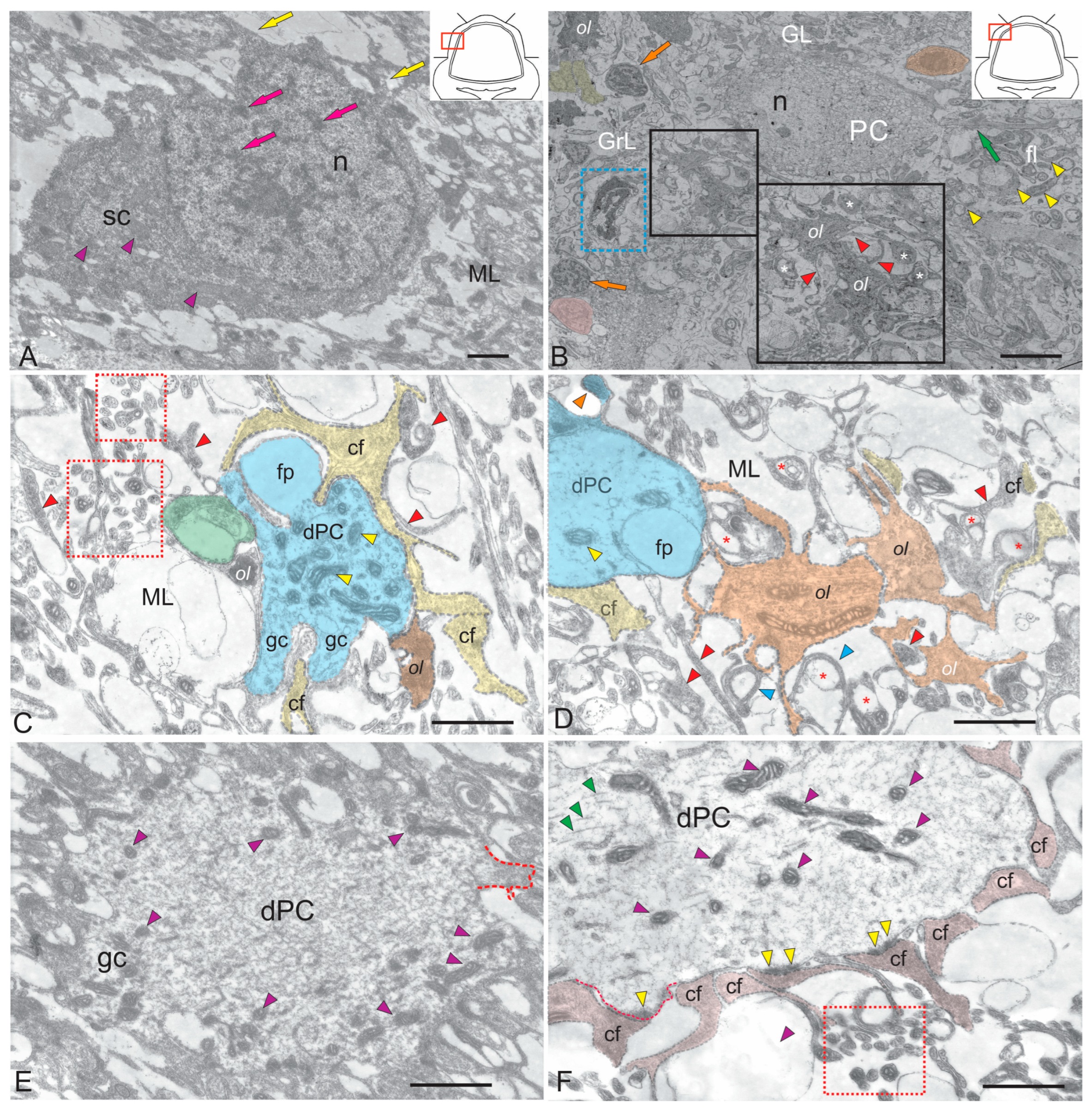
Figure 1.
Ultrastructural organization of cells in the dorsal region of the molecular layer of the cerebellar body of juvenile chum salmon Oncorhynchus keta. (A) Spherical microneuron of the stellate type (SC) with short dendrites (yellow arrows); n—nucleus, pink arrows indicate blocks of heterochromatin, mitochondria are indicated by purple triangular arrows. (B) General view of the dorsal region, including the molecular layer (ML), containing parallel fibers (green arrow), fibrous layer fibers (fl, indicated by yellow triangular arrows); pear-shaped PCs are indicated by the arrow in the GL; n—nucleus; oligodendrocytes (ol) are shown in the black rectangle and inset; the processes are indicated by red triangular arrows; a white asterisk indicates forming myelin fibers, the blue dotted rectangle shows the ends of cf; in the granule cells (GrCs), the orange arrows indicate type IV glial precursors; the oligodendrocyte in a state of mitosis is highlighted in yellow; granule cells are indicated by pink arrow. (C) Growth cones (gc) and filopodia (fp) on PC dendrites (dPC, shown in blue) climbing fibers (cf) adjacent to dPC indicated by yellow arrows, oligodendrocyte (ol) is shown in orange, mitochondria are indicated by yellow triangular arrows; cf terminal branches are shown in red dotted rectangles; cf endings indicated by red triangular arrows; myelin fiber is shown in green. (D) Cluster of oligodendrocytes (shown in orange) forming myelin sheaths (triangular blue arrows) on the cf surface (indicated by red asterisks); filopodia is indicated by orange triangular arrows; mitochondria are indicated by yellow triangular arrows; cf endings are indicated by red triangular arrows. (E) The dendritic bouquets of PCs covered with spikes (highlighted by red dotted line), mitochondria are indicated by purple triangular arrows, the remaining designations are as in (C). (F) Terminations (shown in pink) on the dPC forming asymmetric excitatory type synapses are indicated by yellow triangular arrows; mitochondria—purple triangular arrows, neurofilaments—green triangular arrows, a spike on the surface of the dPC is highlighted with a red dotted line, cf terminal branches are shown in the red dotted rectangle. Scale: (A) 2 µm; (B) 10 µm; (C–F) 1 µm.
Figure 1.
Ultrastructural organization of cells in the dorsal region of the molecular layer of the cerebellar body of juvenile chum salmon Oncorhynchus keta. (A) Spherical microneuron of the stellate type (SC) with short dendrites (yellow arrows); n—nucleus, pink arrows indicate blocks of heterochromatin, mitochondria are indicated by purple triangular arrows. (B) General view of the dorsal region, including the molecular layer (ML), containing parallel fibers (green arrow), fibrous layer fibers (fl, indicated by yellow triangular arrows); pear-shaped PCs are indicated by the arrow in the GL; n—nucleus; oligodendrocytes (ol) are shown in the black rectangle and inset; the processes are indicated by red triangular arrows; a white asterisk indicates forming myelin fibers, the blue dotted rectangle shows the ends of cf; in the granule cells (GrCs), the orange arrows indicate type IV glial precursors; the oligodendrocyte in a state of mitosis is highlighted in yellow; granule cells are indicated by pink arrow. (C) Growth cones (gc) and filopodia (fp) on PC dendrites (dPC, shown in blue) climbing fibers (cf) adjacent to dPC indicated by yellow arrows, oligodendrocyte (ol) is shown in orange, mitochondria are indicated by yellow triangular arrows; cf terminal branches are shown in red dotted rectangles; cf endings indicated by red triangular arrows; myelin fiber is shown in green. (D) Cluster of oligodendrocytes (shown in orange) forming myelin sheaths (triangular blue arrows) on the cf surface (indicated by red asterisks); filopodia is indicated by orange triangular arrows; mitochondria are indicated by yellow triangular arrows; cf endings are indicated by red triangular arrows. (E) The dendritic bouquets of PCs covered with spikes (highlighted by red dotted line), mitochondria are indicated by purple triangular arrows, the remaining designations are as in (C). (F) Terminations (shown in pink) on the dPC forming asymmetric excitatory type synapses are indicated by yellow triangular arrows; mitochondria—purple triangular arrows, neurofilaments—green triangular arrows, a spike on the surface of the dPC is highlighted with a red dotted line, cf terminal branches are shown in the red dotted rectangle. Scale: (A) 2 µm; (B) 10 µm; (C–F) 1 µm.
![Ijms 26 09267 g001 Ijms 26 09267 g001]()
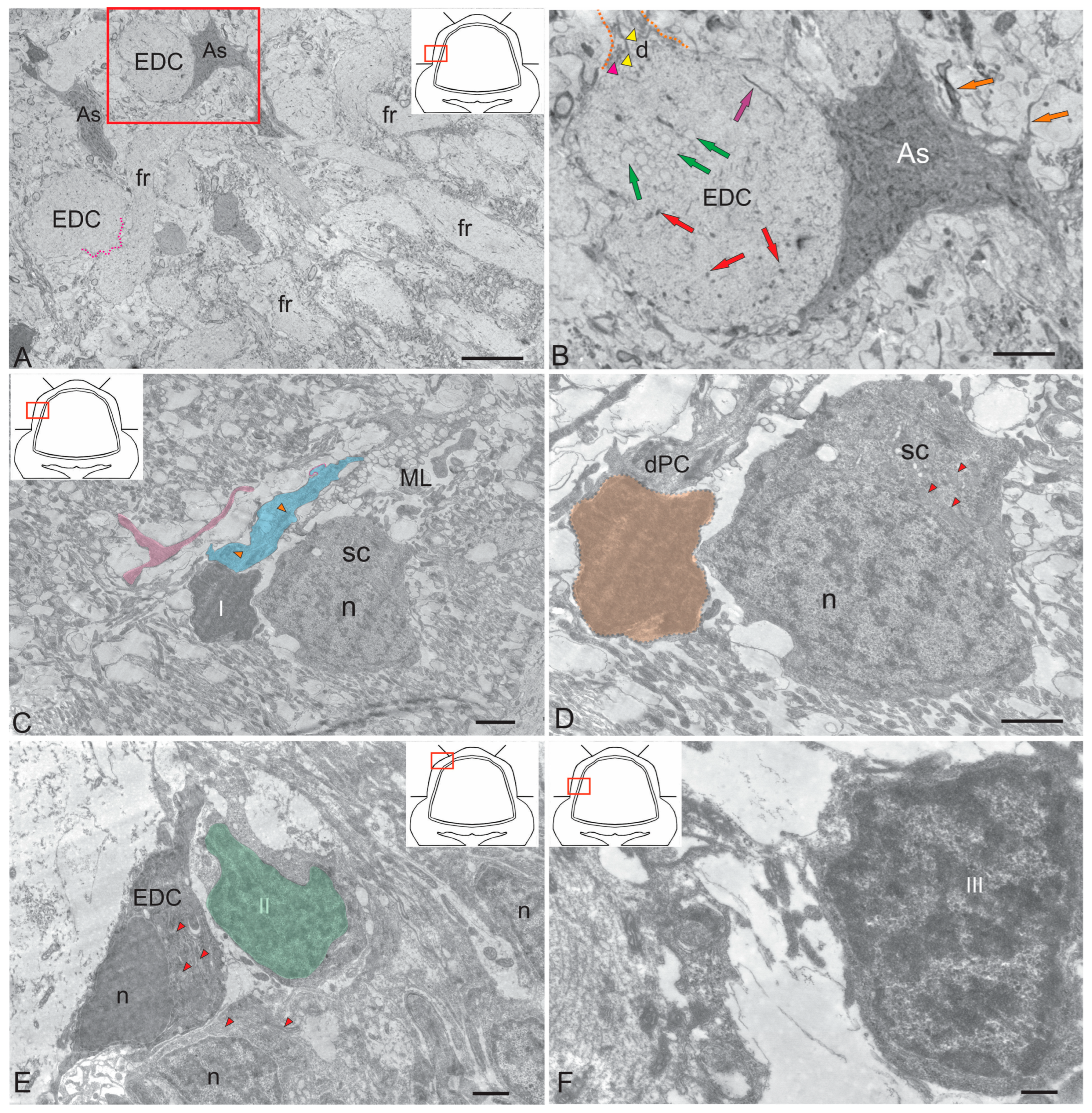
Figure 2.
