Anthracycline Treatments and the Presence of Tumor Cells Synergistically Modify the Composition of Macrophage Subpopulations in the Co-Culture System
Abstract
1. Introduction
2. Results
2.1. The Presence of Tumor Cells Increases CD206 Expression on Macrophages
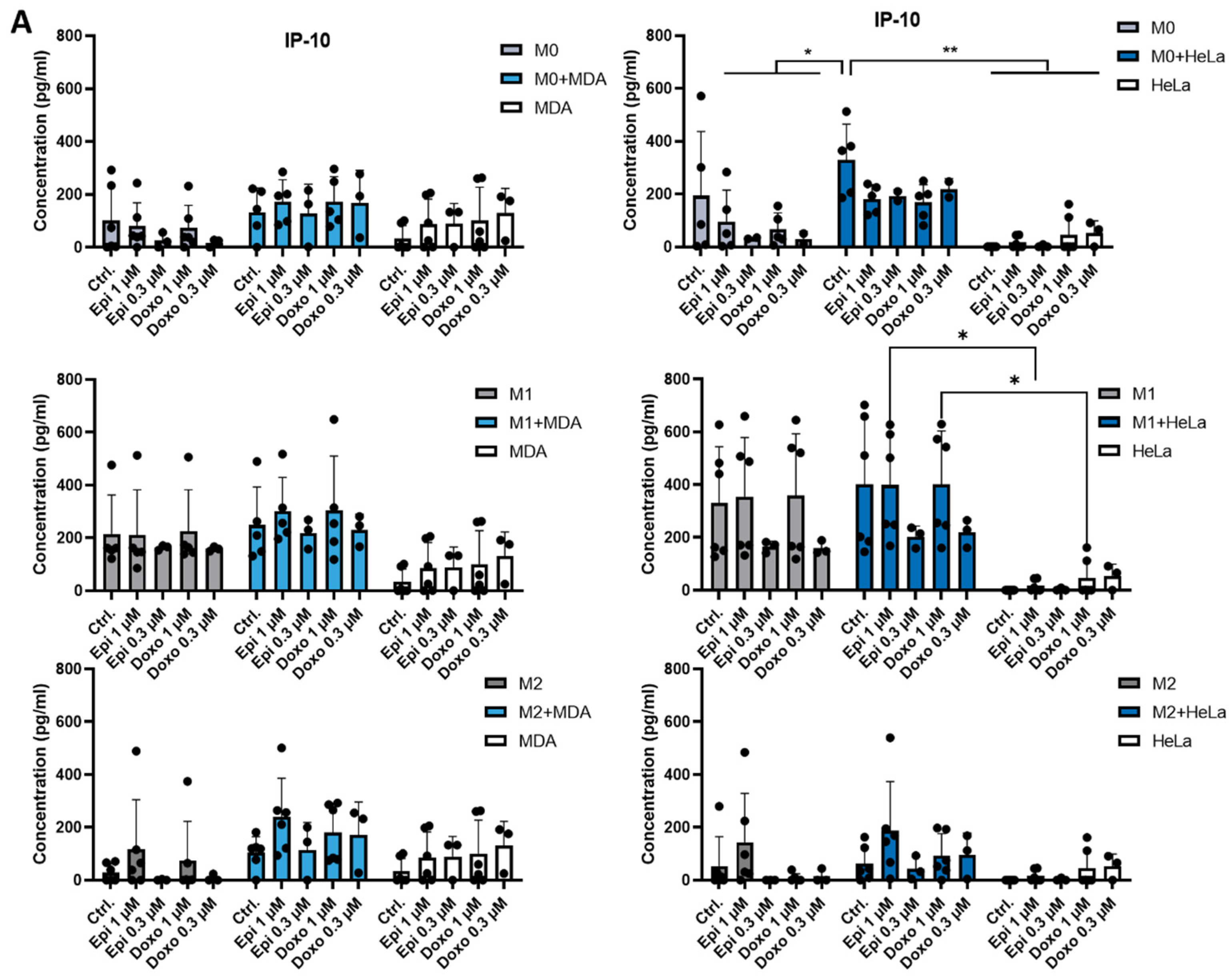
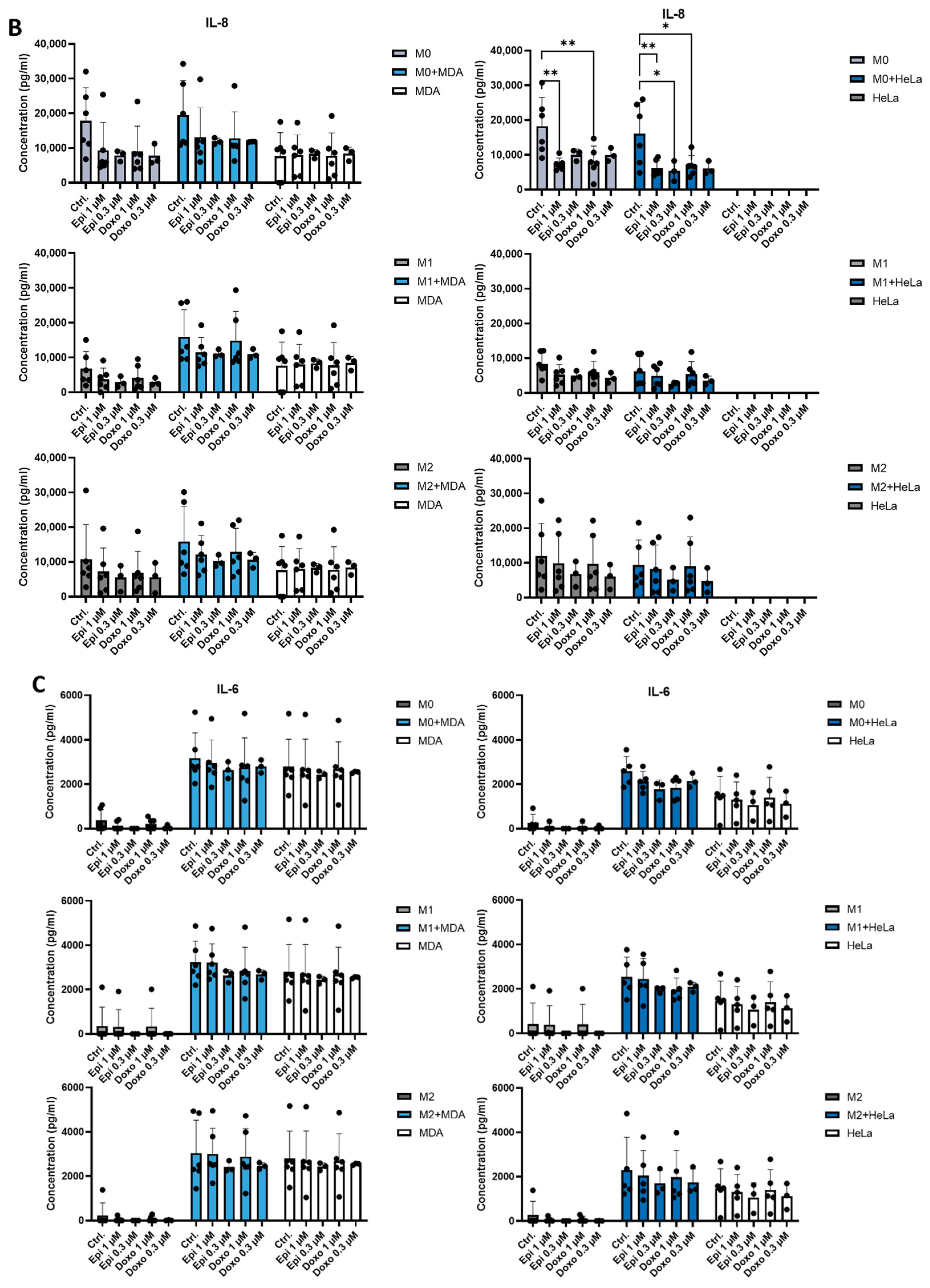
2.2. The Presence of Tumor Cells or Chemotherapeutic Agents Regulates the Production of IL-8 and IP-10 by Macrophages
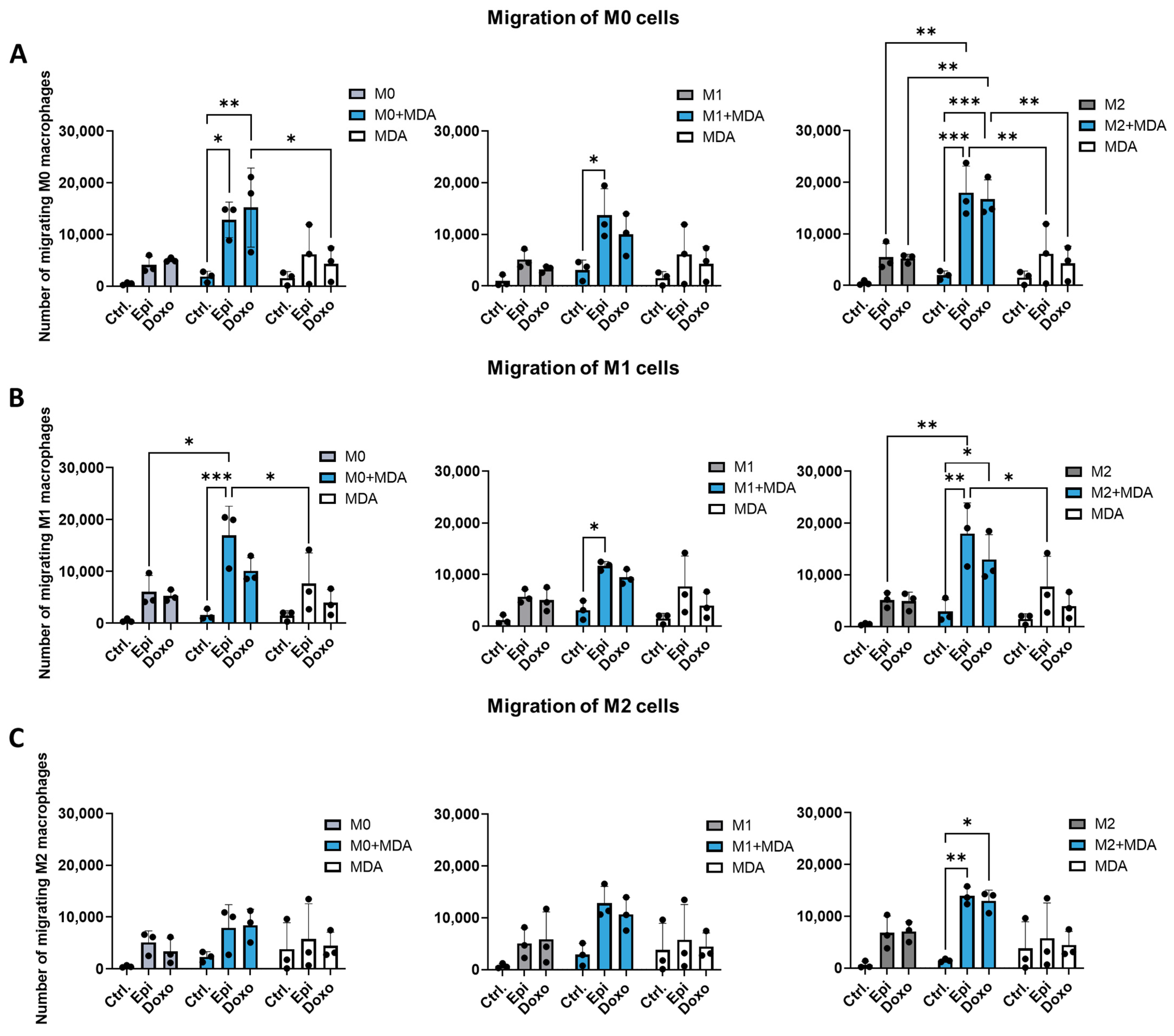
2.3. Anthracyclines Enhance the Migratory Potential of Macrophage Subpopulations Towards Tumor Co-Cultures
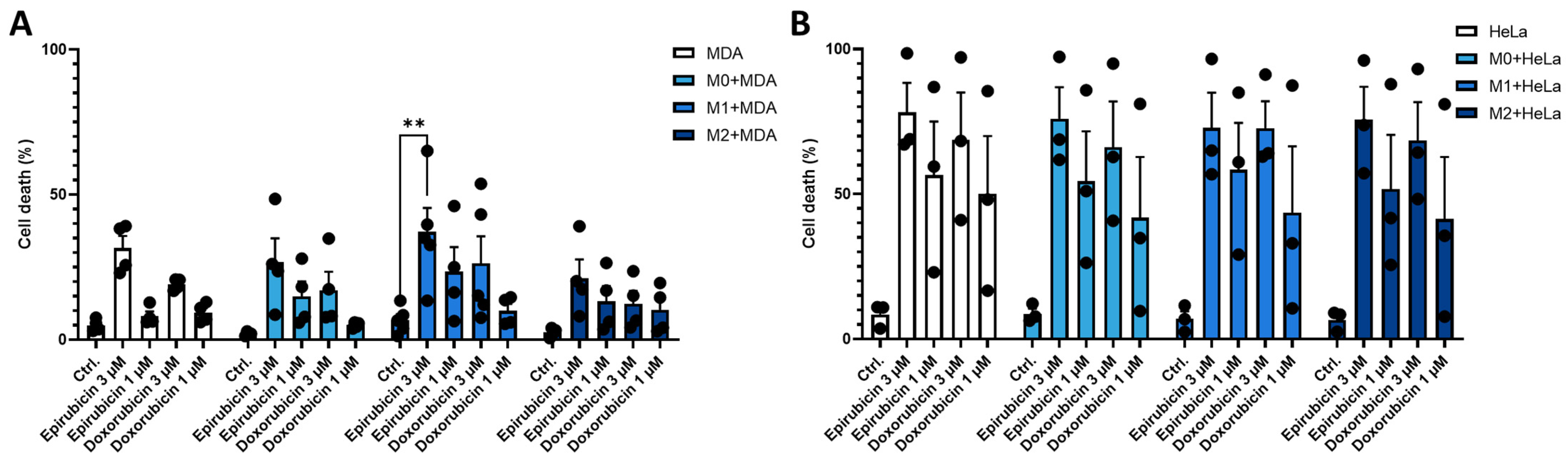
2.4. Co-Culture with MDA-MB-231 Cells Reduces the Sensitivity of M1 Macrophages to Anthracycline Treatment
3. Discussion
4. Materials and Methods
4.1. Differentiation of Human Macrophages
4.2. Cell Lines
4.3. Direct Co-Culture
4.4. Detection of Cell Surface Markers
4.5. Quantitative Real-Time PCR
4.6. ELISA
4.7. Boyden-Chamber Migration Assay
4.8. Determination of Cell Viability
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018 8, 49. [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Zou, Z.; Lin, H.; Li, M.; Lin, B. Tumor-associated macrophage polarization in the inflammatory tumor microenvironment. Front. Oncol. 2023, 13, 1103149. [Google Scholar] [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Liu, L.; Wang, J.; Wu, J.; Sun, C. Role of macrophages in tumor progression and therapy (Review). Int. J. Oncol. 2022, 60, 57. [Google Scholar] [CrossRef]
