Autophagy-Related Proteins in Triple-Negative Breast Cancer: From Molecular Insights to Therapeutic Applications
Abstract
1. Introduction
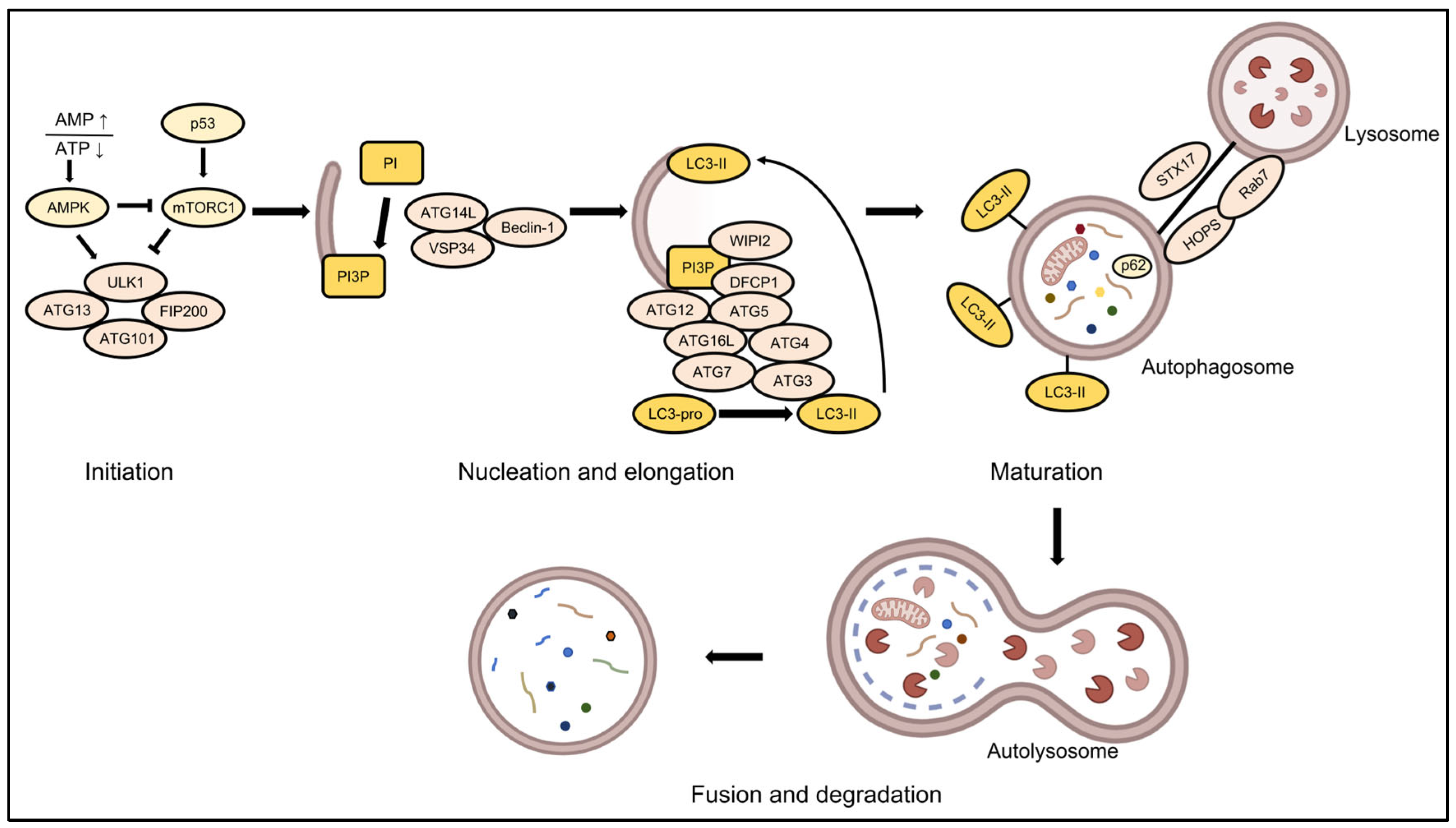
2. Molecular Regulatory Network of Autophagy
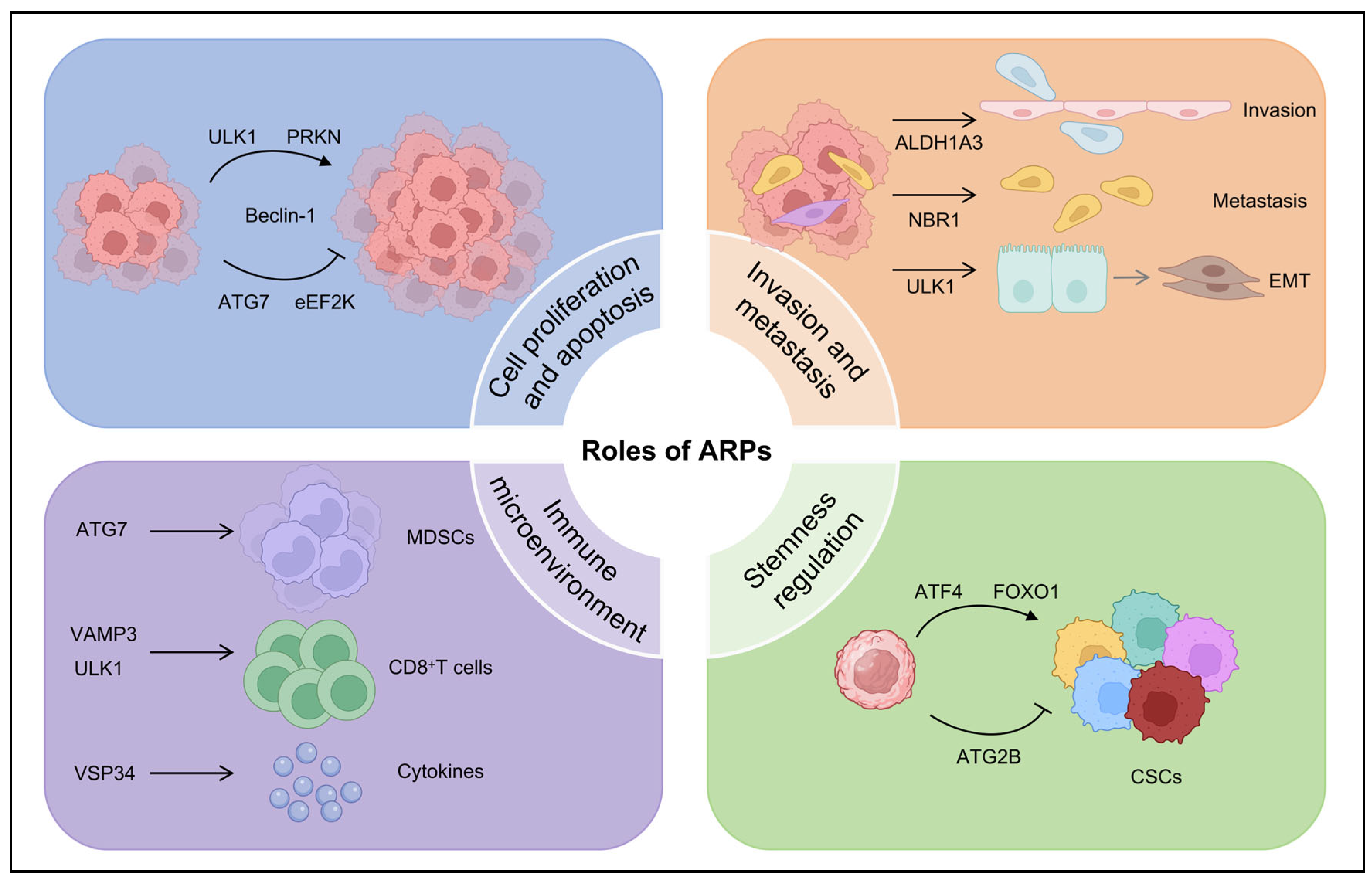
3. Context-Dependent Roles of ARPs in TNBC Progression
3.1. Regulation of Cell Proliferation and Apoptosis by ARPs in TNBC
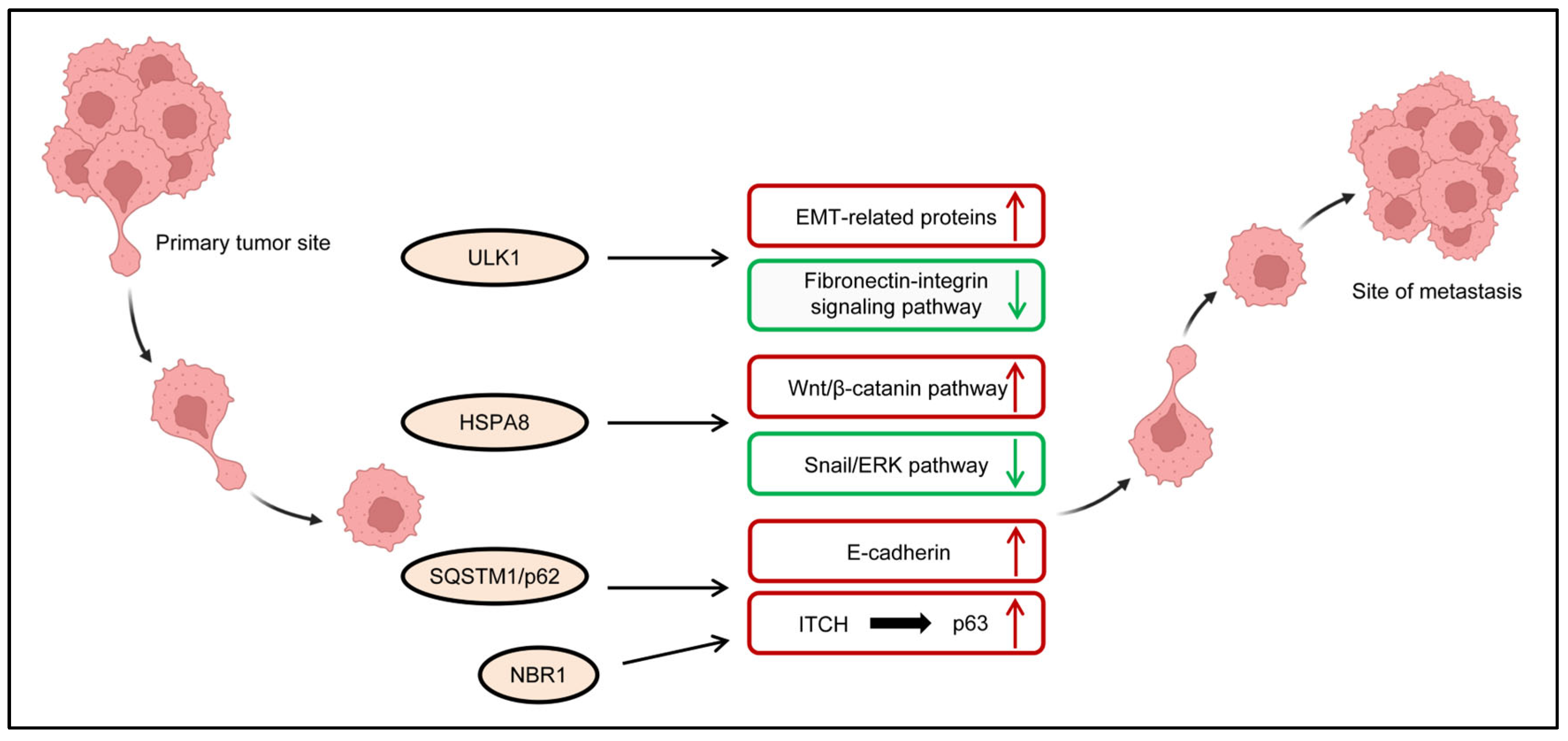
3.2. Regulation of Invasive and Metastatic Potential of TNBC Cells by ARPs
3.3. Regulation of Cancer Cell Stemness by ARPs in TNBC
3.4. Regulation of Tumor Immune Microenvironment by ARPs in TNBC
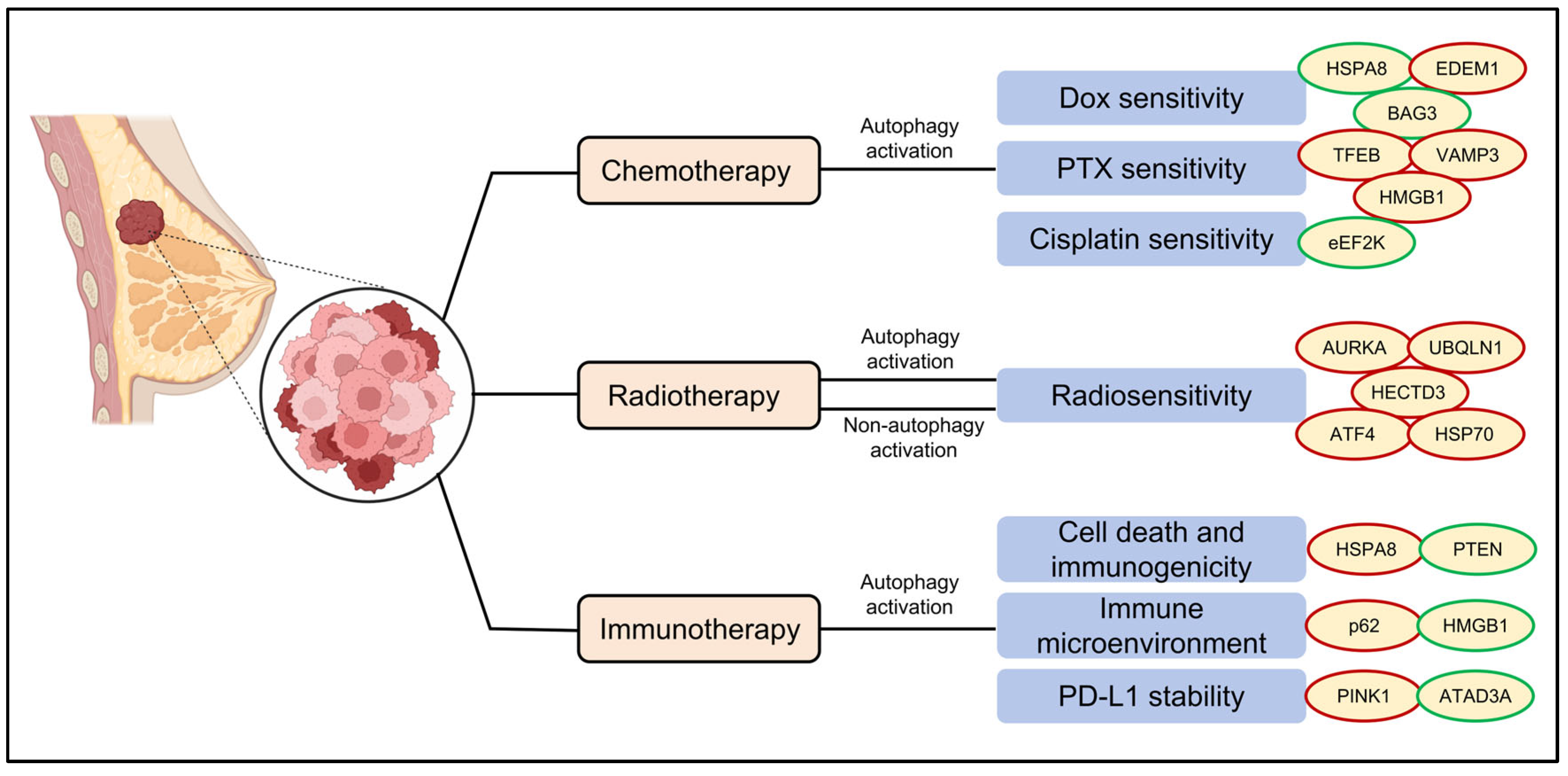
4. Regulation of Therapeutic Responsiveness by ARPs in TNBC
4.1. Chemotherapy
4.2. Radiotherapy
4.3. Immunotherapy
5. ARPs as Biomarkers in TNBC
5.1. Diagnostic Value of ARPs in TNBC
5.2. Prognostic Significance of ARPs in TNBC
5.3. Predictive Value of ARPs for Therapy in TNBC
6. Therapeutic Strategies Targeting ARPs in TNBC
7. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer-expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Vodnala, S.K.; Nini, R.; Hunter, K.W.; Green, J.E. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 2018, 9, 1944. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef]
- Niu, X.; You, Q.; Hou, K.; Tian, Y.; Wei, P.; Zhu, Y.; Gao, B.; Ashrafizadeh, M.; Aref, A.R.; Kalbasi, A.; et al. Autophagy in cancer development, immune evasion, and drug resistance. Drug Resist. Updat. 2025, 78, 101170. [Google Scholar] [CrossRef]
- Abd El-Aziz, Y.S.; Gillson, J.; Jansson, P.J.; Sahni, S. Autophagy: A promising target for triple negative breast cancers. Pharmacol. Res. 2022, 175, 106006. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Piezzo, M.; Caputo, R.; Di Lauro, V.; Di Rella, F.; Fusco, G.; Capozzi, M.; Gioia, G.D.; Budillon, A.; et al. Targeting Autophagy in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 7836. [Google Scholar] [CrossRef]
- Song, H.; Zhao, Z.; Ma, L.; Zhao, W.; Hu, Y.; Song, Y. Novel exosomal circEGFR facilitates triple negative breast cancer autophagy via promoting TFEB nuclear trafficking and modulating miR-224-5p/ATG13/ULK1 feedback loop. Oncogene 2024, 43, 821–836. [Google Scholar] [CrossRef]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2023, 24, 186–203. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Zhang, S.; Mizushima, N. Autophagy genes in biology and disease. Nat. Rev. Genet. 2023, 24, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Young, L.N. Mechanisms of Autophagy Initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Diao, J.; Liu, R.; Rong, Y.; Zhao, M.; Zhang, J.; Lai, Y.; Zhou, Q.; Wilz, L.M.; Li, J.; Vivona, S.; et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 2015, 520, 563–566. [Google Scholar] [CrossRef]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef]
- Shatz, O.; Elazar, Z. The physiological relevance of autophagosome morphogenesis. Trends Biochem. Sci. 2024, 49, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Itoh, T.; Omori, H.; Fukuda, M.; Noda, T.; Yoshimori, T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 2008, 19, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Otomo, C.; Metlagel, Z.; Takaesu, G.; Otomo, T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat. Struct. Mol. Biol. 2013, 20, 59–66. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Reggiori, F.; Ungermann, C. A novel in vitro assay reveals SNARE topology and the role of Ykt6 in autophagosome fusion with vacuoles. J. Cell Biol. 2018, 217, 3670–3682. [Google Scholar] [CrossRef]
- Tian, X.; Teng, J.; Chen, J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy 2021, 17, 2680–2688. [Google Scholar] [CrossRef]
- Shang, J.N.; Yu, C.G.; Li, R.; Xi, Y.; Jian, Y.J.; Xu, N.; Chen, S. The nonautophagic functions of autophagy-related proteins. Autophagy 2024, 20, 720–734. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y. HMGB1 in inflammation and cancer. J. Hematol. Oncol. 2020, 13, 116. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, Z.; Delibaşı, N.; Geçene, S.; Şener, E.F.; Dönmez-Altuntaş, H.; Özkul, Y.; Canatan, H.; Ozpolat, B. Targeting LC3 and Beclin-1 autophagy genes suppresses proliferation, survival, migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin β1/ Src signaling in triple negative breast cancer cells. J. Cancer Res. Clin. Oncol. 2018, 144, 415–430. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.M.; Lin, L.; Gao, S.S.; Fu, K.F.; Liu, X.D.; Liu, Y.; Zhou, L.J.; Zhou, P.K. BECN1-knockout impairs tumor growth, migration and invasion by suppressing the cell cycle and partially suppressing the epithelial-mesenchymal transition of human triple-negative breast cancer cells. Int. J. Oncol. 2018, 53, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Bijian, K.; Wernic, D.; Su, J.; da Silva, S.D.; Yu, H.; Qiu, D.; Asslan, M.; Alaoui-Jamali, M.A. A novel orally available seleno-purine molecule suppresses triple-negative breast cancer cell proliferation and progression to metastasis by inducing cytostatic autophagy. Autophagy 2019, 15, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Tian, W.; Zhu, L.; Luo, Y.; Tang, Y.; Tan, Q.; Zou, Y.; Chen, K.; Deng, X.; Tang, H.; Li, H.; et al. Autophagy Deficiency Induced by SAT1 Potentiates Tumor Progression in Triple-Negative Breast Cancer. Adv. Sci. 2024, 11, e2309903. [Google Scholar] [CrossRef]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Nowosad, A.; Jeannot, P.; Callot, C.; Creff, J.; Perchey, R.T.; Joffre, C.; Codogno, P.; Manenti, S.; Besson, A. p27 controls Ragulator and mTOR activity in amino acid-deprived cells to regulate the autophagy-lysosomal pathway and coordinate cell cycle and cell growth. Nat. Cell Biol. 2020, 22, 1076–1090. [Google Scholar] [CrossRef]