Ultrastructural organization of neurons and glia in the molecular layer of the cerebellar body of juvenile chum salmon Oncorhynchus keta. (A) An astrocyte (As) of the protoplasmic type in contact with a eurydendroid cell (EDC); dark pink dotted line indicates the perisomatic process of the EDC; fr—fibrous fibers. (B) The fragment indicated by the red rectangle in (A) at higher magnification; a thin filamentous fibrillation in the cytoplasm of the EDC is indicated by a purple arrow, glycogen granules (red arrows), lipid droplets (green arrows); the origin of the proximal dendrite (d) is outlined by an orange dotted line; microtubules (yellow triangular arrows), subsurface cisterns (dark pink triangular arrow), orange arrows indicate processes of the astrocyte. (C) A dark cell (I) in contact with stellate cells (SC) in the molecular layer (ML); a T-shaped fiber is shown in pink; n—nucleus, PC dendrites (dPC) are highlighted in blue; dPC mitochondria are indicated by orange triangular arrows. (D) SC in contact with the dark cell I (highlighted in orange) at higher magnification; n—nucleus, mitochondria are indicated by red triangular arrows. (E) A type II glial cell (shown in green) located next to the developing EDC; mitochondria are indicated by red triangular arrows, other designations are as in (D). (F) An adult type glial precursor (aNSPCs, III) according to Lindsay’s classification. TEM. Scale: (A) 10 µm; (B–D) 2 µm; (E) 1 µm; (F) 400 nm.
Figure 2.
Ultrastructural organization of neurons and glia in the molecular layer of the cerebellar body of juvenile chum salmon Oncorhynchus keta. (A) An astrocyte (As) of the protoplasmic type in contact with a eurydendroid cell (EDC); dark pink dotted line indicates the perisomatic process of the EDC; fr—fibrous fibers. (B) The fragment indicated by the red rectangle in (A) at higher magnification; a thin filamentous fibrillation in the cytoplasm of the EDC is indicated by a purple arrow, glycogen granules (red arrows), lipid droplets (green arrows); the origin of the proximal dendrite (d) is outlined by an orange dotted line; microtubules (yellow triangular arrows), subsurface cisterns (dark pink triangular arrow), orange arrows indicate processes of the astrocyte. (C) A dark cell (I) in contact with stellate cells (SC) in the molecular layer (ML); a T-shaped fiber is shown in pink; n—nucleus, PC dendrites (dPC) are highlighted in blue; dPC mitochondria are indicated by orange triangular arrows. (D) SC in contact with the dark cell I (highlighted in orange) at higher magnification; n—nucleus, mitochondria are indicated by red triangular arrows. (E) A type II glial cell (shown in green) located next to the developing EDC; mitochondria are indicated by red triangular arrows, other designations are as in (D). (F) An adult type glial precursor (aNSPCs, III) according to Lindsay’s classification. TEM. Scale: (A) 10 µm; (B–D) 2 µm; (E) 1 µm; (F) 400 nm.
![Ijms 26 09267 g002 Ijms 26 09267 g002]()
Figure 3.
Ultrastructural organization of the dorsal matrix zone of the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) A general view of the dorsal matrix zone (DMZ), shown in the red square of the pictogram, which includes a cluster of non-glial adult-type neural stem/progenitor cells (aNSPCs, yellow arrows) in the dorsomedial region (blue dotted inset); a ventral cluster of aNSPCs (in the blue dotted square); individual aNSPCs are indicated by blue arrows; patterns of sprouting myelin fibers (fb, red arrows); neuroepithelial cells (NECs, red arrows). (B) An enlarged fragment (in orange inset) showing details of the ultrastructure of the NECs (red arrows), surrounded by type III aNSPCs (blue arrows); non-glial adult-type neural stem/progenitor cells (yellow arrows) Purkinje cells (PCs) are indicated. (C) An enlarged fragment showing details of the ultrastructure of a heterogeneous cluster in the blue dotted square in (A); type III aNSPCs are indicated by blue arrows; non-glial type aNSPCs are indicated by yellow arrows; embryonic-type intermediate precursors are indicated by orange arrows; myelin fibers (fb, green arrows); Purkinje cells (PCs). (D) Organization patterns of perivascular astroglia (as), indicated by red arrows; fibers are indicated by green arrows. (E) aNSPCs of non-glial type (yellow arrows) form clusters in the ML (in a black dotted rectangle) and at the border of granular eminence (GrEm), type III aNSPCs—blue arrows, type IV aNSPCs crimson arrows, cf (climbing fibers)—green arrows. (F) An enlarged fragment in a black dotted rectangle in (E), myelin fibers—green arrows; non-glial type aNSPCs are highlighted in pink; type III aNSPCs (III); type IV aNSPCs (IV); secretory granules are indicated by red arrows; a cluster of secretory granules is outlined by a red dotted oval. TEM. Scale: (A,B) 20 µm; (inset); (E) 10 µm; (C,D,F) 5 µm.
Figure 3.
Ultrastructural organization of the dorsal matrix zone of the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) A general view of the dorsal matrix zone (DMZ), shown in the red square of the pictogram, which includes a cluster of non-glial adult-type neural stem/progenitor cells (aNSPCs, yellow arrows) in the dorsomedial region (blue dotted inset); a ventral cluster of aNSPCs (in the blue dotted square); individual aNSPCs are indicated by blue arrows; patterns of sprouting myelin fibers (fb, red arrows); neuroepithelial cells (NECs, red arrows). (B) An enlarged fragment (in orange inset) showing details of the ultrastructure of the NECs (red arrows), surrounded by type III aNSPCs (blue arrows); non-glial adult-type neural stem/progenitor cells (yellow arrows) Purkinje cells (PCs) are indicated. (C) An enlarged fragment showing details of the ultrastructure of a heterogeneous cluster in the blue dotted square in (A); type III aNSPCs are indicated by blue arrows; non-glial type aNSPCs are indicated by yellow arrows; embryonic-type intermediate precursors are indicated by orange arrows; myelin fibers (fb, green arrows); Purkinje cells (PCs). (D) Organization patterns of perivascular astroglia (as), indicated by red arrows; fibers are indicated by green arrows. (E) aNSPCs of non-glial type (yellow arrows) form clusters in the ML (in a black dotted rectangle) and at the border of granular eminence (GrEm), type III aNSPCs—blue arrows, type IV aNSPCs crimson arrows, cf (climbing fibers)—green arrows. (F) An enlarged fragment in a black dotted rectangle in (E), myelin fibers—green arrows; non-glial type aNSPCs are highlighted in pink; type III aNSPCs (III); type IV aNSPCs (IV); secretory granules are indicated by red arrows; a cluster of secretory granules is outlined by a red dotted oval. TEM. Scale: (A,B) 20 µm; (inset); (E) 10 µm; (C,D,F) 5 µm.
![Ijms 26 09267 g003 Ijms 26 09267 g003]()
Figure 4.
Ultrastructural organization of the granular layer of the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Cells of the granular layer (GrL) of the dorsomedial part of the cerebellar body; granule cells (GrCs); type III adult-type neural stem/progenitor cells (aNSPCs)—III, type IV aNSPCs—IV; climbing fibers (cf)—orange arrows; non-glial progenitor cells are highlighted in green. (B) In deeper layers of GrL, type IV aNSPCs (IV); unmyelinated fibers are indicated by orange arrows. (C) Diffuse distribution patterns of GrCs and aNSPCs of types III and IV in the lateral part of the cerebellar body; myelinated fibers are indicated by orange arrows; mixed fibers are shown in the orange rectangle. (D) Cluster distribution patterns of types III and IV aNSPCs in the ventrolateral part of the cerebellar body; climbing fibers (cf); myelinated cf are indicated by orange arrows. (E) Constitutive neurogenic niches of mixed adult type (in a dark red dotted rectangle) type III aNSPCs (III) in the medio-basal zone of the cerebellar body; a cluster of climbing fibers (cf, in a green dotted rectangle); myelinated cf are indicated by orange arrows. (F) Adult neurogenic niche formed by type III aNSPCs from the ventrolateral part of the cerebellar body; myelin fibers—orange arrows, other designations are as in (E). TEM. Scale: (A–C) 3 µm; (D–F) 2 µm.
Figure 4.
Ultrastructural organization of the granular layer of the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Cells of the granular layer (GrL) of the dorsomedial part of the cerebellar body; granule cells (GrCs); type III adult-type neural stem/progenitor cells (aNSPCs)—III, type IV aNSPCs—IV; climbing fibers (cf)—orange arrows; non-glial progenitor cells are highlighted in green. (B) In deeper layers of GrL, type IV aNSPCs (IV); unmyelinated fibers are indicated by orange arrows. (C) Diffuse distribution patterns of GrCs and aNSPCs of types III and IV in the lateral part of the cerebellar body; myelinated fibers are indicated by orange arrows; mixed fibers are shown in the orange rectangle. (D) Cluster distribution patterns of types III and IV aNSPCs in the ventrolateral part of the cerebellar body; climbing fibers (cf); myelinated cf are indicated by orange arrows. (E) Constitutive neurogenic niches of mixed adult type (in a dark red dotted rectangle) type III aNSPCs (III) in the medio-basal zone of the cerebellar body; a cluster of climbing fibers (cf, in a green dotted rectangle); myelinated cf are indicated by orange arrows. (F) Adult neurogenic niche formed by type III aNSPCs from the ventrolateral part of the cerebellar body; myelin fibers—orange arrows, other designations are as in (E). TEM. Scale: (A–C) 3 µm; (D–F) 2 µm.
![Ijms 26 09267 g004 Ijms 26 09267 g004]()
Figure 5.
