FN9-10ELP, an ECM-Mimetic Fusion Protein, Protects Human Mesenchymal Stem Cells from Etoposide-Induced Senescence
Abstract
1. Introduction
2. Results
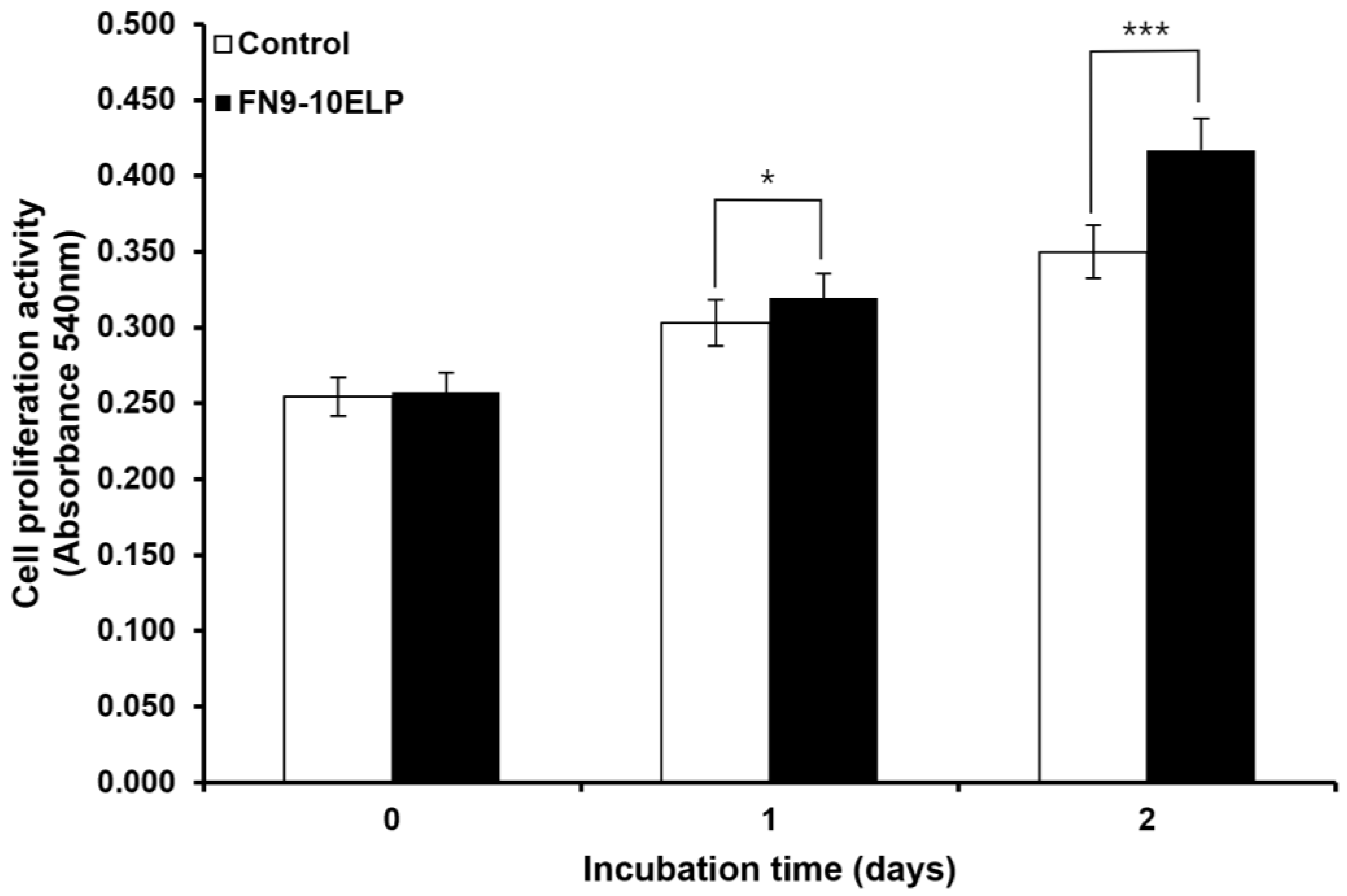
2.1. FN9-10ELP Protects hMSCs from Etoposide-Induced Cellular Senescence by Promoting Proliferation
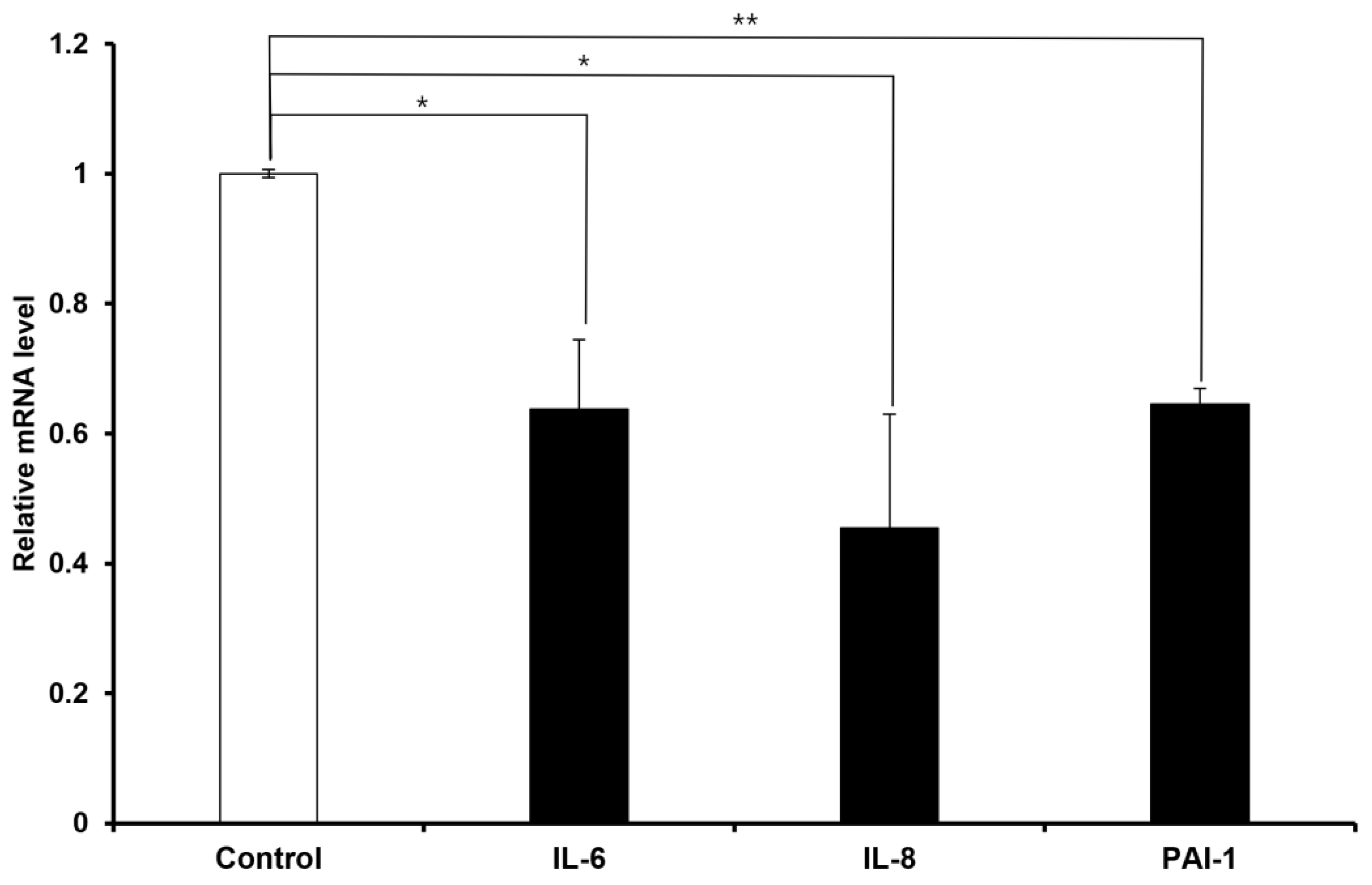
2.2. FN9-10ELP Suppresses the Expression of Senescence-Associated Genes in Etoposide-Treated hMSCs
2.3. FN9-10ELP Attenuates Nuclear Enlargement Induced by Etoposide
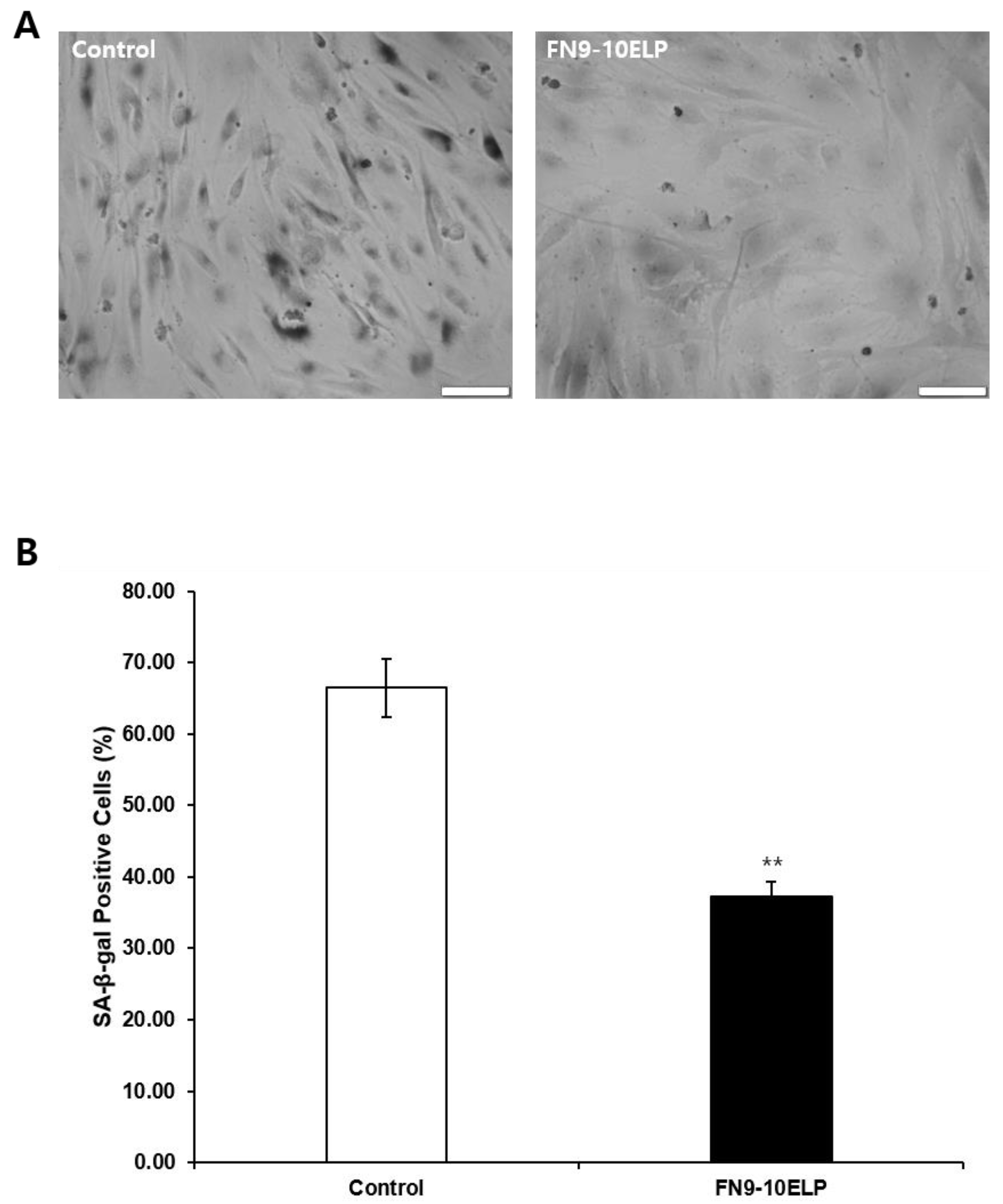
2.4. FN9-10ELP Significantly Suppresses Etoposide-Induced SA-β-Galactosidase Activity in Senescent hMSCs
3. Discussion
4. Materials and Methods
4.1. Construction and Expression of Recombinant FN9-10ELP Fusion Protein
4.2. hMSC Culture and Preparation
4.3. Cell Proliferation Assay
4.4. RNA Extraction and cDNA Synthesis
4.5. Quantitative PCR Analysis
4.6. DAPI Staining and Nuclear Size Analysis
4.7. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAPI | 4′,6-diamidino-2-phenylindole |
| DDR | DNA damage response |
| DMSO | Dimethyl Sulfoxide |
| DNA | Deoxyribonucleic Acid |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| DSBs | DNA double-strand breaks |
| ECM | Extracellular Matrix |
| ELPs | Elastin-like polypeptides |
| FAK | Focal Adhesion Kinase |
| FN | Fibronectin |
| FN9-10ELP | fusion protein of fibronectin type III domains 9-10 and elastin-like polypeptide |
| hMSCs | Human mesenchymal stem cells |
| hTMSCs | Human turbinate-derived mesenchymal stem cells |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| LB-Amp | Luria Broth containing ampicillin |
| MAPK | Mitogen-Activated Protein Kinase |
| mRNA | Messenger Ribonucleic Acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PI3K | phosphatidylinositol 4,5-bisphosphate 3-kinase |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| SA-β-gal | Senescence-Associated β-Galactosidase |
| SASP | Senescence-Associated Secretory Phenotype |
| SD | Standard deviation |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| TLRs | Toll-like receptors |
| TNF-α | Tumor Necrosis Factor-Alpha |
| α-MEM | α-minimal essential medium |