- Collier, J.J.; Guissart, C.; Oláhová, M.; Sasorith, S.; Piron-Prunier, F.; Suomi, F.; Zhang, D.; Martinez-Lopez, N.; Leboucq, N.; Bahr, A.; et al. Developmental Consequences of Defective ATG7-Mediated Autophagy in Humans. N. Engl. J. Med. 2021, 384, 2406–2417. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Li, S.; Feng, Y.; Yi, F.; Wang, L.; Wei, S.; Cao, L. Autophagy-related 7 modulates tumor progression in triple-negative breast cancer. Lab. Investig. 2019, 99, 1266–1274. [Google Scholar] [CrossRef]
- Maycotte, P.; Gearheart, C.M.; Barnard, R.; Aryal, S.; Mulcahy Levy, J.M.; Fosmire, S.P.; Hansen, R.J.; Morgan, M.J.; Porter, C.C.; Gustafson, D.L.; et al. STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res. 2014, 74, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kim, Y.K.; Kim, H.; Lee, J.; Oh, M.J.; Kim, S.B.; Kim, M.; Kim, K.H.; Yoon, H.J.; Lee, M.S.; et al. Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells. Cancer Commun. 2022, 42, 716–749. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, T.; Tu, B.; Yuan, M.; Shu, Z.; Fan, M.; Huo, S.; Guo, Y.; Wang, L.; Wang, H.; et al. Autophagy loss impedes cancer-associated fibroblast activation via downregulating proline biosynthesis. Autophagy 2023, 19, 632–643. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Li, S.; Chen, Y.; Wang, M.; Wu, Z.; Sun, X.; Yao, L.; Dong, H.; Song, Y.; et al. HIF-1-induced mitochondrial ribosome protein L52: A mechanism for breast cancer cellular adaptation and metastatic initiation in response to hypoxia. Theranostics 2021, 11, 7337–7359. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, J.; Wu, Y.; Zheng, S.; Xu, Z.; Yang, S.; Wang, J.; Ma, S.; Xiao, L.; Hu, T.; et al. An ULK1/2-PXN mechanotransduction pathway suppresses breast cancer cell migration. EMBO Rep. 2023, 24, e56850. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Yang, M.; Li, Q.; Weng, S.; Kou, S.; Liu, X.; Jiang, G.; Liu, H. H3K36me2 methyltransferase NSD2/WHSC1 promotes triple-negative breast cancer metastasis via activation of ULK1-dependent autophagy. Autophagy 2025, 21, 1824–1842. [Google Scholar] [CrossRef]
- Dower, C.M.; Bhat, N.; Wang, E.W.; Wang, H.-G. Selective Reversible Inhibition of Autophagy in Hypoxic Breast Cancer Cells Promotes Pulmonary Metastasis. Cancer Res. 2017, 77, 646–657. [Google Scholar] [CrossRef]
- Ying, B.; Xu, W.; Nie, Y.; Li, Y. HSPA8 Is a New Biomarker of Triple Negative Breast Cancer Related to Prognosis and Immune Infiltration. Dis. Markers 2022, 2022, 8446857. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Zhou, L.; Huang, Z.; Jin, P.; Peng, L.; Luo, M.; Zhang, T.; Wang, K.; et al. HSPA8 Activates Wnt/β-Catenin Signaling to Facilitate BRAF V600E Colorectal Cancer Progression by CMA-Mediated CAV1 Degradation. Adv. Sci. 2024, 11, e2306535. [Google Scholar] [CrossRef]
- Ryu, K.-J.; Lee, K.W.; Park, S.-H.; Kim, T.; Hong, K.-S.; Kim, H.; Kim, M.; Ok, D.W.; Kwon, G.N.B.; Park, Y.-J.; et al. Chaperone-mediated autophagy modulates Snail protein stability: Implications for breast cancer metastasis. Mol. Cancer 2024, 23, 227. [Google Scholar] [CrossRef]
- Liu, Q.W.; Fan, Q.L.; Chen, J.Y.; Liu, J.X.; Li, Y.; Luo, Q.; Chen, Y.P.; Wu, H.T.; Xu, A.Q.; Wang, S.; et al. Pristimerin Promotes Ubiquitination of HSPA8 and Activates the VAV1/ERK Pathway to Suppress TNBC Proliferation. Adv. Sci. 2025, 12, e2413174. [Google Scholar] [CrossRef] [PubMed]
- Mondal, G.; Gonzalez, H.; Marsh, T.; Leidal, A.M.; Vlahakis, A.; Phadatare, P.R.; Bustamante Eguiguren, S.a.; Bruck, M.; Naik, A.; Magbanua, M.J.M.; et al. Autophagy-targeted NBR1–p62/SQSTM1 complexes promote breast cancer metastasis by sequestering ITCH. Nat. Cell Biol. 2025, 27, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Damiano, V.; Spessotto, P.; Vanin, G.; Perin, T.; Maestro, R.; Santarosa, M. The Autophagy Machinery Contributes to E-cadherin Turnover in Breast Cancer. Front. Cell Dev. Biol. 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; White, J.; Zhou, J. Cancer stem cells in TNBC. Semin. Cancer Biol. 2022, 82, 26–34. [Google Scholar] [CrossRef]
- Li, D.; Peng, X.; He, G.; Liu, J.; Li, X.; Lin, W.; Fang, J.; Li, X.; Yang, S.; Yang, L.; et al. Crosstalk between autophagy and CSCs: Molecular mechanisms and translational implications. Cell Death Dis. 2023, 14, 409. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.; Lee, S.B.; Oh, C.W.; Lee, E.J.; Ko, J.Y.; Park, J.H. Autophagy inhibits cancer stemness in triple-negative breast cancer via miR-181a-mediated regulation of ATG5 and/or ATG2B. Mol. Oncol. 2022, 16, 1857–1875. [Google Scholar] [CrossRef]
- Xiong, Z.; Yang, L.; Li, N.; Fu, J.; Liu, P.; Sun, P.; Wei, W.; Xie, X. DAB2IP attenuates chemoresistance of triple-negative breast cancer through sequestration of RAC1 to prevent β-catenin nuclear accumulation. Clin. Transl. Med. 2022, 12, e1133. [Google Scholar] [CrossRef]
