Preparation of a Micronutrient-Enriched Apricot Kernel Oil and Assessment of In Vitro Chemopreventive Properties †
Abstract
1. Introduction
2. Results
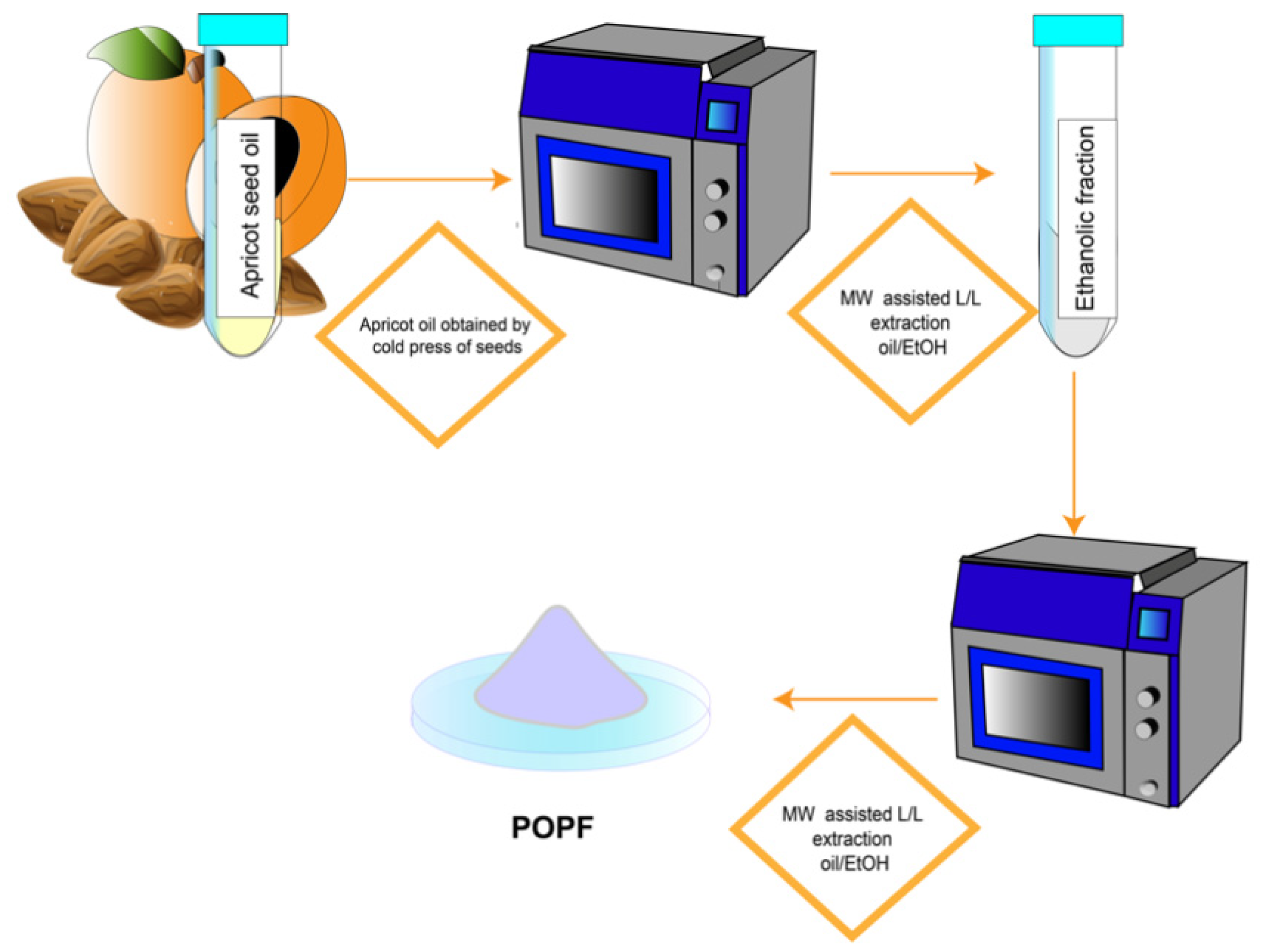
2.1. Apricot Kernel Oil Purification
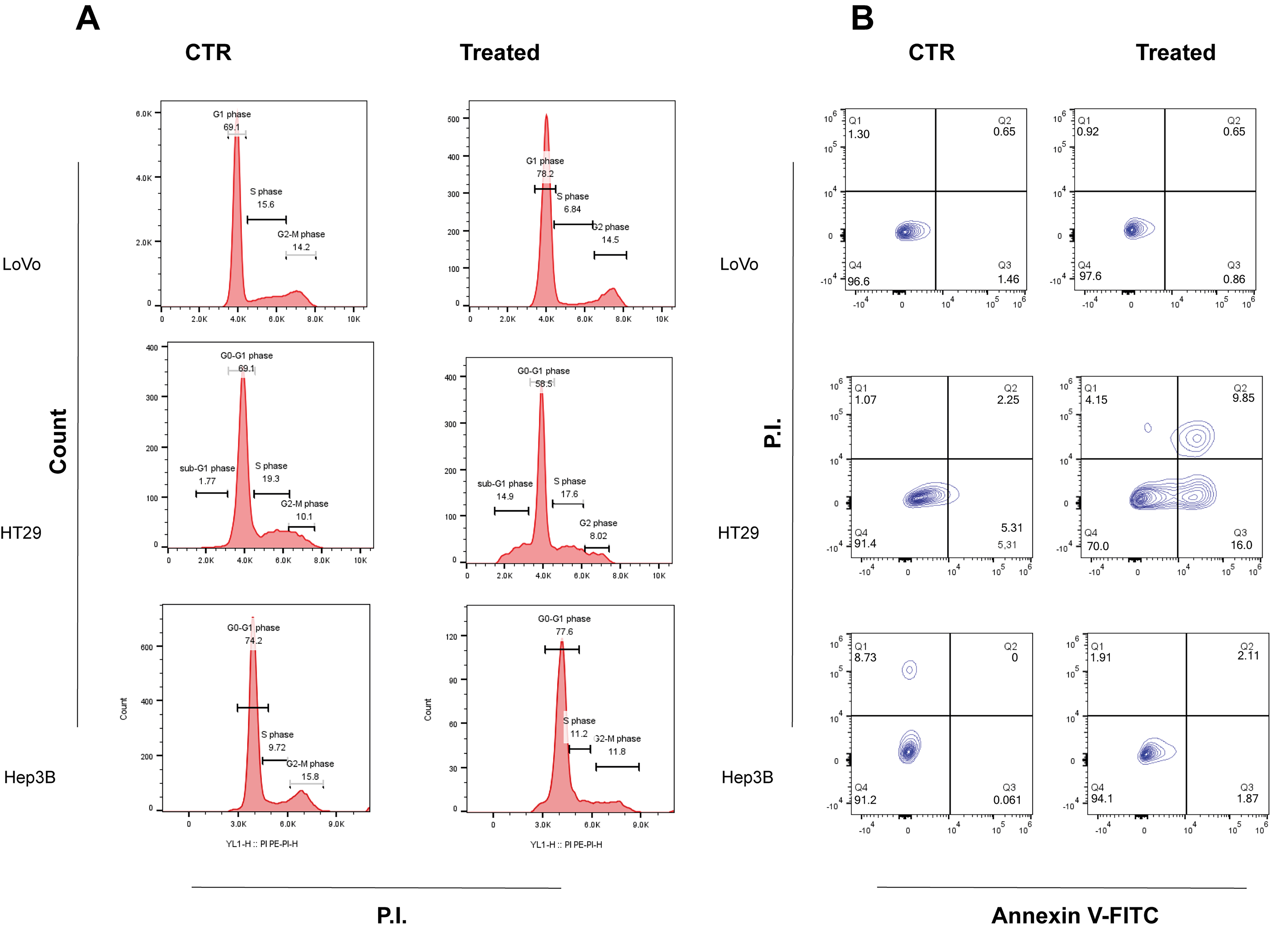
2.2. Purified Apricot Seed Oil Inhibits In Vitro Cancer Cell Growth
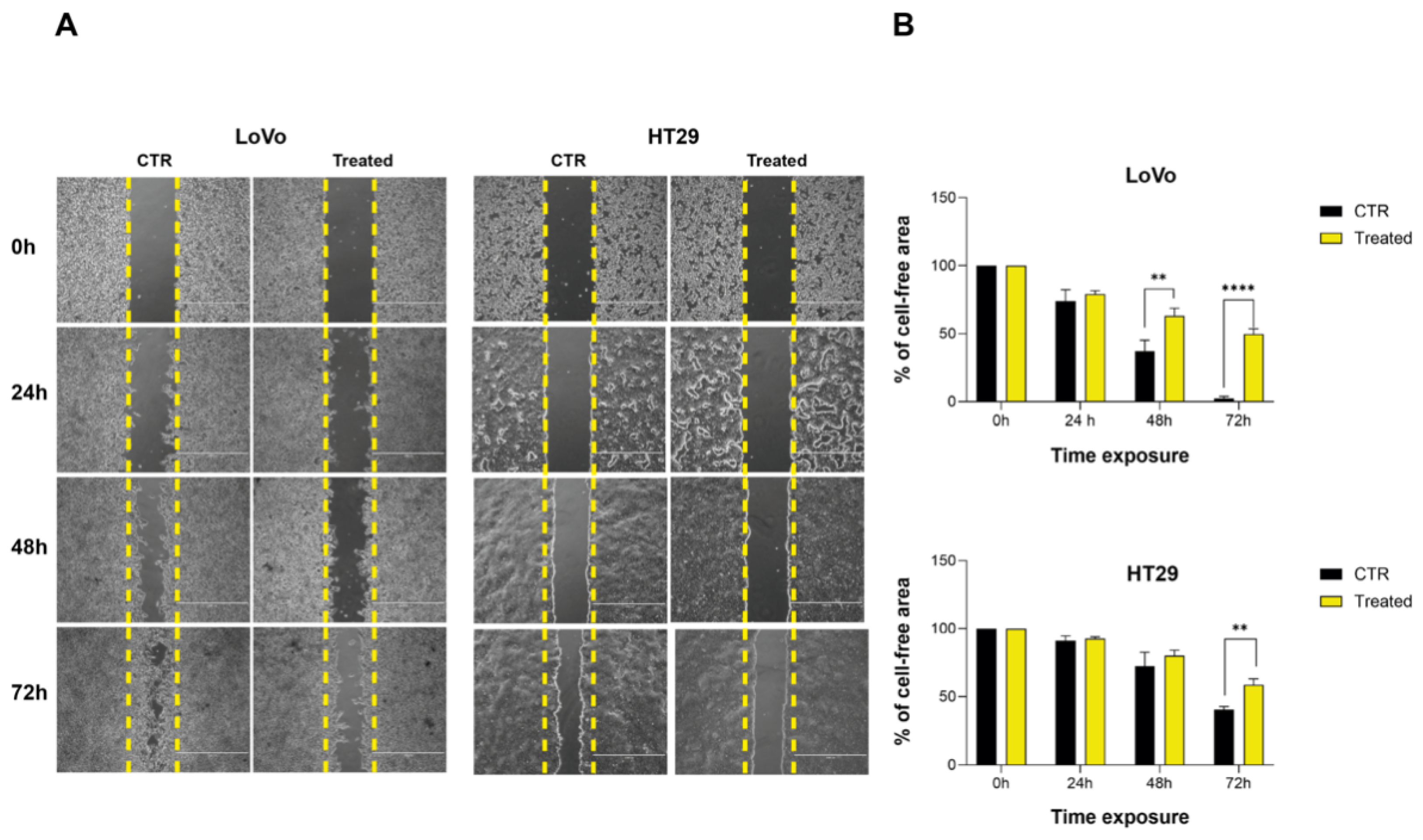
2.3. Purified Apricot Seed Oil Hampers Cancer Cell Migration Rate
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. Extraction and Fractionation
4.4. Gas Chromatography
4.5. In Vitro Experiments
4.5.1. Cell Lines Culture
4.5.2. Cell Growth Assay
4.5.3. Wound-Healing Assay
4.5.4. Annexin V/FITC
4.5.5. Cell Cycle Analysis