Stereoscopic organization of adult neural stem and progenitor cells (aNSPCs) in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) aNSPCs are fixed by microfibrils (red arrows) on the surface of the molecular layer. (B) Cluster of paired aNSPCs. (C) Large stromal clusters of aNSPCs (in a red oval) in the dorsolateral regions of the cerebellum. (D) Diffuse patterns of aNSPCs distribution (yellow arrows) in the granular eminence (GrEm) associated with the surface matrix. (E) aNSPCs are fixed by microfibrils (in the red dotted rectangle) on the surface of the molecular layer; an erythrocyte (Er). (F) An enlarged fragment from the red dotted rectangle in (E); a single cluster of aNSPCs (bounded by a red oval). SEM. Scale: (A) 1 µm; (B,D) 2 µm; (C) 10 µm; (E,F) 3 µm.
Figure 5.
Stereoscopic organization of adult neural stem and progenitor cells (aNSPCs) in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) aNSPCs are fixed by microfibrils (red arrows) on the surface of the molecular layer. (B) Cluster of paired aNSPCs. (C) Large stromal clusters of aNSPCs (in a red oval) in the dorsolateral regions of the cerebellum. (D) Diffuse patterns of aNSPCs distribution (yellow arrows) in the granular eminence (GrEm) associated with the surface matrix. (E) aNSPCs are fixed by microfibrils (in the red dotted rectangle) on the surface of the molecular layer; an erythrocyte (Er). (F) An enlarged fragment from the red dotted rectangle in (E); a single cluster of aNSPCs (bounded by a red oval). SEM. Scale: (A) 1 µm; (B,D) 2 µm; (C) 10 µm; (E,F) 3 µm.
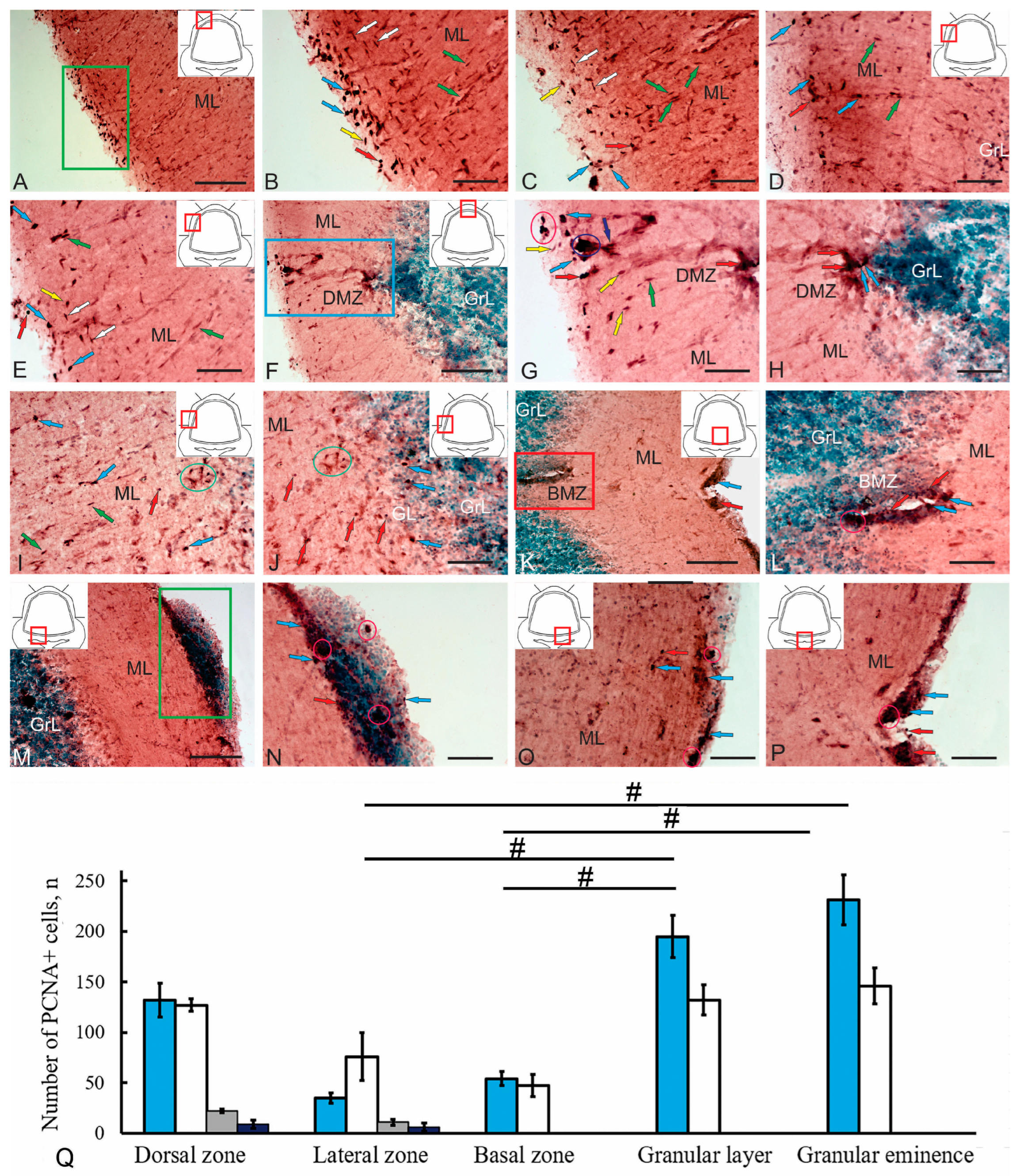
Figure 6.
Immunohistochemical labeling of PCNA in the cerebellum of juvenile Oncorhynchus keta. (A) PCNA immunolocalization in the dorsal region (topography shown in the red square in the pictogram) of the molecular layer (ML); the surface population of PCNA+ cells shown in a green rectangle. (B) An enlarged fragment of a green rectangle in (A); oval cells (blue arrows), rounded cells (red arrows), intensely labeled cells, tangentially migrating surface cells (yellow arrows), deep cells (white arrows), radially migrating cells (green arrows). (C) Localization of PCNA in the dorsal region of the cerebellum, immunolocalization in the upper third of the ML, designations are as in (B). (D) Localization of PCNA in the dorsolateral region of the cerebellum (topography shown in the pictogram in the red square) in ML; patterns of radial migration to the granular layer (GrL); designations are as in (B). (E) Localization of PCNA in the dorsolateral region, patterns of tangential migration; designations are as in (B). (F) A general view of PCNA immunolocalization in the dorsal matrix zone (DMZ), shown in a blue rectangle. (G) An enlarged fragment of the dorsal part of the DMZ; the red oval shows an accumulation of adult-type PCNA+ precursors; the blue oval shows an accumulation of PCNA+ neuroepithelial cells (NECs); individual PCNA+ NECs (dark blue arrow); the remaining designations are as in (B). (H) Enlarged fragment of the ventral part of the DMZ; the red and blue arrows indicate PCNA+ adult-type neural stem/progenitor cells (aNSPCs). (I) Patterns of tangential migration in the lateral zone of PCNA+ cells and an accumulation of aNSPCs (in a green oval) in the lower third of the ML, designations are as in (B). (J) Localization of PCNA+ cells (in green oval) in the lower third of the ML and GrL of the lateral zone of the cerebellum. (K) A general view of PCNA immunolocalization in the basal matrix zone (BMZ), shown in the red rectangle, designations are as in (B). (L) An enlarged fragment of BMZ, an accumulation of aNSPCs shown in the pink oval; designations are as in (B). (M) An accumulation of PCNA+ cells on the ventral wall of the cerebellum (in the green rectangle). (N) A heterogeneous neurogenic cluster at a higher magnification (in pink oval), designations are as in (B,L). (O) Localization of PCNA+ cells and their clusters in the ventrolateral zone of the cerebellum, designations are as in (B,N). (P) Localization of PCNA+ cells and their clusters in the ventromedial zone, designations are as in (B,L,N). (Q) Comparative distribution of PCNA+ cells in various regions of the cerebellum of Oncorhynchus keta (M ± SD); significant intergroup differences between the granular layer and the lateral and basal zones, and the granular eminences and the lateral and basal zones indicated by #—(p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). Blue columns: type I cells; white: type II; gray: type III; black: type IV. Scale: (A,F,K,M) 100 µm; (B–E,G–J,L,N–P) 50 µm.
Figure 6.
Immunohistochemical labeling of PCNA in the cerebellum of juvenile Oncorhynchus keta. (A) PCNA immunolocalization in the dorsal region (topography shown in the red square in the pictogram) of the molecular layer (ML); the surface population of PCNA+ cells shown in a green rectangle. (B) An enlarged fragment of a green rectangle in (A); oval cells (blue arrows), rounded cells (red arrows), intensely labeled cells, tangentially migrating surface cells (yellow arrows), deep cells (white arrows), radially migrating cells (green arrows). (C) Localization of PCNA in the dorsal region of the cerebellum, immunolocalization in the upper third of the ML, designations are as in (B). (D) Localization of PCNA in the dorsolateral region of the cerebellum (topography shown in the pictogram in the red square) in ML; patterns of radial migration to the granular layer (GrL); designations are as in (B). (E) Localization of PCNA in the dorsolateral region, patterns of tangential migration; designations are as in (B). (F) A general view of PCNA immunolocalization in the dorsal matrix zone (DMZ), shown in a blue rectangle. (G) An enlarged fragment of the dorsal part of the DMZ; the red oval shows an accumulation of adult-type PCNA+ precursors; the blue oval shows an accumulation of PCNA+ neuroepithelial cells (NECs); individual PCNA+ NECs (dark blue arrow); the remaining designations are as in (B). (H) Enlarged fragment of the ventral part of the DMZ; the red and blue arrows indicate PCNA+ adult-type neural stem/progenitor cells (aNSPCs). (I) Patterns of tangential migration in the lateral zone of PCNA+ cells and an accumulation of aNSPCs (in a green oval) in the lower third of the ML, designations are as in (B). (J) Localization of PCNA+ cells (in green oval) in the lower third of the ML and GrL of the lateral zone of the cerebellum. (K) A general view of PCNA immunolocalization in the basal matrix zone (BMZ), shown in the red rectangle, designations are as in (B). (L) An enlarged fragment of BMZ, an accumulation of aNSPCs shown in the pink oval; designations are as in (B). (M) An accumulation of PCNA+ cells on the ventral wall of the cerebellum (in the green rectangle). (N) A heterogeneous neurogenic cluster at a higher magnification (in pink oval), designations are as in (B,L). (O) Localization of PCNA+ cells and their clusters in the ventrolateral zone of the cerebellum, designations are as in (B,N). (P) Localization of PCNA+ cells and their clusters in the ventromedial zone, designations are as in (B,L,N). (Q) Comparative distribution of PCNA+ cells in various regions of the cerebellum of Oncorhynchus keta (M ± SD); significant intergroup differences between the granular layer and the lateral and basal zones, and the granular eminences and the lateral and basal zones indicated by #—(p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). Blue columns: type I cells; white: type II; gray: type III; black: type IV. Scale: (A,F,K,M) 100 µm; (B–E,G–J,L,N–P) 50 µm.