- Wan, X.; Gong, R.; Zhao, X.; Li, Y.; Shan, T.; Zhong, C.; Zhu, R.; Chen, Z.; Jiang, S.; He, L.; et al. Identification of a Novel Substrate for eEF2K and the AURKA-SOX8 as the Related Pathway in TNBC. Adv. Sci. 2025, 12, e2412985. [Google Scholar] [CrossRef]
- Harris, M.A.; Savas, P.; Virassamy, B.; O’Malley, M.M.R.; Kay, J.; Mueller, S.N.; Mackay, L.K.; Salgado, R.; Loi, S. Towards targeting the breast cancer immune microenvironment. Nat. Rev. Cancer 2024, 24, 554–577. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, D.; Zhao, S.; Suo, C.; Shi, J.; Xue, M.-Z.; Ruan, M.; Wang, H.; Zhao, J.; Li, Q.; et al. Multi-Omics Profiling Reveals Distinct Microenvironment Characterization and Suggests Immune Escape Mechanisms of Triple-Negative Breast Cancer. Clin. Cancer Res. 2019, 25, 5002–5014. [Google Scholar] [CrossRef]
- Li, W.; Tanikawa, T.; Kryczek, I.; Xia, H.; Li, G.; Wu, K.; Wei, S.; Zhao, L.; Vatan, L.; Wen, B.; et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018, 28, 87–103.e106. [Google Scholar] [CrossRef]
- Cotzomi-Ortega, I.; Nieto-Yañez, O.; Juárez-Avelar, I.; Rojas-Sanchez, G.; Montes-Alvarado, J.B.; Reyes-Leyva, J.; Aguilar-Alonso, P.; Rodriguez-Sosa, M.; Maycotte, P. Autophagy inhibition in breast cancer cells induces ROS-mediated MIF expression and M1 macrophage polarization. Cell. Signal. 2021, 86, 110075. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.D.; Templeton, D.J.; Cross, J.V. Macrophage Migration Inhibitory Factor Promotes Tumor Growth and Metastasis by Inducing Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. J. Immunol. 2012, 189, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Zhang, H.-L.; Huang, Y.; Huang, J.-H.; Sun, P.; Zhou, N.-N.; Chen, Y.-H.; Mai, J.; Wang, Y.; Yu, Y.; et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat. Commun. 2020, 11, 3806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.; Li, X.; Guo, A. CCAAT enhancer binding protein delta activates vesicle associated membrane protein 3 transcription to enhance chemoresistance and extracellular PD-L1 expression in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 115. [Google Scholar] [CrossRef]
- Fader, C.M.; Sánchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta (BBA)–Mol. Cell Res. 2009, 1793, 1901–1916. [Google Scholar] [CrossRef]
- Fischer, P.; Schmid, M.; Ohradanova-Repic, A.; Schneeweiss, R.; Hadatsch, J.; Grünert, O.; Benedum, J.; Röhrer, A.; Staudinger, F.; Schatzlmaier, P.; et al. Molecular features of TNBC govern heterogeneity in the response to radiation and autophagy inhibition. Cell Death Dis. 2025, 16, 540. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Pandy, J.G.P.; Balolong-Garcia, J.C.; Cruz-Ordinario, M.V.B.; Que, F.V.F. Triple negative breast cancer and platinum-based systemic treatment: A meta-analysis and systematic review. BMC Cancer 2019, 19, 1065. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Luo, D.; Ye, F.; Jin, Y.; Wang, L.; Li, Y.; Han, D.; Wang, Z.; Chen, B.; et al. EDEM1 Inhibits Endoplasmic Reticulum Stress to Induce Doxorubicin Resistance through Accelerating ERAD and Activating Keap1/Nrf2 Antioxidant Pathway in Triple-Negative Breast Cancer. Research 2025, 8, 0797. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Gestwicki, J.E.; Murphy, L.O.; Klionsky, D.J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007, 6, 304–312. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Lefort, S.; Joffre, C.; Kieffer, Y.; Givel, A.M.; Bourachot, B.; Zago, G.; Bieche, I.; Dubois, T.; Meseure, D.; Vincent-Salomon, A.; et al. Inhibition of autophagy as a new means of improving chemotherapy efficiency in high-LC3B triple-negative breast cancers. Autophagy 2014, 10, 2122–2142. [Google Scholar] [CrossRef]
- Das, C.K.; Linder, B.; Bonn, F.; Rothweiler, F.; Dikic, I.; Michaelis, M.; Cinatl, J.; Mandal, M.; Kögel, D. BAG3 Overexpression and Cytoprotective Autophagy Mediate Apoptosis Resistance in Chemoresistant Breast Cancer Cells. Neoplasia 2018, 20, 263–279. [Google Scholar] [CrossRef]
- Qiao, J.X.; Guo, D.Y.; Tian, H.; Wang, Z.P.; Fan, Q.Q.; Tian, Y.; Sun, J.; Zhang, X.F.; Zou, J.B.; Cheng, J.X.; et al. Research progress of paclitaxel nanodrug delivery system in the treatment of triple-negative breast cancer. Mater. Today Bio 2024, 29, 101358. [Google Scholar] [CrossRef]
- Zheng, B.; Qian, F.; Wang, X.; Wang, Y.; Zhou, B.; Fang, L. Neddylation activated TRIM25 desensitizes triple-negative breast cancer to paclitaxel via TFEB-mediated autophagy. J. Exp. Clin. Cancer Res. 2024, 43, 177. [Google Scholar] [CrossRef]
- Tang, X.; Gong, J.; Ren, L.; Wang, Z.; Yang, B.; Wang, W.; Wang, N. Tanshinone I improves TNBC chemosensitivity by suppressing late-phase autophagy through AKT/p38 MAPK signaling pathway. Biomed. Pharmacother. 2024, 177, 117037. [Google Scholar] [CrossRef]
