Neuroinflammation in CTLA-4 Haploinsufficiency: Case Report of a New Variant with Remarkable Response to Targeted Therapy
Abstract
1. Introduction
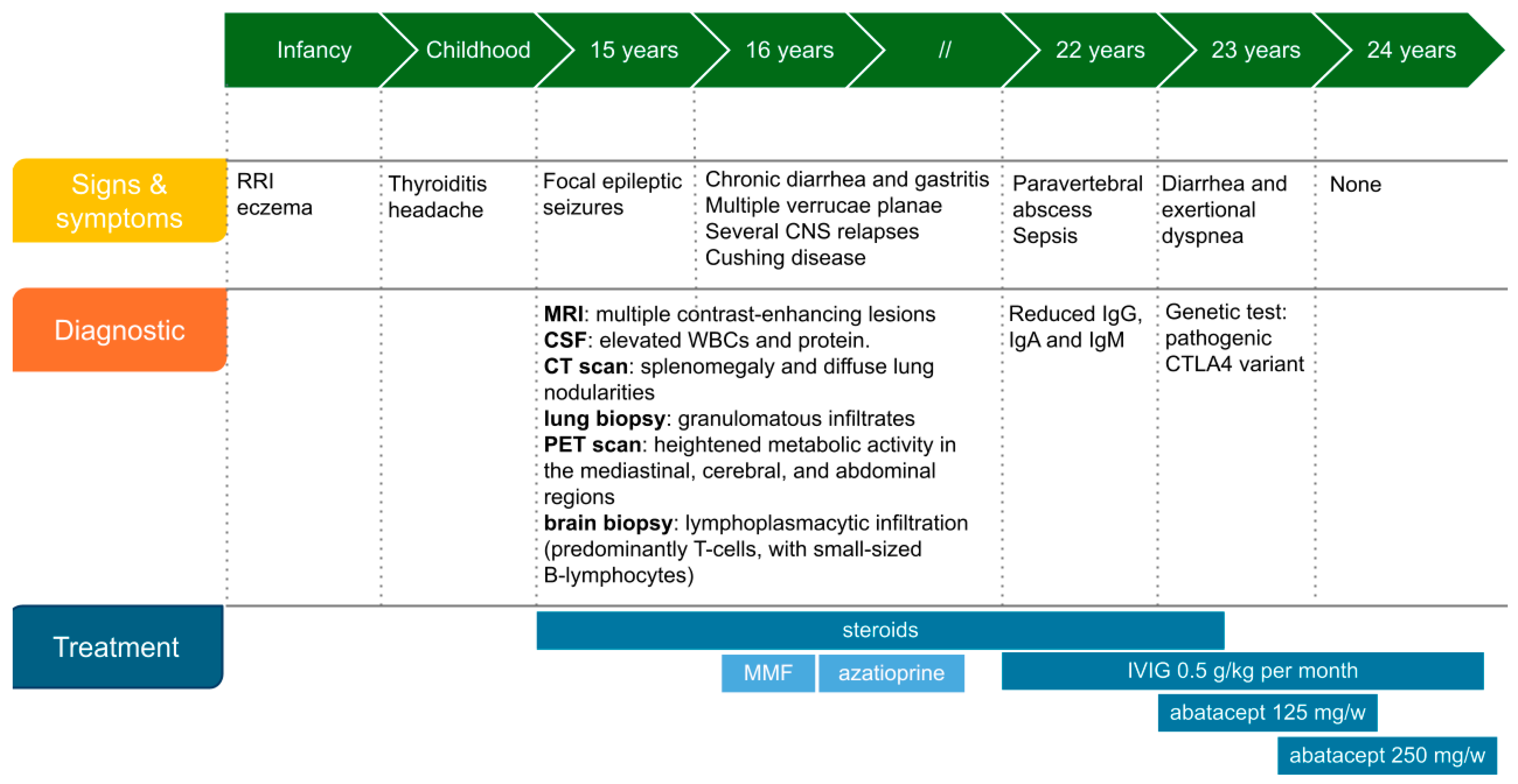
2. Case Report
2.1. Immunologic Workup
2.2. Genetic Diagnosis and Targeted Therapy
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CT | cerebrospinal fluid |
| CTLA-4d | CTLA-4 deficiency |
| IEI | inborn errors of immunity |
| IV | intravenous |
| MRI | magnetic resonance imaging |
| MS | multiple sclerosis |
| NGS | next generation sequencing |
| PET | positron-emission tomography |
| VUS | variant of unknown significance |
References
- Schubert, D.; Bode, C.; Kenefeck, R.; Hou, T.Z.; Wing, J.B.; Kennedy, A.; Bulashevska, A.; Petersen, B.S.; Schäffer, A.A.; Grüning, B.A.; et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014, 20, 1410–1416. [Google Scholar] [CrossRef]
- Egg, D.; Rump, I.C.; Mitsuiki, N.; Rojas-Restrepo, J.; Maccari, M.E.; Schwab, C.; Gabrysch, A.; Warnatz, K.; Goldacker, S.; Patiño, V.; et al. Therapeutic options for CTLA-4 insufficiency. J. Allergy Clin. Immunol. 2022, 149, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, R.; Woon, S.T.; Gillis, D.; Koopmans, W.; Steele, R. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin. Exp. Immunol. 2013, 174, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Z.; Qureshi, O.S.; Wang, C.J.; Baker, J.; Young, S.P.; Walker, L.S.; Sansom, D.M. A Transendocytosis Model of CTLA-4 Function Predicts Its Suppressive Behavior on Regulatory T Cells. J. Immunol. 2015, 194, 2148–2159. [Google Scholar] [CrossRef]
- Rojas-Restrepo, J.; Sindram, E.; Zenke, S.; Haberstroh, H.; Mitsuiki, N.; Gabrysch, A.; Huebscher, K.; Posadas-Cantera, S.; Krausz, M.; Kobbe, R.; et al. Functional Relevance of CTLA4 Variants: An Upgraded Approach to Assess CTLA4-Dependent Transendocytosis by Flow Cytometry. J. Clin. Immunol. 2023, 43, 2076–2089, Erratum in J. Clin. Immunol. 2023, 43, 2090. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, A.; Johnston, S.; Rice, C.M.; Gompels, M. A Family with a Novel CTLA4 Haploinsufficiency Mutation and Neurological Symptoms. J. Clin. Immunol. 2021, 41, 1411–1416. [Google Scholar] [CrossRef]
- Schindler, M.K.; Pittaluga, S.; Enose-Akahata, Y.; Su, H.C.; Rao, V.K.; Rump, A.; Jacobson, S.; Cortese, I.; Reich, D.S.; Uzel, G. Haploinsufficiency of immune checkpoint receptor CTLA4 induces a distinct neuroinflammatory disorder. J. Clin. Investig. 2020, 130, 5551–5561. [Google Scholar] [CrossRef]
- ‘CTLA4’[GENE]-ClinVar-NCBI [Internet]. Available online: https://www-ncbi-nlm-nih-gov.bvsp.idm.oclc.org/clinvar/?term=%22ctla4%22%5BGENE%5D&redir=gene (accessed on 20 May 2025).