![Ijms 26 09267 g006 Ijms 26 09267 g006]()
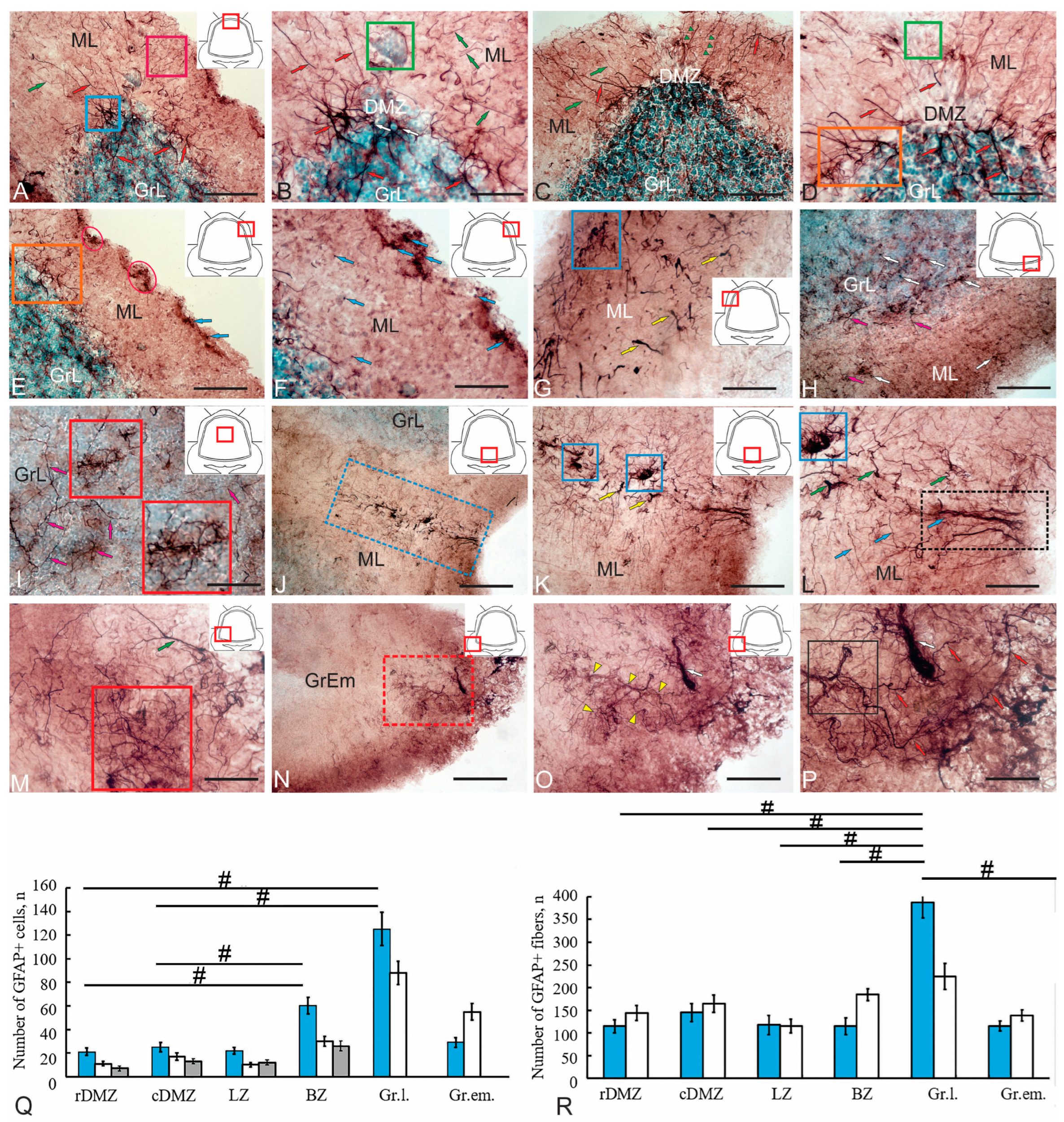
Figure 7.
Immunohistochemical labeling of GFAP in the cerebellum of juvenile Oncorhynchus keta. (A) A general view of GFAP immunolocalization in the rostral part of the dorsal matrix zone (DMZ) (pictogram in the red square); thick radial fibers (red arrows), thin radial fibers (green arrows); clusters of Bergmann glia fibers (in the pink rectangle), thickened plexus of climbing fibers (cf) at the border between molecular layer (ML) and granular layer (GrL) (in the blue rectangle). (B) An enlarged fragment of the rostral DMZ; an accumulation of GFAP-negative neuroepithelial cells (NEC) in the green square; GFAP+ cells in the DMZ (white arrows), other designations are as in (A). (C) A general view of GFAP immunolocalization in the caudal part of the DMZ; bundles of Bergmann glia fibers (green triangular arrows); the remaining designations are as in (A,B). (D) An enlarged fragment of the caudal DMZ, abundant, reticulated branching of GFAP+ fibers at the border of ML and GrL (in the orange rectangle), other designations are as in (A). (E) Clusters of GFAP+ adult-type neural stem/progenitor cells (aNSPCs) in the lateral subpial region of the cerebellum (in pink ovals); the blue arrows indicate individual GFAP+ aNSPCs; designations are as in (D). (F) An enlarged fragment showing the distribution of individual GFAP+ aNSPCs (indicated by blue arrows). (G) The GFAP+ ends of mossy fibers (mf) (indicated by yellow arrows); a cluster of mf endings in a blue rectangle. (H) The individual GFAP+ cells (white arrows) and GFAP+ cf endings (pink arrows) in the basolateral parts of the cerebellum. (I) Separate collaterals of GFAP+ cf in the GrL (pink arrows); terminal extensions of mf, and central parts of the mossy rosette (shown in the red inset). (J) Distribution patterns of GFAP+ mf (in a blue dotted rectangle) in the mediobasal area of the cerebellum. (K) GFAP+ rosettes of mf (blue rectangles) in the mediobasal GFAP+ ascending tract, GFAP+ ends of mf (indicated by yellow arrows). (L) A bundle of GFAP+ cf at the base of the cerebellar body (shown in a black dotted rectangle); thin cf (blue arrows), thick cf (green arrows). (M) Sockets of GFAP+ mf with numerous filamentous appendages (in a red square) in the basolateral regions of the cerebellum, designations are as in (L). (N) GrEm of GFAP+ mf formed large rosettes with an uneven surface (in the red dotted rectangle). (O) The central part of the GFAP+ rosette formed numerous thin filamentous appendages (indicated by yellow triangular arrows) forming a wide arborization, the glomerulus indicated by a white arrow. (P) An enlarged fragment of the central part of the rosette (in a black square); glomerulus (white arrow) and terminal arborizations (indicated by red arrows). (Q) Comparative distribution of GFAP+ cells in various regions of the cerebellum of Oncorhynchus keta (M ± SD); significant intergroup differences between the granular layer (GrL) and rostral (rDMZ) and caudal parts of the DMZ (cDMZ), basal zone (BZ) and cDMZ, BZ and lateral zone (LZ)—# (p < 0.05); (n = 5 in each group), one-way ANOVA. The blue columns represent type I cells, the white ones represent type II cells, and the gray ones represent type III cells. (R) Comparative distribution of GFAP+ fibers in various regions of the cerebellum of Oncorhynchus keta (mean ± SD); significant intergroup differences between the granular layer (GrL) and the rostral (rDMZ) and caudal parts of the DMZ (cDMZ), lateral (LZ), basal (BZ) zones and granular eminence (GrEm)—# (p < 0.05); (n = 5 in each group), one-way analysis of variance (ANOVA). The blue columns represent thin fibers, the white ones represent thick fibers. Scale: (A,C,E,H,K,O) 100 µm; (B,D,F,G,I,L,M,P) 50 µm; (J,N) 200 µm.
Figure 7.
Immunohistochemical labeling of GFAP in the cerebellum of juvenile Oncorhynchus keta. (A) A general view of GFAP immunolocalization in the rostral part of the dorsal matrix zone (DMZ) (pictogram in the red square); thick radial fibers (red arrows), thin radial fibers (green arrows); clusters of Bergmann glia fibers (in the pink rectangle), thickened plexus of climbing fibers (cf) at the border between molecular layer (ML) and granular layer (GrL) (in the blue rectangle). (B) An enlarged fragment of the rostral DMZ; an accumulation of GFAP-negative neuroepithelial cells (NEC) in the green square; GFAP+ cells in the DMZ (white arrows), other designations are as in (A). (C) A general view of GFAP immunolocalization in the caudal part of the DMZ; bundles of Bergmann glia fibers (green triangular arrows); the remaining designations are as in (A,B). (D) An enlarged fragment of the caudal DMZ, abundant, reticulated branching of GFAP+ fibers at the border of ML and GrL (in the orange rectangle), other designations are as in (A). (E) Clusters of GFAP+ adult-type neural stem/progenitor cells (aNSPCs) in the lateral subpial region of the cerebellum (in pink ovals); the blue arrows indicate individual GFAP+ aNSPCs; designations are as in (D). (F) An enlarged fragment showing the distribution of individual GFAP+ aNSPCs (indicated by blue arrows). (G) The GFAP+ ends of mossy fibers (mf) (indicated by yellow arrows); a cluster of mf endings in a blue rectangle. (H) The individual GFAP+ cells (white arrows) and GFAP+ cf endings (pink arrows) in the basolateral parts of the cerebellum. (I) Separate collaterals of GFAP+ cf in the GrL (pink arrows); terminal extensions of mf, and central parts of the mossy rosette (shown in the red inset). (J) Distribution patterns of GFAP+ mf (in a blue dotted rectangle) in the mediobasal area of the cerebellum. (K) GFAP+ rosettes of mf (blue rectangles) in the mediobasal GFAP+ ascending tract, GFAP+ ends of mf (indicated by yellow arrows). (L) A bundle of GFAP+ cf at the base of the cerebellar body (shown in a black dotted rectangle); thin cf (blue arrows), thick cf (green arrows). (M) Sockets of GFAP+ mf with numerous filamentous appendages (in a red square) in the basolateral regions of the cerebellum, designations are as in (L). (N) GrEm of GFAP+ mf formed large rosettes with an uneven surface (in the red dotted rectangle). (O) The central part of the GFAP+ rosette formed numerous thin filamentous appendages (indicated by yellow triangular arrows) forming a wide arborization, the glomerulus indicated by a white arrow. (P) An enlarged fragment of the central part of the rosette (in a black square); glomerulus (white arrow) and terminal arborizations (indicated by red arrows). (Q) Comparative distribution of GFAP+ cells in various regions of the cerebellum of Oncorhynchus keta (M ± SD); significant intergroup differences between the granular layer (GrL) and rostral (rDMZ) and caudal parts of the DMZ (cDMZ), basal zone (BZ) and cDMZ, BZ and lateral zone (LZ)—# (p < 0.05); (n = 5 in each group), one-way ANOVA. The blue columns represent type I cells, the white ones represent type II cells, and the gray ones represent type III cells. (R) Comparative distribution of GFAP+ fibers in various regions of the cerebellum of Oncorhynchus keta (mean ± SD); significant intergroup differences between the granular layer (GrL) and the rostral (rDMZ) and caudal parts of the DMZ (cDMZ), lateral (LZ), basal (BZ) zones and granular eminence (GrEm)—# (p < 0.05); (n = 5 in each group), one-way analysis of variance (ANOVA). The blue columns represent thin fibers, the white ones represent thick fibers. Scale: (A,C,E,H,K,O) 100 µm; (B,D,F,G,I,L,M,P) 50 µm; (J,N) 200 µm.
