Tumor Innervation: From Bystander to Emerging Therapeutic Target for Cancer
Abstract
1. Introduction
2. Tumor Innervation
2.1. Tumor-Associated Nerves
2.2. Origins of Tumor-Associated Neurons
2.2.1. PNI
2.2.2. SNF
2.3. Tumor–Nerve Interaction
3. Functional Roles of Neuronal Effectors in Cancer
3.1. NGF
3.2. BDNF
3.3. CGRP
3.4. NE
3.5. SP
4. Therapeutic Strategies for Tumor Denervation
4.1. Neurotrophic Signaling Blockade
4.2. Exosome Depletion
4.3. Axon Modulation
4.4. Targeting TRPV1
4.5. Targeting Adrenergic Signaling
4.6. Direct Denervation
4.7. Fiber-Specific Denervation and Imaging-Guided Nerve Mapping
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| ANO1 | Anoctamin-1 (calcium-activated chloride channel) |
| APOE | Apolipoprotein E |
| BDNF | Brain-derived neurotrophic factor |
| CAFs | Cancer-associated fibroblasts |
| cAMP | Cyclic adenosine monophosphate |
| CGRP | Calcitonin gene-related peptide |
| DCX | Doublecortin |
| DCC | Deleted in colorectal carcinoma |
| GDNF | Glial cell line-derived neurotrophic factor |
| GPCR | G protein–coupled receptor |
| ICD | Intracellular domain |
| MAP1B | Microtubule-associated protein 1B |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| MNT | Macrophage to neuron-like cell transition |
| NE | Norepinephrine |
| NeuN | Neuronal nuclei |
| NGF | Nerve growth factor |
| NK1R | Neurokinin-1 receptor |
| p75NTR | p75 neurotrophin receptor |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| PNI | Perineural invasion |
| POU4F1 | POU domain, class 4, transcription factor 1 (BRN3A) |
| RET | Rearranged during transfection (RET receptor tyrosine kinase) |
| RAMP1 | Receptor activity-modifying protein 1 |
| SHANK | SH3 and multiple ankyrin repeat domains protein |
| Smad3 | Mothers against decapentaplegic homolog 3 |
| SNF | Spontaneous nerve formation |
| SP | Substance P |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAMs | Tumor-associated macrophages |
| TGF-β1 | Transforming growth factor beta 1 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| TRPV4 | Transient receptor potential vanilloid 4 |
| TrkA | Tropomyosin receptor kinase A |
| TrkB | Tropomyosin receptor kinase B |
| TUBB3 | β3-tubulin |
| VEGF | Vascular endothelial growth factor |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.T.; Bhattarai, T.S.; Schram, A.M.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; Chakravarty, D.; Phillips, S.; Kandoth, C.; Penson, A.; et al. Accelerating Discovery of Functional Mutant Alleles in Cancer. Cancer Discov. 2018, 8, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Tetzlaff, S.K.; Reyhan, E.; Layer, N.; Bengtson, C.P.; Heuer, A.; Schroers, J.; Faymonville, A.J.; Langeroudi, A.P.; Drewa, N.; Keifert, E.; et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell 2025, 188, 390–411.e336. [Google Scholar] [CrossRef]
- Hanahan, D.; Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 2023, 41, 573–580. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wissmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor Innervation: Cancer Has Some Nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Tang, P.C.; Chung, J.Y.; Liao, J.; Chan, M.K.; Chan, A.S.; Cheng, G.; Li, C.; Huang, X.R.; Ng, C.S.; Lam, E.W.; et al. Single-cell RNA sequencing uncovers a neuron-like macrophage subset associated with cancer pain. Sci. Adv. 2022, 8, eabn5535. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Albores-Garcia, D.; Cervantes-Villagrana, A.R.; Garcia-Acevez, S.J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Entschladen, F.; Palm, D.; Lang, K.; Drell, T.L.t.; Zaenker, K.S. Neoneurogenesis: Tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med. Hypotheses 2006, 67, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Lolas, G.; Bianchi, A.; Syrigos, K.N. Tumour-induced neoneurogenesis and perineural tumour growth: A mathematical approach. Sci. Rep. 2016, 6, 20684. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, Z.; Jiang, X.; Zhao, Y.; Wen, P.; Wang, J.; Li, J.; Tanaka, M.; Dan, S.; Zhang, Y.; et al. Regulating tumor innervation by nanodrugs potentiates cancer immunochemotherapy and relieve chemotherapy-induced neuropathic pain. Biomaterials 2024, 309, 122603. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Brundl, J.; Schneider, S.; Weber, F.; Zeman, F.; Wieland, W.F.; Ganzer, R. Computerized quantification and planimetry of prostatic capsular nerves in relation to adjacent prostate cancer foci. Eur. Urol. 2014, 65, 802–808. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Korber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galvan, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Shalabi, S.; Belayachi, A.; Larrivee, B. Involvement of neuronal factors in tumor angiogenesis and the shaping of the cancer microenvironment. Front. Immunol. 2024, 15, 1284629. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S.S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huo, R.; He, K.; Li, W.; Gao, Y.; He, W.; Yu, M.; Jiang, S.H.; Xue, J. Increased nerve density adversely affects outcome in colorectal cancer and denervation suppresses tumor growth. J. Transl. Med. 2025, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, D.; Mattson, B.; Talbot, S.; Gleber-Netto, F.O.; Amit, M. Targeting the peripheral neural-tumour microenvironment for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 780–796. [Google Scholar] [CrossRef]
- Di Chiaro, P.; Nacci, L.; Arco, F.; Brandini, S.; Polletti, S.; Palamidessi, A.; Donati, B.; Soriani, C.; Gualdrini, F.; Frige, G.; et al. Mapping functional to morphological variation reveals the basis of regional extracellular matrix subversion and nerve invasion in pancreatic cancer. Cancer Cell 2024, 42, 662–681.e10. [Google Scholar] [CrossRef]
- Kannan, A.; Clouston, D.; Frydenberg, M.; Ilic, D.; Karim, M.N.; Evans, S.M.; Toivanen, R.; Risbridger, G.P.; Taylor, R.A. Neuroendocrine cells in prostate cancer correlate with poor outcomes: A systematic review and meta-analysis. BJU Int. 2022, 130, 420–433. [Google Scholar] [CrossRef]
- Rutledge, A.; Jobling, P.; Walker, M.M.; Denham, J.W.; Hondermarck, H. Spinal Cord Injuries and Nerve Dependence in Prostate Cancer. Trends Cancer 2017, 3, 812–815. [Google Scholar] [CrossRef]
- Massen, M.; Thijssen, M.S.; Rademakers, G.; Idris, M.; Wouters, K.A.D.; van der Meer, J.R.M.; Buekers, N.; Huijgen, D.; Samarska, I.V.; Weijenberg, M.P.; et al. Neuronal Distribution in Colorectal Cancer: Associations With Clinicopathological Parameters and Survival. Mod. Pathol. 2024, 37, 100565. [Google Scholar] [CrossRef]
- Chen, X.; Geng, Y.; Wei, G.; He, D.; Lv, J.; Wen, W.; Xiang, F.; Tao, K.; Wu, C. Neural Circuitries between the Brain and Peripheral Solid Tumors. Cancer Res. 2024, 84, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front. Physiol. 2012, 3, 97. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.O.; Bergmann, F.; Kadihasanoglu, M.; Altintas, B.; Demir, I.E.; Hinz, U.; Muller, M.W.; Giese, T.; Buchler, M.W.; Giese, N.A.; et al. Pancreatic neuropathy and neuropathic pain—A comprehensive pathomorphological study of 546 cases. Gastroenterology 2009, 136, 177–186.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Istvanffy, R.; Ye, L.; Teller, S.; Laschinger, M.; Diakopoulos, K.N.; Gorgulu, K.; Li, Q.; Ren, L.; Jager, C.; et al. Phenotype screens of murine pancreatic cancer identify a Tgf-alpha-Ccl2-paxillin axis driving human-like neural invasion. J. Clin. Investig. 2023, 133, e166333. [Google Scholar] [CrossRef]
- Niu, Y.; Forster, S.; Muders, M. The Role of Perineural Invasion in Prostate Cancer and Its Prognostic Significance. Cancers 2022, 14, 4065. [Google Scholar] [CrossRef]
- Wang, H.; Huo, R.; He, K.; Cheng, L.; Zhang, S.; Yu, M.; Zhao, W.; Li, H.; Xue, J. Perineural invasion in colorectal cancer: Mechanisms of action and clinical relevance. Cell. Oncol. 2024, 47, 1–17. [Google Scholar] [CrossRef]