- Schwab, C.; Gabrysch, A.; Olbrich, P.; Patiño, V.; Warnatz, K.; Wolff, D.; Hoshino, A.; Kobayashi, M.; Imai, K.; Takagi, M.; et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4–insufficient subjects. J. Allergy Clin. Immunol. 2018, 142, 1932–1946. [Google Scholar] [CrossRef]
- Ayrignac, X.; Goulabchand, R.; Jeziorski, E.; Rullier, P.; Carra-Dallière, C.; Lozano, C.; Portales, P.; Vincent, T.; Viallard, J.F.; de Champfleur, N.M. Two neurologic facets of CTLA4-related haploinsufficiency. Neurol. Neuroimmunol. NeuroInflamm. 2020, 7, e751. [Google Scholar] [CrossRef]
- Coustal, C.; Goulabchand, R.; Labauge, P.; Guilpain, P.; Carra-Dallière, C.; Januel, E.; Jeziorski, E.; Salle, V.; Viallard, J.F.; Boutboul, D.; et al. Clinical, Radiologic, and Immunologic Features of Patients With CTLA4 Deficiency With Neurologic Involvement. Neurology 2023, 101, e1560–e1566. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000207609 (accessed on 20 May 2025). [CrossRef]
- Kolcava, J.; Litzman, J.; Bednarik, J.; Stulik, J.; Stourac, P. Neurological manifestation of immune system dysregulation resulting from CTLA-4 receptor mutation: A case report. Mult. Scler. Relat. Disord. 2020, 45, 102313. [Google Scholar] [CrossRef]
- Kaninia, S.; Grammatikos, A.; Urankar, K.; Renowden, S.A.; Patel, N.K.; Gompels, M.M.; Rice, C.M. CNS demyelination associated with immune dysregulation and a novel CTLA-4 variant. Mult. Scler. J. 2021, 27, 1464–1467. [Google Scholar] [CrossRef]
- Lin, T.W.; Hu, Y.C.; Yang, Y.H.; Chien, Y.H.; Lee, N.C.; Yu, H.H.; Chiang, B.L.; Wang, L.C. CTLA-4 gene mutation and multiple sclerosis: A case report and literature review. J. Microbiol. Immunol. Infect. 2022, 55, 545–548. [Google Scholar] [CrossRef]
- Van Leeuwen, E.M.; Cuadrado, E.; Gerrits, A.M.; Witteveen, E.; De Bree, G.J. Treatment of Intracerebral Lesions with Abatacept in a CTLA4-Haploinsufficient Patient. J. Clin. Immunol. 2018, 38, 464–467. [Google Scholar] [CrossRef]
- Liston, A.; Dooley, J.; Yshii, L. Brain-resident regulatory T cells and their role in health and disease. Immunol. Lett. 2022, 248, 26–30. [Google Scholar] [CrossRef]
- Roth, P.; Winklhofer, S.; Müller, A.M.; Dummer, R.; Mair, M.J.; Gramatzki, D.; Le Rhun, E.; Manz, M.G.; Weller, M.; Preusser, M. Neurological complications of cancer immunotherapy. Cancer Treat. Rev. 2021, 97, 102189. [Google Scholar] [CrossRef]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muñiz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef]
