]
- Kobak, S.; Bes, C. An Autumn Tale: Geriatric Rheumatoid Arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 3–11. [Google Scholar] [CrossRef]
- Schaeverbeke, T.; Fatout, E.; Marcé, S.; Vernhes, J.P.; Hallé, O.; Antoine, J.F.; Lequen, L.; Bannwarth, B.; Dehais, J. Remitting Seronegative Symmetrical Synovitis with Pitting Oedema: Disease or Syndrome? Ann. Rheum. Dis. 1995, 54, 681–684. [Google Scholar] [CrossRef]
- Fukushima, Y.; Ueno, R.; Minato, N.; Hattori, M. Senescence-Associated T Cells in Immunosenescence and Diseases. Int. Immunol. 2025, 37, 143–152. [Google Scholar] [CrossRef]
- Targońska-Stępniak, B.; Grzechnik, K.; Kolarz, K.; Gągoł, D.; Majdan, M. Systemic Inflammatory Parameters in Patients with Elderly-Onset Rheumatoid Arthritis (EORA) and Young-Onset Rheumatoid Arthritis (YORA)—An Observational Study. J. Clin. Med. 2021, 10, 1204. [Google Scholar] [CrossRef]
- Nawata, M.; Someya, K.; Kosaka, S.; Aritomi, T.; Funada, M.; Fujita, Y.; Nagayasu, A.; Fujino, Y.; Saito, K.; Tanaka, Y. Usefulness of Ultrasound as a Predictor of Elderly-Onset Rheumatoid Arthritis with Polymyalgia Rheumatica-like Onset. Mod. Rheumatol. 2023, 33, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Fulbright, J.W.; Goronzy, J.J. Immunosenescence, Autoimmunity, and Rheumatoid Arthritis. Exp. Gerontol. 2003, 38, 833–841. [Google Scholar] [CrossRef]
- Tekeoglu, S. Comprehensive Analysis of Rheumatic Diseases, Comorbidities, and Mortality in Geriatric Population: Real-World Data of 515 Patients in a Single Rheumatology Clinic. Medicine 2024, 103, e40753. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, C.; Tatar, Z.; Dubost, J.-J.; Tournadre, A.; Soubrier, M. Management of Inflammatory Rheumatic Conditions in the Elderly. Rheumatology 2019, 58, 748–764. [Google Scholar] [CrossRef]
- Jones, C.A.; Guy, P.; Xie, H.; Sayre, E.C.; Zhao, K.; Lacaille, D. Incidence of and Risk of Mortality After Hip Fractures in Rheumatoid Arthritis Relative to the General Population. Arthritis Care Res. 2025, 77, 604–613. [Google Scholar] [CrossRef]
- Avalos-Salgado, F.A.; Gonzalez-Lopez, L.; Gonzalez-Vazquez, S.; Ponce-Guarneros, J.M.; Santiago-Garcia, A.P.; Amaya-Cabrera, E.L.; Arellano-Cervantes, R.; Gutiérrez-Aceves, J.A.; Alcaraz-Lopez, M.F.; Nava-Valdivia, C.A.; et al. Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 1863. [Google Scholar] [CrossRef]
- Drosos, A.A. Methotrexate Intolerance in Elderly Patients with Rheumatoid Arthritis. Drugs Aging 2003, 20, 723–736. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Tian, Y.; Gu, H.; Meng, Q.; Cui, J.; Ma, J. The Research Progress of Biologics in Elderly-Onset Rheumatoid Arthritis (EORA). Front. Aging 2025, 5, 1511812. [Google Scholar] [CrossRef]
- Harigai, M.; Fujii, T.; Sakai, R.; Igarashi, A.; Shoji, A.; Yamaguchi, H.; Iwasaki, K.; Makishima, M.; Yoshida, A.; Okada, N.; et al. Risk of Hospitalized Infections in Older Elderly Patients with Rheumatoid Arthritis Treated with Tocilizumab or Other Biological/Targeted Synthetic Disease-Modifying Antirheumatic Drugs: Evaluation of Data from a Japanese Claims Database. Mod. Rheumatol. 2024, 34, 287–296, Erratum in Mod. Rheumatol. 2024, 34, 437. [Google Scholar] [CrossRef]