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent Cells: An Emerging Target for Diseases of Ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Blokland, K.E.C.; Pouwels, S.D.; Schuliga, M.; Knight, D.A.; Burgess, J.K. Regulation of Cellular Senescence by Extracellular Matrix during Chronic Fibrotic Diseases. Clin. Sci. 2020, 134, 2681–2706. [Google Scholar] [CrossRef]
- Jean, C.; Gravelle, P.; Fournie, J.J.; Laurent, G. Influence of Stress on Extracellular Matrix and Integrin Biology. Oncogene 2011, 30, 2697–2706. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review. Polymers 2021, 13, 2950. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, X.; Huang, B.; Zhou, W.; Cui, X.; Zheng, C.; Liu, F.; Bi, J.; Zhang, Y.; Luo, H.; et al. Inhibition of Matrix Stiffness Relating Integrin Beta1 Signaling Pathway Inhibits Tumor Growth in Vitro and in Hepatocellular Cancer Xenografts. BMC Cancer 2021, 21, 1276. [Google Scholar] [CrossRef]
- Choi, H.R.; A Cho, K.; Kang, H.T.; Bin Lee, J.; Kaeberlein, M.; Suh, Y.; Chung, I.K.; Park, S.C. Restoration of Senescent Human Diploid Fibroblasts by Modulation of the Extracellular Matrix. Aging Cell 2011, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular Matrix and Cell Signalling: The Dynamic Cooperation of Integrin, Proteoglycan and Growth Factor Receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef]
- Altroff, H.; Schlinkert, R.; van der Walle, C.F.; Bernini, A.; Campbell, I.D.; Werner, J.M.; Mardon, H.J. Interdomain Tilt Angle Determines Integrin-Dependent Function of the Ninth and Tenth FIII Domains of Human Fibronectin. J. Biol. Chem. 2004, 279, 55995–56003. [Google Scholar] [CrossRef]
- Aota, S.; Nomizu, M.; Yamada, K.M. The Short Amino Acid Sequence Pro-His-Ser-Arg-Asn in Human Fibronectin Enhances Cell-Adhesive Function. J. Biol. Chem. 1994, 269, 24756–24761. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Dzuricky, M.; Chilkoti, A. Elastin-Like Polypeptides as Models of Intrinsically Disordered Proteins. FEBS Lett. 2015, 589, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Despanie, J.; Dhandhukia, J.P.; Hamm-Alvarez, S.F.; MacKay, J.A. Elastin-Like Polypeptides: Therapeutic Applications for an Emerging Class of Nanomedicines. J. Control Release 2016, 240, 93–108. [Google Scholar] [CrossRef]