- Chen, R.; Zou, J.; Zhong, X.; Li, J.; Kang, R.; Tang, D. HMGB1 in the interplay between autophagy and apoptosis in cancer. Cancer Lett. 2024, 581, 216494. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, G.; Wang, S.; Zheng, Y.; Zhang, J.; Pan, B.; Wang, N.; Wang, Z. Tumor-associated macrophages/C-X-C motif chemokine ligand 1 promotes breast cancer autophagy-mediated chemoresistance via IGF1R/STAT3/HMGB1 signaling. Cell Death Dis. 2024, 15, 743. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Liu, X.-M.; Zhu, Z.-S.; Li, Z.; Xie, C.-Z.; Qiao, X.; Feng, Y.-K.; Xu, J.-Y. Fluoxetine-Conjugated Platinum(IV) Prodrugs Targeting eEF2K and Conquering Multidrug Resistance against Triple-Negative Breast Cancer. J. Med. Chem. 2025, 68, 9661–9680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhu, J.; Jiang, S.; Chen, Z.; Xu, P.; Gong, R.; Zhong, C.; Cheng, Y.; Sun, X.; Yi, W.; et al. Targeting lncRNA DDIT4-AS1 Sensitizes Triple Negative Breast Cancer to Chemotherapy via Suppressing of Autophagy. Adv. Sci. 2023, 10, e2207257. [Google Scholar] [CrossRef]
- Xia, M.; Zu, X.; Chen, Z.; Wen, G.; Zhong, J. Noncoding RNAs in triple negative breast cancer: Mechanisms for chemoresistance. Cancer Lett. 2021, 523, 100–110. [Google Scholar] [CrossRef]
- Sarlak, S.; Pagès, G.; Luciano, F. Enhancing radiotherapy techniques for Triple-Negative breast cancer treatment. Cancer Treat. Rev. 2025, 136, 102939. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, S.; Han, Y.; Xie, D.; Xuan, L.; Huang, X.; Luo, J.; Ran, Q.; Li, G.; Guo, H.; et al. Conversion of Ku80 K568 crotonylation to SUMOylation facilitates DNA non-homologous end joining and cancer radioresistance. Signal Transduct. Target. Ther. 2025, 10, 127. [Google Scholar] [CrossRef]
- Pan, P.; Dong, X.; Chen, Y.; Zeng, X.; Zhang, X.Z. Engineered Bacteria for Enhanced Radiotherapy against Breast Carcinoma. ACS Nano 2022, 16, 801–812. [Google Scholar] [CrossRef]
- Kim, B.M.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.H.; Lee, Y.H.; Lee, T.H.; Chang, K.T.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913. [Google Scholar] [CrossRef]
- Huang, M.; Liu, W.; Cheng, Z.; Li, F.; Kong, Y.; Yang, C.; Tang, Y.; Jiang, D.; Li, W.; Hu, Y.; et al. Targeting the HECTD3-p62 axis increases the radiosensitivity of triple negative breast cancer cells. Cell Death Discov. 2024, 10, 462. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, T.; Yuan, Y.; Guo, Z.; Xie, G.; Du, S.; Lin, X.; Xu, Z.; Liu, M.; Wang, W.; et al. MiR-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1. Int. J. Cancer 2015, 136, 1003–1012. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Yang, Z.Z.; Wang, S.J.; Zhang, L.; Luo, J.R.; Feng, Y.; Yu, X.L.; Chen, X.X.; Guo, X.M. The Chk1 inhibitor MK-8776 increases the radiosensitivity of human triple-negative breast cancer by inhibiting autophagy. Acta Pharmacol. Sin. 2017, 38, 513–523. [Google Scholar] [CrossRef]
- Bouchard, G.; Therriault, H.; Geha, S.; Bérubé-Lauzière, Y.; Bujold, R.; Saucier, C.; Paquette, B. Stimulation of triple negative breast cancer cell migration and metastases formation is prevented by chloroquine in a pre-irradiated mouse model. BMC Cancer 2016, 16, 361. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Wasinger, V.C.; Wang, S.; Zhu, Y.; Graham, P.; Li, Y. Activation of the eIF2α/ATF4 axis drives triple-negative breast cancer radioresistance by promoting glutathione biosynthesis. Redox Biol. 2021, 43, 101993. [Google Scholar] [CrossRef]
- Yang, M.; Shi, D.; Lyu, J.; Pan, Y.; Lyv, Y.; Chen, X.; Ouyang, Y.; Liu, Y.; Li, Y.; Song, L. Supplementing sialic acid analogs overcomes radiotherapy resistance in triple-negative breast cancer by exacerbating ER stress. Redox Biol. 2025, 85, 103712. [Google Scholar] [CrossRef]
- Wu, Z.; Stangl, S.; Hernandez-Schnelzer, A.; Wang, F.; Hasanzadeh Kafshgari, M.; Bashiri Dezfouli, A.; Multhoff, G. Functionalized Hybrid Iron Oxide-Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis. Cancers 2023, 15, 1167. [Google Scholar] [CrossRef] [PubMed]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Zheng, P.; Hu, Z.; Shen, Y.; Gu, L.; Ouyang, Y.; Duan, Y.; Ji, G.; Dong, B.; Lin, Y.; Wen, T.; et al. PSAT1 impairs ferroptosis and reduces immunotherapy efficacy via GPX4 hydroxylation. Nat. Chem. Biol. 2025, 21, 1420–1432. [Google Scholar] [CrossRef]
- Hubert, P.; Roncarati, P.; Demoulin, S.; Pilard, C.; Ancion, M.; Reynders, C.; Lerho, T.; Bruyere, D.; Lebeau, A.; Radermecker, C.; et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J. Immunother. Cancer 2021, 9, e001966. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-Q.; Yang, Y.; Wang, Q.; Liu, H.-F.; Fang, X.-Y.; Li, C.-L.; Jiang, Y.-Z.; Wang, S.; Zhao, H.-Y.; Miao, J.-Y.; et al. Targeting ATAD3A-PINK1-mitophagy axis overcomes chemoimmunotherapy resistance by redirecting PD-L1 to mitochondria. Cell Res. 2023, 33, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, J.; Kim, E.H.; Park, W.; Jang, H.; Jang, Y.; Chi, S.G.; Kweon, D.H.; Lee, K.; Kim, S.H.; et al. Design of PD-L1-Targeted Lipid Nanoparticles to Turn on PTEN for Efficient Cancer Therapy. Adv. Sci. 2024, 11, e2309917. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.; Minata, M.; Ibrahim, A.N.; Yamaguchi, S.; Coviello, V.; Bernstock, J.D.; Harada, S.; Cerione, R.A.; Tannous, B.A.; La Motta, C.; et al. Identification of ALDH1A3 as a Viable Therapeutic Target in Breast Cancer Metastasis-Initiating Cells. Mol. Cancer Ther. 2020, 19, 1134–1147. [Google Scholar] [CrossRef]