- Mielnik, P.; Sexton, J.; Lie, E.; Bakland, G.; Loli, L.P.; Kristianslund, E.K.; Rødevand, E.; Lexberg, Å.S.; Kvien, T.K. Does Older Age Have an Impact on Rituximab Efficacy and Safety? Results from the NOR-DMARD Register. Drugs Aging 2020, 37, 617–626. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Saag, K.; Cascino, M.D.; Pei, J.; John, A.; Jahreis, A.; Haselkorn, T.; Furst, D.E. Long-Term Safety of Rituximab in Patients With Rheumatoid Arthritis: Results of a Five-Year Observational Study. Arthritis Care Res. 2019, 71, 993–1003. [Google Scholar] [CrossRef]
- Lahaye, C.; Soubrier, M.; Mulliez, A.; Bardin, T.; Cantagrel, A.; Combe, B.; Dougados, M.; Flipo, R.-M.; Le Loët, X.; Shaeverbeke, T.; et al. Effectiveness and Safety of Abatacept in Elderly Patients with Rheumatoid Arthritis Enrolled in the French Society of Rheumatology’s ORA Registry. Rheumatology 2016, 55, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Xie, F.; Delzell, E.; Levitan, E.B.; Chen, L.; Lewis, J.D.; Saag, K.G.; Beukelman, T.; Winthrop, K.L.; Baddley, J.W.; et al. Comparative Risk of Hospitalized Infection Associated With Biologic Agents in Rheumatoid Arthritis Patients Enrolled in Medicare. Arthritis Rheumatol. 2016, 68, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L. The Emerging Safety Profile of JAK Inhibitors in Rheumatic Disease. Nat. Rev. Rheumatol. 2017, 13, 234–243. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Serhal, L.; Lwin, M.N.; Holroyd, C.; Edwards, C.J. Rheumatoid Arthritis in the Elderly: Characteristics and Treatment Considerations. Autoimmun. Rev. 2020, 19, 102528. [Google Scholar] [CrossRef]
- Kim, S.C.; Glynn, R.J.; Giovannucci, E.; Hernández-Díaz, S.; Liu, J.; Feldman, S.; Karlson, E.W.; Schneeweiss, S.; Solomon, D.H. Risk of High-Grade Cervical Dysplasia and Cervical Cancer in Women with Systemic Inflammatory Diseases: A Population-Based Cohort Study. Ann. Rheum. Dis. 2015, 74, 1360–1367. [Google Scholar] [CrossRef]
- Wadström, H.; Frisell, T.; Askling, J. FRI0061 Cervical Dysplasia and Cervical Cancer in Women with Rheumatoid Arthritis. Ann. Rheum. Dis. 2015, 74, 441. [Google Scholar] [CrossRef]
- Rojo Contreras, W.; Montoya Fuentes, H.; Gámez Nava, J.I.; Suárez Rincón, A.E.; Vázquez Salcedo, J.; Padilla Rosas, M.; Baltazar Rodríguez, L.M.; Trujillo, X.; Ramírez Flores, M.; Trujillo Hernández, B.; et al. Prevalence and Cervical Human Papilloma Virus Associated Factors in Patients with Rheumatoid Arthritis. Ginecol. Obstet. Mex. 2008, 76, 9–17. [Google Scholar]
- Kim, S.C.; Schneeweiss, S.; Liu, J.; Karlson, E.W.; Katz, J.N.; Feldman, S.; Solomon, D.H. Biologic Disease-Modifying Antirheumatic Drugs and Risk of High-Grade Cervical Dysplasia and Cervical Cancer in Rheumatoid Arthritis: A Cohort Study. Arthritis Rheumatol. 2016, 68, 2106–2113. [Google Scholar] [CrossRef]
- Wadström, H.; Frisell, T.; Askling, J. Malignant Neoplasms in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitors, Tocilizumab, Abatacept, or Rituximab in Clinical Practice. JAMA Intern. Med. 2017, 177, 1605. [Google Scholar] [CrossRef]
