Enhanced Kidney Damage in Individuals with Diabetes Who Are Chronically Exposed to Cadmium and Lead: The Emergent Role for β2-Microglobulin
Abstract
1. Introduction
2. Results
2.1. Description of Participants
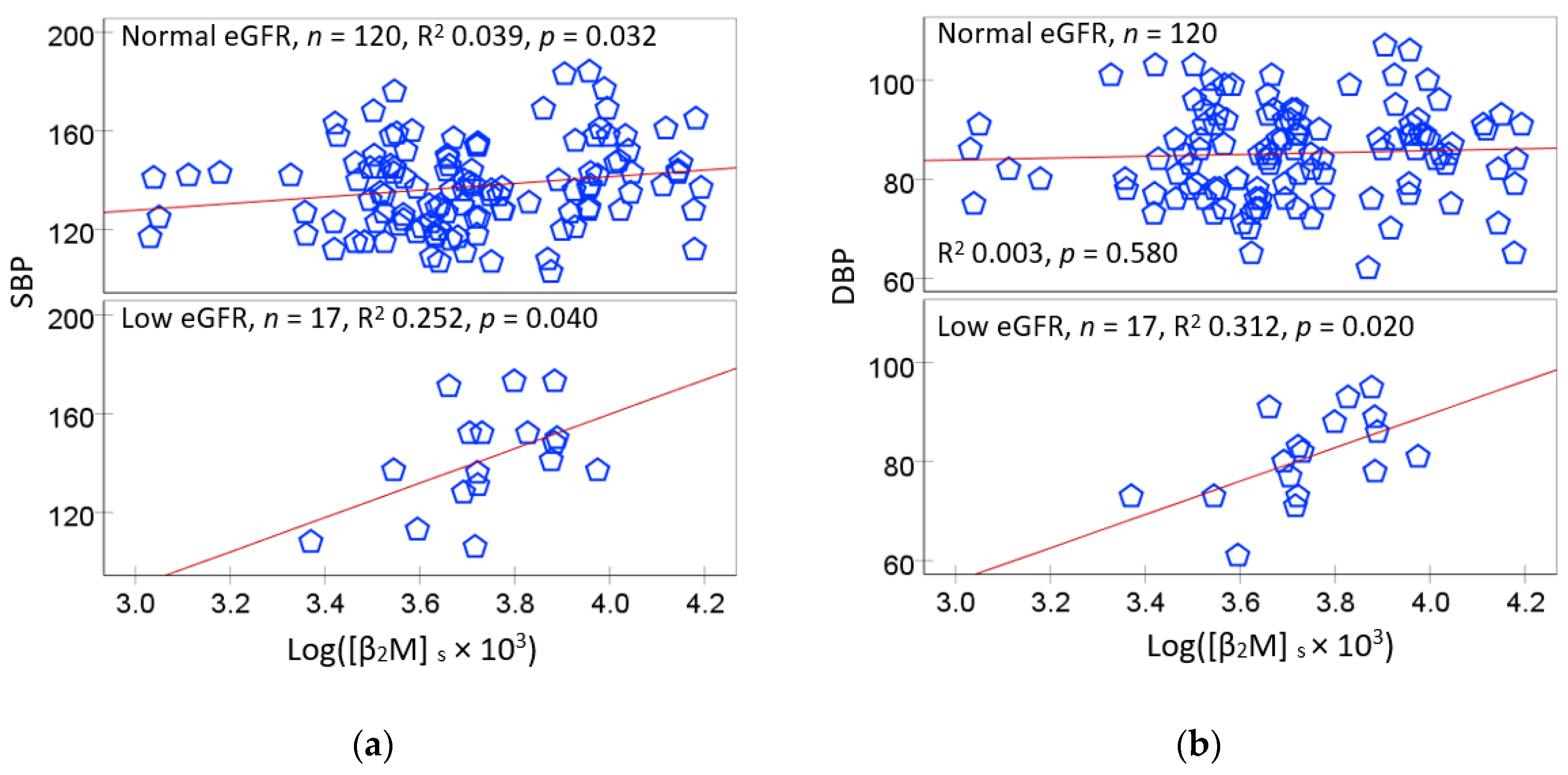
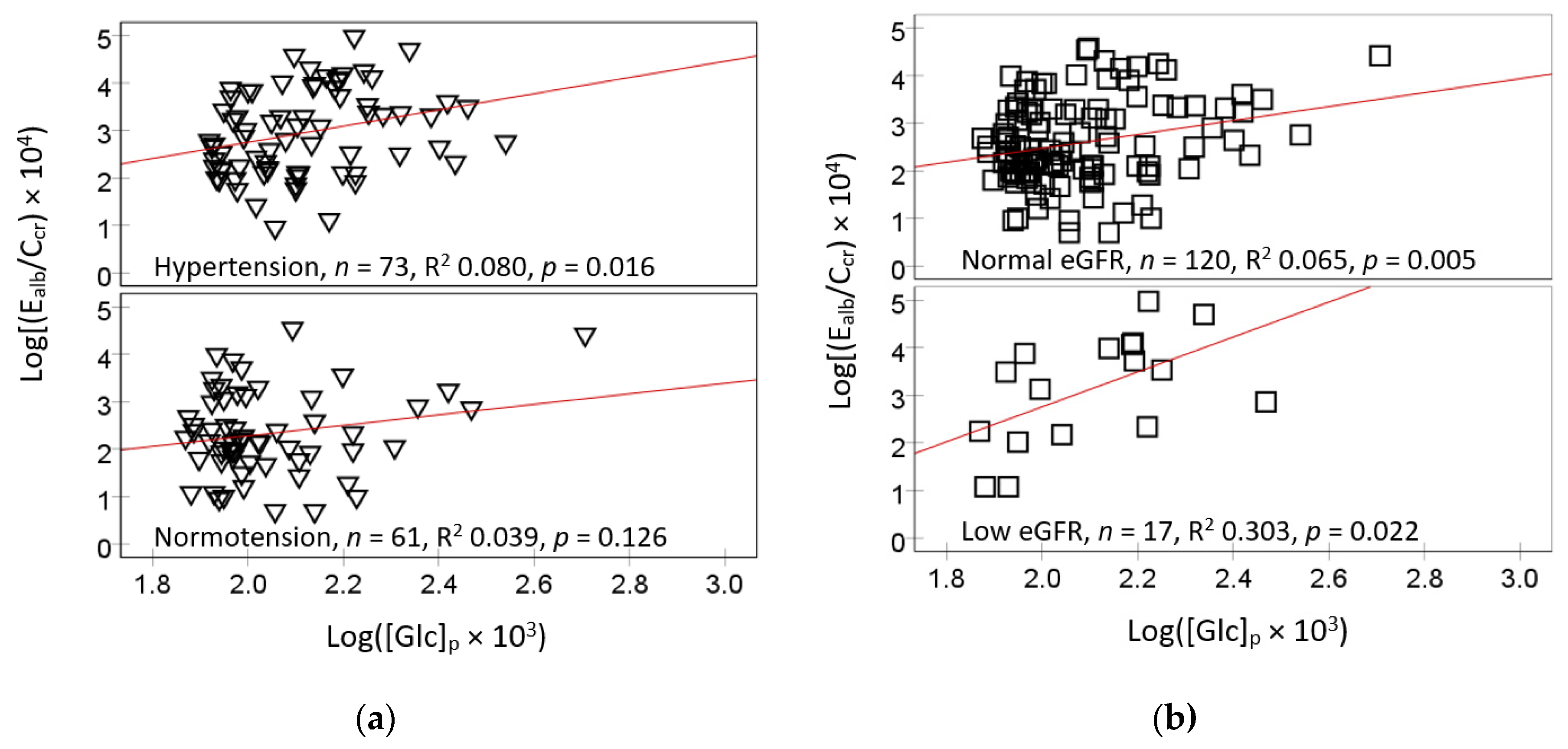
2.2. Bivariate Correlation Analysis
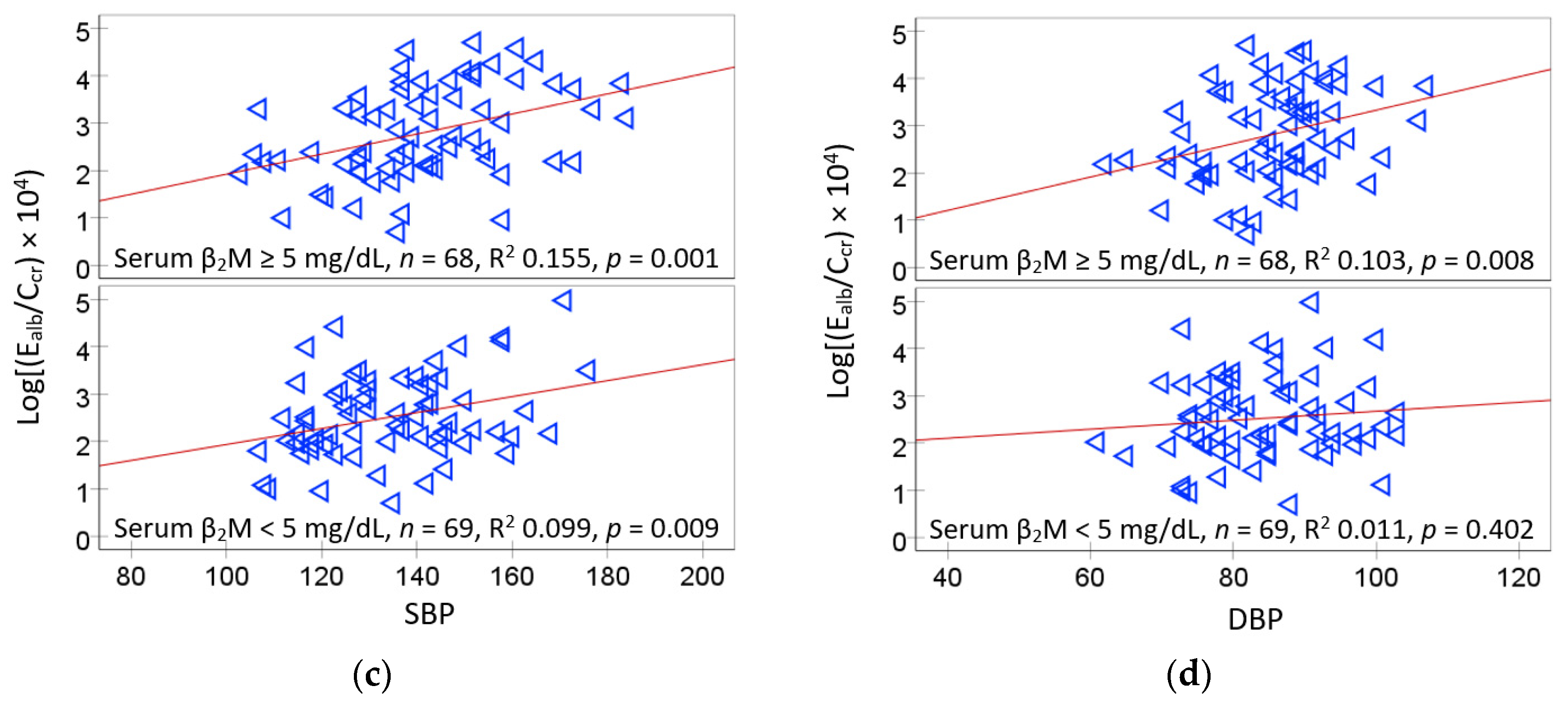
2.3. Logistic Regresssion Model for Serum β2M Higher than the Median 5 mg/L
2.4. Logistic Regresssion Model for Hyperglycemia
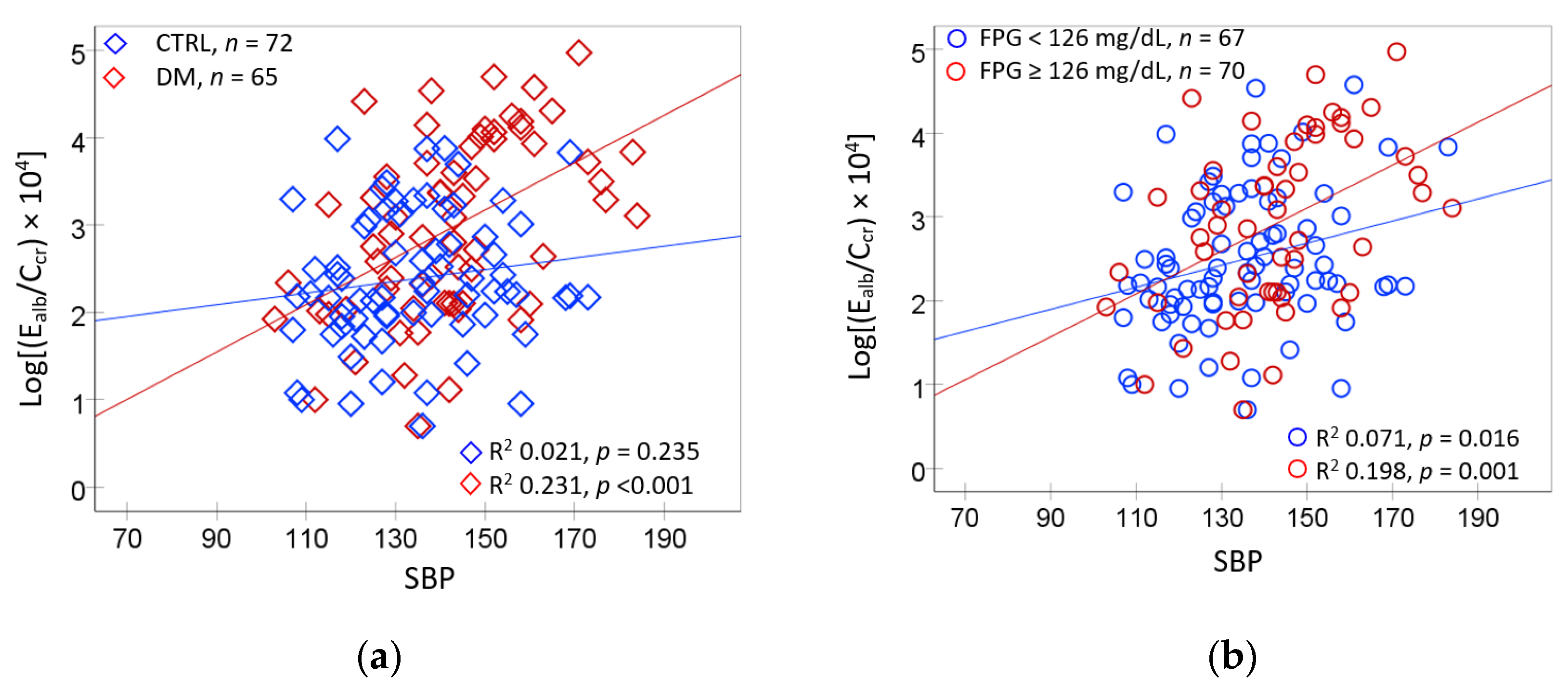
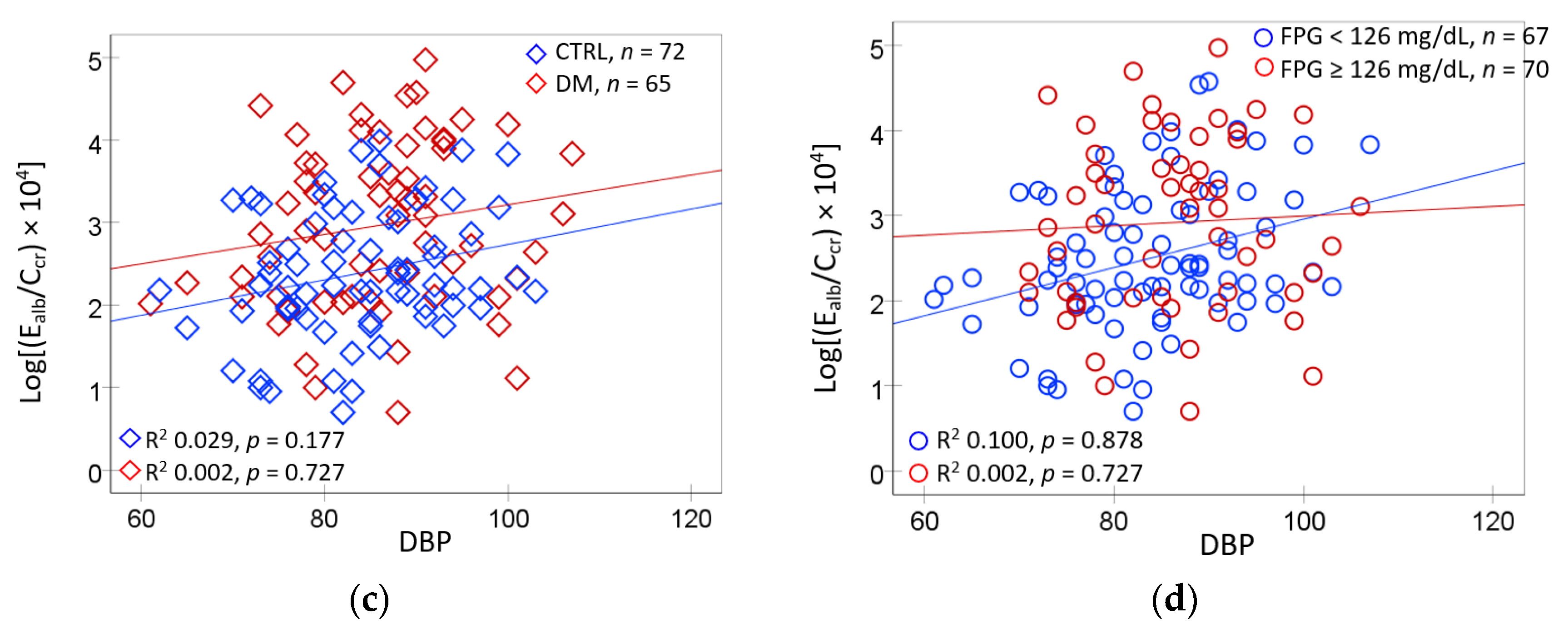
2.5. Logistic Regresssion Model for Hypertension and Albuminuria
3. Discussion
3.1. Hypertension Associated with Hyperglycemia and Environmental Cd and Pb
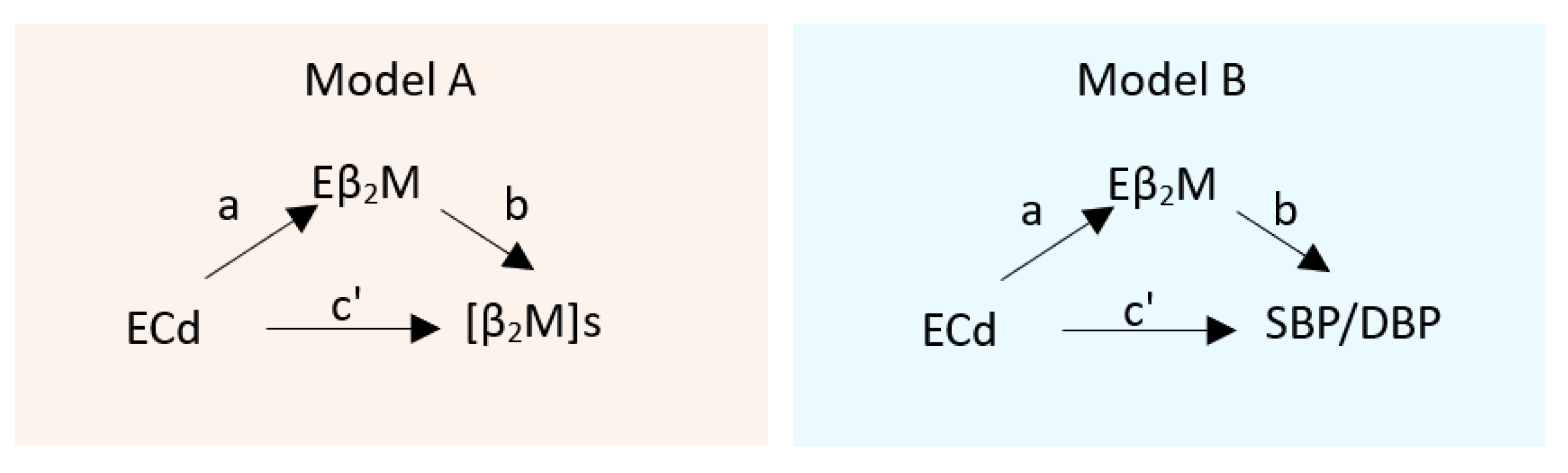
3.2. The SH3B-β2M Axis: A Novel Blood Pressure Regulator
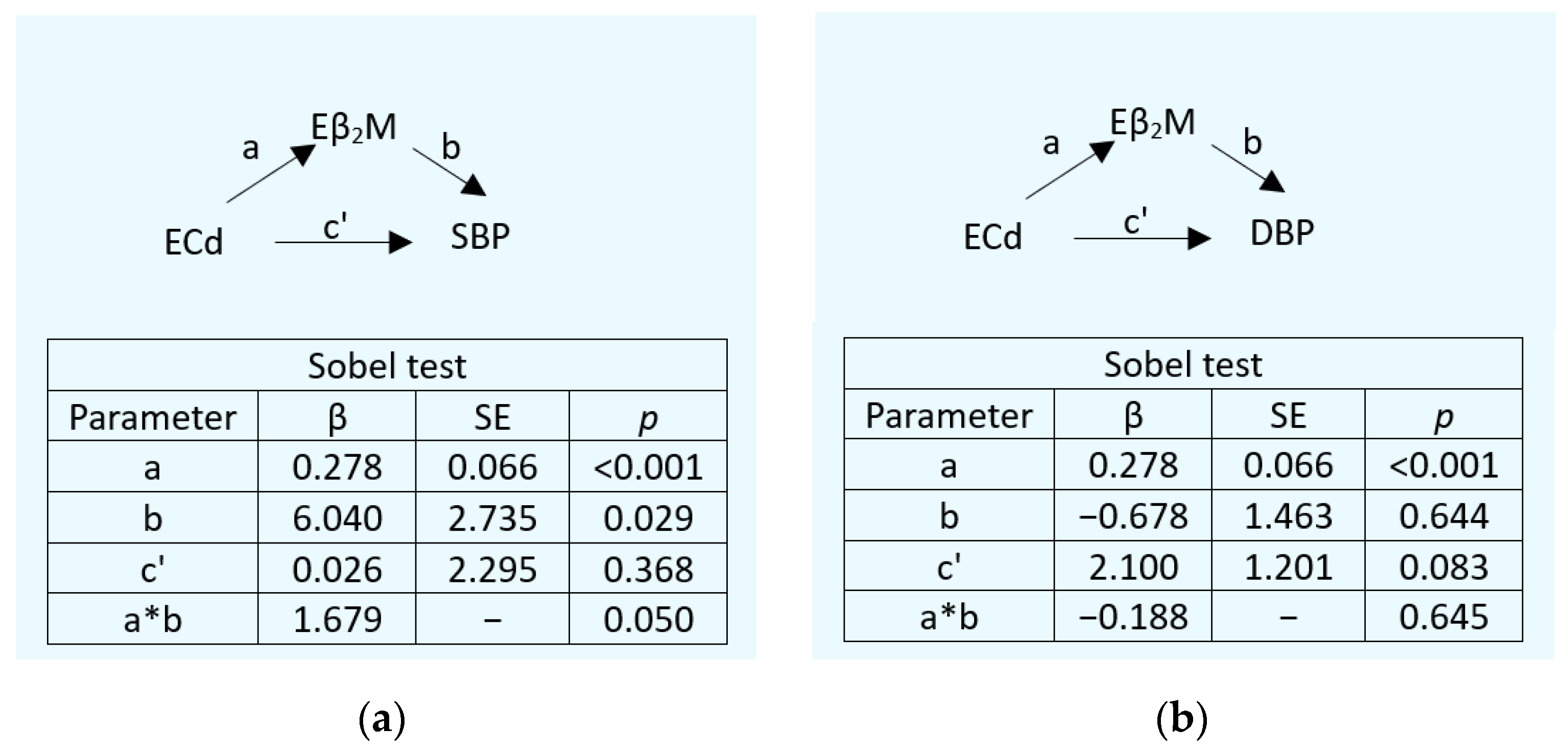
3.3. Mediating Effects of Cd on Serum β2M and SBP
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Data Sourcing
4.2. Collection of Blood and Urine Samples
4.3. Quantification of Exposure to Cd, Pb, and Biomarkers of Kidney Effects
4.4. Assessment of Simultaneous Cd/Pb Exposure
4.5. Calculation and Cut-Off Values for Albuminuria
4.6. The Causal Inference Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hyperten-sion Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D.; et al. Resistant hypertension: Diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008, 51, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shin, J.; Ihm, S.H.; Kim, K.I.; Kim, H.L.; Kim, H.C.; Lee, E.M.; Lee, J.H.; Ahn, S.Y.; Cho, E.J.; et al. Resistant hyper-tension: Consensus document from the Korean society of hypertension. Clin. Hypertens. 2023, 29, 30. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C. Blood pressure control-special role of the kidneys and body fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef]
- Leite, A.P.O.; Li, X.C.; Nwia, S.M.; Hassan, R.; Zhuo, J.L. Angiotensin II and AT1a Receptors in the Proximal Tubules of the Kidney: New Roles in Blood Pressure Control and Hypertension. Int. J. Mol. Sci. 2022, 23, 2402. [Google Scholar] [CrossRef]
- Bloch, M.J.; Basile, J.N. Review of recent literature in hypertension: Updated clinical practice guidelines for chronic kidney disease now include albuminuria in the classification system. J. Clin. Hypertens. 2013, 15, 865–867. [Google Scholar] [CrossRef]