- Qin, L.; Heng, Y.; Deng, S.; Gu, J.; Mao, F.; Xue, Y.; Jiang, Z.; Wang, J.; Cheng, D.; Wu, K.; et al. Perineural invasion affects prognosis of patients undergoing colorectal cancer surgery: A propensity score matching analysis. BMC Cancer 2023, 23, 452. [Google Scholar] [CrossRef]
- Wu, M.P.; Reinshagen, K.L.; Cunnane, M.B.; Shalhout, S.Z.; Kaufman, H.L.; Miller, D.; Emerick, K.S. Clinical Perineural Invasion and Immunotherapy for Head and Neck Cutaneous Squamous Cell Carcinoma. Laryngoscope 2022, 132, 1213–1218. [Google Scholar] [CrossRef]
- Itami, T.; Kurokawa, Y.; Hagi, T.; Nagano, S.; Nakamoto, R.; Kamakura, Y.; Takahashi, T.; Saito, T.; Yamamoto, K.; Momose, K.; et al. Sympathetic innervation induced by nerve growth factor promotes malignant transformation in gastric cancer. Sci. Rep. 2025, 15, 3824. [Google Scholar] [CrossRef]
- Chen, X.; Duan, H.; Zhao, H.; He, F.; Yin, L.; Liu, Y.; Wang, L.; Chen, C. Perineural invasion in cervical cancer: A multicenter retrospective study. Eur. J. Surg. Oncol. 2024, 50, 108313. [Google Scholar] [CrossRef]
- Barnkob, M.B.; Michaels, Y.S.; Andre, V.; Macklin, P.S.; Gileadi, U.; Valvo, S.; Rei, M.; Kulicke, C.; Chen, J.L.; Jain, V.; et al. Semmaphorin 3 A causes immune suppression by inducing cytoskeletal paralysis in tumour-specific CD8(+) T cells. Nat. Commun. 2024, 15, 3173. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, J.D.; Tseveleki, V.; Dimitrakopoulos, F.I.; Konstantinidis, K.; Kalofonos, H. Radical Tumor Denervation Activates Potent Local and Global Cancer Treatment. Cancers 2023, 15, 3758. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.; Chung, J.Y.; Tang, P.C.; Chan, A.S.; Ho, J.Y.; Lin, T.P.; Chen, J.; Leung, K.T.; To, K.F.; Lan, H.Y.; et al. TGF-beta signaling networks in the tumor microenvironment. Cancer Lett. 2022, 550, 215925. [Google Scholar] [CrossRef]
- Thiel, V.; Renders, S.; Panten, J.; Dross, N.; Bauer, K.; Azorin, D.; Henriques, V.; Vogel, V.; Klein, C.; Leppa, A.M.; et al. Characterization of single neurons reprogrammed by pancreatic cancer. Nature 2025, 640, 1042–1051. [Google Scholar] [CrossRef]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct. Target. Ther. 2017, 2, 16036. [Google Scholar] [CrossRef]
- Schmitd, L.B.; Perez-Pacheco, C.; D’Silva, N.J. Nerve density in cancer: Less is better. FASEB BioAdvances 2021, 3, 773–786. [Google Scholar] [CrossRef]
- Hondermarck, H.; Jiang, C.C. Time to Introduce Nerve Density in Cancer Histopathologic Assessment. Clin. Cancer Res. 2023, 29, 2342–2344. [Google Scholar] [CrossRef]
- Venkatesh, H.S. The neural regulation of cancer. Science 2019, 366, 965. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun. 2021, 41, 642–660. [Google Scholar] [CrossRef]
- Bapat, A.A.; Hostetter, G.; Von Hoff, D.D.; Han, H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer 2011, 11, 695–707. [Google Scholar] [CrossRef]
- Sone, Y.; Takatori, S.; Ochi, E.; Zamami, Y.; Matsuyama, A.; Fukuhara, S.; Goda, M.; Kitamura, Y.; Kawasaki, H. Nerve Growth Factor Facilitates the Innervation of Perivascular Nerves in Tumor-Derived Neovasculature in the Mouse Cornea. Pharmacology 2017, 99, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Repasky, E.; Mohammadpour, H. Targeting nerve growth factor: An Achilles’ heel for tumors? J. Immunother. Cancer 2025, 13, e011609. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H. p53, apoptosis and axon-guidance molecules. Cell Death Differ. 2005, 12, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Chedotal, A.; Kerjan, G.; Moreau-Fauvarque, C. The brain within the tumor: New roles for axon guidance molecules in cancers. Cell Death Differ. 2005, 12, 1044–1056. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhou, S.L.; Zhou, Z.J.; Luo, C.B.; Chen, E.B.; Zhan, H.; Wang, P.C.; Dai, Z.; Zhou, J.; Fan, J.; et al. Overexpression of semaphorin 3A promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma after curative resection. Oncotarget 2016, 7, 51733–51746. [Google Scholar] [CrossRef]
- Lupo, F.; Pezzini, F.; Pasini, D.; Fiorini, E.; Adamo, A.; Veghini, L.; Bevere, M.; Frusteri, C.; Delfino, P.; D’Agosto, S.; et al. Axon guidance cue SEMA3A promotes the aggressive phenotype of basal-like PDAC. Gut 2024, 73, 1321–1335. [Google Scholar] [CrossRef]
- Tamagnone, L.; Franzolin, G. Targeting Semaphorin 4D in Cancer: A Look from Different Perspectives. Cancer Res. 2019, 79, 5146–5148. [Google Scholar] [CrossRef]
- Jurcak, N.R.; Rucki, A.A.; Muth, S.; Thompson, E.; Sharma, R.; Ding, D.; Zhu, Q.; Eshleman, J.R.; Anders, R.A.; Jaffee, E.M.; et al. Axon Guidance Molecules Promote Perineural Invasion and Metastasis of Orthotopic Pancreatic Tumors in Mice. Gastroenterology 2019, 157, 838–850.e6. [Google Scholar] [CrossRef]
- Jiang, S.H.; Hu, L.P.; Wang, X.; Li, J.; Zhang, Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, B.; Zhang, P.; Yuan, Y.; Yue, S.; Chen, X.; Liang, J.; Tang, Z.; Zhang, B. Neuroscience of cancer: Unraveling the complex interplay between the nervous system, the tumor and the tumor immune microenvironment. Mol. Cancer 2025, 24, 24. [Google Scholar] [CrossRef]
- Yin, T.; Wang, G.; Wang, L.; Mudgal, P.; Wang, E.; Pan, C.C.; Alexander, P.B.; Wu, H.; Cao, C.; Liang, Y.; et al. Breaking NGF-TrkA immunosuppression in melanoma sensitizes immunotherapy for durable memory T cell protection. Nat. Immunol. 2024, 25, 268–281. [Google Scholar] [CrossRef]
- Jin, Z.; Lu, Y.; Wu, X.; Pan, T.; Yu, Z.; Hou, J.; Wu, A.; Li, J.; Yang, Z.; Li, C.; et al. The cross-talk between tumor cells and activated fibroblasts mediated by lactate/BDNF/TrkB signaling promotes acquired resistance to anlotinib in human gastric cancer. Redox Biol. 2021, 46, 102076. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.M.; Feng, L.; Suthe, S.R.; Weng, T.H.; Hu, C.Y.; Liu, Y.Z.; Wu, Z.G.; Wang, M.H.; Yao, H.P. Therapeutic efficacy of a novel humanized antibody-drug conjugate recognizing plexin-semaphorin-integrin domain in the RON receptor for targeted cancer therapy. J. Immunother. Cancer 2019, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, M.; Feng, K.; Lv, Q.; Zhang, Y. Semaphorin 3C (Sema3C) reshapes stromal microenvironment to promote hepatocellular carcinoma progression. Signal Transduct. Target. Ther. 2024, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Giovannelli, P.; Migliaccio, A.; Castoria, G. The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: When the dialogue replaces the monologue. Cell Biosci. 2023, 13, 60. [Google Scholar] [CrossRef]
- Ayanlaja, A.A.; Zhang, B.; Ji, G.; Gao, Y.; Wang, J.; Kanwore, K.; Gao, D. The reversible effects of glial cell line-derived neurotrophic factor (GDNF) in the human brain. Semin. Cancer Biol. 2018, 53, 212–222. [Google Scholar] [CrossRef]
- Wu, X.; Rauch, T.A.; Zhong, X.; Bennett, W.P.; Latif, F.; Krex, D.; Pfeifer, G.P. CpG island hypermethylation in human astrocytomas. Cancer Res. 2010, 70, 2718–2727. [Google Scholar] [CrossRef]
- Weng, W.C.; Lin, K.H.; Wu, P.Y.; Ho, Y.H.; Liu, Y.L.; Wang, B.J.; Chen, C.C.; Lin, Y.C.; Liao, Y.F.; Lee, W.T.; et al. VEGF expression correlates with neuronal differentiation and predicts a favorable prognosis in patients with neuroblastoma. Sci. Rep. 2017, 7, 11212. [Google Scholar] [CrossRef]
- Lu, K.V.; Chang, J.P.; Parachoniak, C.A.; Pandika, M.M.; Aghi, M.K.; Meyronet, D.; Isachenko, N.; Fouse, S.D.; Phillips, J.J.; Cheresh, D.A.; et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012, 22, 21–35. [Google Scholar] [CrossRef]
- Regan, J.L.; Schumacher, D.; Staudte, S.; Steffen, A.; Lesche, R.; Toedling, J.; Jourdan, T.; Haybaeck, J.; Golob-Schwarzl, N.; Mumberg, D.; et al. Identification of a neural development gene expression signature in colon cancer stem cells reveals a role for EGR2 in tumorigenesis. iScience 2022, 25, 104498. [Google Scholar] [CrossRef]