![Ijms 26 09267 g007 Ijms 26 09267 g007]()
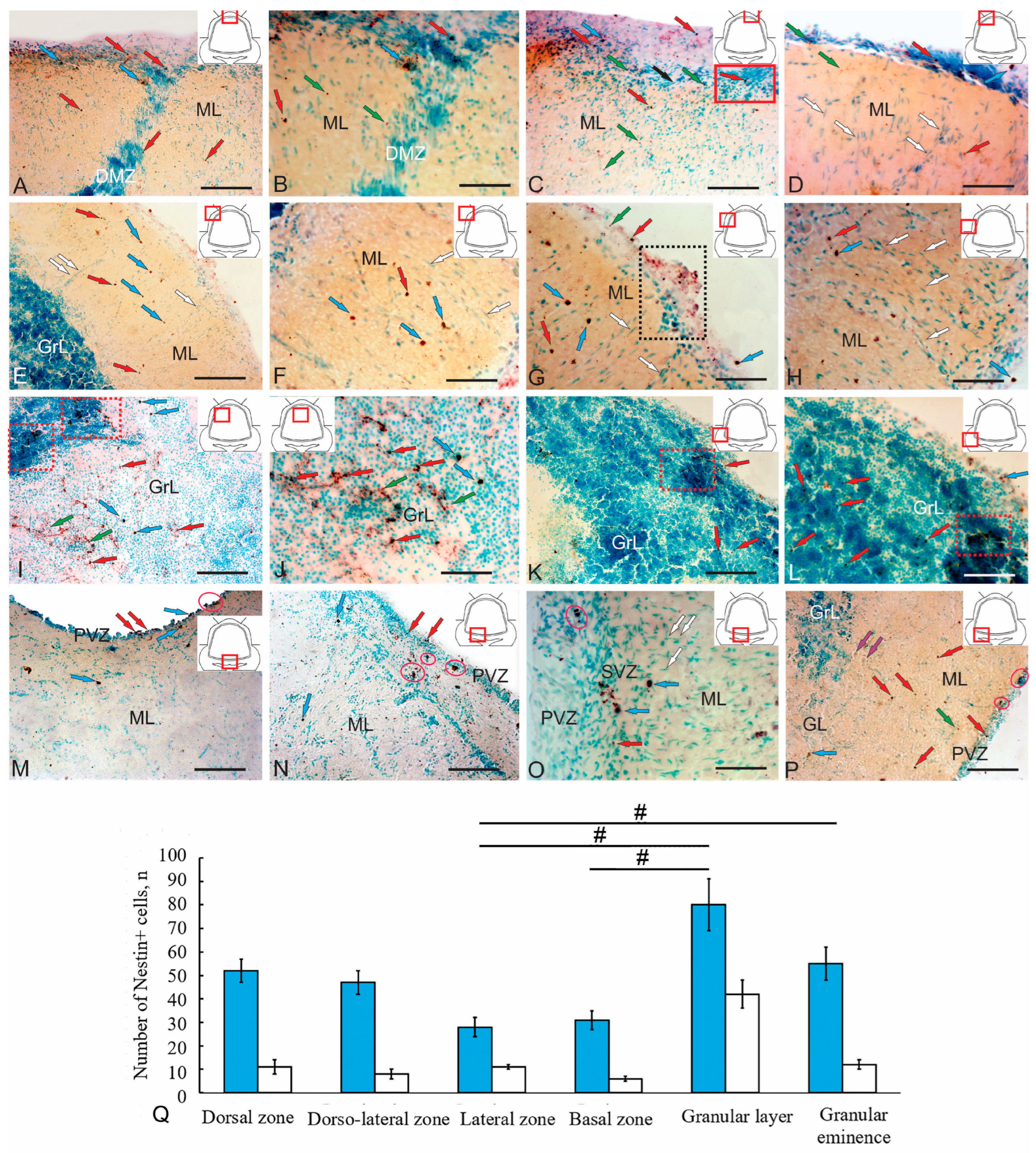
Figure 8.
Immunohistochemical labeling of nestin in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Immunolocalization of nestin (Nes) in the dorsal part of the cerebellum (pictogram in the red square); small oval intensely labeled non-glial aNSPCs (blue arrows), round non-glial adult-type neural stem/progenitor cells (aNSPCs) (red arrows). (B) An enlarged fragment of the dorsal matrix zone (DMZ), nestin+ granules (green arrows), other designations are as in (A). (C) Immunolocalization of nestin in the dorsolateral subpial part of the cerebellum (pictogram in the red square); black arrows indicate nestin-negative tangentially migrating cells; an accumulation of nestin-negative cells in the surface layers of the molecular layer (ML) (in the red rectangle); the remaining designations are as in (A). (D) Patterns of radial migration of nestin-negative cells (white arrows) in the dorsolateral part of the cerebellum. (E) Patterns of distribution of nestin+ aNSPCs in the lateral part of the cerebellar body; nestin-negative cells migrating along blood vessels (white arrows); designations are as in (A). (F) An enlarged fragment of the dorsolateral region showing non-glial nestin+ aNSPCs (blue and red arrows), nestin-negative cells (white arrows). (G) A thickening containing clusters of nestin+ granules (in a black dotted rectangle) in the subpial parts of the lateral region; other designations are as in (D). (H) Patterns of radial migration of nestin-negative cells, designations are as in (D). (I) A large population of nestin+ cells in the GrL forming clusters (in red dotted rectangles); a heterogeneous population of oval and round nestin+ non-glial precursors (indicated by blue and red arrows); nestin+ granules (green arrows). (J) An enlarged fragment showing patterns of extracellular localization of nestin and immunopositive granules (green arrows) adjacent to the DMZ; other designations are as in (I). (K) Foci of nestin+ type II aNSPCs (in the red dotted rectangle) in the granular eminences. (L) An enlarged fragment showing individual nestin+ cells and granules in the GrL of the granular eminences (GrEm), designations are as in I. (M) A general view of the paramedian basal zone of the cerebellum; nestin+ aNSPCs of types I and II (blue and red arrows) forming small clusters (in pink ovals) were identified in the periventricular zone (PVZ), bordering the fourth ventricle (IV). (N) In the lateral part of the basal zone of the cerebellum, the PVZ included several single and paired nestin+ aNSPCs of type II (red arrows); other designations are as in (M). (O) An enlarged fragment of PVZ and subventricular zone (SVZ) in the basal part of the cerebellum; nestin-negative tangentially migrating cells indicated by white arrows; other designations are as in (M). (P) The most lateral areas of the BZ; nestin+ types I and II aNSPCs (blue and red arrows), nestin+ granules (green arrows), PCs (purple arrows); the aNSPC clusters in the PVZ are outlined in red ovals. (Q) Comparative distribution of Nes+ cells in various regions of the cerebellum of O. keta (M ± SD); significant intergroup differences between the granular layer and the lateral, basal, and granular eminences are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The blue columns—type I cells, the white ones—type II cells. Scale: (A,E,I,K,M,N,P) 100 µm; (B–D,F–H,J,L,O) 50 µm.
Figure 8.
Immunohistochemical labeling of nestin in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Immunolocalization of nestin (Nes) in the dorsal part of the cerebellum (pictogram in the red square); small oval intensely labeled non-glial aNSPCs (blue arrows), round non-glial adult-type neural stem/progenitor cells (aNSPCs) (red arrows). (B) An enlarged fragment of the dorsal matrix zone (DMZ), nestin+ granules (green arrows), other designations are as in (A). (C) Immunolocalization of nestin in the dorsolateral subpial part of the cerebellum (pictogram in the red square); black arrows indicate nestin-negative tangentially migrating cells; an accumulation of nestin-negative cells in the surface layers of the molecular layer (ML) (in the red rectangle); the remaining designations are as in (A). (D) Patterns of radial migration of nestin-negative cells (white arrows) in the dorsolateral part of the cerebellum. (E) Patterns of distribution of nestin+ aNSPCs in the lateral part of the cerebellar body; nestin-negative cells migrating along blood vessels (white arrows); designations are as in (A). (F) An enlarged fragment of the dorsolateral region showing non-glial nestin+ aNSPCs (blue and red arrows), nestin-negative cells (white arrows). (G) A thickening containing clusters of nestin+ granules (in a black dotted rectangle) in the subpial parts of the lateral region; other designations are as in (D). (H) Patterns of radial migration of nestin-negative cells, designations are as in (D). (I) A large population of nestin+ cells in the GrL forming clusters (in red dotted rectangles); a heterogeneous population of oval and round nestin+ non-glial precursors (indicated by blue and red arrows); nestin+ granules (green arrows). (J) An enlarged fragment showing patterns of extracellular localization of nestin and immunopositive granules (green arrows) adjacent to the DMZ; other designations are as in (I). (K) Foci of nestin+ type II aNSPCs (in the red dotted rectangle) in the granular eminences. (L) An enlarged fragment showing individual nestin+ cells and granules in the GrL of the granular eminences (GrEm), designations are as in I. (M) A general view of the paramedian basal zone of the cerebellum; nestin+ aNSPCs of types I and II (blue and red arrows) forming small clusters (in pink ovals) were identified in the periventricular zone (PVZ), bordering the fourth ventricle (IV). (N) In the lateral part of the basal zone of the cerebellum, the PVZ included several single and paired nestin+ aNSPCs of type II (red arrows); other designations are as in (M). (O) An enlarged fragment of PVZ and subventricular zone (SVZ) in the basal part of the cerebellum; nestin-negative tangentially migrating cells indicated by white arrows; other designations are as in (M). (P) The most lateral areas of the BZ; nestin+ types I and II aNSPCs (blue and red arrows), nestin+ granules (green arrows), PCs (purple arrows); the aNSPC clusters in the PVZ are outlined in red ovals. (Q) Comparative distribution of Nes+ cells in various regions of the cerebellum of O. keta (M ± SD); significant intergroup differences between the granular layer and the lateral, basal, and granular eminences are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The blue columns—type I cells, the white ones—type II cells. Scale: (A,E,I,K,M,N,P) 100 µm; (B–D,F–H,J,L,O) 50 µm.