- Sarangthem, V.; Sharma, H.; Goel, R.; Ghose, S.; Park, R.W.; Mohanty, S.; Chaudhuri, T.K.; Dinda, A.K.; Singh, T.D. Application of Elastin-Like Polypeptide (ELP) Containing Extra-Cellular Matrix (ECM) Binding Ligands in Regenerative Medicine. Int. J. Biol. Macromol. 2022, 207, 443–453. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.E.; Seo, H.J.; Jang, J.H. Design of Fibronectin Type Iii Domains Fused to an Elastin-Like Polypeptide for the Osteogenic Differentiation of Human Mesenchymal Stem Cells. Acta Biochim. Biophys. Sin. 2019, 51, 856–863. [Google Scholar] [CrossRef]
- Park, B.H.; Jeong, E.S.; Lee, S.; Jang, J.H. Bio-Functionalization and in-Vitro Evaluation of Titanium Surface with Recombinant Fibronectin and Elastin Fragment in Human Mesenchymal Stem Cell. PLoS ONE 2021, 16, e0260760. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hwang, S.H. Human Nasal Turbinate-Derived Stem Cells for Tissue Engineering and Regenerative Medicine. J. Rhinol. 2024, 31, 133–137. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, S.W.; Kwon, D.Y.; Park, S.H.; Son, A.R.; Kim, J.H.; Kim, M.S. In Vivo Osteogenic Differentiation of Human Turbinate Mesenchymal Stem Cells in an Injectable in Situ-Forming Hydrogel. Biomaterials 2014, 35, 5337–5346. [Google Scholar] [CrossRef]
- Hwang, S.H.; Cho, H.K.; Park, S.H.; Lee, W.; Lee, H.J.; Lee, D.C.; Oh, J.H.; Park, S.H.; Kim, T.G.; Sohn, H.J.; et al. Toll Like Receptor 3 & 4 Responses of Human Turbinate Derived Mesenchymal Stem Cells: Stimulation by Double Stranded RNA and Lipopolysaccharide. PLoS ONE 2014, 9, e101558. [Google Scholar] [CrossRef]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.X.; Eckstein, V.; et al. Aging and Replicative Senescence Have Related Effects on Human Stem and Progenitor Cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef]
- te Poele, R.H.; Okorokov, A.L.; Jardine, L.; Cummings, J.; Joel, S.P. DNA Damage Is Able to Induce Senescence in Tumor Cells in Vitro and in Vivo. Cancer Res. 2002, 62, 1876–1883. [Google Scholar] [PubMed]