- Bharadwaj, A.G.; McLean, M.E.; Dahn, M.L.; Cahill, H.F.; Wasson, M.D.; Arun, R.P.; Walker, O.L.; Cruickshank, B.M.; Fernando, W.; Venkatesh, J.; et al. ALDH1A3 promotes invasion and metastasis in triple-negative breast cancer by regulating the plasminogen activation pathway. Mol. Oncol. 2024, 18, 91–112. [Google Scholar] [CrossRef]
- Vidovic, D.; Huynh, T.T.; Konda, P.; Dean, C.; Cruickshank, B.M.; Sultan, M.; Coyle, K.M.; Gujar, S.; Marcato, P. ALDH1A3-regulated long non-coding RNA NRAD1 is a potential novel target for triple-negative breast tumors and cancer stem cells. Cell Death Differ. 2020, 27, 363–378. [Google Scholar] [CrossRef]
- González-González, A.; Muñoz-Muela, E.; Marchal, J.A.; Cara, F.E.; Molina, M.P.; Cruz-Lozano, M.; Jiménez, G.; Verma, A.; Ramírez, A.; Qian, W.; et al. Activating Transcription Factor 4 Modulates TGFβ-Induced Aggressiveness in Triple-Negative Breast Cancer via SMAD2/3/4 and mTORC2 Signaling. Clin. Cancer Res. 2018, 24, 5697–5709. [Google Scholar] [CrossRef]
- Bai, X.; Ali, A.; Lv, Z.; Wang, N.; Zhao, X.; Hao, H.; Zhang, Y.; Rahman, F.-U. Platinum complexes inhibit HER-2 enriched and triple-negative breast cancer cells metabolism to suppress growth, stemness and migration by targeting PKM/LDHA and CCND1/BCL2/ATG3 signaling pathways. Eur. J. Med. Chem. 2021, 224, 113689. [Google Scholar] [CrossRef]
- Shang, F.; Nie, H.; Du, L.; Shang, J.; Song, X.; Chen, Y.; Li, H.; Wang, Z.; Qi, Y.; Zhao, L. Inhibition of ATG5-mediated autophagy maintains PMAIP1 stability to promote cell apoptosis and suppress triple-negative breast cancer progression. Discov. Oncol. 2025, 16, 687. [Google Scholar] [CrossRef]
- Koh, M.; Lim, H.; Jin, H.; Kim, M.; Hong, Y.; Hwang, Y.K.; Woo, Y.; Kim, E.S.; Kim, S.Y.; Kim, K.M.; et al. ANXA2 (annexin A2) is crucial to ATG7-mediated autophagy, leading to tumor aggressiveness in triple-negative breast cancer cells. Autophagy 2024, 20, 659–674. [Google Scholar] [CrossRef]
- Claude-Taupin, A.; Fonderflick, L.; Gauthier, T.; Mansi, L.; Pallandre, J.R.; Borg, C.; Perez, V.; Monnien, F.; Algros, M.P.; Vigneron, M.; et al. ATG9A Is Overexpressed in Triple Negative Breast Cancer and Its In Vitro Extinction Leads to the Inhibition of Pro-Cancer Phenotypes. Cells 2018, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Sun, K.; Xia, W.; Li, Y.; Zhong, M.; Lei, K. Autophagy-related prognostic signature for survival prediction of triple negative breast cancer. PeerJ 2022, 10, e12878. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Qian, C.; Liu, M.Y.; Jiang, F.; Jiang, X.; Liu, H.; Zhang, Z.; Sun, F.; Fu, N.; Hou, Z.; et al. PRKAA/AMPKα phosphorylation switches the role of RASAL2 from a suppressor to an activator of autophagy. Autophagy 2021, 17, 3607–3621. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhou, W.; Li, X.; Peng, F.; Yan, M.; Zhan, Y.; An, F.; Li, X.; Liu, Y.; Liu, Q.; et al. Nuclear Aurora kinase A triggers programmed death-ligand 1-mediated immune suppression by activating MYC transcription in triple-negative breast cancer. Cancer Commun. 2021, 41, 851–866. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wang, X.; Chen, K.; Feng, L. Enhancing radiotherapy in triple-negative breast cancer with hesperetin-induced ferroptosis via AURKA targeting nanocomposites. J. Nanobiotechnol. 2024, 22, 744. [Google Scholar] [CrossRef]
- Li, C.; Liao, J.; Wang, X.; Chen, F.X.; Guo, X.; Chen, X. Combined Aurora Kinase A and CHK1 Inhibition Enhances Radiosensitivity of Triple-Negative Breast Cancer Through Induction of Apoptosis and Mitotic Catastrophe Associated With Excessive DNA Damage. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 1241–1254. [Google Scholar] [CrossRef]
- Contreras-Zárate, M.J.; Day, N.L.; Ormond, D.R.; Borges, V.F.; Tobet, S.; Gril, B.; Steeg, P.S.; Cittelly, D.M. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene 2019, 38, 4685–4699. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.; Zhao, Y.; Jiang, Y.; Chen, L.; Yu, Y.; Ouyang, L. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018, 51, e12402. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Liu, Y.; Zhang, S.; Su, P.; Wang, L.; Li, Y.; Liang, Y.; Wang, X.; Zhao, W.; et al. Hypoxia-induced GPCPD1 depalmitoylation triggers mitophagy via regulating PRKN-mediated ubiquitination of VDAC1. Autophagy 2023, 19, 2443–2463. [Google Scholar] [CrossRef]
- Yu, T.-J.; Liu, Y.-Y.; Li, X.-G.; Lian, B.; Lu, X.-X.; Jin, X.; Shao, Z.-M.; Hu, X.; Di, G.-H.; Jiang, Y.-Z. PDSS1-Mediated Activation of CAMK2A-STAT3 Signaling Promotes Metastasis in Triple-Negative Breast Cancer. Cancer Res. 2021, 81, 5491–5505. [Google Scholar] [CrossRef]