![Ijms 26 09267 g008 Ijms 26 09267 g008]()
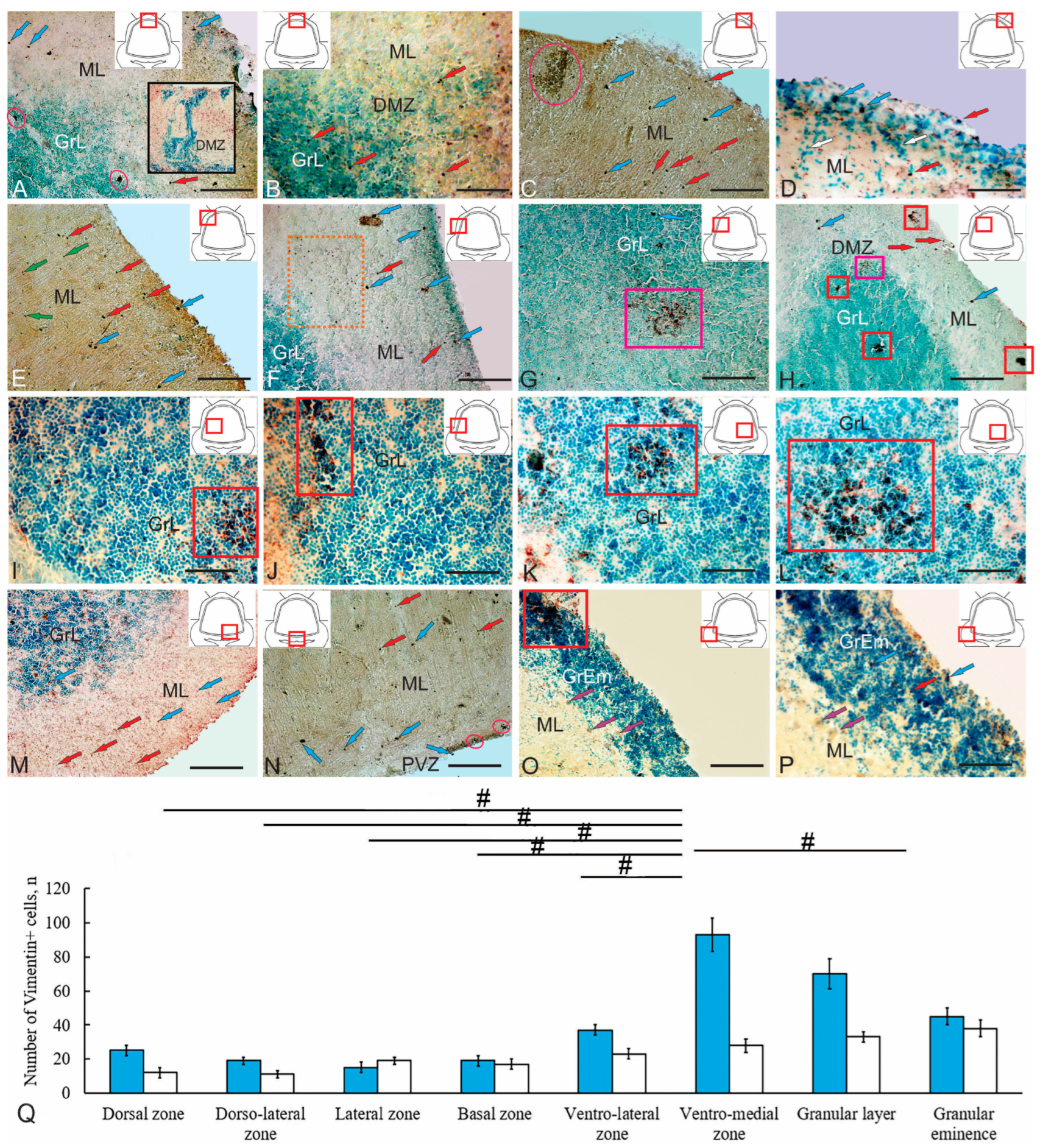
Figure 9.
Immunohistochemical labeling of vimentin in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Vimentin (Vim) expression in the dorsal zone of the cerebellum (shown in the pictogram) of juvenile chum salmon in adult-type neural stem/progenitor cells (aNSPCs) of types I and II (blue and red arrows); the dorsal matrix zone (DMZ) is shown in a black rectangle; aNSPC clusters in pink ovals. (B) An enlarged fragment of the DMZ, rounded aNSPCs are indicated by red arrows. (C) Vimentin+ cluster of aNSPCs in the dorsolateral region (shown in a dark pink oval) types I and II; other designations are as in (A). (D) An enlarged fragment of the surface zone in the dorsolateral region containing vimentin+ type I aNSPCs (blue arrows) and type II in the upper third of the molecular layer (ML) (red arrows); patterns of radial migration of vimentin-negative cells (white arrows). (E) Separate subcellular vimentin+ granules (green arrows) in the dorsolateral areas, other designations are as in (A). (F) An accumulation of vimentin+ granules (in the orange dotted rectangle) in the lateral zone of the cerebellum, other designations are as in (A). (G) Sparse clusters of vimentin+ aNSPCs in the dorsal part of the granular layer (GrL) (in the pink rectangle); single vimentin+ aNSPCs of type I (blue arrow). (H) Local dense clusters of vimentin+ aNSPCs (in red squares); other designations are as in (A,G). (I) Heterogeneous clusters of vimentin+ aNSPCs; immunopositive neuropil and extracellular deposits of vimentin (in the red rectangle) in the GrL. (J) Similar structures (in the red rectangle) at the border of the ML and GrL (see pictogram). (K) In the ventrolateral part of the GrL (see pictogram). (L) In the ventromedial part of the GrL (see pictogram). (M) Distribution of vimentin+ aNSPCs of type I and type II (blue and red arrows) in the ventrolateral part of the basal zone (BZ). (N) Distribution of vimentin+ aNSPCs of type I and type II in the ventromedial part of the BZ (blue and red arrows), pink ovals show clusters of vimentin+ aNSPCs in the periventricular zone (PVZ) of the IV ventricle. (O) Local neurogenic niche with vimentin+ aNSPCs (in the red rectangle) in the granular eminence (GrEm), vimentin-negative ML (purple arrows). (P) An enlarged GrEm fragment showing vimentin+ types I and II aNSPCs (blue and red arrows); vimentin–negative ML (purple arrows). (Q) Comparative distribution of Vim+ cells in various regions of the cerebellum of Oncorhynchus keta (M ± SD); significant intergroup differences between the ventromedial region of the granular layer and the granular layer, dorsal, dorsolateral, lateral, basal and ventrolateral zones are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The blue columns represent type I cells, the white ones represent type 2 cells. Scale: (A,C,E–H,M–O) 100 µm; (B,D,I–L,P) 50 µm.
Figure 9.
Immunohistochemical labeling of vimentin in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Vimentin (Vim) expression in the dorsal zone of the cerebellum (shown in the pictogram) of juvenile chum salmon in adult-type neural stem/progenitor cells (aNSPCs) of types I and II (blue and red arrows); the dorsal matrix zone (DMZ) is shown in a black rectangle; aNSPC clusters in pink ovals. (B) An enlarged fragment of the DMZ, rounded aNSPCs are indicated by red arrows. (C) Vimentin+ cluster of aNSPCs in the dorsolateral region (shown in a dark pink oval) types I and II; other designations are as in (A). (D) An enlarged fragment of the surface zone in the dorsolateral region containing vimentin+ type I aNSPCs (blue arrows) and type II in the upper third of the molecular layer (ML) (red arrows); patterns of radial migration of vimentin-negative cells (white arrows). (E) Separate subcellular vimentin+ granules (green arrows) in the dorsolateral areas, other designations are as in (A). (F) An accumulation of vimentin+ granules (in the orange dotted rectangle) in the lateral zone of the cerebellum, other designations are as in (A). (G) Sparse clusters of vimentin+ aNSPCs in the dorsal part of the granular layer (GrL) (in the pink rectangle); single vimentin+ aNSPCs of type I (blue arrow). (H) Local dense clusters of vimentin+ aNSPCs (in red squares); other designations are as in (A,G). (I) Heterogeneous clusters of vimentin+ aNSPCs; immunopositive neuropil and extracellular deposits of vimentin (in the red rectangle) in the GrL. (J) Similar structures (in the red rectangle) at the border of the ML and GrL (see pictogram). (K) In the ventrolateral part of the GrL (see pictogram). (L) In the ventromedial part of the GrL (see pictogram). (M) Distribution of vimentin+ aNSPCs of type I and type II (blue and red arrows) in the ventrolateral part of the basal zone (BZ). (N) Distribution of vimentin+ aNSPCs of type I and type II in the ventromedial part of the BZ (blue and red arrows), pink ovals show clusters of vimentin+ aNSPCs in the periventricular zone (PVZ) of the IV ventricle. (O) Local neurogenic niche with vimentin+ aNSPCs (in the red rectangle) in the granular eminence (GrEm), vimentin-negative ML (purple arrows). (P) An enlarged GrEm fragment showing vimentin+ types I and II aNSPCs (blue and red arrows); vimentin–negative ML (purple arrows). (Q) Comparative distribution of Vim+ cells in various regions of the cerebellum of Oncorhynchus keta (M ± SD); significant intergroup differences between the ventromedial region of the granular layer and the granular layer, dorsal, dorsolateral, lateral, basal and ventrolateral zones are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The blue columns represent type I cells, the white ones represent type 2 cells. Scale: (A,C,E–H,M–O) 100 µm; (B,D,I–L,P) 50 µm.
![Ijms 26 09267 g009 Ijms 26 09267 g009]()
Figure 10.