- Qiu, L.; Wu, B. The relationship between the age of onset of hypertension and chronic kidney disease: A cross-sectional study of the American population. Front. Cardiovasc. Med. 2024, 11, 1426953. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Leng, X.; Huang, F.; Huang, R.; Wijayabahu, A.; Chen, X.; Xu, Y. Associations of blood lead, cadmium, and mercury with resistant hypertension among adults in NHANES, 1999–2018. Environ. Health Prev. Med. 2023, 28, 66. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Ma, Z.; Dang, Y.; Yang, Y.; Cao, S.; Ouyang, C.; Shi, X.; Pan, J.; Hu, X. Associations of urinary and blood cadmium concentrations with all-cause mortality in US adults with chronic kidney disease: A prospective cohort study. Environ. Sci. Pollut. Res. Int. 2023, 30, 61659–61671. [Google Scholar] [CrossRef]
- Yin, G.; Zhao, S.; Zhao, M.; Xu, J.; Ge, X.; Wu, J.; Zhou, Y.; Liu, X.; Wei, L.; Xu, Q. Complex interplay of heavy metals and renal injury: New perspectives from longitudinal epidemiological evidence. Ecotoxicol. Environ. Saf. 2024, 278, 116424. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Xin, M.; Zhao, S.; Zhao, M.; Xu, J.; Chen, X.; Xu, Q. Heavy metals and elderly kidney health: A multidimensional study through Enviro-target Mendelian Randomization. Ecotoxicol. Environ. Saf. 2024, 281, 116659. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, S.; Jang, M.; Kim, K. Effect of combined exposure to lead, mercury, and cadmium on hypertension: The 2008-2013 Korean National Health and Nutrition Examination Surveys. Int. J. Occup. Med. Environ. Health 2025, 28, 264. [Google Scholar] [CrossRef]
- Yeon, J.; Kang, S.; Park, J.; Ahn, J.H.; Cho, E.A.; Lee, S.H.; Ryu, K.H.; Shim, J.G. Association between blood cadmium levels and the risk of chronic kidney disease in Korea, based on the Korea National Health and Nutrition Examination Survey 2016–2017. Asian Biomed. (Res. Rev. News) 2025, 19, 60–66. [Google Scholar] [CrossRef]