- Mukherjee, D.; Previs, R.A.; Haines, C.; Al Abo, M.; Juras, P.K.; Strickland, K.C.; Chakraborty, B.; Artham, S.; Whitaker, R.S.; Hebert, K.; et al. Targeting CaMKK2 Inhibits Actin Cytoskeletal Assembly to Suppress Cancer Metastasis. Cancer Res. 2023, 83, 2889–2907. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sun, H.; Liu, S.; Liao, L.; Song, X.; Wu, Y.; Hou, Y.; Jin, W. IFI35 limits antitumor immunity in triple-negative breast cancer via CCL2 secretion. Oncogene 2024, 43, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, M.; Yin, J.; Li, P.; Zeng, S.; Zheng, G.; He, Z.; Liu, H.; Wang, Q.; Zhang, F.; et al. Tumor-associated macrophages promote epithelial–mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun. Signal. 2022, 20, 92. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Hughes, C.J.; Fields, K.M.; Purdy, S.C.; Gustafson, A.L.; Wolin, A.; Hampton, D.; Shrivastava, N.M.; Turner, N.; Danis, E.; et al. EYA3 regulation of NF-κB and CCL2 suppresses cytotoxic NK cells in the premetastatic niche to promote TNBC metastasis. Sci. Adv. 2025, 11, eadt0504. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, J.; Ma, T.; Chen, D.; Lu, D. miR-1205/DNAJB1 reverses docetaxel chemoresistance in human triple negative breast carcinoma cells via regulation of mutp53/TAp63 signaling. Acta Biochim. Biophys. Sin. 2022, 54, 37–46. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.M.; Hong, S. DNAJB9 suppresses the metastasis of triple-negative breast cancer by promoting FBXO45-mediated degradation of ZEB1. Cell Death Dis. 2021, 12, 461. [Google Scholar] [CrossRef]
- Huang, L.; Wei, B.; Zhao, Y.; Gong, X.; Chen, L. DYNLT1 promotes mitochondrial metabolism to fuel breast cancer development by inhibiting ubiquitination degradation of VDAC1. Mol. Med. 2023, 29, 72. [Google Scholar] [CrossRef]
- Zhong, C.; Zhu, R.; Jiang, T.; Tian, S.; Zhao, X.; Wan, X.; Jiang, S.; Chen, Z.; Gong, R.; He, L.; et al. Design and Characterization of a Novel eEF2K Degrader with Potent Therapeutic Efficacy Against Triple-Negative Breast Cancer. Adv. Sci. 2024, 11, e2305035. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wu, Z.X.; Chen, J.W.; Li, H.F.; Wu, H.D.; Bao, J.X.; Cheng, Y.; Dai, Y.W.; Wang, O.C.; Dai, X.X. Cyclovirobuxine D inhibits triple-negative breast cancer via YAP/TAZ suppression and activation of the FOXO3a/PINK1-Parkin pathway-induced mitophagy. Phytomedicine 2025, 136, 156287. [Google Scholar] [CrossRef]
- Yang, A.; Peng, F.; Zhu, L.; Li, X.; Ou, S.; Huang, Z.; Wu, S.; Peng, C.; Liu, P.; Kong, Y. Melatonin inhibits triple-negative breast cancer progression through the Lnc049808-FUNDC1 pathway. Cell Death Dis. 2021, 12, 712. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Zhang, M.; Jiang, Y.; Ng, A.S.; Bridges, E.; Zhang, W.; Zeng, X.; Luo, Q.; Liang, J.; et al. GTP Cyclohydrolase Drives Breast Cancer Development and Promotes EMT in an Enzyme-Independent Manner. Cancer Res. 2023, 83, 3400–3413. [Google Scholar] [CrossRef] [PubMed]
- Brasher, M.I.; Chafe, S.C.; McDonald, P.C.; Nemirovsky, O.; Gorshtein, G.; Gerbec, Z.J.; Brown, W.S.; Grafinger, O.R.; Marchment, M.; Matus, E.; et al. Syntaxin4-Munc18c Interaction Promotes Breast Tumor Invasion and Metastasis by Regulating MT1-MMP Trafficking. Mol. Cancer Res. 2022, 20, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Gao, J.; Zhou, L.; Xue, W.; Wang, Y.; Chen, J.; Li, W.; Yu, Y.; Liu, B.; Shen, Y.; et al. Highly expressed SERCA2 triggers tumor cell autophagy and is a druggable vulnerability in triple-negative breast cancer. Acta Pharm. Sin. B 2022, 12, 4407–4423. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Ivan, C.; Bayraktar, E.; Kanlikilicer, P.; Kabil, N.N.; Kahraman, N.; Mokhlis, H.A.; Karakas, D.; Rodriguez-Aguayo, C.; Arslan, A.; et al. Dual Suppressive Effect of miR-34a on the FOXM1/eEF2-Kinase Axis Regulates Triple-Negative Breast Cancer Growth and Invasion. Clin. Cancer Res. 2018, 24, 4225–4241. [Google Scholar] [CrossRef]
- Egan, D.F.; Chun, M.G.; Vamos, M.; Zou, H.; Rong, J.; Miller, C.J.; Lou, H.J.; Raveendra-Panickar, D.; Yang, C.C.; Sheffler, D.J.; et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell 2015, 59, 285–297. [Google Scholar] [CrossRef]
- Yu, L.; Shi, Q.; Jin, Y.; Liu, Z.; Li, J.; Sun, W. Blockage of AMPK-ULK1 pathway mediated autophagy promotes cell apoptosis to increase doxorubicin sensitivity in breast cancer (BC) cells: An in vitro study. BMC Cancer 2021, 21, 195. [Google Scholar] [CrossRef]
- Desai, J.M.; Karve, A.S.; Gudelsky, G.A.; Gawali, M.V.; Seibel, W.; Sallans, L.; DasGupta, B.; Desai, P.B. Brain pharmacokinetics and metabolism of the AMP-activated protein kinase selective inhibitor SBI-0206965, an investigational agent for the treatment of glioblastoma. Investig. New Drugs 2022, 40, 944–952. [Google Scholar] [CrossRef]
- Ren, H.; Bakas, N.A.; Vamos, M.; Chaikuad, A.; Limpert, A.S.; Wimer, C.D.; Brun, S.N.; Lambert, L.J.; Tautz, L.; Celeridad, M.; et al. Design, Synthesis, and Characterization of an Orally Active Dual-Specific ULK1/2 Autophagy Inhibitor that Synergizes with the PARP Inhibitor Olaparib for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2020, 63, 14609–14625. [Google Scholar] [CrossRef]
- Petherick, K.J.; Conway, O.J.; Mpamhanga, C.; Osborne, S.A.; Kamal, A.; Saxty, B.; Ganley, I.G. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J. Biol. Chem. 2015, 290, 28726. [Google Scholar] [CrossRef]
- Xue, S.-T.; Li, K.; Gao, Y.; Zhao, L.-Y.; Gao, Y.; Yi, H.; Jiang, J.-D.; Li, Z.-R. The role of the key autophagy kinase ULK1 in hepatocellular carcinoma and its validation as a treatment target. Autophagy 2020, 16, 1823–1837. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Li, S.Y.; Zhou, Z.Y.; Han, X.Y.; Li, K.; Xue, S.T.; Jiang, J.D. Substituted indole derivatives as UNC-51-like kinase 1 inhibitors: Design, synthesis and anti-hepatocellular carcinoma activity. Biomed. Pharmacother. 2024, 178, 117260. [Google Scholar] [CrossRef]