- Zhong, Z.Y.; Shi, B.J.; Zhou, H.; Wang, W.B. CD133 expression and MYCN amplification induce chemoresistance and reduce average survival time in pediatric neuroblastoma. J. Int. Med. Res. 2018, 46, 1209–1220. [Google Scholar] [CrossRef]
- Vaidya, M.; Sreerama, S.; Gonzalez-Vega, M.; Smith, J.; Field, M.; Sugaya, K. Coculture with Neural Stem Cells May Shift the Transcription Profile of Glioblastoma Multiforme towards Cancer-Specific Stemness. Int. J. Mol. Sci. 2023, 24, 3242. [Google Scholar] [CrossRef] [PubMed]
- Di Tomaso, T.; Mazzoleni, S.; Wang, E.; Sovena, G.; Clavenna, D.; Franzin, A.; Mortini, P.; Ferrone, S.; Doglioni, C.; Marincola, F.M.; et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 2010, 16, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, E.; Persano, L.; Pistollato, F.; Moro, E.; Frasson, C.; Porazzi, P.; Della Puppa, A.; Bresolin, S.; Battilana, G.; Indraccolo, S.; et al. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis. 2013, 4, e500. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, T.; Liu, A.Y.; Ouyang, G. Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget 2015, 6, 39550–39563. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e569. [Google Scholar] [CrossRef]
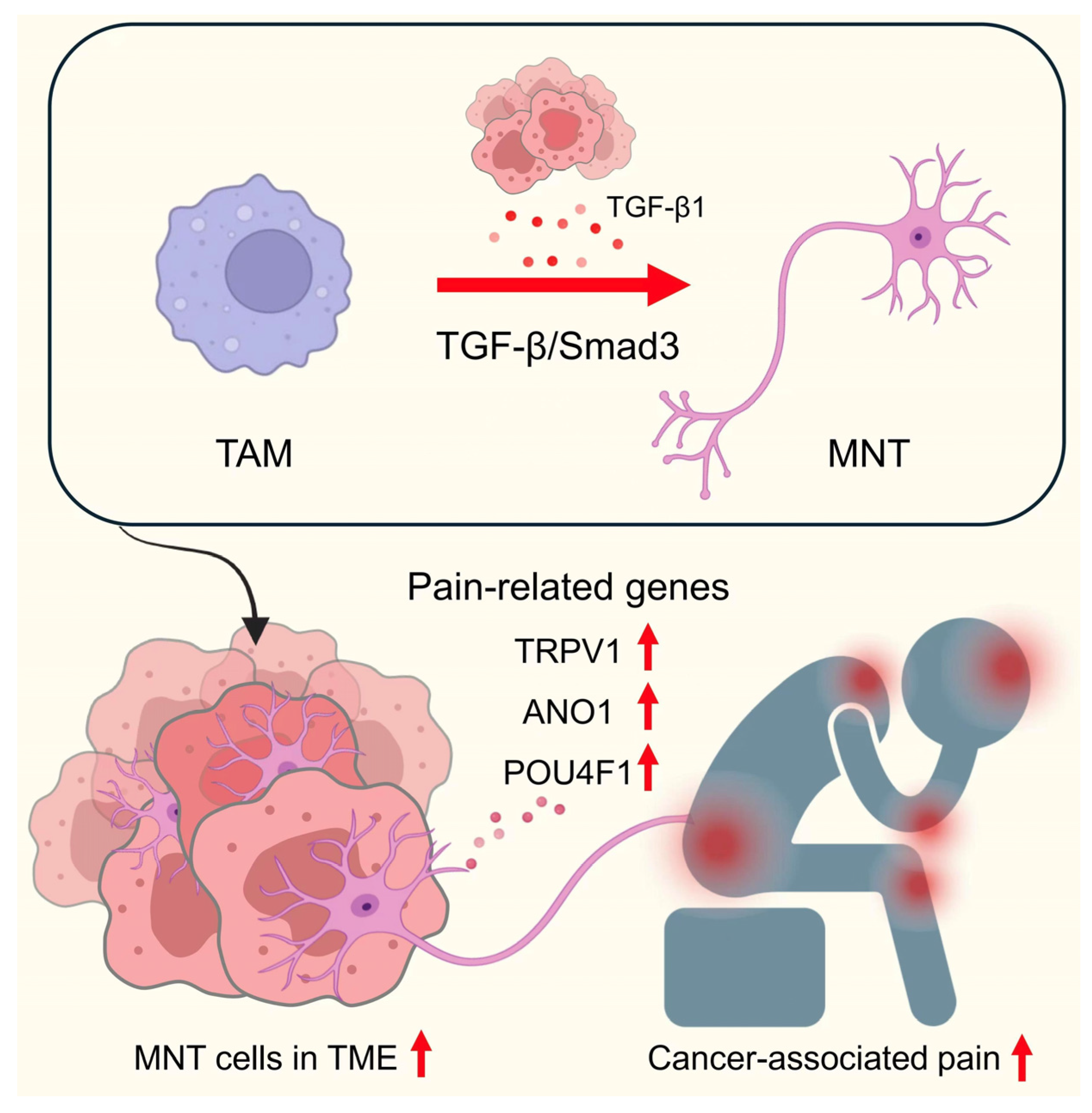
- Li, H.; Zhao, L.; Li, J.; Zhang, K.; Bai, W.; Chen, Y. Neuron-like macrophage differentiation via the APOE-TREM2 axis contributes to chronic pain in nasopharyngeal carcinoma. Cell Biol. Toxicol. 2025, 41, 86. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, J.; Yu, L.; Huang, Y.; Hu, Y. Cancer-nervous system crosstalk: From biological mechanism to therapeutic opportunities. Mol. Cancer 2025, 24, 133. [Google Scholar] [CrossRef]
- Schmitd, L.B.; Perez-Pacheco, C.; Bellile, E.L.; Wu, W.; Casper, K.; Mierzwa, M.; Rozek, L.S.; Wolf, G.T.; Taylor, J.M.G.; D’Silva, N.J. Spatial and Transcriptomic Analysis of Perineural Invasion in Oral Cancer. Clin. Cancer Res. 2022, 28, 3557–3572. [Google Scholar] [CrossRef]
- Brown, I.S. Pathology of Perineural Spread. J. Neurol. Surg. B Skull Base 2016, 77, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Hu, X.F.; Feng, X.S.; Gao, S.G. Pleiotrophin promotes perineural invasion in pancreatic cancer. World J. Gastroenterol. 2013, 19, 6555–6558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Tao, L.Y.; Yang, M.W.; Xu, D.P.; Jiang, S.H.; Fu, X.L.; Liu, D.J.; Huo, Y.M.; Liu, W.; Yang, J.Y.; et al. CD74 promotes perineural invasion of cancer cells and mediates neuroplasticity via the AKT/EGR-1/GDNF axis in pancreatic ductal adenocarcinoma. Cancer Lett. 2021, 508, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Balood, M.; Ahmadi, M.; Eichwald, T.; Ahmadi, A.; Majdoubi, A.; Roversi, K.; Roversi, K.; Lucido, C.T.; Restaino, A.C.; Huang, S.; et al. Nociceptor neurons affect cancer immunosurveillance. Nature 2022, 611, 405–412. [Google Scholar] [CrossRef]
- Guo, X.; Pan, Y.; Xiong, M.; Sanapala, S.; Anastasaki, C.; Cobb, O.; Dahiya, S.; Gutmann, D.H. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat. Commun. 2020, 11, 2177. [Google Scholar] [CrossRef]
- Perez-Pacheco, C.; Schmitd, L.B.; Furgal, A.; Bellile, E.L.; Liu, M.; Fattah, A.; Gonzalez-Maldonado, L.; Unsworth, S.P.; Wong, S.Y.; Rozek, L.S.; et al. Increased Nerve Density Adversely Affects Outcome in Oral Cancer. Clin. Cancer Res. 2023, 29, 2501–2512. [Google Scholar] [CrossRef]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Ali, S.R.; Jordan, M.; Nagarajan, P.; Amit, M. Nerve Density and Neuronal Biomarkers in Cancer. Cancers 2022, 14, 4817. [Google Scholar] [CrossRef]
- Chen, X.; Cao, Y.; Zhao, Y.; Ma, Y.; Shi, X.; Wang, J.; Jiang, Z.; Luo, R.; Deng, Z.; Zhou, X.; et al. Neurodegeneration of local sympathetic inputs promotes colorectal cancer progression. Cancer Lett. 2025, 625, 217817. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.T.; Liu, J.; Gan, L.; Jiang, Y. Role of transforming growth factor-beta1 pathway in angiogenesis induced by chronic stress in colorectal cancer. Cancer Biol. Ther. 2024, 25, 2366451. [Google Scholar] [CrossRef]
- Zeng, Z.; Cai, S.; Ye, C.; Li, T.; Tian, Y.; Liu, E.; Cai, J.; Yuan, X.; Yang, H.; Liang, Q.; et al. Neural influences in colorectal cancer progression and therapeutic strategies. Int. J. Color. Dis. 2025, 40, 120. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jiang, C.; Tian, Y.; Liu, Y.; Zhang, H.; Xiang, Z.; Xue, H.; Gu, L.; Xu, Q. Nervous system in colorectal cancer. Cancer Lett. 2024, 611, 217431. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, N.J.; Perez-Pacheco, C.; Schmitd, L.B. The 3D’s of Neural Phenotypes in Oral Cancer: Distance, Diameter, and Density. Adv. Biol. 2023, 7, e2200188. [Google Scholar] [CrossRef] [PubMed]
- Zareba, P.; Flavin, R.; Isikbay, M.; Rider, J.R.; Gerke, T.A.; Finn, S.; Pettersson, A.; Giunchi, F.; Unger, R.H.; Tinianow, A.M.; et al. Perineural Invasion and Risk of Lethal Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 719–726. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef]
- Sinha, S.; Fu, Y.Y.; Grimont, A.; Ketcham, M.; Lafaro, K.; Saglimbeni, J.A.; Askan, G.; Bailey, J.M.; Melchor, J.P.; Zhong, Y.; et al. PanIN Neuroendocrine Cells Promote Tumorigenesis via Neuronal Cross-talk. Cancer Res. 2017, 77, 1868–1879. [Google Scholar] [CrossRef]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl. Med. 2018, 6, 89. [Google Scholar] [CrossRef]
- Molloy, N.H.; Read, D.E.; Gorman, A.M. Nerve growth factor in cancer cell death and survival. Cancers 2011, 3, 510–530. [Google Scholar] [CrossRef]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Friess, H.; Ceyhan, G.O. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim. Biophys. Acta 2016, 1866, 37–50. [Google Scholar] [CrossRef]
- Tacconelli, A.; Farina, A.R.; Cappabianca, L.; Desantis, G.; Tessitore, A.; Vetuschi, A.; Sferra, R.; Rucci, N.; Argenti, B.; Screpanti, I.; et al. TrkA alternative splicing: A regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 2004, 6, 347–360. [Google Scholar] [CrossRef]
- Singh, R.; Karri, D.; Shen, H.; Shao, J.; Dasgupta, S.; Huang, S.; Edwards, D.P.; Ittmann, M.M.; O’Malley, B.W.; Yi, P. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J. Clin. Investig. 2018, 128, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Oikawa, M.; Misaka, T.; Ishida, T.; Takeishi, Y. Heart Failure Post-Myocardial Infarction Promotes Mammary Tumor Growth Through the NGF-TRKA Pathway. JACC CardioOncology 2024, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, G.; Liu, Y.; Feng, L.; Wang, M.; Liu, J.; Chen, Y.; Ouyang, L. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm. Sin. B 2021, 11, 355–372, Correction in Acta Pharm. Sin. B 2022, 12, 2963–2964. [Google Scholar] [CrossRef] [PubMed]
- Romon, R.; Adriaenssens, E.; Lagadec, C.; Germain, E.; Hondermarck, H.; Le Bourhis, X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol. Cancer 2010, 9, 157. [Google Scholar] [CrossRef]
- Taylor, K.R.; Barron, T.; Hui, A.; Spitzer, A.; Yalcin, B.; Ivec, A.E.; Geraghty, A.C.; Hartmann, G.G.; Arzt, M.; Gillespie, S.M.; et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature 2023, 623, 366–374. [Google Scholar] [CrossRef]
- Contreras-Zarate, M.J.; Day, N.L.; Ormond, D.R.; Borges, V.F.; Tobet, S.; Gril, B.; Steeg, P.S.; Cittelly, D.M. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene 2019, 38, 4685–4699. [Google Scholar] [CrossRef]
- Pearse, R.N.; Swendeman, S.L.; Li, Y.; Rafii, D.; Hempstead, B.L. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood 2005, 105, 4429–4436. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Liu, Z.; Woo, C.W.; Thiele, C.J. Downregulation of Bim by brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from paclitaxel but not etoposide or cisplatin-induced cell death. Cell Death Differ. 2007, 14, 318–326. [Google Scholar] [CrossRef]