- Verzelloni, P.; Giuliano, V.; Wise, L.A.; Urbano, T.; Baraldi, C.; Vinceti, M.; Filippini, T. Cadmium exposure and risk of hy-pertension: A systematic review and dose-response meta-analysis. Environ. Res. 2024, 263 Pt 1, 120014. [Google Scholar] [CrossRef]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Farrell, D.R.; Vassalotti, J.A. Screening, identifying, and treating chronic kidney disease: Why, who, when, how, and what? BMC Nephrol. 2024, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Oosterwijk, M.M.; Hagedoorn, I.J.M.; Maatman, R.G.H.J.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Cadmium, active smoking and renal function deterioration in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2023, 38, 876–883. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, R.; Wang, Y.; Wang, Z.; Zhang, L.; Xu, Y.; Wang, D.; Wu, J.; Wei, L.; Yang, L.; et al. Association between blood heavy metals and diabetic kidney disease among type 2 diabetic patients: A cross-sectional study. Sci. Rep. 2024, 14, 26823. [Google Scholar] [CrossRef]
- Barregard, L.; Bergström, G.; Fagerberg, B. Cadmium, type 2 diabetes, and kidney damage in a cohort of middle-aged women. Environ. Res. 2014, 135, 311–316. [Google Scholar] [CrossRef]
- Liao, K.W.; Chien, L.C.; Chen, Y.C.; Kao, H.C. Sex-specific differences in early renal impairment associated with arsenic, lead, and cadmium exposure among young adults in Taiwan. Environ. Sci. Pollut. Res. Int. 2022, 29, 52655–52664. [Google Scholar] [CrossRef]
- Huan, T.; Meng, Q.; Saleh, M.A.; Norlander, A.E.; Joehanes, R.; Zhu, J.; Chen, B.H.; Zhang, B.; Johnson, A.D.; Ying, S.; et al. Integrative network analysis reveals molecular mechanisms of blood pressure regulation. Mol. Syst. Biol. 2015, 11, 799. [Google Scholar] [CrossRef]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhang, H.; Shen, J. In vivo heavy metal and diabetes association: A cross-sectional interpretable machine learning analysis of NHANES. Int. J. Diabetes Develop. Ctries. 2025, 1–15. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Yimthiang, S.; Khamphaya, T.; Pouyfung, P.; Đorđević, A.B. Environmental Cadmium Exposure Induces an Increase in Systolic Blood Pressure by Its Effect on GFR. Stresses 2024, 4, 436–451. [Google Scholar] [CrossRef]