Immunohistochemical labeling of HuCD in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) The localization of HuCD in the dorsal part of the cerebellum (pictogram), the HuCD-negative dorsal matrix zone (DMZ) is shown in a blue rectangle, paired HuCD+ neurons are indicated by yellow arrows. (B) An enlarged fragment of the DMZ, HuCD+ cells are localized at the border of the granular layer (GrL) and molecular layer (ML), eurydendroid cells (EDCs) with axonal arborization (white arrows), undifferentiated HuCD+ cells in the ML are indicated by yellow arrows. (C) A fragment of the dorsolateral part of the ML containing intensely labeled HuCD+ cells (yellow arrows), superficially located HuCD+ cells with an immunonegative nucleus (orange arrows), HuCD-negative adult-type neural stem/progenitor cells (aNSPCs) (blue arrows), HuCD-negative radially migrating cells (green arrows). (D) The dorsal region adjacent to the DMZ, containing HuCD+ cells (yellow arrows). (E) HuCD+ cells in the dorsal part of the cerebellum: superficial small undifferentiated cells (red arrows), oval single cells or forming small clusters of cells (yellow arrows), small moderately labeled bipolar cells (blue arrows); HuCD+ neurons forming connections in the ML (in the blue dotted rectangle); axonal afferent HuCD+ connections, at the border of the ML and GL (white arrow). (F) Morphogenetic patterns of HuCD expression in cells of various types, designations are as in (C,E). (G) Mass labeling of HuCD in cells of the surface layers of the dorsolateral zone of ML; in the green dotted rectangle HuCD+ EDCs are indicated. (H) An enlarged fragment in the green dotted rectangle in (G), the EDC axon with varicose microcytosculpture is indicated by a white arrow. (I) Heterogeneous HuCD+ cells in the lateral part of the superficial and middle layers of the ML; the purple dotted rectangle shows an accumulation of immunopositive cells in the middle part of the ML; the orange arrow indicates the EDC, the blue arrows indicate the surface of an undifferentiated HuCD+ cell. (J) An enlarged fragment in the purple rectangle in I, designations are as in (C,E). (K) An accumulation of HuCD+ cells in the deep layers of the ML; forming foci of neuronal differentiation (in white dotted rectangles) in the lateral zone; the dark blue arrows indicate developing projection neurons. (L) An enlarged fragment of the ML, HuCD+ cells with an immunonegative nucleus (orange arrows), other designations are as in (E,K). (M) Small HuCD+ cells and their clusters (in green ovals) in the surface layers of granular eminences (GrEm). (N) An enlarged fragment of HuCD+ clusters in the surface layers of GrEm. (O) Immunolocalization of HuCD in the ventromedial part of the BZ, EDC is indicated by orange arrows. (P) An enlarged fragment showing the morphology of HuCD+ EDCs (orange arrows). (Q) Comparative distribution of HuCD+ cells in various regions of the cerebellum of O. keta (M ± SD); significant intergroup differences between the granular layer, dorsal, dorsolateral, lateral and basal zones are indicated by # (p < 0.05); ## (p < 0.01); (n = 5 in each group); one-way analysis of variance (ANOVA). The blue columns represent type I cells, the white ones—type II, the gray ones—type III, the black ones—type IV, the red ones—type V, and the yellow ones—type VI. Scale: (A,E,G,I,K,M,O) 100 µm; (B–C,F,H,J,L,N,P) 50 µm.
Figure 10.
Immunohistochemical labeling of HuCD in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) The localization of HuCD in the dorsal part of the cerebellum (pictogram), the HuCD-negative dorsal matrix zone (DMZ) is shown in a blue rectangle, paired HuCD+ neurons are indicated by yellow arrows. (B) An enlarged fragment of the DMZ, HuCD+ cells are localized at the border of the granular layer (GrL) and molecular layer (ML), eurydendroid cells (EDCs) with axonal arborization (white arrows), undifferentiated HuCD+ cells in the ML are indicated by yellow arrows. (C) A fragment of the dorsolateral part of the ML containing intensely labeled HuCD+ cells (yellow arrows), superficially located HuCD+ cells with an immunonegative nucleus (orange arrows), HuCD-negative adult-type neural stem/progenitor cells (aNSPCs) (blue arrows), HuCD-negative radially migrating cells (green arrows). (D) The dorsal region adjacent to the DMZ, containing HuCD+ cells (yellow arrows). (E) HuCD+ cells in the dorsal part of the cerebellum: superficial small undifferentiated cells (red arrows), oval single cells or forming small clusters of cells (yellow arrows), small moderately labeled bipolar cells (blue arrows); HuCD+ neurons forming connections in the ML (in the blue dotted rectangle); axonal afferent HuCD+ connections, at the border of the ML and GL (white arrow). (F) Morphogenetic patterns of HuCD expression in cells of various types, designations are as in (C,E). (G) Mass labeling of HuCD in cells of the surface layers of the dorsolateral zone of ML; in the green dotted rectangle HuCD+ EDCs are indicated. (H) An enlarged fragment in the green dotted rectangle in (G), the EDC axon with varicose microcytosculpture is indicated by a white arrow. (I) Heterogeneous HuCD+ cells in the lateral part of the superficial and middle layers of the ML; the purple dotted rectangle shows an accumulation of immunopositive cells in the middle part of the ML; the orange arrow indicates the EDC, the blue arrows indicate the surface of an undifferentiated HuCD+ cell. (J) An enlarged fragment in the purple rectangle in I, designations are as in (C,E). (K) An accumulation of HuCD+ cells in the deep layers of the ML; forming foci of neuronal differentiation (in white dotted rectangles) in the lateral zone; the dark blue arrows indicate developing projection neurons. (L) An enlarged fragment of the ML, HuCD+ cells with an immunonegative nucleus (orange arrows), other designations are as in (E,K). (M) Small HuCD+ cells and their clusters (in green ovals) in the surface layers of granular eminences (GrEm). (N) An enlarged fragment of HuCD+ clusters in the surface layers of GrEm. (O) Immunolocalization of HuCD in the ventromedial part of the BZ, EDC is indicated by orange arrows. (P) An enlarged fragment showing the morphology of HuCD+ EDCs (orange arrows). (Q) Comparative distribution of HuCD+ cells in various regions of the cerebellum of O. keta (M ± SD); significant intergroup differences between the granular layer, dorsal, dorsolateral, lateral and basal zones are indicated by # (p < 0.05); ## (p < 0.01); (n = 5 in each group); one-way analysis of variance (ANOVA). The blue columns represent type I cells, the white ones—type II, the gray ones—type III, the black ones—type IV, the red ones—type V, and the yellow ones—type VI. Scale: (A,E,G,I,K,M,O) 100 µm; (B–C,F,H,J,L,N,P) 50 µm.
![Ijms 26 09267 g010 Ijms 26 09267 g010]()
Figure 11.
Comparative distribution of immunohistochemical markers in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Comparative distribution of PCNA+, Nes+ and Vim+ cells in different regions of the cerebellum (M ± SD); significant intergroup differences are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The green columns represent PCNA+ cells, the yellow columns represent Nes+ cells, and the white columns—Vim+ cells. (B) Comparative distribution of PCNA+, HuCD+, Nes+ cells in various regions of the cerebellum (M ± SD); significant intergroup differences are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The green columns represent PCNA+ cells, the white columns—Vim+ cells, and the yellow columns—Nes+ cells.
Figure 11.
Comparative distribution of immunohistochemical markers in the cerebellum of juvenile chum salmon Oncorhynchus keta. (A) Comparative distribution of PCNA+, Nes+ and Vim+ cells in different regions of the cerebellum (M ± SD); significant intergroup differences are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The green columns represent PCNA+ cells, the yellow columns represent Nes+ cells, and the white columns—Vim+ cells. (B) Comparative distribution of PCNA+, HuCD+, Nes+ cells in various regions of the cerebellum (M ± SD); significant intergroup differences are indicated by # (p < 0.05); (n = 5 in each group); one-way analysis of variance (ANOVA). The green columns represent PCNA+ cells, the white columns—Vim+ cells, and the yellow columns—Nes+ cells.
Table 1.
Ultrastructural characteristics of cerebellum in juvenile chum salmon, O. keta.
Table 1.
Ultrastructural characteristics of cerebellum in juvenile chum salmon, O. keta.
| Cells of Molecular Layer |
|---|
| Type of Cells | Stellate Cells | Non-glial aNSPCs (DMZ) |
|---|
| Long axis of cells soma (µm) | 8.47 ± 0.66 | 7.71 ± 0.45 |
| Short axis of cells soma (µm) | 7.48 ± 0.78 | 6.06 ± 0.53 |
| Sample size | N = 22 | N = 18 |
| Nucleus |
| Contour | Round or oval | Round, smooth |
| Long axis (µm) | 6.79 ± 0.76 | 6.65 ± 0.36 |
| Short axis (µm) | 5.02 ± 0.21 | 5.46 ± 0.54 |
| Chromatin | Denser euchromatin and heterochromatin | reticular euchromatin |
| Color | medium | light |
| Nucleoli | 1–2 are rarely found | No or 1 |
| Cytoplasm |
| Percentage/color | medium | light |
| Mitochondria | many | few |
| Vacuoles | some | no |
| Lipid droplets | Yes | no |
| Dense bodies | No | no |
| Cell contacts | No | Non-glial aNSPCs, glial aNSPCs III |
| glial aNSPCs (GrL, ML) |
| Type of Cells | III | IV | DMZ |
| Long axis of cells soma (µm) | 5.55 ± 0.6 | 5.67 ± 1.13 | 5.32 ± 0.92 |
| Short axis of cells soma (µm) | 3.87 ± 0.67 | 3.81 ± 0.42 | 3.07 ± 0.66 |
| Sample size | N = 15 | N = 15 | N = 20 |
| Nucleus |
| Contour | elongated; irregular ± invaginations | ovoid,
irregular ± invaginations | irregular ± invaginations |
| Long axis (µm) | 4.81 ± 0.48 | 4.87 ± 1.21 | 4.73 ± 0.9 |
| Short axis (µm) | 3.39 ± 0.63 | 3.39 ± 0.35 | 2.62 ± 0.67 |
| Chromatin | evenly distributed; non-clumped | reticulated; clumped hetero | reticulated; clumped hetero |
| Color | medium | dark | dark |
| Nucleoli | 1–2 | 1 or 2, rarely visible | No visible |
| Cytoplasm |
| Percentage/color | scanty, dark | scanty,
medium | scanty,
medium |
| Mitochondria | few | few | single |
| Vacuoles | no | several, rarely encountered | no |
| Lipid droplets | 0–1 | no | no |
| Dense bodies | yes | no | no |
| Localization | ML, GrL | ML, GL, GrL | ML |
| Cell contacts | III, IV | III, IV | III, NEC |
Table 2.
Morphometric and densitometric characteristics of PCNA-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
Table 2.
Morphometric and densitometric characteristics of PCNA-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
| Brain Areas | Type of Cells | Cell Size, µm * | Optical Density **, UOD |
|---|
| Dorsal zone | Oval | 5.2 ± 0.8/3.4 ± 0.6 | +++ |
| Oval | 7.0 ± 0.6/5.4 ± 0.8 | +++ |
| Oval | 7.4 ± 0.9/3.2 ± 0.4 | ++/+++ |
| Elongated | 10.9 ± 1.1/3.6 ± 0.5 | ++/+++ |
| Elongated | 16.4 ± 1.1/3.1 ± 0.3 | ++ |
| Lateral zone | Oval | 4.7 ± 0.5/3.5 ± 0.5 | +++ |
| Oval | 7.1 ± 0.7/3.6 ± 0.7 | ++/+++ |
| Elongated | 8.5 ± 0.8/3.3 ± 0.6 | +++ |
| Elongated | 15.5 ± 1.3/3 ± 0.3 | ++/+++ |
| Basal zone | Oval | 4.4 ± 0.7/3.4 ± 0.6 | ++/+++ |
| Oval | 6.9 ± 0.8/3.9 ± 0.6 | ++/+++ |
| Granular layer | Round | 4.5 ± 0.1/3.7 ± 0.6 | ++/+++ |
| Oval | 6.1 ± 0.5/4.3 ± 0.4 | ++/+++ |
| Granular eminence | Round | 5.3 ± 0.5/3.6 ± 0.7 | ++/+++ |
| Oval | 6.2 ± 0.2/4.2 ± 0.8 | +++ |
Table 3.