- D’Arcy, M.E.; Beachler, D.C.; Pfeiffer, R.M.; Curtis, J.R.; Mariette, X.; Seror, R.; Mahale, P.; Rivera, D.R.; Yanik, E.L.; Engels, E.A. Tumor Necrosis Factor Inhibitors and the Risk of Cancer among Older Americans with Rheumatoid Arthritis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2059–2067. [Google Scholar] [CrossRef]
- Schieferdecker, A.; Voigt, M.; Riecken, K.; Braig, F.; Schinke, T.; Loges, S.; Bokemeyer, C.; Fehse, B.; Binder, M. Denosumab Mimics the Natural Decoy Receptor Osteoprotegerin by Interacting with Its Major Binding Site on RANKL. Oncotarget 2014, 5, 6647–6653. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Choi, Y. Regulation of T Cell-Associated Tissues and T Cell Activation by RANKL-RANK-OPG. J. Bone Miner. Metab. 2021, 39, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.K.; Hao, B.B.; McCarthy, L.; Guilcher, S.J.T.; Cadarette, S.M. Denosumab Utilization among Older Adults in Ontario: Patient Characteristics, Persistence with Therapy, and Return to Therapy after an Extended Gap. Osteoporos. Int. 2019, 30, 1865–1872. [Google Scholar] [CrossRef]
- Clemens, K.K.; Jeyakumar, N.; Ouédraogo, A.M.; Thain, J.; Khan, T. Bisphosphonate and Denosumab Initiation in Older Adults in Ontario, Canada: A Population-Based Cohort Study. Arch. Osteoporos. 2020, 15, 133. [Google Scholar] [CrossRef]
- Watts, N.B.; Roux, C.; Modlin, J.F.; Brown, J.P.; Daniels, A.; Jackson, S.; Smith, S.; Zack, D.J.; Zhou, L.; Grauer, A.; et al. Infections in Postmenopausal Women with Osteoporosis Treated with Denosumab or Placebo: Coincidence or Causal Association? Osteoporos. Int. 2012, 23, 327–337. [Google Scholar] [CrossRef]
- Ha, J.; Lee, Y.-J.; Kim, J.; Jeong, C.; Lim, Y.; Lee, J.; Baek, K.-H. Long-Term Efficacy and Safety of Denosumab: Insights beyond 10 Years of Use. Endocrinol. Metab. 2025, 40, 47–56. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ando, K.; Machino, M.; Morozumi, M.; Kanbara, S.; Ito, S.; Inoue, T.; Yamaguchi, H.; Ishiguro, N.; Imagama, S. Persistence of Denosumab Therapy among Patients with Osteoporosis. Asian Spine J. 2020, 14, 453–458. [Google Scholar] [CrossRef]
- Silverman, S.L.; Siris, E.; Belazi, D.; Recknor, C.; Papaioannou, A.; Brown, J.P.; Gold, D.T.; Lewiecki, E.M.; Quinn, G.; Balasubramanian, A.; et al. Persistence at 24 Months with Denosumab among Postmenopausal Women with Osteoporosis: Results of a Prospective Cohort Study. Arch. Osteoporos. 2018, 13, 85. [Google Scholar] [CrossRef]
- Iwamoto, N.; Sato, S.; Sumiyoshi, R.; Chiba, K.; Miyamoto, N.; Arinaga, K.; Kobayashi, M.; Yamamoto, H.; Osaki, M.; Kawakami, A. Comparative Study of the Inhibitory Effect on Bone Erosion Progression with Denosumab Treatment and Conventional Treatment in Rheumatoid Arthritis Patients: Study Protocol for an Open-Label Randomized Controlled Trial by HR-PQCT. Trials 2019, 20, 494. [Google Scholar] [CrossRef]
- Yee, A.J.; Raje, N.S. Denosumab for The Treatment of Bone Disease in Solid Tumors and Multiple Myeloma. Future Oncol. 2018, 14, 195–203. [Google Scholar] [CrossRef]