- Malaise, O.; Tachikart, Y.; Constantinides, M.; Mumme, M.; Ferreira-Lopez, R.; Noack, S.; Krettek, C.; Noël, D.; Wang, J.; Jorgensen, C.; et al. Mesenchymal Stem Cell Senescence Alleviates Their Intrinsic and Seno-Suppressive Paracrine Properties Contributing to Osteoarthritis Development. Aging 2019, 11, 9128–9146. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Hu, D.; Zhang, L.; Shi, S. Alkbh5 Regulates Etoposide-Induced Cellular Senescence and Osteogenic Differentiation in Osteoporosis through Mediating the M(6)a Modification of Vdac3. Sci. Rep. 2024, 14, 23461. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, M.; Barr, P.; Ricci, B.; Yan, H. Replication-Dependent and Transcription-Dependent Mechanisms of DNA Double-Strand Break Induction by the Topoisomerase 2-Targeting Drug Etoposide. PLoS ONE 2013, 8, e79202. [Google Scholar] [CrossRef] [PubMed]
- Muslimović, A.; Nyström, S.; Gao, Y.; Hammarsten, O. Numerical Analysis of Etoposide Induced DNA Breaks. PLoS ONE 2009, 4, e5859, Erratum in PLoS ONE 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Khoddam, A.; Vaughan, D.; Wilsbacher, L. Role of Plasminogen Activator Inhibitor-1 (Pai-1) in Age-Related Cardiovascular Pathophysiology. J. Cardiovasc. Aging 2025, 5, 1–13. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory Networks during Cellular Senescence: Causes and Consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Menendez, J.A.; Alarcón, T. Senescence-Inflammatory Regulation of Reparative Cellular Reprogramming in Aging and Cancer. Front. Cell Dev. Biol. 2017, 5, 49. [Google Scholar] [CrossRef]
- Biton, S.; Ashkenazi, A. Nemo and Rip1 Control Cell Fate in Response to Extensive DNA Damage Via TNF-Alpha Feedforward Signaling. Cell 2011, 145, 92–103. [Google Scholar] [CrossRef]
- Kang, K.W.; Lee, S.J.; Kim, J.H.; Lee, B.H.; Kim, S.J.; Park, Y.; Kim, B.S. Etoposide-Mediated Interleukin-8 Secretion from Bone Marrow Stromal Cells Induces Hematopoietic Stem Cell Mobilization. BMC Cancer 2020, 20, 619. [Google Scholar] [CrossRef]
