From Early Diagnoses to New Treatments for Liver, Pancreatic, Gastric, and Colorectal Cancers Using Carbon Nanotubes: New Chances Still Underexplored
Abstract
1. Introduction
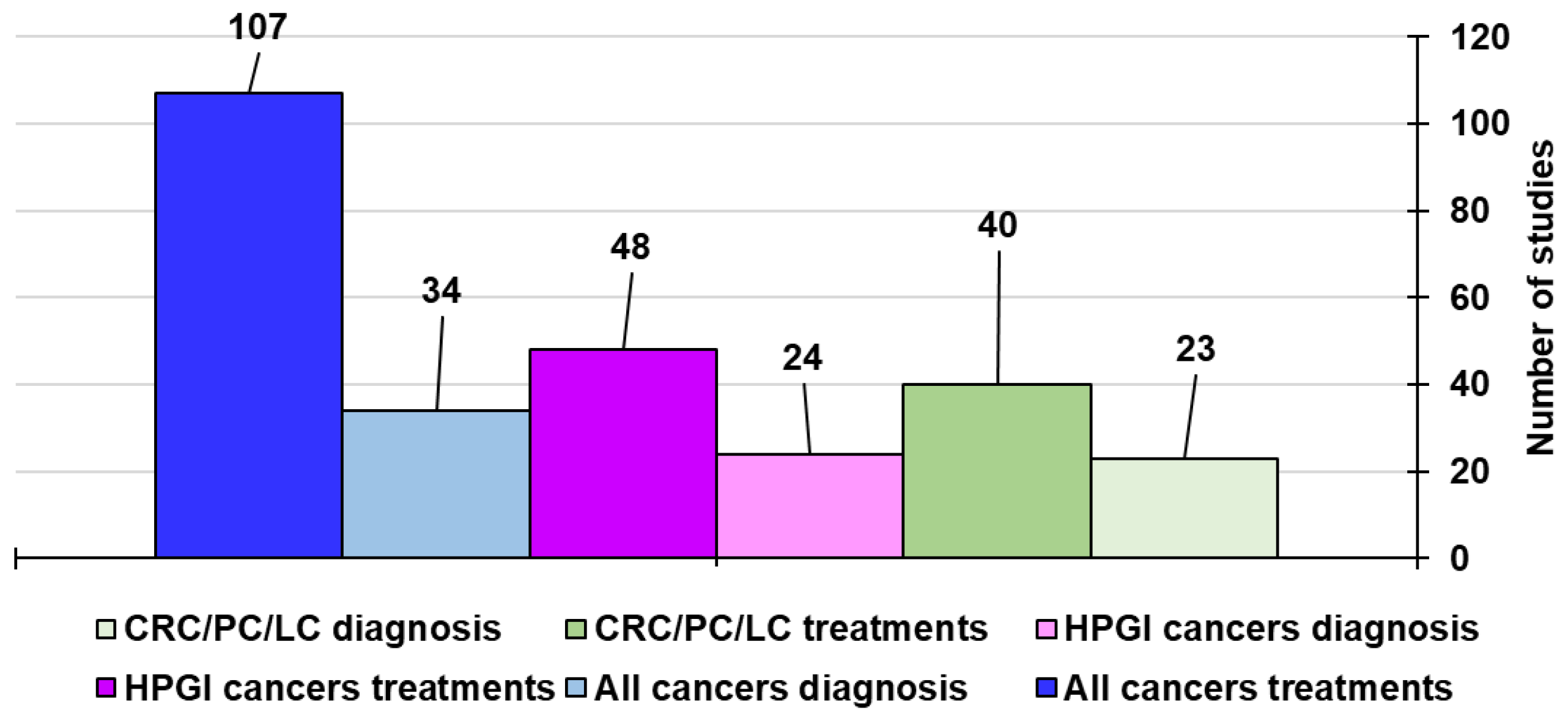
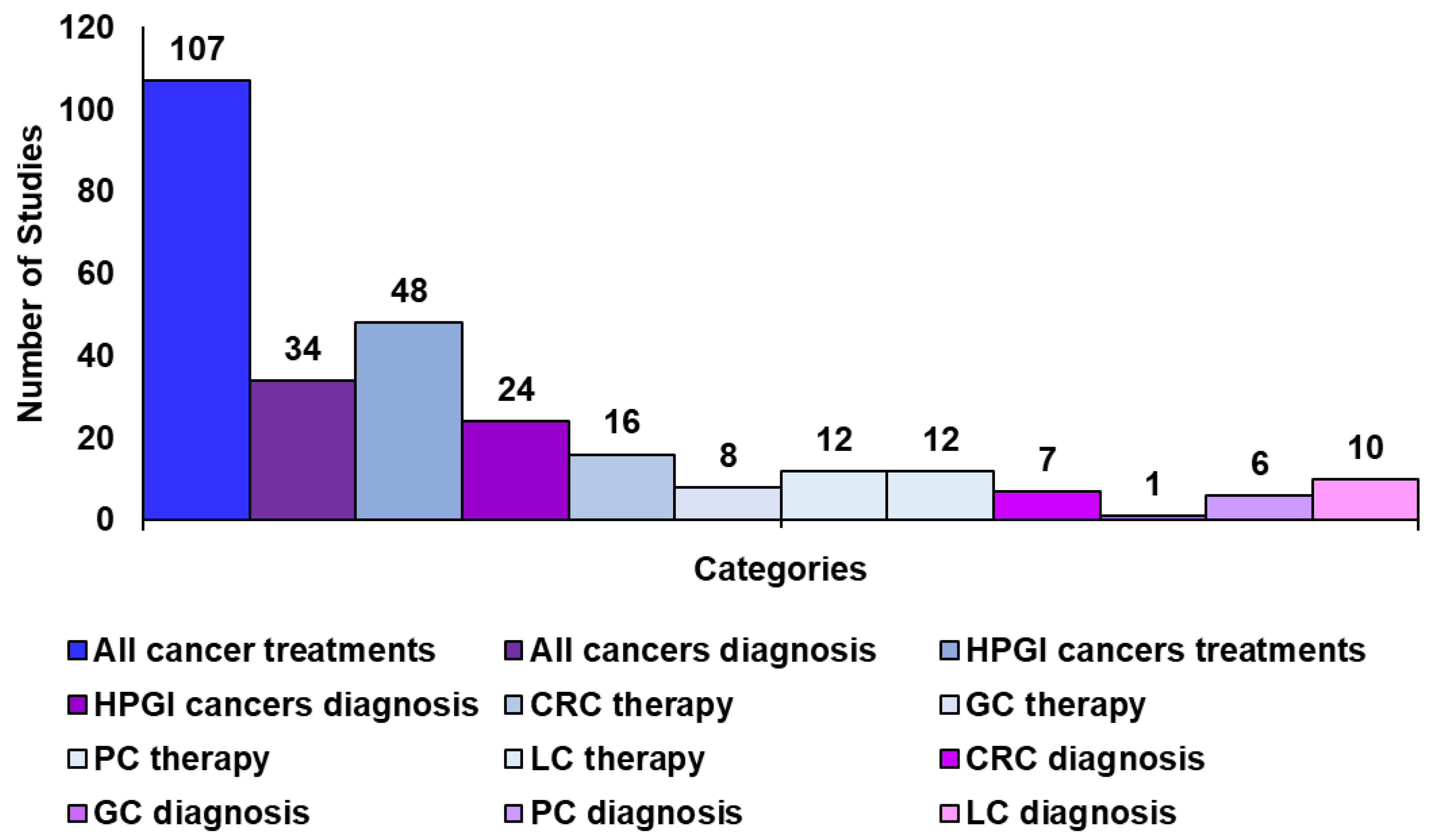
Methodology to Create This Review and Related Tables
2. Nanotechnology for Improved Cancer Treatment and Diagnosis