- Larionova, I.; Cherdyntseva, N.; Liu, T.; Patysheva, M.; Rakina, M.; Kzhyshkowska, J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 2019, 8, 1596004. [Google Scholar] [CrossRef]
- Jenei, V.; Burai, S.; Molnár, T.; Kardos, B.; Mácsik, R.; Tóth, M.; Debreceni, Z.; Bácsi, A.; Mázló, A.; Koncz, G. Comparison of the immunomodulatory potential of platinum-based anti-cancer drugs and anthracyclins on human monocyte-derived cells. Cancer Chemother. Pharmacol. 2023, 91, 53–66. [Google Scholar] [CrossRef]
- Liston, D.R.; Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef]
- Sun, X.; Qu, Q.; Lao, Y.; Zhang, M.; Yin, X.; Zhu, H.; Wang, Y.; Yang, J.; Yi, J.; Hao, M. Tumor suppressor HIC1 is synergistically compromised by cancer-associated fibroblasts and tumor cells through the IL-6/pSTAT3 axis in breast cancer. BMC Cancer 2019, 19, 1180. [Google Scholar] [CrossRef]
- Artaza-Irigaray, C.; Molina-Pineda, A.; Aguilar-Lemarroy, A.; Ortiz-Lazareno, P.; Limón-Toledo, L.P.; Pereira-Suárez, A.L.; Rojo-Contreras, W.; Jave-Suárez, L.F. E6/E7 and E6(*) From HPV16 and HPV18 Upregulate IL-6 Expression Independently of p53 in Keratinocytes. Front. Immunol. 2019, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Jaillon, S.; Garlanda, C.; Allavena, P. Tumor-associated myeloid cells: Diversity and therapeutic targeting. Cell. Mol. Immunol. 