- Irelli, A.; Sirufo, M.; Scipioni, T.; Pietro, F.; Pancotti, A.; Ginaldi, L.; De Martinis, M. Denosumab in Breast Cancer Patients Receiving Aromatase Inhibitors: A Single-Center Observational Study of Effectiveness in Adjuvant Setting. Indian J. Cancer 2021, 58, 136. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Cipriani, C.; Palermo, A.; Viapiana, O.; Zavatta, G.; Mazziotti, G. A Practical Approach for Anabolic Treatment of Bone Fragility with Romosozumab. J. Endocrinol. Investig. 2024, 47, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef]
- Gupta, N.; Kanwar, N.; Arora, A.; Khatri, K.; Kanwal, A. The Interplay of Rheumatoid Arthritis and Osteoporosis: Exploring the Pathogenesis and Pharmacological Approaches. Clin. Rheumatol. 2024, 43, 1421–1433. [Google Scholar] [CrossRef]
- Zheng, M.; Wan, Y.; Liu, G.; Gao, Y.; Pan, X.; You, W.; Yuan, D.; Shen, J.; Lu, J.; Wang, X.; et al. Differences in the Prevalence and Risk Factors of Osteoporosis in Chinese Urban and Rural Regions: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2023, 24, 46. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Polsinelli, M.; Placidi, G.; Di Silvestre, D.; Ginaldi, L. Gender Differences in Osteoporosis: A Single-Center Observational Study. World J. Mens. Health 2021, 39, 750. [Google Scholar] [CrossRef]
- Blake, J.; Cosman, F.A.; Lewiecki, E.M.; McClung, M.R.; Pinkerton, J.; Shapiro, M. Management of Osteoporosis in Postmenopausal Women: The 2021 Position Statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Newey, P.J.; Hannan, F.M.; Wilson, A.; Thakker, R.V. Genetics of Monogenic Disorders of Calcium and Bone Metabolism. Clin. Endocrinol. 2022, 97, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.; Kumar, P.; Rai, V. Vitamin D Receptor (VDR) Gene FokI, BsmI, ApaI, and TaqI Polymorphisms and Osteoporosis Risk: A Meta-Analysis. Egypt. J. Med. Hum. Genet. 2020, 21, 15. [Google Scholar] [CrossRef]
- Colombini, A.; Brayda-Bruno, M.; Lombardi, G.; Croiset, S.J.; Vrech, V.; Maione, V.; Banfi, G.; Cauci, S. FokI Polymorphism in the Vitamin D Receptor Gene (VDR) and Its Association with Lumbar Spine Pathologies in the Italian Population: A Case-Control Study. PLoS ONE 2014, 9, e97027. [Google Scholar] [CrossRef]
- Man, S. Association of COL1A1 SP1 and FOK-I VDR Genetic Polymorphisms in Young Male Idiopathic Osteoporosis. Acta Endocrinol. 2017, 13, 224–227. [Google Scholar] [CrossRef]
- Kinga, S.; Marciniak, M.D.; Michalak, M.; Zawada, A.; Ratajczak-Pawłowska, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. The Other Side of Celiac Disease—Assessment of Bone Mineral Density and Body Composition in Patients with Celiac Disease. Gastroenterol. Rev. 2024, 19, 434–438. [Google Scholar] [CrossRef]
- Aitella, E.; Cozzolino, D.; Ginaldi, L.; Romano, C. Celiac Disease: A Transitional Point of View. Nutrients 2025, 17, 234. [Google Scholar] [CrossRef]
- Degboé, Y.; Nezzar, C.; Alary, P.; Maëva, M.; Bulai Livideanu, C.; Laroche, M. Management of Bone Health in Adult Mastocytosis. Curr. Osteoporos. Rep. 2025, 23, 10. [Google Scholar] [CrossRef]
- Olstad, O.K.; Gautvik, V.T.; LeBlanc, M.; Kvernevik, K.J.; Utheim, T.P.; Runningen, A.; Wiig, H.; Kirkegaard, C.; Raastad, T.; Reppe, S.; et al. Postmenopausal Osteoporosis Is a Musculoskeletal Disease with a Common Genetic Trait Which Responds to Strength Training: A Translational Intervention Study. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20929443. [Google Scholar] [CrossRef]
