Human Leukocyte Antigen-DR Expression on Monocytes Is a Useful Predictor in a Systemic Inflammation Response-Based Prognostic Model in Advanced Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
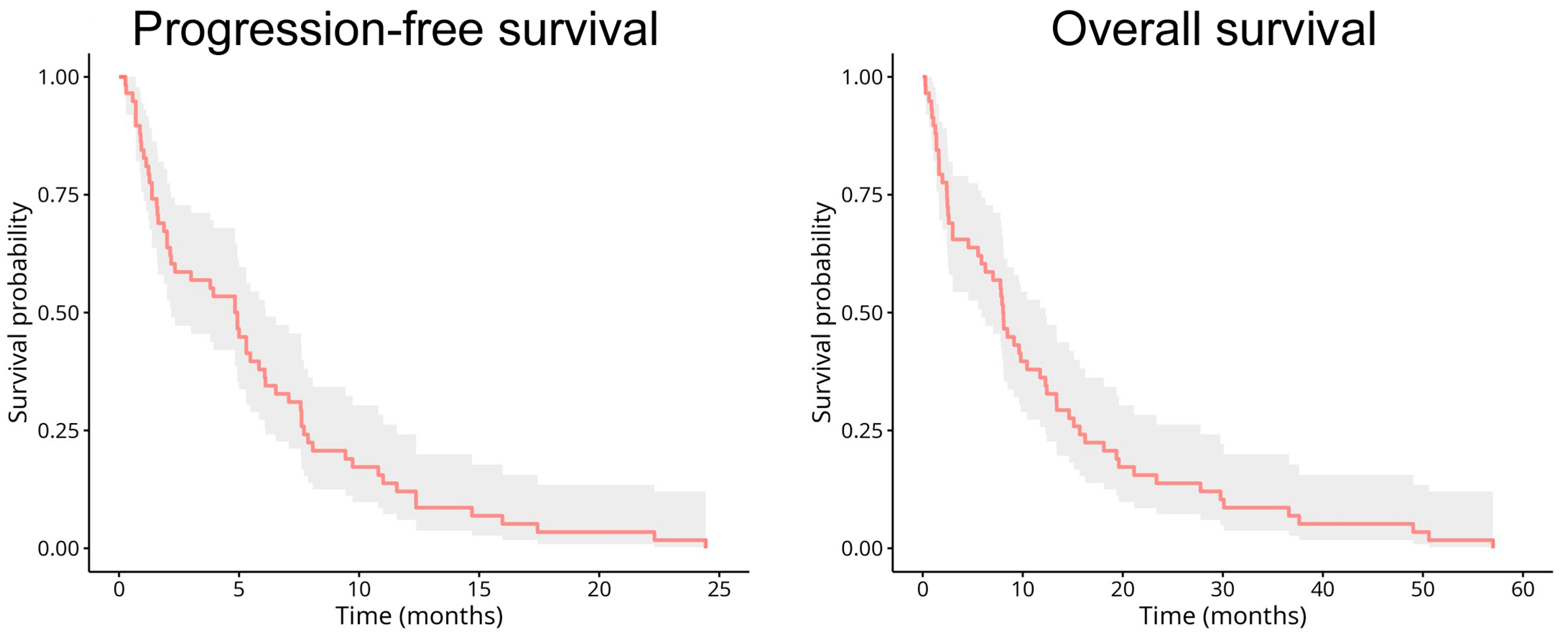
2.1. Patient Characteristics
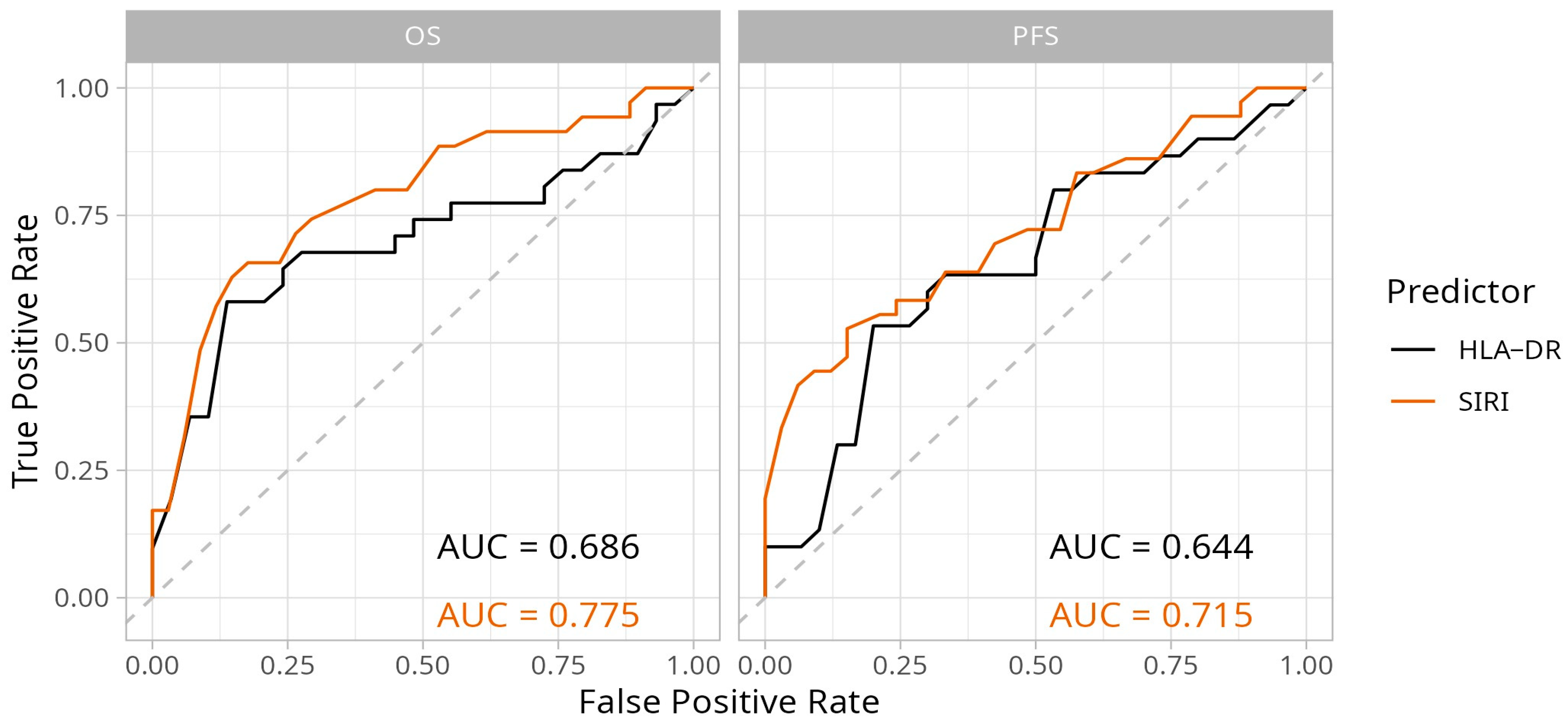