Morphometric and densitometric characteristics of GFAP-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
Table 3.
Morphometric and densitometric characteristics of GFAP-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
| Brain Areas | Type of Cells | Cell Size, µm * | Optical Density **, UOD |
|---|
| Dorsal zone rostral | Round | 4.0 ± 0.4/3.4 ± 0.5 | +++ |
| Round | 4.9 ± 0.3/3.8 ± 0.4 | +++ |
| Round | 6.5 ± 0.7/4.5 ± 0.5 | +++ |
| Fibers | |
| 143.6 ± 14.2/1.7 ± 0.4 | ++/+++ |
| 158.4 ± 16.1/2.5 ± 0.2 | +++ |
| Dorsal zone caudal | Round | 4.0 ± 0.4/3.4 ± 0.5 | +++ |
| Round | 4.9 ± 0.3/3.8 ± 0.4 | +++ |
| Round | 6.5 ± 0.7/4.5 ± 0.5 | +++ |
| Fibers | |
| 123.6 ± 34.2/1.6 ± 0.3 | ++/+++ |
| 167.1 ± 10.9/2.5 ± 0.2 | +++ |
| Lateral zone | Round | 6.5 ± 0.4/4.8 ± 0.2 | ++/+++ |
| Round | 7.5 ± 0.7/5.7 ± 0.5 | +++ |
| Round | 8.7 ± 0.7/6.9 ± 0.4 | +++ |
| Fibers | |
| 112.4 ± 14.1/1.7 ± 0.2 | ++/+++ |
| 157 ± 14.4/2.6 ± 0.3 | +++ |
| Basal zone | Round | 3.5 ± 0.3/3.0 ± 0.3 | ++/+++ |
| Round | 4.5 ± 0.3/3.7 ± 0.5 | +++ |
| Round | 5.7 ± 0.4/4.4 ± 0.9 | +++ |
| Fibers | |
| 95.5 ± 34.6/2.1 ± 0.3 | ++/+++ |
| 109.0 ± 28.6/1.3 ± 0.3 | +++ |
| Granular layer | Round | 3.8 ± 0.5/3 ± 0.3 | ++/+++ |
| Round | 5.4 ± 0.6/3.1 ± 0.3 | +++ |
| Fibers | |
| 133.7 ± 24.1/1.7± 0.4 | ++/+++ |
| 147.4 ± 16.3/2.2 ± 0.4 | +++ |
| Granular eminence | Round | 5.4 ± 0.1/3.9 ± 0.8 | ++/+++ |
| Round | 3.7 ± 0.5/3.4 ± 0.6 | +++ |
| Fibers | |
| 111.5 ± 18.3/1.8 ± 0.4 | ++/+++ |
| 151.2 ± 59.9/2.6 ± 0.2 | +++ |
Table 4.
Morphometric and densitometric characteristics of nestin-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
Table 4.
Morphometric and densitometric characteristics of nestin-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
| Brain Areas | Type of Cells | Cell Size, µm * | Optical Density **, UOD |
|---|
| Dorsal zone | Granules | 3 ± 0.3/2.6 ± 0.4 | ++/+++ |
| Round | 4.4 ± 0.5/3.7 ± 0.4 | ++/+++ |
| Oval | 6.8 ± 0.6/5.2 ± 0.6 | +++ |
| Dorso-lateral zone | Granules | 2.9 ± 0.2/2.6 ± 0.2 | ++ |
| Round | 4.4 ± 0.2/3.3 ± 0.5 | ++/+++ |
| Oval | 6.4 ± 0.5/5.7 ± 0.5 | ++/+++ |
| Lateral zone | Granules | 2.8 ± 0.3/2.7 ± 0.2 | ++ |
| Round | 4.1 ± 0.4/3.3 ± 0.4 | ++/+++ |
| Oval | 6.7 ± 0.9/4.8 ± 0.4 | ++/+++ |
| Basal zone | Granules | 2.9 ± 0.2/2.7 ± 0.2 | ++/+++ |
| Round | 4.2 ± 0.5/3.7 ± 0.4 | ++/+++ |
| Oval | 6.4 ± 0.3/5.6 ± 0.5 | +++ |
| Granular layer | Granules | 3.1 ± 0.2/2.6 ± 0.2 | ++/+++ |
| Round | 3.6 ± 0.2/3.2 ± 0.3 | +++ |
| Round | 6.4 ± 0.3/4.9 ± 0.5 | ++/+++ |
| Granular eminence | Granules | 3 ± 0.3/2.6 ± 0.3 | ++/+++ |
| Round | 3.9 ± 0.5/3.5 ± 0.5 | ++/+++ |
| Oval | 6.5 ± 0.4/4.6 ± 0.7 | ++/+++ |
Table 5.
Morphometric and densitometric characteristics of vimentin-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
Table 5.
Morphometric and densitometric characteristics of vimentin-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
| Brain Areas | Type of Cells | Cell Size, µm * | Optical Density **, UOD |
|---|
| Dorsal zone | Granules | 3.1 ± 0.3/2.9 ± 0.3 | ++/+++ |
| Round | 4.5 ± 0.3/3.5 ± 0.5 | +++ |
| Oval | 5.8 ± 0.4/3.8 ± 0.5 | +++ |
| Dorso-lateral zone | Granules | 3 ± 0.3/2.8 ± 0.3 | ++ |
| Round | 4.3 ± 0.4/3.4 ± 0.3 | +++ |
| Oval | 5.6 ± 0.5/3.9 ± 0.5 | ++/+++ |
| Lateral zone | Granules | 3 ± 0.2/2.4 ± 0.2 | ++/+++ |
| Round | 4.4 ± 0.4/3.6 ± 0.2 | ++/+++ |
| Oval | 5.2 ± 0.2/3.6 ± 0.5 | +++ |
| Ventro-lateral zone | Granules | 2.9 ± 0.1/2.5 ± 0.3 | ++/+++ |
| Round | 4.3 ± 0.4/3.4 ± 0.4 | +++ |
| Oval | 5.6 ± 0.3/3.4 ± 0.4 | +++ |
| Basal zone | Granules | 2.8 ± 0.3/2.6 ± 0.4 | ++/+++ |
| Round | 4.2 ± 0.3/3.4 ± 0.5 | +++ |
| Oval | 5.5 ± 0.3/3.8 ± 0.5 | +++ |
| Ventro-medial zone | Granules | 3 ± 0.3/2.7 ± 0.4 | ++/+++ |
| Round | 4.4 ± 0.3/3.5 ± 0.4 | +++ |
| Oval | 5.6 ± 0.4/3.7 ± 0.3 | +++ |
| Granular layer | Granules | 2.8 ± 0.3/2.6 ± 0.3 | ++ |
| Round | 4.3 ± 0.3/3.6 ± 0.5 | +++ |
| Oval | 6.2 ± 0.6/3.5 ± 0.5 | ++/+++ |
| Granular eminence | Granules | 2.7 ± 0.3/2.8 ± 0.4 | ++/+++ |
| Round | 4.3 ± 0.2/3.5± 0.4 | +++ |
| Oval | 5.2 ± 0.6/3.2 ± 0.2 | +++ |
Table 6.
Morphometric and densitometric characteristics of HuCd-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
Table 6.
Morphometric and densitometric characteristics of HuCd-labeled cells (M ± SD) in the intact cerebellum of juvenile chum salmon, O. keta.
| Brain Areas | Type of Cells | Cell Size, µm * | Optical Density **, UOD |
|---|
| Dorsal zone | Round | 6.9 ± 0.5/6.6 ± 0.6 | + |
| Oval | 13.7 ± 1.4/11.7 ± 1 | ++/+++ |
| Bipolar | 11.3 ± 1.4/6.5 ± 0.6 | ++/+++ |
| Oval | 13.4 ± 1.1/11.2 ± 1 | +++ |
| EDC | 27.5 ± 1.3/15.7 ± 1.5 | +++ |
| Dorso-lateral zone | Round | 7.9 ± 0.7/7.6 ± 0.6 | + |
| Oval | 14.2 ± 1.2/11.3 ± 1 | ++/+++ |
| Bipolar | 11.7 ± 1.2/6.7 ± 0.6 | ++/+++ |
| Oval | 15.2 ± 1.3/10.3 ± 1.2 | ++/+++ |
| EDC | 25.4 ± 2.3/14.6 ± 1.8 | +++ |
| Lateral zone | Round | 8.5 ± 0.5/7.3 ± 0.6 | + |
| Oval | 15.4 ± 1.2/11.5 ± 0.9 | ++/+++ |
| Bipolar | 13.4 ± 1.4/7.1 ± 0.6 | ++/+++ |
| Oval | 16.8 ± 1.1/10.2 ± 1.4 | +++ |
| EDC | 28.3 ± 1.3/13.5 ± 1.7 | +++ |
| Basal zone | Round | 8.6 ± 0.5/8.4 ± 0.6 | + |
| Oval | 12.5 ± 1/10.8 ± 0.9 | ++/+++ |
| Oval | 14.9 ± 1.1/9.3 ± 1.1 | +++ |
| EDC | 29.6 ± 2.8/15.3 ± 1.4 | +++ |
| Granular layer | Oval | 4.1 ± 0.3/3.2 ± 0.4 | ++/+++ |
| Round | 5.9 ± 0.6/5.1 ± 0.6 | ++/+++ |
| Granular eminence | Oval | 5.3 ± 0.5/4.6 ± 0.6 | ++/+++ |
| Oval | 6.7 ± 0.6/5.3 ± 0.8 | ++/+++ |
Table 7.
Characteristics of primary antibodies used in immunohistochemical studies.
Table 7.
Characteristics of primary antibodies used in immunohistochemical studies.
| No. | Antibodies | Manufacturer | Dilution | Catalog Number | Marker |
|---|
| 1 | GFAP | Abcam, Cambridge CB2 0AX, UK. | 1:300 | GF5 Catalog No. ab10062 | GFAP |
| 2 | PCNA | Novus Biologicals, Centennial, CO, USA | 1:300 | Catalog No. NB500-106, Lot A2, | PCNA |
| 3 | Vimentin | Abcam, Cambridge CB2 0AX, UK. | 1:300 | Catalog No. ab28028 | Vimentin |
| 4 | Nestin | Abcam, Cambridge CB2 0AX, UK. | 1:300 | clone 2C1.3A11; Catalog No. ab18102 | Nestin |
| 5 | HuCD | Invitrogen™, Thermo Fisher Scientific, Waltham, MA, USA. | 1:300 | clone 16A11; Catalog No. A21271; | HuCD |