- Smit, A.E.; Meijer, O.C.; Winter, E.M. The Multi-Faceted Nature of Age-Associated Osteoporosis. Bone Rep. 2024, 20, 101750. [Google Scholar] [CrossRef]
- Liang, X.; Shi, W.; Zhang, X.; Pang, R.; Zhang, K.; Xu, Q.; Xu, C.; Wan, X.; Cui, W.; Li, D.; et al. Causal Association of Epigenetic Aging and Osteoporosis: A Bidirectional Mendelian Randomization Study. BMC Med. Genom. 2023, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, T.-S.; Choi, Y.-W.; Lorenzo, J. Osteoimmunology: Cytokines and the Skeletal System. BMB Rep. 2008, 41, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Mangion, D.; Pace, N.P.; Formosa, M.M. The Relationship between Adipokine Levels and Bone Mass—A Systematic Review. Endocrinol. Diabetes Metab. 2023, 6, e408. [Google Scholar] [CrossRef] [PubMed]
- Deepika, F.; Bathina, S.; Armamento-Villareal, R. Novel Adipokines and Their Role in Bone Metabolism: A Narrative Review. Biomedicines 2023, 11, 644. [Google Scholar] [CrossRef]
- Ginaldi, L.; De Martinis, M. Osteoimmunology and Beyond. Curr. Med. Chem. 2016, 23, 3754–3774. [Google Scholar] [CrossRef]
- Chiarito, M.; Piacente, L.; Chaoul, N.; Pontrelli, P.; D’Amato, G.; Grandone, A.; Russo, G.; Street, M.E.; Wasniewska, M.G.; Brunetti, G.; et al. Role of Wnt-Signaling Inhibitors DKK-1 and Sclerostin in Bone Fragility Associated with Turner Syndrome. J. Endocrinol. Investig. 2022, 45, 1255–1263. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL Biology: Bone Metabolism, the Immune System, and Beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica 2013, 2013, 125705. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Wang, Y. Wnt and the Wnt Signaling Pathway in Bone Development and Disease. Front. Biosci. 2014, 19, 379. [Google Scholar] [CrossRef]
- Manolagas, S.C. Wnt Signaling and Osteoporosis. Maturitas 2014, 78, 233–237. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, N.; Fu, Z.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Pioggia, G.; Calapai, G.; Gangemi, S.; Mannucci, C. Alarmins in Osteoporosis, RAGE, IL-1, and IL-33 Pathways: A Literature Review. Medicina 2020, 56, 138. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; De Martinis, M.; Ciccarelli, F.; Saitta, S.; Imbesi, S.; Mannucci, C.; Gangemi, S. Increased Levels of Interleukin 31 (IL-31) in Osteoporosis. BMC Immunol. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; De Pietro, F.; Bassino, E.M.; Ginaldi, L.; De Martinis, M. Osteoporosis in Skin Diseases. Int. J. Mol. Sci. 2020, 21, 4749. [Google Scholar] [CrossRef]
- Aitella, E.; De Martinis, M.; Romano, C.; Azzellino, G.; Ginaldi, L. Neurogenic Inflammation in Allergic Contact Dermatitis. Biomedicines 2025, 13, 656. [Google Scholar] [CrossRef]
- Valdes-Rodriguez, R.; Stull, C.; Yosipovitch, G. Chronic Pruritus in the Elderly: Pathophysiology, Diagnosis and Management. Drugs Aging 2015, 32, 201–215. [Google Scholar] [CrossRef]
- Ginaldi, L.; De Martinis, M.; Saitta, S.; Sirufo, M.M.; Mannucci, C.; Casciaro, M.; Ciccarelli, F.; Gangemi, S. Interleukin-33 Serum Levels in Postmenopausal Women with Osteoporosis. Sci. Rep. 2019, 9, 3786. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef]