- Xie, S.; Perrais, M.; Golshayan, D.; Wuerzner, G.; Vaucher, J.; Thomas, A.; Marques-Vidal, P. Association between urinary heavy metal/trace element concentrations and kidney function: A prospective study. Clin. Kidney J. 2024, 18, sfae378. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, P.; Li, H. U-shaped relationship between fasting blood glucose and urinary albumin-to-creatinine ratio in the general United States population. Front. Endocrinol. 2024, 15, 1334949. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jia, J.; Li, J.; Huo, Y.; Fan, F.; Zhang, Y. Impaired fasting blood glucose is associated with incident albuminuria: Data from a Chinese community-based cohort. J. Diabetes Complicat. 2022, 36, 108125. [Google Scholar] [CrossRef]
- Ren, F.; Li, M.; Xu, H.; Qin, X.; Teng, Y. Urine albumin-to-creatinine ratio within the normal range and risk of hypertension in the general population: A meta-analysis. J. Clin. Hypertens. 2021, 23, 1284–1290. [Google Scholar] [CrossRef]
- Mashima, Y.; Konta, T.; Kudo, K.; Takasaki, S.; Ichikawa, K.; Suzuki, K.; Shibata, Y.; Watanabe, T.; Kato, T.; Kawata, S.; et al. Increases in urinary albumin and beta2-microglobulin are independently associated with blood pressure in the Japanese general population: The Takahata Study. Hypertens. Res. 2011, 34, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Konta, T.; Mashima, Y.; Ichikawa, K.; Takasaki, S.; Ikeda, A.; Hoshikawa, M.; Suzuki, K.; Shibata, Y.; Watanabe, T.; et al. The association between renal tubular damage and rapid renal deterioration in the Japanese population: The Takahata study. Clin. Exp. Nephrol. 2011, 15, 235–241. [Google Scholar] [CrossRef]
- Kaneda, M.; Wai, K.M.; Kanda, A.; Ando, M.; Murashita, K.; Nakaji, S.; Ihara, K. Low Level of Serum Cadmium in Relation to Blood Pressures Among Japanese General Population. Biol. Trace Elem. Res. 2022, 200, 67–75. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Keefe, J.A.; Hwang, S.J.; Huan, T.; Mendelson, M.; Yao, C.; Courchesne, P.; Saleh, M.A.; Madhur, M.S.; Levy, D. Evidence for a causal role of the SH2B3-β2M axis in blood pressure regulation. Hypertension 2019, 73, 497–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, G.; Leng, W.; Li, Y.; Li, H.; Zhou, L.; Ge, L.; Shao, J.; Li, X.; Long, M. Association between serum β2-microglobulin and left ventricular hypertrophy in patients with type 2 diabetes mellitus: A cross-sectional study. J. Diabetes 2024, 16, e13599. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.L.; Lam, K.S.; Cheung, B.M. Serum beta-2 microglobulin predicts mortality in people with diabetes. Eur. J. Endocrinol. 2013, 169, 1–7. [Google Scholar] [CrossRef]