2021, 18, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Wei, S.; Jiang, P.; Xue, L.; Wang, J. Tumor-associated macrophages: Potential therapeutic strategies and future prospects in cancer. J. Immunother. Cancer 2021, 9, e001341. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Ji, C.; Liu, X.; Gu, B.; Dong, T. Macrophage polarization in the tumor microenvironment: Emerging roles and therapeutic potentials. Biomed. Pharmacother. 2024, 177, 116930. [Google Scholar] [CrossRef] [PubMed]
- Unver, N. Macrophage chemoattractants secreted by cancer cells: Sculptors of the tumor microenvironment and another crucial piece of the cancer secretome as a therapeutic target. Cytokine Growth Factor Rev. 2019, 50, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Molnár, T.; Mázló, A.; Kovács, R.; Jenei, V.; Kerekes, K.; Bácsi, A.; Koncz, G. Differences in the sensitivity of classically and alternatively activated macrophages to TAK1 inhibitor-induced necroptosis. Cancer Immunol. Immunother. 2020, 69, 2193–2207. [Google Scholar] [CrossRef]
- Hollmen, M.; Roudnicky, F.; Karaman, S.; Detmar, M. Characterization of macrophage—Cancer cell crosstalk in estrogen receptor positive and triple-negative breast cancer. Sci. Rep. 2015, 5, 9188. [Google Scholar] [CrossRef]