- Pasquier, B. SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells. Autophagy 2015, 11, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Wang, H.L.; Lu, G.; Zhang, H.; Wang, L.; Li, Z.Y.; Wang, L.; Wu, Y.; Xia, D.; Fang, E.F.; et al. Spautin-1 promotes PINK1-PRKN-dependent mitophagy and improves associative learning capability in an alzheimer disease animal model. Autophagy 2024, 20, 2655–2676. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yeo, S.; Wang, C.; Chen, S.; Sun, S.; Haas, M.A.; Tu, W.; Jin, F.; Guan, J.-L. Autophagy inhibition re-sensitizes pulse stimulation-selected paclitaxel-resistant triple negative breast cancer cells to chemotherapy-induced apoptosis. Breast Cancer Res. Treat. 2015, 149, 619–629. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Guo, H.; Zhang, B.; Zhang, X.B.; Shi, Z.J.; Yu, L. Synthesis and screening of 3-MA derivatives for autophagy inhibitors. Autophagy 2013, 9, 595–603. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, X.; Li, Y.; Fan, J.; Zeng, X.; Xian, Z.; Wang, Z.; Sun, Y.; Wang, S.; Song, P.; et al. Blocking autophagy enhanced cytotoxicity induced by recombinant human arginase in triple-negative breast cancer cells. Cell Death Dis. 2014, 5, e1563. [Google Scholar] [CrossRef]
- Nirk, E.L.; Reggiori, F.; Mauthe, M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol. Med. 2020, 12, e12476. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Sousa, R.W.R.; Ferreira, J.R.O.; Militão, G.C.G.; Bezerra, D.P. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef]
- Liang, D.H.; Choi, D.S.; Ensor, J.E.; Kaipparettu, B.A.; Bass, B.L.; Chang, J.C. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016, 376, 249–258. [Google Scholar] [CrossRef]
- Sun, R.; Shen, S.; Zhang, Y.J.; Xu, C.F.; Cao, Z.T.; Wen, L.P.; Wang, J. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Roca, M.S.; Lombardi, R.; Piezzo, M.; Caputo, R.; Ciardiello, C.; Costantini, S.; Bruzzese, F.; Sisalli, M.J.; et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J. Transl. Med. 2022, 20, 290. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Jiang, X.; Zhang, H.; Gao, Z.; Li, Y.; Fu, R.; Li, L.; Li, J.; Cui, H.; et al. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J. Exp. Clin. Cancer Res. 2019, 38, 225. [Google Scholar] [CrossRef]
- Pelt, J.; Busatto, S.; Ferrari, M.; Thompson, E.A.; Mody, K.; Wolfram, J. Chloroquine and nanoparticle drug delivery: A promising combination. Pharmacol. Ther. 2018, 191, 43–49. [Google Scholar] [CrossRef]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef]
- Chu, H.Y.; Wang, W.; Chen, X.; Jiang, Y.E.; Cheng, R.; Qi, X.; Zhong, Z.M.; Zeng, M.S.; Zhu, X.F.; Sun, C.Z. Bafilomycin A1 increases the sensitivity of tongue squamous cell carcinoma cells to cisplatin by inhibiting the lysosomal uptake of platinum ions but not autophagy. Cancer Lett. 2018, 423, 105–112. [Google Scholar] [CrossRef]
| Protein | Change | Phenotype | Mechanism | Reference |
|---|---|---|---|---|
| ALDH1A3 | Upregulation | Promotes adhesion and migration, facilitating the initiation of early metastasis | Enhances fibrinolytic activity through transcriptional regulation mediated by retinoic acid (ATRA), promoting extracellular matrix degradation | [104,105] |
| Promotes tumor growth and progression, increasing the proportion of cancer stem cells | Induces the expression of lncRNA NRAD1 | [106] | ||
| ATF4 | Upregulation | Radiation resistance | Activates transcription of GSH synthesis genes, inhibiting ROS accumulation | [94,95] |
| Promotes proliferation and invasion | Regulates through the TGFβ/SMAD2/3/4 and PI3K/mTORC2 pathways | [107] | ||
| ATG2B | Downregulation | Reduces cancer stem cell proportion and malignant tumor phenotype | Regulates by enhancing autophagy | [57] |
| ATG3 | Downregulation | Induces cell death, inhibits tumor stem cell traits and migratory ability | Activates autophagy to promote apoptosis | [108] |
| ATG5 | Upregulation | Maintains cell survival, proliferation, migration, and invasion capabilities | Activates autophagy to degrade pro-apoptotic protein PMAIP1 | [95,109] |
| Radiation resistance | Activates autophagy, alleviates DNA damage | [92] | ||
| ATG7 | Downregulation | Inhibits proliferation, migration, EMT | Downregulates expression of key EMT transcription factors and mesenchymal markers | [40,110] |
| Enhances chemotherapy-induced apoptosis | Activates autophagy and inhibits aerobic glycolysis | [110] | ||
| ATG9A | Upregulation | Promotes cell proliferation and invasion | Unknown | [111] |
| ATG12 | Upregulation | Promotes tumor stemness and malignant progression | Regulates via enhanced autophagy | [112] |
| ATG13 | Upregulation | Promotes cell proliferation, migration, invasion, and autophagy activation | Via circEGFR → TFEB → ATG13/ULK1 positive feedback loop, enhancing autophagy and thereby promoting malignant phenotype | [11] |
| ATG14 | Unknown | Promotes cell survival | Regulates via enhanced autophagy | [113] |
| ULK1 | Upregulation | Promotes cell proliferation, migration, and invasion | Via circEGFR → TFEB → ATG13/ULK1 positive feedback loop, enhancing autophagy and thereby promoting malignant phenotype | [11] |
| AURKA | Upregulation | Promotes cell proliferation, metastasis, and stemness | Regulates transcription factor SOX8, activating downstream target genes | [59] |