- Kimura, S.; Harada, T.; Ijichi, K.; Tanaka, K.; Liu, R.; Shibahara, D.; Kawano, Y.; Otsubo, K.; Yoneshima, Y.; Iwama, E.; et al. Expression of brain-derived neurotrophic factor and its receptor TrkB is associated with poor prognosis and a malignant phenotype in small cell lung cancer. Lung Cancer 2018, 120, 98–107. [Google Scholar] [CrossRef]
- Smeele, P.; d’Almeida, S.M.; Meiller, C.; Chene, A.L.; Liddell, C.; Cellerin, L.; Montagne, F.; Deshayes, S.; Benziane, S.; Copin, M.C.; et al. Brain-derived neurotrophic factor, a new soluble biomarker for malignant pleural mesothelioma involved in angiogenesis. Mol. Cancer 2018, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- McIlvried, L.A.; Atherton, M.A.; Horan, N.L.; Goch, T.N.; Scheff, N.N. Sensory Neurotransmitter Calcitonin Gene-Related Peptide Modulates Tumor Growth and Lymphocyte Infiltration in Oral Squamous Cell Carcinoma. Adv. Biol. 2022, 6, e2200019. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Heintz, O.K.; Jain, A.; Sun, L.; Seshan, M.L.; Peterse, D.; Lindholm, A.E.; Anchan, R.M.; et al. Nociceptor-to-macrophage communication through CGRP/RAMP1 signaling drives endometriosis-associated pain and lesion growth in mice. Sci. Transl. Med. 2024, 16, eadk8230. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, B.; Liu, J.; Li, Y.; Liao, Y.; Wang, S.; Tao, S.; Hu, S.; He, W.; Shu, Q.; et al. The CGRP/macrophage axis signal facilitates inflammation recovery in the intestine. Clin. Immunol. 2022, 245, 109154. [Google Scholar] [CrossRef]
- Darragh, L.B.; Nguyen, A.; Pham, T.T.; Idlett-Ali, S.; Knitz, M.W.; Gadwa, J.; Bukkapatnam, S.; Corbo, S.; Olimpo, N.A.; Nguyen, D.; et al. Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs. Med 2024, 5, 254–270.e8. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Hu, J.; Lu, R.; Zhang, Y.; Li, W.; Hu, Q.; Chen, C.; Liu, Z.; Zhang, W.; Chen, L.; Xu, R.; et al. beta-adrenergic receptor inhibition enhances oncolytic herpes virus propagation through STAT3 activation in gastric cancer. Cell Biosci. 2021, 11, 174. [Google Scholar] [CrossRef]
- Feistritzer, C.; Clausen, J.; Sturn, D.H.; Djanani, A.; Gunsilius, E.; Wiedermann, C.J.; Kahler, C.M. Natural killer cell functions mediated by the neuropeptide substance P. Regul. Pept. 2003, 116, 119–126. [Google Scholar] [CrossRef]
- Esteban, F.; Munoz, M.; Gonzalez-Moles, M.A.; Rosso, M. A role for substance P in cancer promotion and progression: A mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer Metastasis Rev. 2006, 25, 137–145. [Google Scholar] [CrossRef]
- Isorna, I.; Gonzalez-Moles, M.A.; Munoz, M.; Esteban, F. Substance P and Neurokinin-1 Receptor System in Thyroid Cancer: Potential Targets for New Molecular Therapies. J. Clin. Med. 2023, 12, 6409. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef]
- Pu, T.; Sun, J.; Ren, G.; Li, H. Neuro-immune crosstalk in cancer: Mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2025, 10, 176. [Google Scholar] [CrossRef]
- Tseng, T.H.; Shen, C.H.; Huang, W.S.; Chen, C.N.; Liang, W.H.; Lin, T.H.; Kuo, H.C. Activation of neutral-sphingomyelinase, MAPKs, and p75 NTR-mediating caffeic acid phenethyl ester-induced apoptosis in C6 glioma cells. J. Biomed. Sci. 2014, 21, 61. [Google Scholar] [CrossRef]
- Zaninelli, T.H.; Fattori, V.; Heintz, O.K.; Wright, K.R.; Bennallack, P.R.; Sim, D.; Bukhari, H.; Terry, K.L.; Vitonis, A.F.; Missmer, S.A.; et al. Targeting NGF but not VEGFR1 or BDNF signaling reduces endometriosis-associated pain in mice. J. Adv. Res. 2024, 73, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, C.; Liu, Z.; Sun, Y.; Chen, M.; Guo, Y.; Liu, W.; Zhang, C.; Chen, W.; Sun, J.; et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 2022, 34, 1999–2017.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ni, B.; Xie, Y.; Li, Z.; Yuan, L.; Meng, C.; Zhao, T.; Gao, S.; Huang, C.; Wang, H.; et al. Nociceptor neurons promote PDAC progression and cancer pain by interaction with cancer-associated fibroblasts and suppression of natural killer cells. Cell Res. 2025, 35, 362–380. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, G.; Thompson, M.L.; Majuta, L.; Fealk, M.N.; Chartier, S.; Longo, G.; Mantyh, P.W. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014, 74, 7014–7023. [Google Scholar] [CrossRef]
- Zhu, M.; Yea, J.H.; Li, Z.; Qin, Q.; Xu, M.; Xing, X.; Negri, S.; Archer, M.; Mittal, M.; Levi, B.; et al. Pharmacologic or genetic targeting of peripheral nerves prevents peri-articular traumatic heterotopic ossification. Bone Res. 2024, 12, 54. [Google Scholar] [CrossRef]
- Zhu, Z.; Friess, H.; diMola, F.F.; Zimmermann, A.; Graber, H.U.; Korc, M.; Buchler, M.W. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J. Clin. Oncol. 1999, 17, 2419–2428. [Google Scholar] [CrossRef]
- Zhang, W.; He, R.; Yang, W.; Zhang, Y.; Yuan, Q.; Wang, J.; Liu, Y.; Chen, S.; Zhang, S.; Zhang, W.; et al. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. J. Exp. Clin. Cancer Res. 2022, 41, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nong, X.; Chen, Q.; Yang, Y.; Li, J.; Li, Y. Nerve growth factor and vascular endothelial growth factor: Retrospective analysis of 63 patients with salivary adenoid cystic carcinoma. Int. J. Oral Sci. 2010, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Botteri, E.; Gillis, R.D.; Lofling, L.; Le, C.P.; Ziegler, A.I.; Chung, N.C.; Rowe, M.C.; Fabb, S.A.; Hartley, B.J.; et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eadf1147. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, S.; Bianca, P.; Sardina, D.S.; Turdo, A.; Gaggianesi, M.; Veschi, V.; Nicotra, A.; Mangiapane, L.R.; Lo Iacono, M.; Pillitteri, I.; et al. Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nat. Commun. 2021, 12, 5006. [Google Scholar] [CrossRef]
- Lasa, M.; Notarfranchi, L.; Agullo, C.; Gonzalez, C.; Castro, S.; Perez, J.J.; Burgos, L.; Guerrero, C.; Calasanz, M.J.; Flores-Montero, J.; et al. Minimally Invasive Assessment of Peripheral Residual Disease During Maintenance or Observation in Transplant-Eligible Patients With Multiple Myeloma. J. Clin. Oncol. 2025, 43, 125–132. [Google Scholar] [CrossRef]
- Fallon, M.; Sopata, M.; Dragon, E.; Brown, M.T.; Viktrup, L.; West, C.R.; Bao, W.; Agyemang, A. A Randomized Placebo-Controlled Trial of the Anti-Nerve Growth Factor Antibody Tanezumab in Subjects with Cancer Pain Due to Bone Metastasis. Oncologist 2023, 28, e1268–e1278. [Google Scholar] [CrossRef]
- Gregorc, V.; Gaafar, R.M.; Favaretto, A.; Grossi, F.; Jassem, J.; Polychronis, A.; Bidoli, P.; Tiseo, M.; Shah, R.; Taylor, P.; et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2018, 19, 799–811. [Google Scholar] [CrossRef]
- Cavaletti, G.; Bogliun, G.; Marzorati, L.; Zincone, A.; Piatti, M.; Colombo, N.; Franchi, D.; La Presa, M.T.; Lissoni, A.; Buda, A.; et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann. Oncol. 2004, 15, 1439–1442. [Google Scholar] [CrossRef]
- Ho, R.; Eggert, A.; Hishiki, T.; Minturn, J.E.; Ikegaki, N.; Foster, P.; Camoratto, A.M.; Evans, A.E.; Brodeur, G.M. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002, 62, 6462–6466. [Google Scholar]
- Vanhecke, E.; Adriaenssens, E.; Verbeke, S.; Meignan, S.; Germain, E.; Berteaux, N.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 2011, 17, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, Y.; He, Z.; An, Y.; Zhao, W.; Wu, J. Autocrine activity of BDNF induced by the STAT3 signaling pathway causes prolonged TrkB activation and promotes human non-small-cell lung cancer proliferation. Sci. Rep. 2016, 6, 30404. [Google Scholar] [CrossRef]