- Vasudevan, K.M.; Gurumurthy, S.; Rangnekar, V.M. Suppression of PTEN Expression by NF-Kappa B Prevents Apoptosis. Mol. Cell. Biol. 2004, 24, 1007–1021. [Google Scholar] [CrossRef]
- Pathak, R.U.; Soujanya, M.; Mishra, R.K. Deterioration of Nuclear Morphology and Architecture: A Hallmark of Senescence and Aging. Ageing Res. Rev. 2021, 67, 101264. [Google Scholar] [CrossRef] [PubMed]
- Heckenbach, I.; Mkrtchyan, G.V.; Ben Ezra, M.; Bakula, D.; Madsen, J.S.; Nielsen, M.H.; Oró, D.; Osborne, B.; Covarrubias, A.J.; Idda, M.L.; et al. Nuclear Morphology Is a Deep Learning Biomarker of Cellular Senescence. Nat. Aging 2022, 2, 742–755. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A Biomarker That Identifies Senescent Human Cells in Culture and in Aging Skin in Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Tragoonlugkana, P.; Pruksapong, C.; Ontong, P.; Kamprom, W.; Supokawej, A. Fibronectin and Vitronectin Alleviate Adipose-Derived Stem Cells Senescence during Long-Term Culture through the Akt/Mdm2/P53 Pathway. Sci. Rep. 2024, 14, 14242. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-Associated Β-Galactosidase Reflects an Increase in Lysosomal Mass during Replicative Ageing of Human Endothelial Cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kwon, H.K.; Lee, J.H.; Anwar, M.A.; Choi, S. Etoposide induced cytotoxicity mediated by ROS and ERK in human kidney proximal tubule cells. Sci. Rep. 2016, 6, 34064. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, J.; Tonnesen, M.G.; Taira, B.R.; McClain, S.A.; Singer, A.J.; Clark, R.A.F. Fibronectin Peptides That Bind PDGF-BB Enhance Survival of Cells and Tissue under Stress. J. Investig. Dermatol. 2014, 134, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Sakurai, H.; Ueno, Y.; Ohtani, O.; Saiki, I. Activation of Mek/Erk and Pi3k/Akt Pathways by Fibronectin Requires Integrin Alphav-Mediated Adam Activity in Hepatocellular Carcinoma: A Novel Functional Target for Gefitinib. Cancer Sci. 2006, 97, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Veevers-Lowe, J.; Ball, S.G.; Shuttleworth, A.; Kielty, C.M. Mesenchymal Stem Cell Migration Is Regulated by Fibronectin through A5β1-Integrin-Mediated Activation of PDGFR-Β and Potentiation of Growth Factor Signals. J. Cell Sci. 2011, 124, 1288–1300. [Google Scholar] [CrossRef]