- Mannucci, C.; Calapai, G.; Gangemi, S. Commentary: Circulatory Pattern of Cytokines, Adipokines and Bone Markers in Postmenopausal Women with Low BMD. Front. Immunol. 2019, 10, 2666. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The Effect of Cytokines on Osteoblasts and Osteoclasts in Bone Remodeling in Osteoporosis: A Review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, T.J.; Keen, J.A.; Wells, L.M.; Roberts, S.J. Novel Insights on the Effect of Sclerostin on Bone and Other Organs. J. Endocrinol. 2023, 257, e220209. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Thiele, S.; Baschant, U.; Rachner, T.D.; Niehrs, C.; Hofbauer, L.C.; Rauner, M. Mice Lacking DKK1 in T Cells Exhibit High Bone Mass and Are Protected from Estrogen-Deficiency-Induced Bone Loss. iScience 2021, 24, 102224. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Dong, Z.; Hui, Z.; Aifei, W.; Lianfu, D.; Youjia, X. Bone Sclerostin and Dickkopf-Related Protein-1 Are Positively Correlated with Bone Mineral Density, Bone Microarchitecture, and Bone Strength in Postmenopausal Osteoporosis. BMC Musculoskelet. Disord. 2021, 22, 480. [Google Scholar] [CrossRef]
- Umur, E.; Bulut, S.B.; Yiğit, P.; Bayrak, E.; Arkan, Y.; Arslan, F.; Baysoy, E.; Kaleli-Can, G.; Ayan, B. Exploring the Role of Hormones and Cytokines in Osteoporosis Development. Biomedicines 2024, 12, 1830. [Google Scholar] [CrossRef]
- Shashidhara, A.; Tahir, S.H.; Syed, Z.A.; Lee, J.; Tahir, H. An Update on the Pharmacotherapy of Osteoporosis. Expert. Opin. Pharmacother. 2025, 26, 821–833. [Google Scholar] [CrossRef]
- Morin, S.N.; Leslie, W.D.; Schousboe, J.T. Osteoporosis. JAMA 2025, 334, 894. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Krautmann, M.; Walters, R.R.; King, V.L.; Esch, K.; Mahabir, S.P.; Gonzales, A.; Dominowski, P.J.; Sly, L.; Mwangi, D.; Foss, D.L.; et al. Laboratory Safety Evaluation of Lokivetmab, a Canine Anti-Interleukin-31 Monoclonal Antibody, in Dogs. Vet. Immunol. Immunopathol. 2023, 258, 110574. [Google Scholar] [CrossRef]
- Keam, S.J. Nemolizumab: First Approval. Drugs 2022, 82, 1143–1150. [Google Scholar] [CrossRef]
- England, E.; Rees, D.G.; Scott, I.C.; Carmen, S.; Chan, D.T.Y.; Chaillan Huntington, C.E.; Houslay, K.F.; Erngren, T.; Penney, M.; Majithiya, J.B.; et al. Tozorakimab (MEDI3506): An Anti-IL-33 Antibody That Inhibits IL-33 Signalling via ST2 and RAGE/EGFR to Reduce Inflammation and Epithelial Dysfunction. Sci. Rep. 2023, 13, 9825. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Guller, P.; Reid, F.; Doffman, S.; Seppälä, U.; Psallidas, I.; Moate, R.; Smith, R.; Kiraga, J.; Jimenez, E.; et al. A Phase 2a Trial of the IL-33 Monoclonal Antibody Tozorakimab in Patients with COPD: FRONTIER-4. Eur. Respir. J. 2025, 66, 2402231. [Google Scholar] [CrossRef] [PubMed]
- Zimba, O.; Baimukhamedov, C.; Kocyigit, B.F. Late-Onset Rheumatoid Arthritis: Clinical Features, Diagnostic Challenges, and Treatment Approaches. Rheumatol. Int. 2025, 45, 152. [Google Scholar] [CrossRef] [PubMed]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility Fractures in Europe: Burden, Management and Opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The Impact of Fragility Fracture and Approaches to Osteoporosis Risk Assessment Worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef]