- Lam, C.T.; Yang, Z.F.; Lau, C.K.; Tam, K.H.; Fan, S.T.; Poon, R.T. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: Implication in hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, Q.; Wang, X.; Ding, J.; Chen, F.; Xiao, Y.; Qin, T.; Qian, W.; Li, J.; Ma, Q.; et al. Nodal Enhances Perineural Invasion in Pancreatic Cancer by Promoting Tumor-Nerve Convergence. J. Healthc. Eng. 2022, 2022, 9658890. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.K.; Armaiz-Pena, G.N.; Nagaraja, A.S.; Sadaoui, N.C.; Ortiz, T.; Dood, R.; Ozcan, M.; Herder, D.M.; Haemmerle, M.; Gharpure, K.M.; et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res. 2018, 78, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shen, K.; Wang, H.; Wang, S.; Wang, X.; Zhu, L.; Zheng, Y.; Zou, T.; Ci, H.; Dong, Q.; et al. Alpha5 nicotine acetylcholine receptor subunit promotes intrahepatic cholangiocarcinoma metastasis. Signal Transduct. Target. Ther. 2024, 9, 63. [Google Scholar] [CrossRef]
- Meng, L.; Liu, B.; Ji, R.; Jiang, X.; Yan, X.; Xin, Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 2019, 17, 2031–2039. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Lin, E.J.; Wang, C.; Choi, E.Y.; Riban, V.; Lin, B.; During, M.J. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 2010, 142, 52–64. [Google Scholar] [CrossRef]
- Wang, X.; Prager, B.C.; Wu, Q.; Kim, L.J.Y.; Gimple, R.C.; Shi, Y.; Yang, K.; Morton, A.R.; Zhou, W.; Zhu, Z.; et al. Reciprocal Signaling between Glioblastoma Stem Cells and Differentiated Tumor Cells Promotes Malignant Progression. Cell Stem Cell 2018, 22, 514–528.e515. [Google Scholar] [CrossRef]
- Yang, X.; Martin, T.A.; Jiang, W.G. Biological influence of brain-derived neurotrophic factor (BDNF) on colon cancer cells. Exp. Ther. Med. 2013, 6, 1475–1481. [Google Scholar] [CrossRef]
- Drexler, R.; Khatri, R.; Sauvigny, T.; Mohme, M.; Maire, C.L.; Ryba, A.; Zghaibeh, Y.; Duhrsen, L.; Salviano-Silva, A.; Lamszus, K.; et al. A prognostic neural epigenetic signature in high-grade glioma. Nat. Med. 2024, 30, 1622–1635. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Liu, Y.; Gao, S.; Yu, Y.; Hu, Z. Serum Levels of BDNF in Patients with Adenoma and Colorectal Cancer. Dis. Markers 2021, 2021, 8867368. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying patients with NTRK fusion cancer. Ann. Oncol. 2019, 30, viii16–viii22. [Google Scholar] [CrossRef]
- Repetto, M.; Chiara Garassino, M.; Loong, H.H.; Lopez-Rios, F.; Mok, T.; Peters, S.; Planchard, D.; Popat, S.; Rudzinski, E.R.; Drilon, A.; et al. NTRK gene fusion testing and management in lung cancer. Cancer Treat. Rev. 2024, 127, 102733. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Suzuki, T.; Hosono, K.; Hayashi, I.; Hashiba, S.; Onuma, Y.; Amano, H.; Kurihara, Y.; Kurihara, H.; Okamoto, H.; et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc. Natl. Acad. Sci. USA 2008, 105, 13550–13555. [Google Scholar] [CrossRef] [PubMed]
- Majima, M.; Ito, Y.; Hosono, K.; Amano, H. CGRP/CGRP Receptor Antibodies: Potential Adverse Effects Due to Blockade of Neovascularization? Trends Pharmacol. Sci. 2019, 40, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, B.; Xu, T.; Jiang, J.; Luo, S.; Chen, W.; Chen, X.; Wang, Y.; Liao, G.; Wang, J.; et al. The neurotransmitter calcitonin gene-related peptide shapes an immunosuppressive microenvironment in medullary thyroid cancer. Nat. Commun. 2024, 15, 5555. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Gao, F.; Liu, G.; Wang, J.; Huang, S.; Ding, F.; Lian, W.; Lv, X.; Guo, Y.; Fan, X.; Zhang, S.; et al. Methylation of CALCA and CALCB in Pancreatic Ductal Adenocarcinoma. Oxidative Med. Cell. Longev. 2021, 2021, 2088345. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.; Wang, X.; Ji, T. Calcitonin gene-related peptide: A promising bridge between cancer development and cancer-associated pain in oral squamous cell carcinoma. Oncol. Lett. 2020, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Wu, F.; Qian, J.; Ochiai, Y.; Lian, G.; Malagola, E.; Zheng, B.; Tu, R.; Zeng, Y.; Kobayashi, H.; et al. Nociceptive neurons promote gastric tumour progression via a CGRP-RAMP1 axis. Nature 2025, 640, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Cesaris, F.; Ferrari, A.; Benemei, S.; Fattori, D.; Chiarugi, A. Effectiveness of anti-CGRP monoclonal antibodies on central symptoms of migraine. Cephalalgia 2022, 42, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, A.; Schenk, H.; Wurthmann, S.; Nsaka, M.; Kleinschnitz, C.; Glas, M.; Holle, D. CGRP antibody therapy in patients with drug resistant migraine and chronic daily headache: A real-world experience. J. Headache Pain 2021, 22, 111. [Google Scholar] [CrossRef]
- Iannone, L.F.; Fattori, D.; Benemei, S.; Chiarugi, A.; Geppetti, P.; De Cesaris, F. Long-Term Effectiveness of Three Anti-CGRP Monoclonal Antibodies in Resistant Chronic Migraine Patients Based on the MIDAS score. CNS Drugs 2022, 36, 191–202. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Y.; Tang, S.; Yang, D.; Zhang, Y.; Zhang, J.; Yang, F.; Zhou, T.; Xia, X.; Chen, Q.; et al. Nociceptive adenosine A(2A) receptor on trigeminal nerves orchestrates CGRP release to regulate the progression of oral squamous cell carcinoma. Int. J. Oral Sci. 2024, 16, 46. [Google Scholar] [CrossRef]
- Deng, G.H.; Liu, J.; Zhang, J.; Wang, Y.; Peng, X.C.; Wei, Y.Q.; Jiang, Y. Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J. Exp. Clin. Cancer Res. 2014, 33, 21. [Google Scholar] [CrossRef]
- Kobayashi, H.; Iida, T.; Ochiai, Y.; Malagola, E.; Zhi, X.; White, R.A.; Qian, J.; Wu, F.; Waterbury, Q.T.; Tu, R.; et al. Neuro-Mesenchymal Interaction Mediated by a beta2-Adrenergic Nerve Growth Factor Feedforward Loop Promotes Colorectal Cancer Progression. Cancer Discov. 2025, 15, 202–226. [Google Scholar] [CrossRef]
- Eng, J.W.; Kokolus, K.M.; Reed, C.B.; Hylander, B.L.; Ma, W.W.; Repasky, E.A. A nervous tumor microenvironment: The impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol. Immunother. 2014, 63, 1115–1128. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Y.; Luo, M.; Hu, X.; Li, H.; Liu, Q.; Zou, Z. Chronic stress in solid tumor development: From mechanisms to interventions. J. Biomed. Sci. 2023, 30, 8. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90.e7. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Park, D.; Zhong, Y.; Lu, Y.; Rycaj, K.; Gong, S.; Chen, X.; Liu, X.; Chao, H.P.; Whitney, P.; et al. Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat. Commun. 2016, 7, 10798. [Google Scholar] [CrossRef]
- Madden, K.S.; Szpunar, M.J.; Brown, E.B. beta-Adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 2011, 130, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, Z.; Lu, L.; Cho, C.H. beta-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin. Cancer Biol. 2013, 23, 533–542. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Q.; Li, J.; Wang, Z.; Shan, T.; Li, W.; Xu, Q.; Xie, K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 2013, 12, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Baghaei, K.; Amani, D.; Ebtekar, M. Tumor-derived exosomes encapsulating miR-34a promote apoptosis and inhibit migration and tumor progression of colorectal cancer cells under in vitro condition. DARU J. Pharm. Sci. 2021, 29, 267–278. [Google Scholar] [CrossRef]
- Higuchi, T.; Klimek, K.; Groener, D.; Chen, X.; Werner, R.A. Norepinephrine Transporter-Targeted Cancer Theranostics-New Horizons. Clin. Nucl. Med. 2025, 50, 44–51. [Google Scholar] [CrossRef]