- Kim, M.K.; Yun, K.J.; Chun, H.J.; Jang, E.H.; Han, K.D.; Park, Y.M.; Baek, K.H.; Song, K.H.; Cha, B.Y.; Park, C.S.; et al. Clinical utility of serum beta-2-microglobulin as a predictor of diabetic complications in patients with type 2 diabetes without renal impairment. Diabetes Metab. 2014, 40, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Scinicariello, F.; Abadin, H.G.; Murray, H.E. Association of low-level blood lead and blood pressure in NHANES 1999–2006. Environ. Res. 2011, 111, 1249–1257. [Google Scholar] [CrossRef]
- Xu, R.; Tan, X.; Li, T.; Liu, S.; Li, Y.; Li, H. Norepinephrine-induced AuPd aerogels with peroxidase- and glucose oxidase-like activity for colorimetric determination of glucose. Mikrochim. Acta 2021, 188, 362. [Google Scholar] [CrossRef]
- Apple, F.; Bandt, C.; Prosch, A.; Erlandson, G.; Holmstrom, V.; Scholen, J.; Googins, M. Creatinine clearance: Enzymatic vs Jaffé determinations of creatinine in plasma and urine. Clin. Chem. 1986, 32, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Bargnoux, A.S.; Barrot, A.; Fesler, P.; Kuster, N.; Badiou, S.; Dupuy, A.M.; Ribstein, J.; Cristol, J.P. Evaluation of five immunoturbidimetric assays for urinary albumin quantification and their impact on albuminuria categorization. Clin. Biochem. 2014, 47, 250–253. [Google Scholar] [CrossRef]
- Kok, M.B.; Tegelaers, F.P.; van Dam, B.; van Rijn, J.L.; van Pelt, J. Carbamylation of albumin is a cause for discrepancies between albumin assays. Clin. Chim. Acta 2014, 434, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Trzcinka-Ochocka, M.; Brodzka, R.; Janasik, B. Useful and Fast Method for Blood Lead and Cadmium Determination Using ICP-MS and GF-AAS; Validation Parameters. J. Clin. Lab. Anal. 2016, 30, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef]
- Preacher, K.J. Advances in mediation analysis: A survey and synthesis of new developments. Annu. Rev. Psychol. 2015, 66, 825–852. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Warsi, G.; Dwyer, J.H. A simulation study of mediated effect measures. Multiv. Behav. Res. 1995, 30, 41–62. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Meth. Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]