- Canbeyli, I.D.; Cirpar, M.; Oktas, B.; Coban, M. Analysis of Factors among 30-Day and 1-Year Mortality Rates in Patients with Borderline Stable-Unstable Intertrochanteric Hip Fracture. Acta Orthop. Traumatol. Turc. 2021, 55, 16–21. [Google Scholar] [CrossRef]
- Morri, M.; Ambrosi, E.; Chiari, P.; Orlandi Magli, A.; Gazineo, D.; D’ Alessandro, F.; Forni, C. One-Year Mortality after Hip Fracture Surgery and Prognostic Factors: A Prospective Cohort Study. Sci. Rep. 2019, 9, 18718. [Google Scholar] [CrossRef]
- Blanco, J.F.; da Casa, C.; Pablos-Hernández, C.; González-Ramírez, A.; Julián-Enríquez, J.M.; Díaz-Álvarez, A. 30-Day Mortality after Hip Fracture Surgery: Influence of Postoperative Factors. PLoS ONE 2021, 16, e0246963. [Google Scholar] [CrossRef]
- Bandeira, L.; Silva, B.C.; Bilezikian, J.P. Male Osteoporosis. Arch. Endocrinol. Metab. 2022, 66, 739–747. [Google Scholar] [CrossRef]
- Misiorowski, W. Osteoporosis in Men. Menopause Rev. 2017, 2, 70–73. [Google Scholar] [CrossRef]
- Corona, G.; Vena, W.; Pizzocaro, A.; Giagulli, V.A.; Francomano, D.; Rastrelli, G.; Mazziotti, G.; Aversa, A.; Isidori, A.M.; Pivonello, R.; et al. Testosterone Supplementation and Bone Parameters: A Systematic Review and Meta-Analysis Study. J. Endocrinol. Investig. 2022, 45, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Balercia, G.; Calogero, A.E.; Corona, G.; Ferlin, A.; Francavilla, S.; Santi, D.; Maggi, M. Outcomes of Androgen Replacement Therapy in Adult Male Hypogonadism: Recommendations from the Italian Society of Endocrinology. J. Endocrinol. Investig. 2015, 38, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Honda, T.; Ishizu, Y.; Imai, N.; Ito, T.; Yamamoto, K.; Kojima, T.; Kariya, N.; Nakamura, M.; Kawashima, H. Impact of Alcohol Consumption and Muscle Mass on Bone Mineral Density in Metabolic Dysfunction and Alcohol-Associated Liver Disease (MetALD) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Clin. Nutr. ESPEN 2025, 69, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Masoni, B.; De Deo, D.; Ferraris, M.; Franchellucci, G.; Hassan, C.; Repici, A. Gender-Related Differences in Celiac Disease Presentation and Follow-up in Adult Patients. Expert Rev. Gastroenterol. Hepatol. 2025, 19, 871–876. [Google Scholar] [CrossRef]
- Jørgensen, M.P.; Øvlisen, A.K.; Jensen, J.F.; El-Galaly, T.C.; Dalager, M.G.; Vestergaard, H.; Broesby-Olsen, S.; Severinsen, M.T. Prevalence and Incidence of Mastocytosis in Adults: A Danish Nationwide Register Study. Eur. J. Epidemiol. 2025, 40, 43–53. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, Inflammation and Ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef]
- Lau, A.N.; Wong-Pack, M.; Rodjanapiches, R.; Ioannidis, G.; Wade, S.; Spangler, L.; Balasubramanian, A.; Pannacciulli, N.; Lin, C.J.F.; Roy-Gayos, P.; et al. Occurrence of Serious Infection in Patients with Rheumatoid Arthritis Treated with Biologics and Denosumab Observed in a Clinical Setting. J. Rheumatol. 2018, 45, 170–176. [Google Scholar] [CrossRef]