4.5.6. qRT-PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Anwar, S.; Yunusa, B.M.; Nayik, G.A.; Mousavi Khaneghah, A. The Potential of Apricot Seed and Oil as Functional Food: Composition, Biological Properties, Health Benefits & Safety. Food Biosci. 2023, 51, 102336. [Google Scholar] [CrossRef]
- Pawar, K.R.; Nema, P.K. Apricot Kernel Characterization, Oil Extraction, and Its Utilization: A Review. Food Sci. Biotechnol. 2023, 32, 249–263. [Google Scholar] [CrossRef]
- Shariatifar, N.; Pourfard, I.M.; Khaniki, G.J.; Nabizadeh, R.; Akbarzadeh, A.; Nejad, A.S.M. Mineral Composition, Physico-Chemical Properties and Fatty Acids Profile of Prunus Armeniaca Apricot Seed Oil. Asian J. Chem. 2017, 29, 2011–2015. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Q.-A.; Fan, X.-H.; Zhang, X.-Y. Effect of debitterizing treatment on the quality of the apricot kernels in the industrial processing. J. Food Process. Preserv. 2018, 42, e13556. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Ubaid, M.; Ahmed, N.; Gul, M.K. Effects of controlled microwave heat treatment on the compositional attributes, antioxidant potential, and anti-nutritional components of apricot kernel flour. J. Food Meas. Charact. 2025, 19, 1859–1873. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Rubin, J.; Kvols, L.K.; Sarna, G.; Koch, R.; Currie, V.E.; Young, C.W.; Jones, S.E.; Davignon, J.P. A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. N. Engl. J. Med. 1982, 306, 201–206. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.-A.; Yao, J.-L.; Zhang, X.-Y. Changes of Amygdalin and Volatile Components of Apricot Kernels during the Ultrasonically-Accelerated Debitterizing. Ultrason. Sonochemistry 2019, 58, 104614. [Google Scholar] [CrossRef]
- Halysh, V.; Romero-García, J.M.; Vidal, A.M.; Kulik, T.; Palianytsia, B.; García, M.; Castro, E. Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources. Molecules 2023, 28, 1455. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and In Vitro Anti-Inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-Biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef]
- Utilization, Valorization and Functional Properties of Wild Apricot Kernels. Available online: https://www.phytojournal.com/archives/2021.v10.i4.14215/utilization-valorization-and-functional-properties-of-wild-apricot-kernels (accessed on 11 August 2025).
- Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. [Google Scholar] [CrossRef]
- Tarek, H.E.; Shalash, H.N.; Ellithy, M.M. Apricot Oil Extract as a Topical Chemopreventive Agent in Induced Tongue Squamous Cell Carcinoma. J. Arab. Soc. Med. Res. 2023, 18, 26–34. [Google Scholar] [CrossRef]
- Cassiem, W.; De Kock, M. The Anti-Proliferative Effect of Apricot and Peach Kernel Extracts on Human Colon Cancer Cells in Vitro. BMC Complement. Altern. Med. 2019, 19, 32. [Google Scholar] [CrossRef]
- Bassi, D.; Foschi, S. Trends in apricot and peach industries in Italy. In Proceedings of the 4th Conference “Innovation in Fruit Growing”, Belgrade, Serbia, 4–6 October 2013. [Google Scholar]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Nassra, M.; Atgié, C.; Plaisancié, P.; Géloën, A. Oleic Acid Uptake Reveals the Rescued Enterocyte Phenotype of Colon Cancer Caco-2 by HT29-MTX Cells in Co-Culture Mode. Int. J. Mol. Sci. 2017, 18, 1573. [Google Scholar] [CrossRef]
- Dailey, O.D., Jr.; Wang, X.; Chen, F.; Huang, G. Anticancer activity of branched-chain derivatives of oleic acid. Anticancer. Res. 2011, 31, 3165–3169. [Google Scholar] [PubMed]
- Pavlović, N.; Vidović, S.; Vladić, J.; Popović, L.; Moslavac, T.; Jakobović, S.; Jokić, S. Recovery of Tocopherols Amygdalin and Fatty Acids from Apricot Kernel Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1800043. [Google Scholar] [CrossRef]
- Jin, F.; Wang, J.; Regenstein, J.M.; Wang, F. Effect of Roasting Temperatures on the Properties of Bitter Apricot (Armeniaca sibirica L.) Kernel Oil. J. Oleo Sci. 2018, 67, 813–822. [Google Scholar] [CrossRef]
- Balamurugan, R.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic Activity of γ-Sitosterol Isolated from Lippia nodiflora L. in Streptozotocin Induced Diabetic Rats. Eur. J. Pharmacol. 2011, 667, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources Extraction Methods and Uses of Squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- Rameshkumar, R.; Satish, L.; Pandian, S.; Rathinapriya, P.; Rency, A.S.; Shanmugaraj, G.; Pandian, S.K.; Leung, D.W.M.; Ramesh, M. Production of Squalene with Promising Antioxidant Properties in Callus Cultures of Nilgirianthus Ciliatus. Ind. Crops Prod. 2018, 126, 357–367. [Google Scholar] [CrossRef]
- Stanley, L.A.; Wolf, C.R. Through a Glass, Darkly? HepaRG and HepG2 Cells as Models of Human Phase I Drug Metabolism. Drug Metab. Rev. 2022, 54, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Guillouzo, A.; Corlu, A.; Aninat, C.; Glaise, D.; Morel, F.; Guguen-Guillouzo, C. The Human Hepatoma HepaRG Cells: A Highly Differentiated Model for Studies of Liver Metabolism and Toxicity of Xenobiotics. Chem. Biol. Interact. 2007, 168, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Catalano, M.G.; Cavalli, R.; Ugazio, E.; Bosco, O.; Canaparo, R.; Muntoni, E.; Frairia, R.; Gasco, M.R.; Eandi, M.; et al. Cytotoxicity of Anticancer Drugs Incorporated in Solid Lipid Nanoparticles on HT-29 Colorectal Cancer Cell Line. Eur. J. Pharm. Biopharm. 2004, 58, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Arimochi, H.; Morita, K. Characterization of Cytotoxic Actions of Tricyclic Antidepressants on Human HT29 Colon Carcinoma Cells. Eur. J. Pharmacol. 2006, 541, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.L.; Wada, K.; Takahashi, H.; Matsuhashi, N.; Ohnishi, S.; Wolfe, M.M.; Turner, J.R.; Nakajima, A.; Borkan, S.C.; Saubermann, L.J. Peroxisome Proliferator-Activated Receptor γ Inhibition Prevents Adhesion to the Extracellular Matrix and Induces Anoikis in Hepatocellular Carcinoma Cells. Cancer Res. 2005, 65, 2251–2259. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, R.; Jiang, H.; Zhang, M.; Guo, W.; Feng, G.; Pan, W.; Xu, H.; Wang, S. Evaluation of [18F]FDG/[18F]FLT/[18F]FMISO-Based Micro-Positron Emission Tomography in Detection of Liver Metastasis in Human Colorectal Cancer. Nucl. Med. Biol. 2019, 72–73, 36–44. [Google Scholar] [CrossRef]