2.2. Predictive Performance and Optimal Cut-Off Value Selection for the Biomarkers
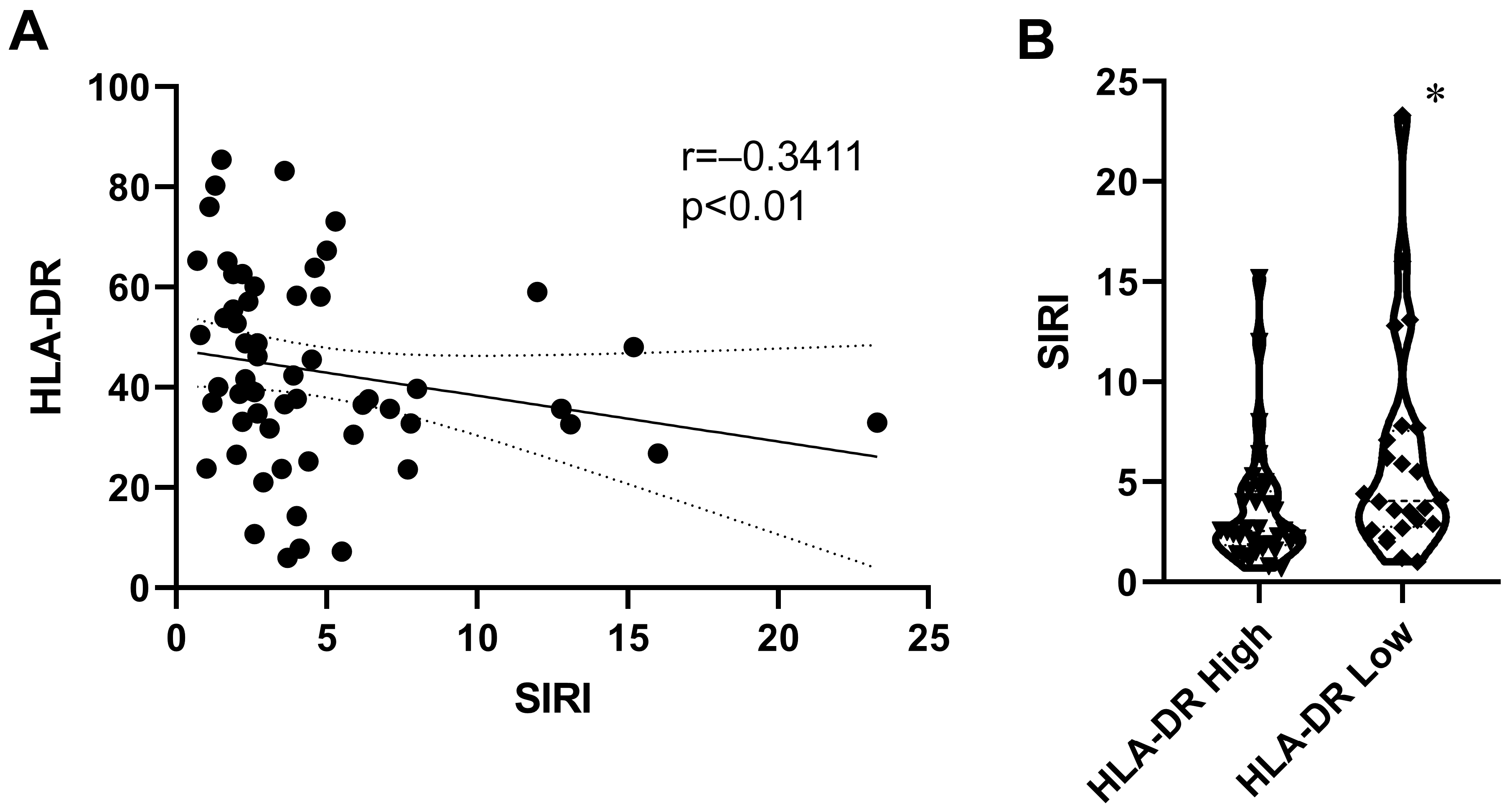
2.3. Relationship Between SIRI and Monocyte HLA-DR Expression
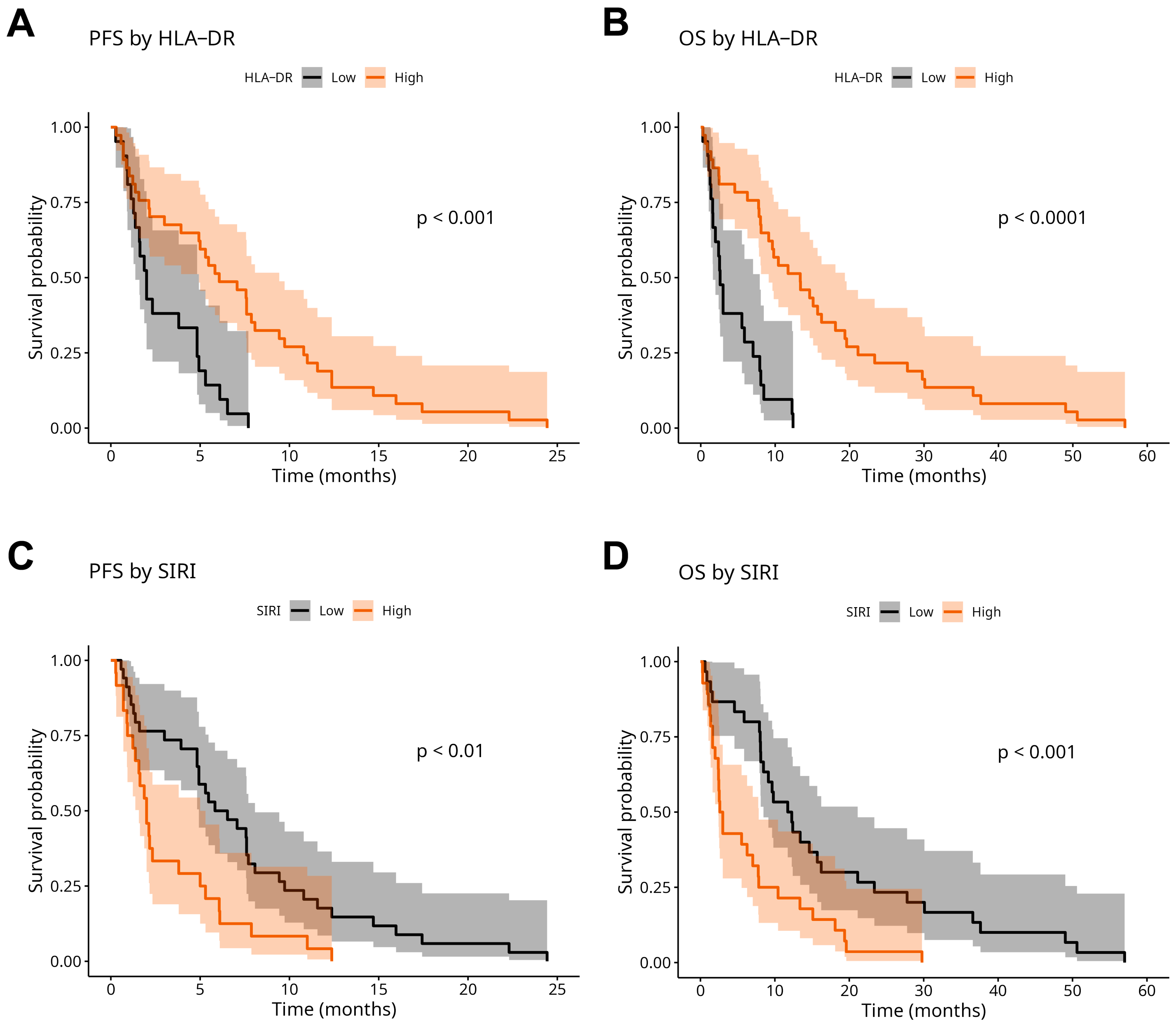
2.4. Prognostic Analysis
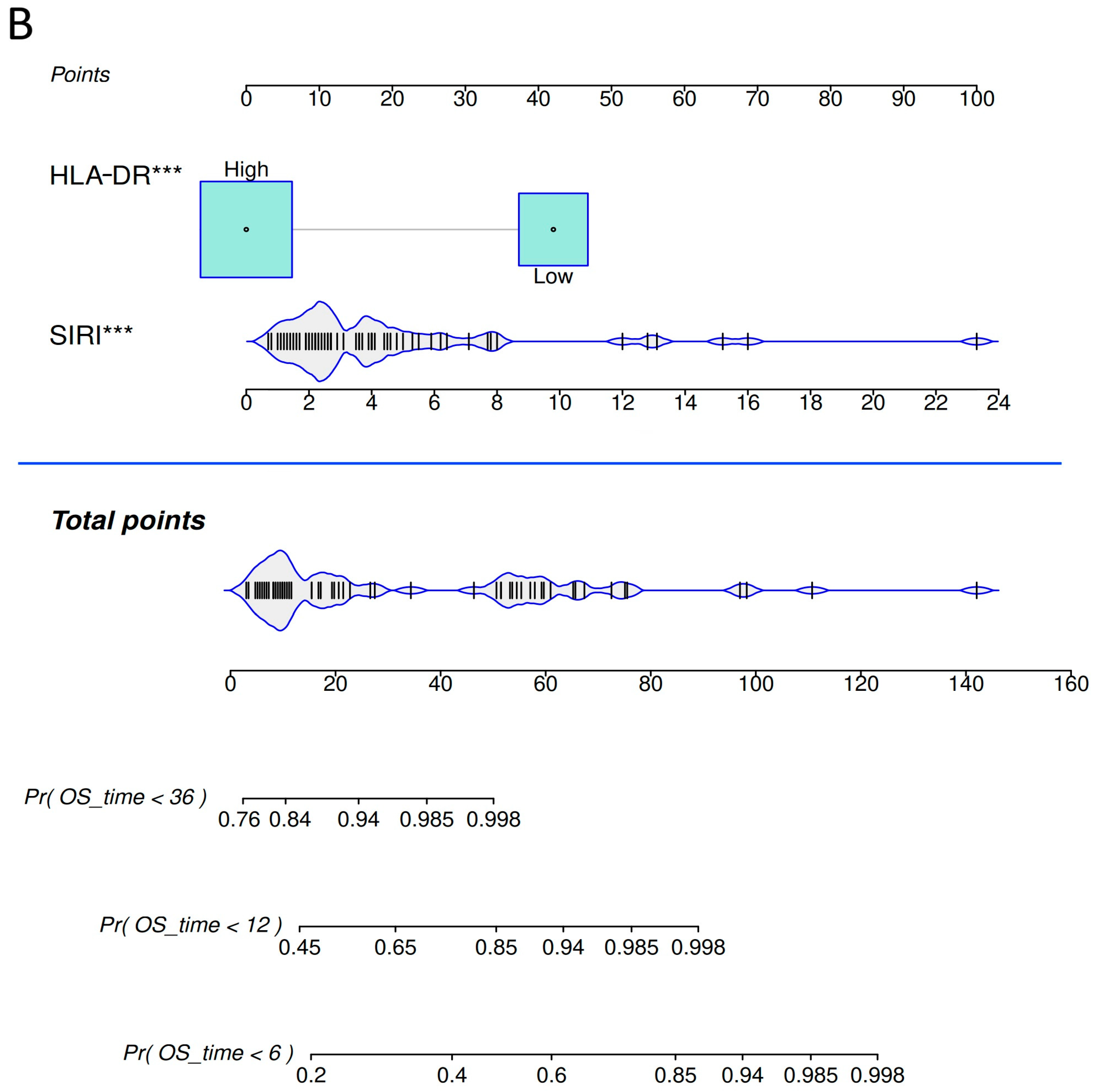
2.5. Nomograms for Predicting Progression-Free Survival and Overall Survival Rates in Advanced NSCLC