| Variables | All Subjects N = 137 | Tertile of Serum β2M Concentration, mg/L | p | ||
|---|---|---|---|---|---|
| T1: 1–3.9 n = 45 | T2: 4.0–5.9 n = 46 | T3: 6.0–16 n = 46 | |||
| Women, % | 78.1 | 80.0 | 82.6 | 71.1 | 0.421 |
| Smoking, % | 10.2 | 4.4 | 8.7 | 17,4 | 0.115 |
| Diagnosed diabetes | 47.4 | 42.2 | 28.3 | 71.7 | <0.001 |
| Age, years | 59.7 (9.1) | 57.2 (9.7) | 60.4 (8.3) | 61.4 (8.9) | 0.060 |
| BMI, kg/m2 | 25.6 (4.8) | 26.3 (5.7) | 25.1 (3.9) | 25.4 (4.6) | 0.751 |
| SBP, mm Hg | 138 (18) | 136 (17) | 133 (15) | 145 (19) | 0.004 |
| DBP, mm Hg | 85 (9) | 84 (10) | 83 (8) | 87 (9) | 0.129 |
| Hypertension, % | 54.5 | 54.5 | 40.0 | 68.9 | 0.023 |
| eGFR, mL/min/1.73 m2 | 79 (16) | 86 (16) | 75 (15) | 77 (15) | 0.002 |
| Low eGFR a, % | 12.7 | 6.7 | 15.2 | 15.2 | 0.326 |
| FPG, mg/dL | 129 (61) | 135 (83) | 119 (51) | 134 (41) | 0.007 |
| [Cd]b, µg/L | 0.57 (0.70) | 0.40 (0.56) | 0.59 (0.69) | 0.70 (0.81) | 0.041 |
| [Pb]b, mg/dL | 4.49 (4.78) | 4.73 (5.11) | 3.07 (2.13) | 5.68 (5.96) | 0.002 |
| ECd/Ecr, µg/g creatinine | 0.98 (1.86) | 1.05 (1.87) | 1.09 (2.14) | 0.79 (1.53) | 0.567 |
| ECd/Ccr, (µg/L filtrate) × 100 | 0.86 (1.68) | 0.86 (1.56) | 1.01 (2.00) | 0.69 (1.45) | 0.796 |
| Eβ2M/Ccr, (µg/L filtrate) × 100 | 99 (114) | 63 (77) | 78 (110) | 155 (129) | <0.001 |
| ACR (Ealb/Ecr), mg/g creatinine | 40 (102) | 26 (70) | 43 (134) | 50 (90) | 0.463 |
| Ealb/Ccr, (mg/L filtrate) × 100 | 37 (106) | 20 (49) | 44 (155) | 47 (84) | 0.366 |
| Ealb/Ccr ≥ 0.2 mg/L filtrate, % | 26.3 | 17.8 | 21.7 | 39.1 | 0.048 |
| FPG ≥ 110 mg/dL, % | 48.9 | 42.2 | 34.8 | 69.6 | 0.002 |
| FPG ≥ 126 mg/dL, % | 39.4 | 35.6 | 23.9 | 58.7 | 0.002 |
| Variables | Spearman’s Correlation Coefficient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [β2M]s | Age | BMI | FPG | SBP | DBP | eGFR | Ealb/Ccr | Eβ2M/Ccr | ECd/Ccr | |
| Age | 0.200 * | |||||||||
| BMI | −0.061 | −0.262 ** | ||||||||
| FPG | 0.210 * | −0.222 ** | 0.184 * | |||||||
| SBP | 0.229 ** | 0.224 ** | 0.072 | 0.250 ** | ||||||
| DBP | 0.117 | −0.123 | 0.043 | 0.168 | 0.552 ** | |||||
| eGFR | −0.265 ** | −0.356 ** | 0.161 | 0.089 | −0.048 | 0.042 | ||||