- Hwang, S.H.; Cho, H.K.; Park, S.H.; Lee, W.; Lee, H.J.; Lee, D.C.; Park, S.H.; Lim, M.H.; Back, S.A.; Yun, B.G.; et al. Characteristics of Human Turbinate-Derived Mesenchymal Stem Cells Are Not Affected by Allergic Condition of Donor. PLoS ONE 2015, 10, e0138041. [Google Scholar] [CrossRef][Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013; Updated 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 27 August 2025).[Green Version]
- Georget, M.; Defois, A.; Guiho, R.; Bon, N.; Allain, S.; Boyer, C.; Halgand, B.; Waast, D.; Grimandi, G.; Fouasson-Chailloux, A.; et al. Development of a DNA Damage-Induced Senescence Model in Osteoarthritic Chondrocytes. Aging 2023, 15, 8576–8593. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | TGGCACCCAGCACAATGAAGAT | TACTCCTGCTTGCTGATCCA |
| IL-6 | CCCCTGACCCAACCACAAAT | GCCCAGTGGACAGGTTTCTG |
| IL-8 | GTCTGCTAGCCAGGATCCAC | AGTGCTTCCACATGTCCTCA |
| PAI-1 | CAGACCAAGAGCCTCTCCAC | GGTTCCATCACTTGGCCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-H.; Jang, J.-H. FN9-10ELP, an ECM-Mimetic Fusion Protein, Protects Human Mesenchymal Stem Cells from Etoposide-Induced Senescence. Int. J. Mol. Sci. 2025, 26, 9218. https://doi.org/10.3390/ijms26189218
Jang S-H, Jang J-H. FN9-10ELP, an ECM-Mimetic Fusion Protein, Protects Human Mesenchymal Stem Cells from Etoposide-Induced Senescence. International Journal of Molecular Sciences. 2025; 26(18):9218. https://doi.org/10.3390/ijms26189218
Chicago/Turabian StyleJang, Su-Hyeon, and Jun-Hyeog Jang. 2025. "FN9-10ELP, an ECM-Mimetic Fusion Protein, Protects Human Mesenchymal Stem Cells from Etoposide-Induced Senescence" International Journal of Molecular Sciences 26, no. 18: 9218. https://doi.org/10.3390/ijms26189218
APA StyleJang, S.-H., & Jang, J.-H. (2025). FN9-10ELP, an ECM-Mimetic Fusion Protein, Protects Human Mesenchymal Stem Cells from Etoposide-Induced Senescence. International Journal of Molecular Sciences, 26(18), 9218. https://doi.org/10.3390/ijms26189218