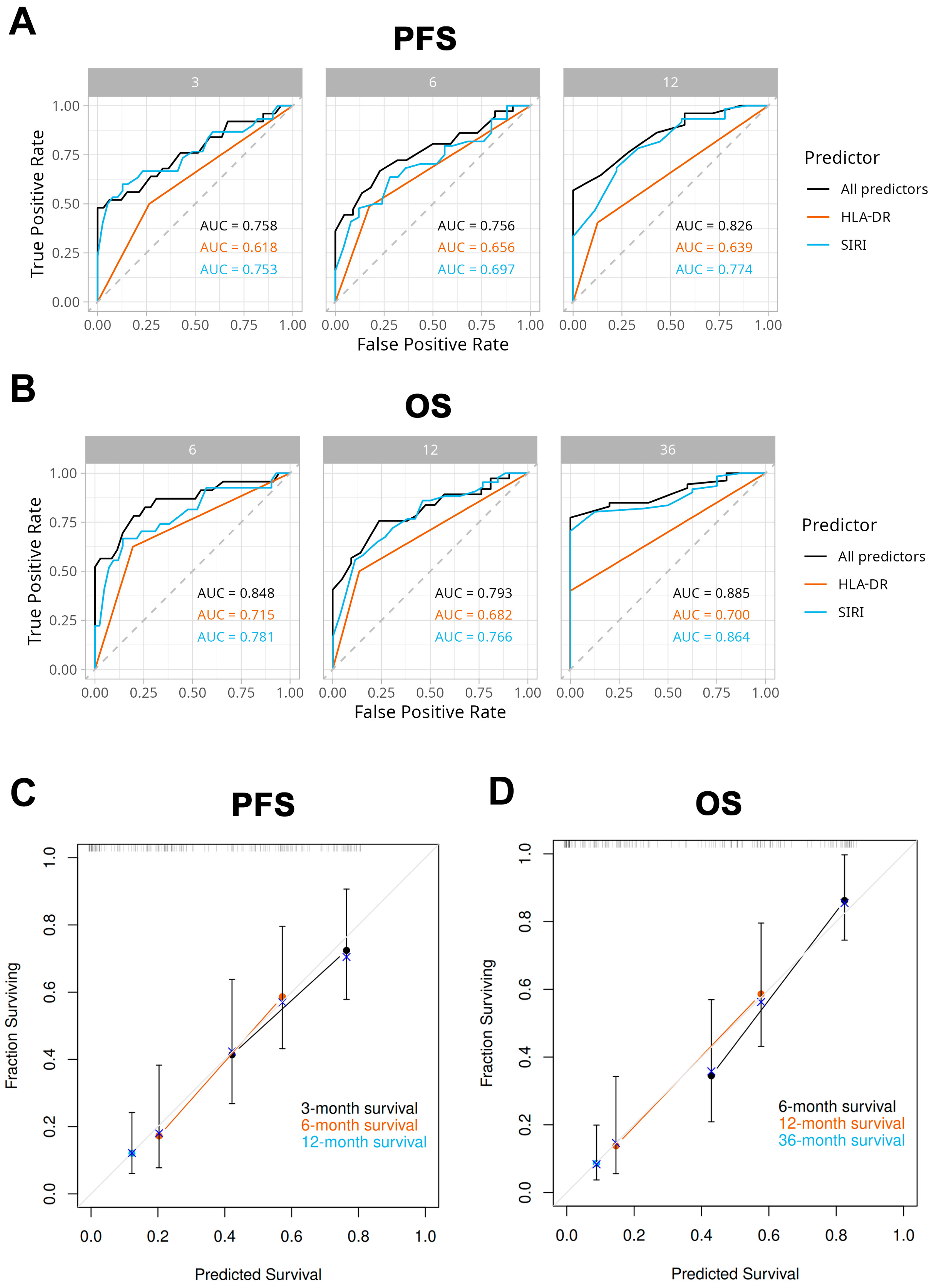
2.6. Model Performance and Validation
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SIRI | Systemic Inflammation Response Index |
| PFS | progression-free survival |
| OS | overall survival |
| NSCLC | non-small cell lung cancer |
| HLA-DR | Human Leukocyte Antigen-DR |
| MHC | major histocompatibility complex |
| APCs | antigen-presenting cells |
| LMR | Lymphocyte-to-Monocyte Ratio |
| BMI | body mass index |
| WBC | white blood cells |
| IQR | interquartile range |
| ROC | Receiver Operating Characteristic |
| AUC | area under the curve |
| CT | computer tomography |
| MRI | magnetic resonance imaging |
| PET/CT | positron emission tomography |
| MFI | median fluorescence intensity |
| PH | proportional hazard |
| PBMC | peripheral blood mononuclear cells |
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Bogos, K.; Ostoros, G. Tüdőrák. Korányi Bull. 2025, 1, 32–41. Available online: https://heyzine.com/flip-book/dd45b25755.html (accessed on 13 May 2025).