| Promotes immune evasion | Through activation of MYC transcription, mediating PD-L1 upregulation | [114] | ||
| Reduces radiosensitivity | By upregulating GPX4, inhibiting ferroptosis, and weakening radiotherapy-induced ROS effects | [115,116] | ||
| BAG3 | Upregulation | Enhances chemotherapy resistance and malignant phenotype | Stabilizes anti-apoptotic BCL-2 family proteins, promotes protective autophagy, inhibits apoptosis, and upregulates EMT transcription factors | [77] |
| BDNF | Upregulation | Promotes colonization and growth of brain metastases | Activates the TrkB receptor on the cell surface | [117] |
| BECLIN 1 | Upregulation | Promotes cell proliferation, migration, and invasion | Induces G0/G1 cell cycle arrest and promotes EMT | [33,34] |
| BNIP1 | Downregulation | Inhibit cell proliferation and enhance autophagic cell death | Activates autophagy | [118] |
| BNIP3 | Upregulation | Supports cell survival, proliferation, and metastasis | Acts synergistically with GPCPD1-mediated mitophagy pathway | [119] |
| CAMK2A | Upregulation | Promotes tumor cell invasion and metastasis | Promotes STAT3 phosphorylation at Tyr705, upregulates downstream target genes, driving EMT and extracellular matrix degradation | [120] |
| CAMKK2 | Upregulation | Promotes cell migration and invasion | Activates the PDE1A-PKG1-VASP axis, promoting actin cytoskeleton assembly | [121] |
| CCL2 | Upregulation | Induces immune evasion and reduces sensitivity to immunotherapy | Recruits MDSCs to remodel the immune microenvironment | [122] |
| Promotes EMT, cell invasion, and stemness | Activates AKT kinase, phosphorylating β-catenin at Ser552 to translocate into the nucleus, upregulating EMT and stem cell markers | [123] | ||
| Promotes lung metastasis | Directly inhibiting CCR2 signaling and recruiting inflammation | [124] | ||
| DAB2IP | Downregulation | Suppresses stemness and chemoresistance | Inhibiting nuclear translocation of β-catenin | [58] |
| DNAJB1 | Upregulation | Promotes docetaxel resistance and reduces cell cycle arrest and apoptosis | Regulating mutp53/TAp63 | [125] |
| DNAJB9 | Downregulation | Inhibits EMT and metastasis | Stabilizing FBXO45 and promoting ubiquitination of ZEB1 | [126] |
| DYNLT1 | Upregulation | Promotes proliferation, colony formation, migration, and invasion | Enhancing mitochondrial metabolism via DYNLT1-Parkin-VDAC1 | [127] |
| EEF2K | Upregulation | Promotes survival, proliferation, invasion, migration, and metastasis | Binding and phosphorylating AURKA at S391 to enhance stability and kinase activity, upregulating SOX8 expression | [59,128] |
| Mediates chemoresistance | Activating DNA damage repair pathways | [83] | ||
| Regulates immune evasion | Phosphorylating GSK3β to inhibit its activity and stabilizing PD-L1 expression | [59,128] | ||
| FOXO3 | Downregulation | Promotes apoptosis | Activating downstream targets PINK1 and Parkin to facilitate mitophagy | [129] |
| FUNDC1 | Upregulation | Promotes proliferation, colony formation, invasion, and metastasis | Mediating hypoxia-induced mitophagy | [130] |
| HSP90 | Upregulation | Promotes proliferation, invasion, EMT, angiogenesis, and immune evasion | Sustaining EGFR/ERK signaling and regulating immunosuppressive molecule expression | [131] |
| STX4 | Upregulation | Promotes migration, invasion, and distant metastasis | Binding Munc18c and forming functional complexes with other SNARE proteins to enhance invadopodia function | [132] |
| NBR1 | Upregulation | Promotes metastatic potential | Stabilizing and activating transcription factor p63, enhancing expression of downstream targets such as CK5 and CK14 | [53] |
| HMGB1 | Upregulation | Promotes immune evasion | Inducing immune tolerance and recruiting immunosuppressive cells via the RAGE pathway | [101] |
| Promotes chemoresistance | Activating autophagy and mediating drug efflux through ABCG2 | [82] | ||
| HSPA8 | Upregulation | Promotes proliferation and inhibits apoptosis | Degrading VAV1 via CMA, suppressing ERK pathway activation | [52] |
| Regulates immunotherapy response | Inhibiting ferroptosis by stabilizing GPX4 | [100] | ||
| WIPI2 | Unknown | Promotes chemoresistance | Activating autophagy | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.-K.; Li, D.-Q. Autophagy-Related Proteins in Triple-Negative Breast Cancer: From Molecular Insights to Therapeutic Applications. Int. J. Mol. Sci. 2025, 26, 9231. https://doi.org/10.3390/ijms26189231
Ma M-K, Li D-Q. Autophagy-Related Proteins in Triple-Negative Breast Cancer: From Molecular Insights to Therapeutic Applications. International Journal of Molecular Sciences. 2025; 26(18):9231. https://doi.org/10.3390/ijms26189231
Chicago/Turabian StyleMa, Meng-Ke, and Da-Qiang Li. 2025. "Autophagy-Related Proteins in Triple-Negative Breast Cancer: From Molecular Insights to Therapeutic Applications" International Journal of Molecular Sciences 26, no. 18: 9231. https://doi.org/10.3390/ijms26189231
APA StyleMa, M.-K., & Li, D.-Q. (2025). Autophagy-Related Proteins in Triple-Negative Breast Cancer: From Molecular Insights to Therapeutic Applications. International Journal of Molecular Sciences, 26(18), 9231. https://doi.org/10.3390/ijms26189231