- Cavalloro, V.; Marrubini, G.; Stabile, R.; Rossi, D.; Linciano, P.; Gheza, G.; Assini, S.; Martino, E.; Collina, S. Microwave-Assisted Extraction and HPLC-UV-CD Determination of (S)-Usnic Acid in Cladonia Foliacea. Molecules 2021, 26, 455. [Google Scholar] [CrossRef]
- Cavalloro, V.; Marrubini, G.; Rossino, G.; Martino, E.; Collina, S. Combining DoE and MASE: A Winning Strategy for the Isolation of Natural Bioactive Compounds from Plant Materials. Green Chem. 2024, 26, 244–258. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Mutoh, M. Induction of Anoikis in Human Colorectal Cancer Cells by Fucoxanthinol. Nutr. Cancer 2017, 69, 1043–1052. [Google Scholar] [CrossRef]
- Blaszczyk, A.; Matysiak, S.; Kula, J.; Szostakiewicz, K.; Karkusiewicz, Z. Cytotoxic and Genotoxic Effects of (R)-and (S)-ricinoleic Acid Derivatives. Chirality 2020, 32, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Zeidooni, L.; Hashemitabar, M.; Razzazzadeh, S.; Mahdavinia, M.; Ghasemi, K. Gamma-Tocopherol Enhances Apoptotic Effects of Lovastatin in Human Colorectal Carcinoma Cell Line (HT29). Nutr. Cancer 2014, 66, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Ouyang, H.; Zuo, F.; Ge, M.; Wu, M.; Zhao, L.; Zhu, Y.; Miao, X.; Bai, Y.; Chang, Y.; et al. Spectrum–Effect Relationship Study Between Ultra-high-performance Liquid Chromatography Fingerprints and Anti-Hepatoma Effect In Vitro of Cnidii Fructus. Biomed. Chromatogr. 2024, 38, e5847. [Google Scholar] [CrossRef] [PubMed]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.N.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010, 31, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Tesei, A.; Ulivi, P.; Fabbri, F.; Rosetti, M.; Leonetti, C.; Scarsella, M.; Zupi, G.; Amadori, D.; Bolla, M.; Zoli, W. In Vitro and in Vivo Evaluation of NCX 4040 Cytotoxic Activity In Human Colon Cancer Cell Lines. J. Transl. Med. 2005, 3, 7. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaccari, M.E.; Cavalloro, V.; Bedeschi, M.; Serra, P.; Simonetti, G.; Casali, E.; Porta, A.; Fossati, A.; Martino, E.; Collina, S.; et al. Preparation of a Micronutrient-Enriched Apricot Kernel Oil and Assessment of In Vitro Chemopreventive Properties. Int. J. Mol. Sci. 2025, 26, 9237. https://doi.org/10.3390/ijms26189237
Vaccari ME, Cavalloro V, Bedeschi M, Serra P, Simonetti G, Casali E, Porta A, Fossati A, Martino E, Collina S, et al. Preparation of a Micronutrient-Enriched Apricot Kernel Oil and Assessment of In Vitro Chemopreventive Properties. International Journal of Molecular Sciences. 2025; 26(18):9237. https://doi.org/10.3390/ijms26189237
Chicago/Turabian StyleVaccari, Melania Elettra, Valeria Cavalloro, Martina Bedeschi, Patrizia Serra, Giorgia Simonetti, Emanuele Casali, Alessio Porta, Alice Fossati, Emanuela Martino, Simona Collina, and et al. 2025. "Preparation of a Micronutrient-Enriched Apricot Kernel Oil and Assessment of In Vitro Chemopreventive Properties" International Journal of Molecular Sciences 26, no. 18: 9237. https://doi.org/10.3390/ijms26189237
APA StyleVaccari, M. E., Cavalloro, V., Bedeschi, M., Serra, P., Simonetti, G., Casali, E., Porta, A., Fossati, A., Martino, E., Collina, S., & Tesei, A. (2025). Preparation of a Micronutrient-Enriched Apricot Kernel Oil and Assessment of In Vitro Chemopreventive Properties. International Journal of Molecular Sciences, 26(18), 9237. https://doi.org/10.3390/ijms26189237