| Ealb/Ccr | 0.138 | 0.085 | 0.076 | 0.273 ** | 0.372 ** | 0.232 ** | −0.136 | |||
| Eβ2M/Ccr | 0.390 ** | 0.170 * | −0.066 | 0.306 ** | 0.237 ** | 0.051 | −0.515 ** | 0.265 ** | ||
| ECd/Ccr | 0.021 | 0.078 | −0.083 | 0.166 | 0.133 | 0.123 | −0.227 ** | 0.106 | 0.496 ** | |
| Cd/Pb exposure a | 0.158 | 0.009 | −0.012 | 0.181 * | 0.114 | 0.194 * | −0.013 | 0.095 | 0.309 ** | 0.301 ** |
| Independent Variables/Factors | [β2M]s ≥ 5 mg/L | ||||
|---|---|---|---|---|---|
| β Coefficients | POR | 95% CI | p | ||
| (SE) | Lower | Upper | |||
| Age, years | 0.025 (0.025) | 1.025 | 0.976 | 1.076 | 0.320 |
| BMI, kg/m2 | −0.026 (0.046) | 0.974 | 0.890 | 1.067 | 0.574 |
| eGFR, mL/min/1.73 m2 | −0.041 (0.014) | 0.960 | 0.933 | 0.988 | 0.005 |
| Log10[(ECd/Ccr) × 105], µg/ L filtrate | 0.257 (0.281) | 1.293 | 0.746 | 2.240 | 0.360 |
| Gender | 0.136 (0.607) | 1.146 | 0.349 | 3.763 | 0.822 |
| Smoking | 1.410 (0.856) | 4.098 | 0.765 | 21.95 | 0.100 |
| Diagnosed diabetes | 1.411 (0.425) | 4.099 | 1.783 | 9.421 | 0.001 |
| Hypertension | 0.436 (0.406) | 1.547 | 0.699 | 3.425 | 0.282 |
| Independent Variables | FPG ≥ 110 mg/dL | FPG ≥ 126 mg/dL | ||
|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | |
| Age, years | 1.046 (0.999, 1.096) | 0.056 | 1.077 (1.023, 1.133) | 0.004 |
| BMI, kg/m2 | 0.929 (0.855, 1.011) | 0.088 | 0.942 (0.865, 1.027) | 0.177 |
| Gender | 1.574 (0.505, 4.910) | 0.434 | 1.338 (0.424, 4.225) | 0.620 |
| Smoking | 3.087 (0.628, 15.18) | 0.165 | 2.881 (0.538, 15.42) | 0.216 |
| [β2M]s ≥ 5 mg/dL | 3.392 (1.554, 7.406) | 0.002 | 3.875 (1.673, 8.977) | 0.002 |
| Cd/Pb exposure category a | ||||
| 1 | Referent | Referent | ||
| 2 | 2.107 (0.825, 5.378) | 0.119 | 3.141 (1.185, 8.328) | 0.021 |
| 3 | 2.802 (1.026, 7.651) | 0.044 | 3.702 (1.299, 10.54) | 0.014 |
| Independent Variables | Hypertension a | Albuminuria b | ||
|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | |
| Age, years | 0.964 (0.920, 1.009) | 0.114 | 0.974 (0.927, 1.023) | 0.295 |
| BMI, kg/m2 | 0.975 (0.894, 1.063) | 0.561 | 0.980 (0.898, 1.070) | 0.653 |
| Gender | 2.299 (0.690, 7.662) | 0.175 | 2.490 (0.763, 8.122) | 0.130 |
| Non-smoker | 7.920 (1.381, 45.42) | 0.020 | 3.187 (0.559, 18.18) | 0.192 |