- Lo, B.; Zhang, K.; Lu, W.; Zheng, L.; Zhang, Q.; Kanellopoulou, C.; Zhang, Y.; Liu, Z.; Fritz, J.M.; Marsh, R.; et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 2015, 349, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Rovshanov, S.; Göçmen, R.; Barişta, İ.; Çağdaş, D.; Üner, A.; Çilingir, V.; Filik, İ.T.; Tan, Ç.; Aytekin, E.S.; Tezcan, I.; et al. A rare case of central nervous system due to CTLA-4 gene defect. Arch. Neuropsychiatry 2022, 59, 248. Available online: https://www.noropsikiyatriarsivi.com/submission/MakaleKontrol?Id=VFdwak5VMUVRVDA9 (accessed on 20 May 2025).
- Quaak, M.S.; Buijze, M.S.; Verhoeven, V.J.; Vermont, C.; Buddingh, E.P.; Heredia, M.; Samsom, J.N.; Titulaer, M.J.; van Rossum, A.M.; Kamphuis, S.; et al. Management of Autoimmune Encephalitis in a 7-Year-Old Child With CTLA-4 Haploinsufficiency and AMPA Receptor Antibodies: A Case Report. Neurol. Neuroimmunol. NeuroInflamm. 2024, 11, e200254. [Google Scholar] [CrossRef] [PubMed]
- Taghizade, N.; Babayeva, R.; Kara, A.; Karakus, I.S.; Catak, M.C.; Bulutoglu, A.; Haskologlu, Z.S.; Haci, I.A.; Dalgic, C.T.; Karabiber, E.; et al. Therapeutic modalities and clinical outcomes in a large cohort with LRBA deficiency and CTLA4 insufficiency. J. Allergy Clin. Immunol. 2023, 152, 1634–1645. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, L.; Del Vecchio, L.; Cerasi, S.; Fetta, A.; Moratti, M.; Bezzi, A.; Ferrari, S.; Di Dalmazi, G.; Rossi, S.; Toni, F.; et al. Neuroinflammation in CTLA-4 Haploinsufficiency: Case Report of a New Variant with Remarkable Response to Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 9230. https://doi.org/10.3390/ijms26189230
Baldini L, Del Vecchio L, Cerasi S, Fetta A, Moratti M, Bezzi A, Ferrari S, Di Dalmazi G, Rossi S, Toni F, et al. Neuroinflammation in CTLA-4 Haploinsufficiency: Case Report of a New Variant with Remarkable Response to Targeted Therapy. International Journal of Molecular Sciences. 2025; 26(18):9230. https://doi.org/10.3390/ijms26189230
Chicago/Turabian StyleBaldini, Letizia, Lucia Del Vecchio, Sara Cerasi, Anna Fetta, Mattia Moratti, Alessandra Bezzi, Simona Ferrari, Guido Di Dalmazi, Simone Rossi, Francesco Toni, and et al. 2025. "Neuroinflammation in CTLA-4 Haploinsufficiency: Case Report of a New Variant with Remarkable Response to Targeted Therapy" International Journal of Molecular Sciences 26, no. 18: 9230. https://doi.org/10.3390/ijms26189230
APA StyleBaldini, L., Del Vecchio, L., Cerasi, S., Fetta, A., Moratti, M., Bezzi, A., Ferrari, S., Di Dalmazi, G., Rossi, S., Toni, F., Cordelli, D. M., Lanari, M., & Conti, F. (2025). Neuroinflammation in CTLA-4 Haploinsufficiency: Case Report of a New Variant with Remarkable Response to Targeted Therapy. International Journal of Molecular Sciences, 26(18), 9230. https://doi.org/10.3390/ijms26189230