- Miao, D.; Zhao, J.; Han, Y.; Zhou, J.; Li, X.; Zhang, T.; Li, W.; Xia, Y. Management of locally advanced non-small cell lung cancer: State of the art and future directions. Cancer Commun. 2024, 44, 23–46. [Google Scholar] [CrossRef]
- Miller, M.; Hanna, N. Advances in systemic therapy for non-small cell lung cancer. BMJ 2021, 375, n2363. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef]
- Xu, W.; Liu, X.; Yan, C.; Abdurahmane, G.; Lazibiek, J.; Zhang, Y.; Cao, M. The prognostic value and model construction of inflammatory markers for patients with non-small cell lung cancer. Sci. Rep. 2024, 14, 7568. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, M.; Jia, H.; Wang, T.; Wu, H.; Xu, K.; Ren, T.; Liang, L. The systemic inflammation response index (SIRI) predicts survival in advanced non-small cell lung cancer patients undergoing immunotherapy and the construction of a nomogram model. Front. Immunol. 2024, 15, 1516737. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Zhao, J. The role of CD4 T cell help for CD8 CTL activation. Biochem. Biophys. Res. Commun. 2009, 384, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Coënon, L.; Geindreau, M.; Ghiringhelli, F.; Villalba, M.; Bruchard, M. Natural Killer cells at the frontline in the fight against cancer. Cell Death Dis. 2024, 15, 614. [Google Scholar] [CrossRef]
- Gossez, M.; Bonnet, B.; Boussaid, I.; Chapuis, N.; Cointe, S.; Cravat, M.; De Carvalho Bittencourt, M.; Dignat-George, F.; Evrard, B.; Jeannet, R.; et al. Multicenter inter-laboratory quality control of monocyte HLA-DR expression by flow cytometry. Cytom. B Clin. Cytom. 2025, 108, 95–97. [Google Scholar] [CrossRef]
- Heng, E.; Neuwirth, M.; Mas, F.; Contant, G.; Mazighi, M.; Feriel, J.; Montpellier, B.; Brumpt, C.; Jourdi, G.; Curis, E.; et al. Assessment of inter-operator variability in peripheral monocyte subset gating strategy using flow cytometry in patients with suspected acute stroke. Cytometry Part A J. Int. Soc. Anal. Cytol. 2024, 105, 171–180. [Google Scholar] [CrossRef]
- Demaret, J.; Walencik, A.; Jacob, M.C.; Timsit, J.F.; Venet, F.; Lepape, A.; Monneret, G. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytom. B Clin. Cytom. 2013, 84, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, W.; Szatmary, P.; Zhang, X.; Lin, J.W.; Chen, L.; Liu, D.; Sutton, R.; Xia, Q.; Jin, T.; et al. Monocytic HLA-DR Expression in Immune Responses of Acute Pancreatitis and COVID-19. Int. J. Mol. Sci. 2023, 24, 3246. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Chuang, L.P.; Liu, P.H.; Chu, C.M.; Yu, C.C.; Lin, S.W.; Kao, K.C.; Li, L.F.; Chuang, D.Y. Decreased Monocyte HLA-DR Expression in Patients with Sepsis and Acute Kidney Injury. Medicina 2022, 58, 1198. [Google Scholar] [CrossRef] [PubMed]
- de Roquetaillade, C.; Dupuis, C.; Faivre, V.; Lukaszewicz, A.C.; Brumpt, C.; Payen, D. Monitoring of circulating monocyte HLA-DR expression in a large cohort of intensive care patients: Relation with secondary infections. Ann. Intensive Care 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Palojärvi, A.; Petäjä, J.; Siitonen, S.; Janér, C.; Andersson, S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr. Res. 2013, 73, 469–475. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, B.; Wang, B.; Zhang, F.; Fan, K.X.; Guo, Y.J. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol. Immunother. 2013, 62, 1439–1451. [Google Scholar] [CrossRef]