- Hirshberg, B. Safety of Low Dose Methotrexate in Elderly Patients with Rheumatoid Arthritis. Postgrad. Med. J. 2000, 76, 787–789. [Google Scholar] [CrossRef][Green Version]
- Fitzcharles, M.-A.; Lussier, D.; Shir, Y. Management of Chronic Arthritis Pain in the Elderly. Drugs Aging 2010, 27, 471–490. [Google Scholar] [CrossRef]
- Yi, S.-J.; Lim, J.; Kim, K. Exploring Epigenetic Strategies for the Treatment of Osteoporosis. Mol. Biol. Rep. 2024, 51, 398, Erratum in Mol. Biol. Rep. 2024, 51, 664. [Google Scholar] [CrossRef]

| Drug | Molecular Target | Immunosenescence Management |
|---|---|---|
| Etanercept | TNF-α (Inflammatory cytokine) | Shorter half-life [66]. |
| Infliximab | TNF-α (Inflammatory cytokine) | Increased risk of cervical cancer (screening) [65,67,68,69,70]. |
| Other TNFi | TNF-α (Inflammatory cytokine) | Increased risk of non-melanoma skin cancer and non-Hodgkin lymphoma in older adults (screening) [71,72]. |
| Tocilizumab | IL-6 (Inflammatory cytokine) | Higher risk of infection [59,71]. |
| Rituximab | CD 20 (B cell surface protein) | Higher risk of infection [60,61]. |
| Abatacept | CD80/CD86 (CTLA-4 immunomodulatory pathway) | Favorable safety and tolerability profile; shorter half-life; increased risk of cutaneous squamous cell carcinoma (screening) [62,71]. |
| Baricitinib Tofacitinib Fingotinib | JAK (JAK/STAT pathway; inflammatory signals transduction) | Considers cardiovascular risk factors, Herpes Zoster infection, and dose adjustments [64,65]. |
| Denosumab | RANKL (Proinflammatory and osteoclastogenic cytokine) | Antiresorptive treatment in OP; reduction of bone erosions progression in RA and tumor metastasis risk; considers infectious risk factors, hypocalcemia, and renal function [73,74,75,76,77,78,79,80,81,82,83,84]. |
| Romosozumab | Sclerostin (Bone formation inhibiting protein) | Bone builder; considers cardiovascular risk factors [85,86,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitella, E.; Azzellino, G.; Romano, C.; Ginaldi, L.; De Martinis, M. Rheumatoid Arthritis and Osteoporosis as Prototypes of Immunosenescence in Osteoimmunology: Molecular Pathways of Inflammaging and Targeted Therapies. Int. J. Mol. Sci. 2025, 26, 9268. https://doi.org/10.3390/ijms26199268
Aitella E, Azzellino G, Romano C, Ginaldi L, De Martinis M. Rheumatoid Arthritis and Osteoporosis as Prototypes of Immunosenescence in Osteoimmunology: Molecular Pathways of Inflammaging and Targeted Therapies. International Journal of Molecular Sciences. 2025; 26(19):9268. https://doi.org/10.3390/ijms26199268
Chicago/Turabian StyleAitella, Ernesto, Gianluca Azzellino, Ciro Romano, Lia Ginaldi, and Massimo De Martinis. 2025. "Rheumatoid Arthritis and Osteoporosis as Prototypes of Immunosenescence in Osteoimmunology: Molecular Pathways of Inflammaging and Targeted Therapies" International Journal of Molecular Sciences 26, no. 19: 9268. https://doi.org/10.3390/ijms26199268
APA StyleAitella, E., Azzellino, G., Romano, C., Ginaldi, L., & De Martinis, M. (2025). Rheumatoid Arthritis and Osteoporosis as Prototypes of Immunosenescence in Osteoimmunology: Molecular Pathways of Inflammaging and Targeted Therapies. International Journal of Molecular Sciences, 26(19), 9268. https://doi.org/10.3390/ijms26199268