- Zhang, H.; Han, J.; Zhang, J.; Miao, J.; Li, F.; Tang, K.; Zhou, K.; Duan, B.; Li, W.; Cheng, J.; et al. Venlafaxine antagonizes the noradrenaline-promoted colon cancer progression by inhibiting the norepinephrine transporter. Cell Death Discov. 2023, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front. Cell. Neurosci. 2016, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Friess, H.; Zhu, Z.; Liard, V.; Shi, X.; Shrikhande, S.V.; Wang, L.; Lieb, K.; Korc, M.; Palma, C.; Zimmermann, A.; et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Lab. Investig. 2003, 83, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Ma, K.; Wu, H.; Cao, T. A Substance P (SP)/Neurokinin-1 Receptor Axis Promotes Perineural Invasion of Pancreatic Cancer and Is Affected by lncRNA LOC389641. J. Immunol. Res. 2022, 2022, 5582811. [Google Scholar] [CrossRef]
- Padmanaban, V.; Keller, I.; Seltzer, E.S.; Ostendorf, B.N.; Kerner, Z.; Tavazoie, S.F. Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis. Nature 2024, 633, 207–215. [Google Scholar] [CrossRef]
- Singh, S.; Kumaravel, S.; Dhole, S.; Roy, S.; Pavan, V.; Chakraborty, S. Neuropeptide Substance P Enhances Inflammation-Mediated Tumor Signaling Pathways and Migration and Proliferation of Head and Neck Cancers. Indian J. Surg. Oncol. 2021, 12, 93–102. [Google Scholar] [CrossRef]
- Ge, C.; Huang, H.; Huang, F.; Yang, T.; Zhang, T.; Wu, H.; Zhou, H.; Chen, Q.; Shi, Y.; Sun, Y.; et al. Neurokinin-1 receptor is an effective target for treating leukemia by inducing oxidative stress through mitochondrial calcium overload. Proc. Natl. Acad. Sci. USA 2019, 116, 19635–19645. [Google Scholar] [CrossRef]
- Maintz, L.; Wardelmann, E.; Walgenbach, K.; Fimmers, R.; Bieber, T.; Raap, U.; Novak, N. Neuropeptide blood levels correlate with mast cell load in patients with mastocytosis. Allergy 2011, 66, 862–869. [Google Scholar] [CrossRef]
- Isidro, R.A.; Cruz, M.L.; Isidro, A.A.; Baez, A.; Arroyo, A.; Gonzalez-Marques, W.A.; Gonzalez-Keelan, C.; Torres, E.A.; Appleyard, C.B. Immunohistochemical expression of SP-NK-1R-EGFR pathway and VDR in colonic inflammation and neoplasia. World J. Gastroenterol. 2015, 21, 1749–1758. [Google Scholar] [CrossRef]
- Erin, N.; Shurin, G.V.; Baraldi, J.H.; Shurin, M.R. Regulation of Carcinogenesis by Sensory Neurons and Neuromediators. Cancers 2022, 14, 2333. [Google Scholar] [CrossRef]
- Li, X.; Ma, G.; Ma, Q.; Li, W.; Liu, J.; Han, L.; Duan, W.; Xu, Q.; Liu, H.; Wang, Z.; et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol. Cancer Res. 2013, 11, 294–302. [Google Scholar] [CrossRef]
- Restaino, A.C.; Walz, A.; Vermeer, S.J.; Barr, J.; Kovacs, A.; Fettig, R.R.; Vermeer, D.W.; Reavis, H.; Williamson, C.S.; Lucido, C.T.; et al. Functional neuronal circuits promote disease progression in cancer. Sci. Adv. 2023, 9, eade4443. [Google Scholar] [CrossRef]
- Kaduri, M.; Sela, M.; Kagan, S.; Poley, M.; Abumanhal-Masarweh, H.; Mora-Raimundo, P.; Ouro, A.; Dahan, N.; Hershkovitz, D.; Shklover, J.; et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases. Sci. Adv. 2021, 7, eabj5435. [Google Scholar] [CrossRef]
- Garrido, M.P.; Vallejos, C.; Girardi, S.; Gabler, F.; Selman, A.; Lopez, F.; Vega, M.; Romero, C. NGF/TRKA Promotes ADAM17-Dependent Cleavage of P75 in Ovarian Cells: Elucidating a Pro-Tumoral Mechanism. Int. J. Mol. Sci. 2022, 23, 2124. [Google Scholar] [CrossRef]
- Van Rijen, P.C.; Luyten, P.R.; van der Sprenkel, J.W.; Kraaier, V.; van Huffelen, A.C.; Tulleken, C.A.; Hollander, J.A.d. 1H and 31P NMR measurement of cerebral lactate, high-energy phosphate levels, and pH in humans during voluntary hyperventilation: Associated EEG, capnographic, and Doppler findings. Magn. Reson. Med. 1989, 10, 182–193. [Google Scholar] [CrossRef]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2018, 15, 151–167, Erratum in Nat. Rev. Clin. Oncol. 2018, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, P.D. Exosomal Induction of Tumor Innervation. Cancer Res. 2019, 79, 3529–3535. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat. Rev. Cancer 2024, 24, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Batson, J.; Kadir, S.; Charlet, J.; Persad, R.A.; Gillatt, D.; Oxley, J.D.; Nobes, C.D. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 2010, 12, 1194–1204. [Google Scholar] [CrossRef]
- Ko, S.Y.; Dass, C.R.; Nurgali, K. Netrin-1 in the developing enteric nervous system and colorectal cancer. Trends Mol. Med. 2012, 18, 544–554. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, P.; Xu, X.; Zhou, B.; Chen, J.; Jiang, X.; Liu, Y.; Wu, Y.; Yue, W.; Xu, H.; et al. Piezoelectric Analgesia Blocks Cancer-Induced Bone Pain. Adv. Mater. 2024, 36, e2403979. [Google Scholar] [CrossRef] [PubMed]
- Carnet Le Provost, K.; Kepp, O.; Kroemer, G.; Bezu, L. Trial watch: Beta-blockers in cancer therapy. Oncoimmunology 2023, 12, 2284486. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Andre, N.; Trahair, T.; Kavallaris, M. Reply: Comment on ‘Beta-blockers increase response to chemotherapy via direct anti-tumour and anti-angiogenic mechanisms in neuroblastoma’—beta-blockers are potent anti-angiogenic and chemo-sensitising agents, rather than cytotoxic drugs. Br. J. Cancer 2013, 109, 2024–2025. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Cole, S.W. Expanding our therapeutic options: Beta blockers for breast cancer? J. Clin. Oncol. 2011, 29, 2612–2616. [Google Scholar] [CrossRef]
- Volenec, A.; Zetterstrom, T.S.; Flanigan, T.P. 6-OHDA denervation substantially decreases DCC mRNA levels in rat substantia nigra compacta. Neuroreport 1998, 9, 3553–3556. [Google Scholar] [CrossRef]
- Zhu, Y.; Meerschaert, K.A.; Galvan-Pena, S.; Bin, N.R.; Yang, D.; Basu, H.; Kawamoto, R.; Shalaby, A.; Liberles, S.D.; Mathis, D.; et al. A chemogenetic screen reveals that Trpv1-expressing neurons control regulatory T cells in the gut. Science 2024, 385, eadk1679. [Google Scholar] [CrossRef]
- Braucke, A.; Frederiksen, N.L.; Berg, L.C.; Aarsvold, S.; Muller, F.C.; Boesen, M.P.; Lindegaard, C. Identification and Quantification of Transient Receptor Potential Vanilloid 1 (TRPV1) in Equine Articular Tissue. Animals 2020, 10, 506. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Friess, H.; Wang, L.; Bogardus, T.; Korc, M.; Kleeff, J.; Buchler, M.W. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin. Cancer Res. 2001, 7, 105–112. [Google Scholar]
- Weeraratna, A.T.; Arnold, J.T.; George, D.J.; DeMarzo, A.; Isaacs, J.T. Rational basis for Trk inhibition therapy for prostate cancer. Prostate 2000, 45, 140–148. [Google Scholar] [CrossRef]
- Ugolini, G.; Marinelli, S.; Covaceuszach, S.; Cattaneo, A.; Pavone, F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 2985–2990. [Google Scholar] [CrossRef]
- Miladinovic, T.; Ungard, R.G.; Linher-Melville, K.; Popovic, S.; Singh, G. Functional effects of TrkA inhibition on system x(C)(-)-mediated glutamate release and cancer-induced bone pain. Mol. Pain 2018, 14, 1744806918776467. [Google Scholar] [CrossRef]
- Huang, S.M.; Chen, T.S.; Chiu, C.M.; Chang, L.K.; Liao, K.F.; Tan, H.M.; Yeh, W.L.; Chang, G.R.; Wang, M.Y.; Lu, D.Y. GDNF increases cell motility in human colon cancer through VEGF-VEGFR1 interaction. Endocr.-Relat. Cancer 2014, 21, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Cavel, O.; Shomron, O.; Shabtay, A.; Vital, J.; Trejo-Leider, L.; Weizman, N.; Krelin, Y.; Fong, Y.; Wong, R.J.; Amit, M.; et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012, 72, 5733–5743. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Santoro, M.; Schlumberger, M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 2021, 17, 296–306. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Takeuchi, K.; Soda, M.; Togashi, Y.; Suzuki, R.; Sakata, S.; Hatano, S.; Asaka, R.; Hamanaka, W.; Ninomiya, H.; Uehara, H.; et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012, 18, 378–381. [Google Scholar] [CrossRef]
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: Physiological and pathophysiological implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Xu, P.; Fan, H.R.; Zhang, E.M.; Zhang, H.N.; Fei, Y. Advances in the Treatment of Neuropathic Pain by Sympathetic Regulation. Curr. Pain Headache Rep. 2024, 28, 1167–1176. [Google Scholar] [CrossRef]