- Jeong, S.; Jang, N.; Kim, M.; Choi, I.K. CD4(+) cytotoxic T cells: An emerging effector arm of anti-tumor immunity. BMB Rep. 2023, 56, 140–144. [Google Scholar] [CrossRef]
- Ezdoglian, A.; Tsang, A.S.M.; Khodadust, F.; Burchell, G.; Jansen, G.; de Gruijl, T.; Labots, M.; van der Laken, C.J. Monocyte-related markers as predictors of immune checkpoint inhibitor efficacy and immune-related adverse events: A systematic review and meta-analysis. Cancer Metastasis Rev. 2025, 44, 35. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wu, B.; Li, J.; Yi, Z.; Chen, C.; Wu, Y.; Liu, G.; Wang, P. AGR, LMR and SIRI are the optimal combinations for risk stratification in advanced patients with non-small cell lung cancer following immune checkpoint blockers. Int. Immunopharmacol. 2025, 149, 114215. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Liu, G. NRI and SIRI are the optimal combinations for prognostic risk stratification in patients with non-small cell lung cancer after EGFR-TKI therapy. Clin. Transl. Oncol. 2025, 27, 1529–1538. [Google Scholar] [CrossRef]
- Ye, X.; Dai, M.; Xiang, Z. Prognostic role of systemic inflammation response index in patients with non-small cell lung cancer: A meta-analysis. BMJ Open 2024, 14, e087841. [Google Scholar] [CrossRef] [PubMed]
- Faivre, V.; Lukaszewicz, A.-C.; Payen, D. Downregulation of Blood Monocyte HLA-DR in ICU Patients Is Also Present in Bone Marrow Cells. PLoS ONE 2016, 11, e0164489. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.-F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Joshi, I.; Carney, W.P.; Rock, E.P. Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis. Front. Immunol. 2023, 14, 1130214. [Google Scholar] [CrossRef]
- Mengos, A.E.; Gastineau, D.A.; Gustafson, M.P. The CD14(+)HLA-DR(lo/neg) Monocyte: An Immunosuppressive Phenotype That Restrains Responses to Cancer Immunotherapy. Front. Immunol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 14 June 2024).
- Marshall, R. regplot: Enhanced Regression Nomogram Plot. Available online: https://CRAN.R-project.org/package=regplot (accessed on 3 July 2025).
- Qiu, W.; Chavarro, J.; Lazarus, R.; Rosner, B.; Ma, J. powerSurvEpi: Power and Sample Size Calculation for Survival Analysis of Epidemiological Studies. 2025. Available online: https://CRAN.R-project.org/package=powerSurvEpi (accessed on 1 August 2025).

| Group | All Patients | HLA-DR Low | HLA-DR High | p Value |
|---|---|---|---|---|
| n = 58 | n = 22 | n = 36 | ||
| Sex | 0.999 | |||
| male, n (%) | 37 (63.8) | 14 (63.6) | 23 (64) | |
| female, n (%) | 21 (36.2) | 8 (36.4) | 13 (36) | |
| Age (years) | 66.08 ± 7.5 | 66.5 ± 7.2 | 66.1 ± 7.6 | 0.642 |
| Smoking status, pack-year (IQR) | 40 (30, 45) | 40 (30, 44) | 41 (37.5, 46.3) | 0.311 |
| Oncology stage, n (%) | 0.266 | |||
| IIIB + IIIC | 22 (37.9) | 6 (27.3) | 16 (44.4) | |
| IV | 36 (62.1) | 16 (72.7) | 20 (55.6) | |
| Tumor histotype, n (%) | 0.774 | |||
| adenocarcinoma | 39 (67.2) | 14 (63.6) | 25 (69.4) | |
| squamous cell carcinoma | 19 (32.8) | 8 (36.4) | 11 (30.6) | |
| ECOG state, n (%) | <0.01 | |||
| 0 | 35 (60.3) | 10 (45.5) | 25 (69.3) | |
| 1 | 15 (25.9) | 5 (22.7) | 10 (28) | |
| 2 | 7 (12.1) | 6 (27.3) | 1 (2.7) | |
| 3 | 1 (1.70) | 1 (4.5) | 0 (0) | |
| Oncology therapy | 0.386 | |||