- Chang, L.; Yuan, Z.; Tian, W. A retrospective study of carboplatin and liposomal doxorubicin in patients with locally advanced or recurrent squamous cell carcinoma of the cervix. J. Clin. Oncol. 2020, 38, e18021. [Google Scholar] [CrossRef]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206(+) tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef]
- Paduch, R.; Klatka, M.; Pieniądz, P.; Wertel, I.; Pawłowska, A.; Klatka, J. Reciprocal Interactions of Human Monocytes and Cancer Cells in Co-Cultures In Vitro. Curr. Issues Mol. Biol. 2024, 46, 6836–6852. [Google Scholar] [CrossRef]
- Luo, X.; Yao, J.; Nie, P.; Yang, Z.; Feng, H.; Chen, P.; Shi, X.; Zou, Z. FOXM1 promotes invasion and migration of colorectal cancer cells partially dependent on HSPA5 transactivation. Oncotarget 2016, 7, 26480–26495. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-induced metastasis in breast cancer. Oncotarget 2017, 8, 110733–110734. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’aLfonso, T.M.; et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 2017, 9, eaan0026. [Google Scholar] [CrossRef]
- Wu, X.; Schulte, B.C.; Zhou, Y.; Haribhai, D.; Mackinnon, A.C.; Plaza, J.A.; Williams, C.B.; Hwang, S.T. Depletion of M2-Like Tumor-Associated Macrophages Delays Cutaneous T-Cell Lymphoma Development In Vivo. J. Investig. Dermatol. 2014, 134, 2814–2822. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenei, V.; Muszka, Z.; Stigelmayer, Á.; Debreceni, Z.; Bácsi, A.; Mázló, A.; Koncz, G. Anthracycline Treatments and the Presence of Tumor Cells Synergistically Modify the Composition of Macrophage Subpopulations in the Co-Culture System. Int. J. Mol. Sci. 2025, 26, 9202. https://doi.org/10.3390/ijms26189202
Jenei V, Muszka Z, Stigelmayer Á, Debreceni Z, Bácsi A, Mázló A, Koncz G. Anthracycline Treatments and the Presence of Tumor Cells Synergistically Modify the Composition of Macrophage Subpopulations in the Co-Culture System. International Journal of Molecular Sciences. 2025; 26(18):9202. https://doi.org/10.3390/ijms26189202
Chicago/Turabian StyleJenei, Viktória, Zsuzsa Muszka, Ádám Stigelmayer, Zsuzsanna Debreceni, Attila Bácsi, Anett Mázló, and Gábor Koncz. 2025. "Anthracycline Treatments and the Presence of Tumor Cells Synergistically Modify the Composition of Macrophage Subpopulations in the Co-Culture System" International Journal of Molecular Sciences 26, no. 18: 9202. https://doi.org/10.3390/ijms26189202
APA StyleJenei, V., Muszka, Z., Stigelmayer, Á., Debreceni, Z., Bácsi, A., Mázló, A., & Koncz, G. (2025). Anthracycline Treatments and the Presence of Tumor Cells Synergistically Modify the Composition of Macrophage Subpopulations in the Co-Culture System. International Journal of Molecular Sciences, 26(18), 9202. https://doi.org/10.3390/ijms26189202