- Doroshenko, M.; Turkot, O.; Dua, A.; Horn, D.B. Sympathetic Nerve Block; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef]
- Gregory, E.; Dugan, R.; David, G.; Song, Y.H. The biology and engineered modeling strategies of cancer-nerve crosstalk. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188406. [Google Scholar] [CrossRef]
- Wu, Y.; Han, W.; Dong, H.; Liu, X.; Su, X. The rising roles of exosomes in the tumor microenvironment reprogramming and cancer immunotherapy. MedComm 2024, 5, e541. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Kurywchak, P.; Tavormina, J.; Kalluri, R. The emerging roles of exosomes in the modulation of immune responses in cancer. Genome Med. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; You, Y.; Xu, X.; Lu, J.; Huang, X.; Zhang, J.; Zhu, L.; Hu, J.; Wu, X.; Xu, X.; et al. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes Biopotentiated Extracellular Matrix Hydrogels Accelerate Diabetic Wound Healing and Skin Regeneration. Adv. Sci. 2023, 10, e2304023. [Google Scholar] [CrossRef]
- Shetgaonkar, G.G.; Marques, S.M.; CEM, D.C.; Vibhavari, R.J.A.; Kumar, L.; Shirodkar, R.K. Exosomes as cell-derivative carriers in the diagnosis and treatment of central nervous system diseases. Drug Deliv. Transl. Res. 2022, 12, 1047–1079. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, W.; Shen, L.; Chen, Z.; Huang, J. Crosstalk between the peripheral nervous system and breast cancer influences tumor progression. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188828. [Google Scholar] [CrossRef]
- Thielman, N.R.J.; Funes, V.; Davuluri, S.; Ibanez, H.E.; Sun, W.C.; Fu, J.; Li, K.; Muth, S.; Pan, X.; Fujiwara, K.; et al. Semaphorin 3D promotes pancreatic ductal adenocarcinoma progression and metastasis through macrophage reprogramming. Sci. Adv. 2024, 10, eadp0684. [Google Scholar] [CrossRef]
- Hung, Y.H.; Hou, Y.C.; Hsu, S.H.; Wang, L.Y.; Tsai, Y.L.; Shan, Y.S.; Su, Y.Y.; Hung, W.C.; Chen, L.T. Pancreatic cancer cell-derived semaphorin 3A promotes neuron recruitment to accelerate tumor growth and dissemination. Am. J. Cancer Res. 2023, 13, 3417–3432. [Google Scholar]
- Peacock, J.W.; Takeuchi, A.; Hayashi, N.; Liu, L.; Tam, K.J.; Al Nakouzi, N.; Khazamipour, N.; Tombe, T.; Dejima, T.; Lee, K.C.; et al. SEMA3C drives cancer growth by transactivating multiple receptor tyrosine kinases via Plexin B1. EMBO Mol. Med. 2018, 10, 219–238. [Google Scholar] [CrossRef]
- Andryszak, N.; Kurzawa, P.; Krzyzaniak, M.; Ruchala, M.; Nowicki, M.; Izycki, D.; Czepczynski, R. Expression of semaphorin 3A (SEMA3A) in breast cancer subtypes. Sci. Rep. 2024, 14, 1969. [Google Scholar] [CrossRef]
- Nakayama, H.; Murakami, A.; Nishida-Fukuda, H.; Fukuda, S.; Matsugi, E.; Nakahara, M.; Kusumoto, C.; Kamei, Y.; Higashiyama, S. Semaphorin 3F inhibits breast cancer metastasis by regulating the Akt-mTOR and TGFbeta signaling pathways via neuropilin-2. Sci. Rep. 2025, 15, 7394. [Google Scholar] [CrossRef]
- Hui, D.H.F.; Tam, K.J.; Jiao, I.Z.F.; Ong, C.J. Semaphorin 3C as a Therapeutic Target in Prostate and Other Cancers. Int. J. Mol. Sci. 2019, 20, 774. [Google Scholar] [CrossRef]
- Maione, F.; Molla, F.; Meda, C.; Latini, R.; Zentilin, L.; Giacca, M.; Seano, G.; Serini, G.; Bussolino, F.; Giraudo, E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J. Clin. Investig. 2009, 119, 3356–3372. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Z.; Pan, S.; Shi, M.; Li, J.; Yang, C.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Overcoming Multidrug Resistance by Codelivery of MDR1-Targeting siRNA and Doxorubicin Using EphA10-Mediated pH-Sensitive Lipoplexes: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 21590–21600. [Google Scholar] [CrossRef] [PubMed]
- Toosi, B.M.; El Zawily, A.; Truitt, L.; Shannon, M.; Allonby, O.; Babu, M.; DeCoteau, J.; Mousseau, D.; Ali, M.; Freywald, T.; et al. EPHB6 augments both development and drug sensitivity of triple-negative breast cancer tumours. Oncogene 2018, 37, 4073–4093. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.; Ryu, H.S.; Theocharis, S. Viewing the Eph receptors with a focus on breast cancer heterogeneity. Cancer Lett. 2018, 434, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Liu, Y.; Yan, W.; Meng, Q.; Song, X.; Cheng, B.; Yao, R. Netrin-4: Focus on Its Role in Axon Guidance, Tissue Stability, Angiogenesis and Tumors. Cell. Mol. Neurobiol. 2023, 43, 1663–1683. [Google Scholar] [CrossRef]
- Yin, K.; Wang, L.; Xia, Y.; Dang, S.; Zhang, X.; He, Z.; Xu, J.; Shang, M.; Xu, Z. Netrin-1 promotes cell neural invasion in gastric cancer via its receptor neogenin. J. Cancer 2019, 10, 3197–3207. [Google Scholar] [CrossRef]
- Eveno, C.; Contreres, J.O.; Hainaud, P.; Nemeth, J.; Dupuy, E.; Pocard, M. Netrin-4 overexpression suppresses primary and metastatic colorectal tumor progression. Oncol. Rep. 2013, 29, 73–78. [Google Scholar] [CrossRef]
- Cassier, P.A.; Navaridas, R.; Bellina, M.; Rama, N.; Ducarouge, B.; Hernandez-Vargas, H.; Delord, J.P.; Lengrand, J.; Paradisi, A.; Fattet, L.; et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 2023, 620, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Alto, L.T.; Terman, J.R. Semaphorins and their Signaling Mechanisms. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1493, pp. 1–25. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. PAIN 2008, 136, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, W.; Zhu, Y.; Zhao, F.; Deng, S.; Tian, M.; Wang, Y.; Gong, Y. TRPV1: The key bridge in neuroimmune interactions. J. Intensive Med. 2024, 4, 442–452. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. Int. J. Biol. Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef]
- Chinreddy, S.R.; Mashozhera, N.T.; Rashrash, B.; Flores-Iga, G.; Nimmakayala, P.; Hankins, G.R.; Harris, R.T.; Reddy, U.K. Unraveling TRPV1’s Role in Cancer: Expression, Modulation, and Therapeutic Opportunities with Capsaicin. Molecules 2024, 29, 4729. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in neural systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef]
- Muller, U.R.; Haeberli, G. Use of beta-blockers during immunotherapy for Hymenoptera venom allergy. J. Allergy Clin. Immunol. 2005, 115, 606–610. [Google Scholar] [CrossRef]
- Farzam, K.; Jan, A. Beta Blockers; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bottasso, E. Toward the Existence of a Sympathetic Neuroplasticity Adaptive Mechanism Influencing the Immune Response. A Hypothetical View—Part II. Front. Endocrinol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Guo, J.A.; Hoffman, H.I.; Su, J.; Mino-Kenudson, M.; Barth, J.L.; Schenkel, J.M.; Loeffler, J.S.; Shih, H.A.; Hong, T.S.; et al. Therapeutic avenues for cancer neuroscience: Translational frontiers and clinical opportunities. Lancet Oncol. 2022, 23, e62–e74. [Google Scholar] [CrossRef]
- Faulkner, S.; Jobling, P.; March, B.; Jiang, C.C.; Hondermarck, H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019, 9, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, J.; Xu, J. Denervation-Related Neuromuscular Junction Changes: From Degeneration to Regeneration. Front. Mol. Neurosci. 2021, 14, 810919. [Google Scholar] [CrossRef]
- Campbell, W.A.t.; Makary, M.S. Advances in Image-Guided Ablation Therapies for Solid Tumors. Cancers 2024, 16, 2560. [Google Scholar] [CrossRef]
- Samaddar, S.; Redhwan, M.A.M.; Eraiah, M.M.; Koneri, R. Neurotrophins in Peripheral Neuropathy: Exploring Pathophysiological Mechanisms and Emerging Therapeutic Opportunities. CNS Neurol. Disord. Drug Targets 2025, 24, 91–101. [Google Scholar] [CrossRef]
- Makhijani, K.; To, T.L.; Ruiz-Gonzalez, R.; Lafaye, C.; Royant, A.; Shu, X. Precision Optogenetic Tool for Selective Single- and Multiple-Cell Ablation in a Live Animal Model System. Cell Chem. Biol. 2017, 24, 110–119. [Google Scholar] [CrossRef]