| chemotherapy | 50 (86.2) | 18 (81.8) | 32 (88.8) | |
| radiotherapy | 20 (34.5) | 7 (31.8) | 13 (36.1) | |
| biological therapy | 24 (41.4) | 12 (54.5) | 12 (33.3) | |
| tyrosine-kinase inhibitor | 4 (6.9) | 0 (0) | 4 (11.1) | |
| best supportive care | 6 (10.3) | 2 (9.1) | 4 (11.1) | |
| BMI (kg/m2) | 26.5 ± 4.8 | 23.78 ± 4.2 | 27.07 ± 4.9 | <0.05 |
| WBC (G/L) | 12.17 ± 5 | 13.3 ± 5 | 11.34 ± 5 | <0.05 |
| Neu. count (G/L) | 9.2 ± 4.6 | 10.4 ± 4.7 | 8.36 ± 4.4 | <0.05 |
| Ly. count (G/L) | 1.87 ± 0.95 | 1.79 ± 1 | 1.9 ± 0.9 | 0.820 |
| Mono. count (G/L) | 0.75 ± 0.33 | 0.82 ± 0.4 | 0.68 ± 0.3 | <0.05 |
| Platelets (G/L) | 356 ± 151 | 406 ± 187 | 320 ± 109 | <0.05 |
| SIRI | 4.62 ± 4.2 | 6.4 ± 5.4 | 3.5 ± 3 | <0.05 |
| Mono. HLA-DR (MFI) | 43.17 ± 18.8 | 25.38 ± 9.8 | 54.22 ± 13.8 | <0.001 |
| Median PFS month (IQR) | 4.88 (1.58, 7.68) | 2 (1.37, 5.2) | 5.83 (1.6, 10.8) | <0.01 |
| Median OS month (IQR) | 8.05 (2.46, 15.8) | 2.8 (1.6, 7.7) | 11.73 (6.27, 21.13) | <0.001 |
| Characteristics | SIRI | HLA-DR (Low vs. High) | ||
|---|---|---|---|---|
| Univariate analysis (PFS) | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| 1.16 (1.08–1.25) | <0.001 | 3.12 (1.67–5.82) | <0.001 | |
| Multivariate analysis (PFS) | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| 1.13 (1.05–1.21) | 0.002 | 2.56 (1.32–4.94) | 0.005 | |
| Univariate analysis (OS) | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| 1.19 (1.10–1.27) | <0.001 | 4.56 (2.35–8.86) | <0.001 | |
| Multivariate analysis (OS) | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| 1.14 (1.06–1.23) | <0.001 | 3.66 (1.81–7.41) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szűcs, G.; Gézsi, A.; Szentkereszty, M.; Losonczy, G.; Barna, G.; Gálffy, G.; Bohács, A.; Tamási, L.; Müller, V.; Buzás, E.I.; et al. Human Leukocyte Antigen-DR Expression on Monocytes Is a Useful Predictor in a Systemic Inflammation Response-Based Prognostic Model in Advanced Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2025, 26, 9226. https://doi.org/10.3390/ijms26189226
Szűcs G, Gézsi A, Szentkereszty M, Losonczy G, Barna G, Gálffy G, Bohács A, Tamási L, Müller V, Buzás EI, et al. Human Leukocyte Antigen-DR Expression on Monocytes Is a Useful Predictor in a Systemic Inflammation Response-Based Prognostic Model in Advanced Non-Small Cell Lung Cancer. International Journal of Molecular Sciences. 2025; 26(18):9226. https://doi.org/10.3390/ijms26189226
Chicago/Turabian StyleSzűcs, Gergő, András Gézsi, Márton Szentkereszty, György Losonczy, Gábor Barna, Gabriella Gálffy, Anikó Bohács, Lilla Tamási, Veronika Müller, Edit I. Buzás, and et al. 2025. "Human Leukocyte Antigen-DR Expression on Monocytes Is a Useful Predictor in a Systemic Inflammation Response-Based Prognostic Model in Advanced Non-Small Cell Lung Cancer" International Journal of Molecular Sciences 26, no. 18: 9226. https://doi.org/10.3390/ijms26189226
APA StyleSzűcs, G., Gézsi, A., Szentkereszty, M., Losonczy, G., Barna, G., Gálffy, G., Bohács, A., Tamási, L., Müller, V., Buzás, E. I., & Komlósi, Z. I. (2025). Human Leukocyte Antigen-DR Expression on Monocytes Is a Useful Predictor in a Systemic Inflammation Response-Based Prognostic Model in Advanced Non-Small Cell Lung Cancer. International Journal of Molecular Sciences, 26(18), 9226. https://doi.org/10.3390/ijms26189226