| FPG ≥ 110 mg/dL | 3.664 (1.658, 8.097) | 0.001 | 2.955 (1.254, 6.965) | 0.013 |
| Cd/Pb exposure category c | ||||
| 1 | Referent | Referent | ||
| 2 | 3.063 (1.022, 9.186) | 0.046 | 1.369 (0.513, 3.650) | 0.530 |
| 3 | 4.413 (1.555, 12.53) | 0.005 | 1.993 (0.664, 5.980) | 0.219 |
| Independent Variables | Hypertension a | Albuminuria b | ||
|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | |
| Age, years | 0.963 (0.920, 1.008) | 0.101 | 0.965 (0.917, 1.016) | 0.172 |
| BMI, kg/m2 | 0.969 (0.880, 1.056) | 0.469 | 0.976 (0/895, 1.065) | 0.586 |
| Gender | 2.356 (0.732, 7.577) | 0.151 | 2.671 (0.817, 8.737) | 0.104 |
| Non-smoker | 8.030 (1.429, 45.11) | 0.018 | 3.275 (0.572, 18.76) | 0.183 |
| FPG ≥ 126 mg/dL | 2.905 (1.275, 6.622) | 0.011 | 3.482 (1.458, 8.312) | 0.005 |
| Cd/Pb exposure category c | ||||
| 1 | Referent | Referent | ||
| 2 | 2.966 (0.998, 8.811) | 0.050 | 1.196 (0.439, 3.260) | 0.726 |
| 3 | 4.053 (1.445, 11.36) | 0.008 | 1.846 (0.603, 5.449) | 0.283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S.; Vesey, D.A.; Waeyeng, D.; Khamphaya, T.; Yimthiang, S. Enhanced Kidney Damage in Individuals with Diabetes Who Are Chronically Exposed to Cadmium and Lead: The Emergent Role for β2-Microglobulin. Int. J. Mol. Sci. 2025, 26, 9208. https://doi.org/10.3390/ijms26189208
Satarug S, Vesey DA, Waeyeng D, Khamphaya T, Yimthiang S. Enhanced Kidney Damage in Individuals with Diabetes Who Are Chronically Exposed to Cadmium and Lead: The Emergent Role for β2-Microglobulin. International Journal of Molecular Sciences. 2025; 26(18):9208. https://doi.org/10.3390/ijms26189208
Chicago/Turabian StyleSatarug, Soisungwan, David A. Vesey, Donrawee Waeyeng, Tanaporn Khamphaya, and Supabhorn Yimthiang. 2025. "Enhanced Kidney Damage in Individuals with Diabetes Who Are Chronically Exposed to Cadmium and Lead: The Emergent Role for β2-Microglobulin" International Journal of Molecular Sciences 26, no. 18: 9208. https://doi.org/10.3390/ijms26189208
APA StyleSatarug, S., Vesey, D. A., Waeyeng, D., Khamphaya, T., & Yimthiang, S. (2025). Enhanced Kidney Damage in Individuals with Diabetes Who Are Chronically Exposed to Cadmium and Lead: The Emergent Role for β2-Microglobulin. International Journal of Molecular Sciences, 26(18), 9208. https://doi.org/10.3390/ijms26189208