- Toi, P.T.; Jang, H.J.; Min, K.; Kim, S.P.; Lee, S.K.; Lee, J.; Kwag, J.; Park, J.Y. In vivo direct imaging of neuronal activity at high temporospatial resolution. Science 2022, 378, 160–168. [Google Scholar] [CrossRef]
- Haas, S.; Bravo, F.; Ionescu, T.M.; Gonzalez-Menendez, I.; Quintanilla-Martinez, L.; Dunkel, G.; Kuebler, L.; Hahn, A.; Lanzenberger, R.; Weigelin, B.; et al. Functional PET/MRI reveals active inhibition of neuronal activity during optogenetic activation of the nigrostriatal pathway. Sci. Adv. 2024, 10, eadn2776. [Google Scholar] [CrossRef] [PubMed]
- Erikainen, S.; Chan, S. Contested futures: Envisioning “Personalized,” “Stratified,” and “Precision” medicine. New Genet. Soc. 2019, 38, 308–330. [Google Scholar] [CrossRef] [PubMed]
- Scherzai, S.; Lennartz, M.; Jacobsen, F.; Viehweger, F.; Dum, D.; Menz, A.; Schlichter, R.; Hinsch, A.; Hoflmayer, D.; Hube-Magg, C.; et al. PGP9.5 expression in human tumors: A tissue microarray study on 13,920 tumors from 120 different tumor entities. Pathol.—Res. Pract. 2024, 264, 155676. [Google Scholar] [CrossRef] [PubMed]
- Catana, C.; Drzezga, A.; Heiss, W.D.; Rosen, B.R. PET/MRI for neurologic applications. J. Nucl. Med. 2012, 53, 1916–1925. [Google Scholar] [CrossRef]

| Cancer Type | Innervation Characteristics | Key Findings and Implications | Reference |
|---|---|---|---|
| Pancreatic Cancer | Nearly 100% of cases exhibit dense nerve infiltration and PNI. | Strong correlation with poor prognosis, recurrence, and pain; neural crosstalk promotes angiogenesis and metastasis. | [17,32,33,34] |
| Prostate Cancer | High nerve density, with sympathetic and parasympathetic involvement. | Sympathetic nerves promote growth; parasympathetic nerves promote metastasis; PNI is a survival risk factor. | [18,19,35] |
| Colorectal Cancer | ~33% show PNI and tumor-associated nerve infiltration. | High nerve density linked to recurrence, poor survival; nerves serve as dissemination routes. | [25,30,36,37] |
| Head and Neck Cancer | Up to 80% exhibit PNI, especially in aggressive subtypes. | Associated with local recurrence, reduced survival, immune suppression, and progression. | [24,38] |
| Gastric Cancer | Exhibits both peripheral nerve infiltration and spontaneous nerve formation. | High nerve density linked to metastasis and poor prognosis; NGF-mediated innervation may drive malignancy. | [39] |
| Cervical Cancer | PNI observed in subsets, often in large or late-stage tumors. | Neural infiltration enhances tumor aggressiveness and worsens clinical outcomes. | [40] |
| Glioblastoma | Tumor cells directly induce spontaneous neurogenesis. | Glioma-neuron networks promote proliferation, angiogenesis, and treatment resistance. | [6,8,20,21] |
| Breast Cancer | Less common neural infiltration, but observed in aggressive subtypes. | Sympathetic signaling drives tumor growth; axon guidance molecules contribute to metastasis. | [41,42] |
| Melanoma | Neural association linked to neural crest origin. | PNI enhances invasion depth and therapy resistance. | [10] |
| NSCLC | Tumors co-opt neural elements via metastatic niche formation (MNT). | Tumor-associated nerves promote immune suppression and angiogenesis. | [3,43] |
| Neuronal Factor | Receptor(s) | Function in TME | Key Tumor-Associated Effects | Reference |
|---|---|---|---|---|
| NGF | TrkA | Promotes tumor innervation, neurogenesis, angiogenesis | Enhances PNI, tumor growth, metastasis, and pain | [99,100,101,102,103,104,105] |
| BDNF | TrkB | Stimulates PI3K/AKT, MAPK, STAT3 pathways | Promotes tumor proliferation, therapy resistance, angiogenesis | [106,107,108,109,110,111] |
| CGRP | RAMP1/CLR complex | Induces vasodilation, suppresses immune cells | Facilitates angiogenesis, immune evasion, metastasis, therapy resistance, pain | [112,113,114,115] |
| NE | β2/β3-AR (Adrenergic Receptors) | Activates cAMP–PKA, induces BDNF and NGF | Enhances DNA repair, immune evasion, angiogenesis, metastasis, pain | [116,117,118] |
| SP | NK-1R | Promotes inflammation, angiogenesis, nerve activity | Enhances proliferation, motility, vascularization, tumor pain | [119,120,121,122] |
| Strategy | Mechanism | Examples | Potential Outcomes | Clinical Status | Reference |
|---|---|---|---|---|---|
| Blocking Neurotrophic Signaling | Inhibits nerve growth and recruitment by targeting neurotrophic factors and their receptors. |
| Reduces nerve density, impairs tumor growth, and alleviates cancer-associated pain. | TrkA: Phase II; RET inhibitors: approved for RET + cancers | [61,194,195,196] |
| Exosome-Based Therapies | Disrupts tumor-derived exosome production, release, or uptake to block neurogenic signaling. |
| Reduces nerve recruitment and spontaneous nerve formation within tumors. | Preclinical | [196,197] |
| Axon Guidance Molecule Modulation | Inhibits pathways involved in nerve growth and integration into tumors. |
| Prevents neural infiltration, reduces tumor-supportive nerve networks, and limits metastasis. | Preclinical | [198,199,200] |
| Targeting TRPV1 | Blocks nociceptive sensory neuron signaling and neuropeptide release by inhibiting TRPV1 channel activity. | TRPV1 antagonists, TRPV1 gene ablation approaches. | Reduces cancer-associated pain and may impair tumor-supportive neural activity; risks include hyperthermia and impaired heat sensation. | Preclinical/early clinical trials | [85,201] |
| Targeting Adrenergic Signaling | Blocks norepinephrine-mediated β2/β3-adrenergic receptor signaling to reduce tumor-promoting neural effects. | β-blockers (e.g., propranolol), adrenergic nerve ablation. | Reduces angiogenesis, immunosuppression, and metastasis; risks include cardiovascular side effects and compensatory sympathetic sprouting. | Propranolol: repurposed in clinical studies; others: preclinical | [202,203,204] |
| Denervation Approaches | Ablates nerves physically or chemically to disrupt tumor–nerve interactions. |
| Reduces tumor progression, enhances therapy response, and alleviates neural contributions to tumor-supportive environments. | Preclinical/limited clinical experience | [200,205] |
| Fiber-Specific Denervation and Imaging-Guided Mapping | Targets specific nerve subtypes based on molecular markers; enables visualization of intratumoral nerve architecture | TRPV1+/β-AR+ fiber ablation, PET imaging, optogenetics | Enhances selectivity, reduces collateral damage, enables precision denervation | / | [206,207] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.Z.; Chan, M.K.-K.; Tang, P.C.-T.; Ng, C.S.-H.; Li, C.; Zhang, D.; Nikolic-Paterson, D.J.; To, K.-F.; Jiang, X.; Tang, P.M.-K. Tumor Innervation: From Bystander to Emerging Therapeutic Target for Cancer. Int. J. Mol. Sci. 2025, 26, 9257. https://doi.org/10.3390/ijms26189257
Ji ZZ, Chan MK-K, Tang PC-T, Ng CS-H, Li C, Zhang D, Nikolic-Paterson DJ, To K-F, Jiang X, Tang PM-K. Tumor Innervation: From Bystander to Emerging Therapeutic Target for Cancer. International Journal of Molecular Sciences. 2025; 26(18):9257. https://doi.org/10.3390/ijms26189257
Chicago/Turabian StyleJi, Zoey Zeyuan, Max Kam-Kwan Chan, Philip Chiu-Tsun Tang, Calvin Sze-Hang Ng, Chunjie Li, Dongmei Zhang, David J. Nikolic-Paterson, Ka-Fai To, Xiaohua Jiang, and Patrick Ming-Kuen Tang. 2025. "Tumor Innervation: From Bystander to Emerging Therapeutic Target for Cancer" International Journal of Molecular Sciences 26, no. 18: 9257. https://doi.org/10.3390/ijms26189257
APA StyleJi, Z. Z., Chan, M. K.-K., Tang, P. C.-T., Ng, C. S.-H., Li, C., Zhang, D., Nikolic-Paterson, D. J., To, K.-F., Jiang, X., & Tang, P. M.-K. (2025). Tumor Innervation: From Bystander to Emerging Therapeutic Target for Cancer. International Journal of Molecular Sciences, 26(18), 9257. https://doi.org/10.3390/ijms26189257