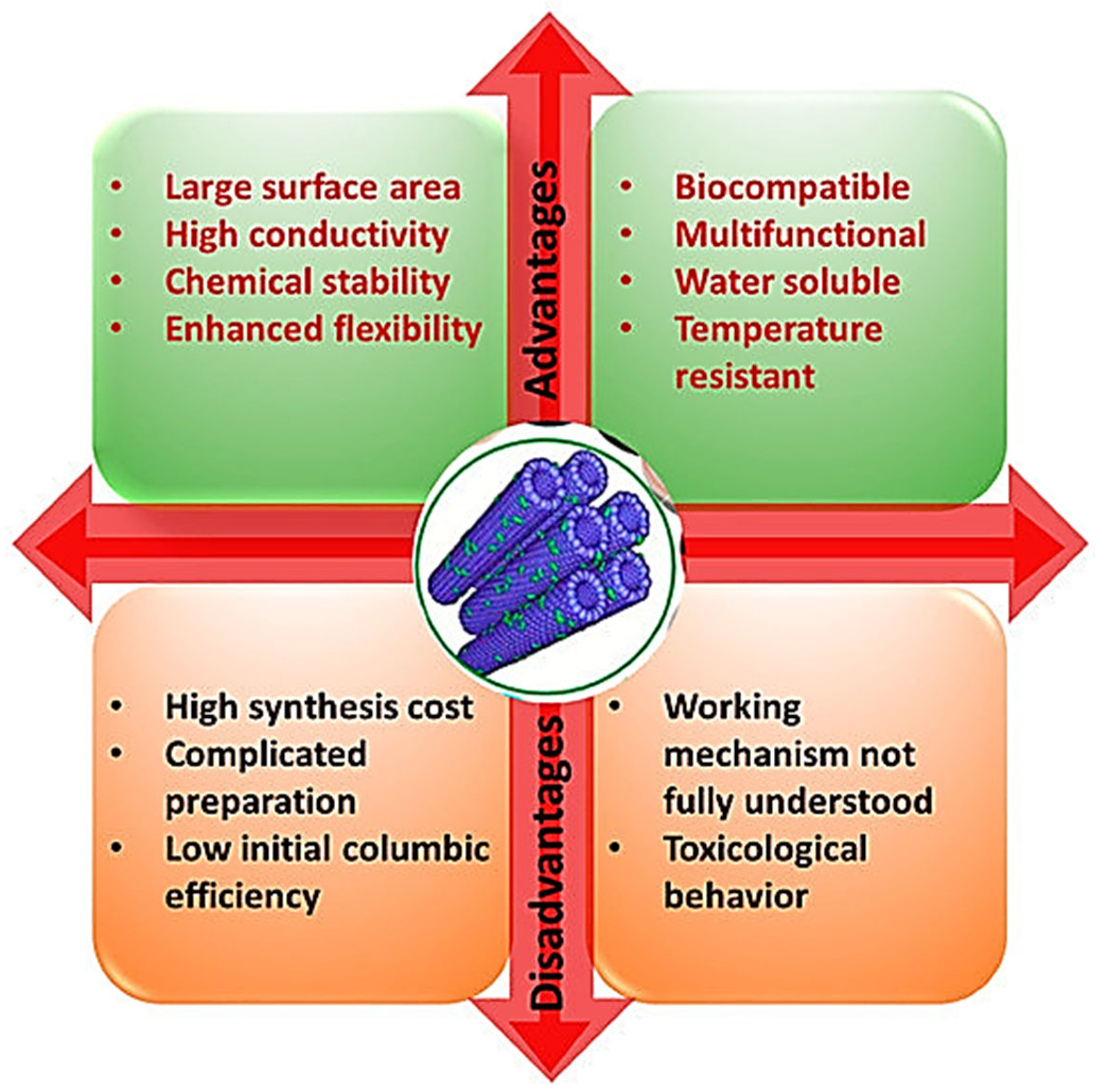
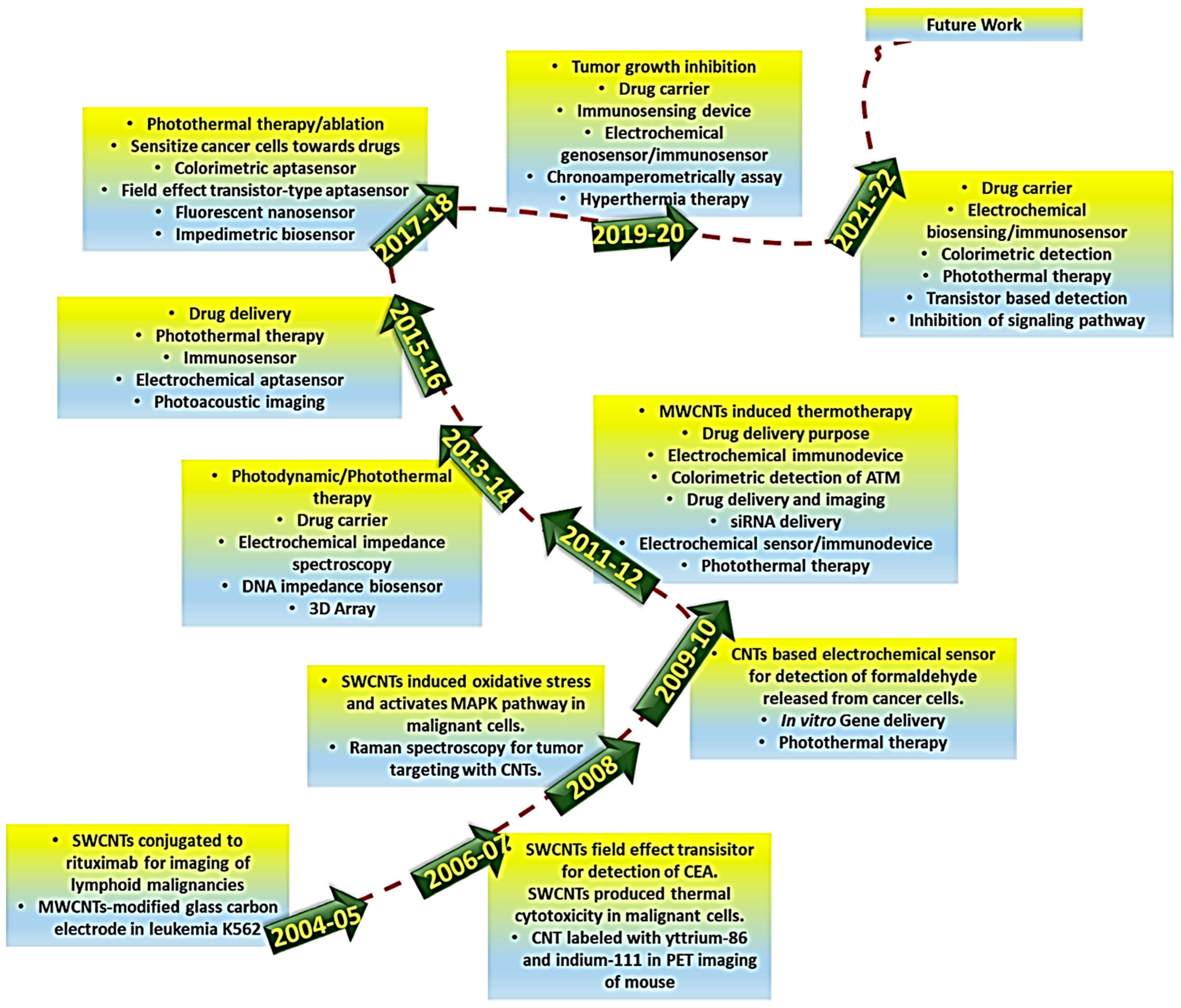
3. Carbon Nanotubes for Anticancer Therapy and Cancer Diagnosis
4. Hepatopancreatic Cancer Therapy and Diagnosis Using CNTs
4.1. Carbon Nanotubes for Pancreatic and Hepatocellular Cancers Diagnosis
4.1.1. Carbon Nanotubes for Pancreatic Cancer Diagnosis
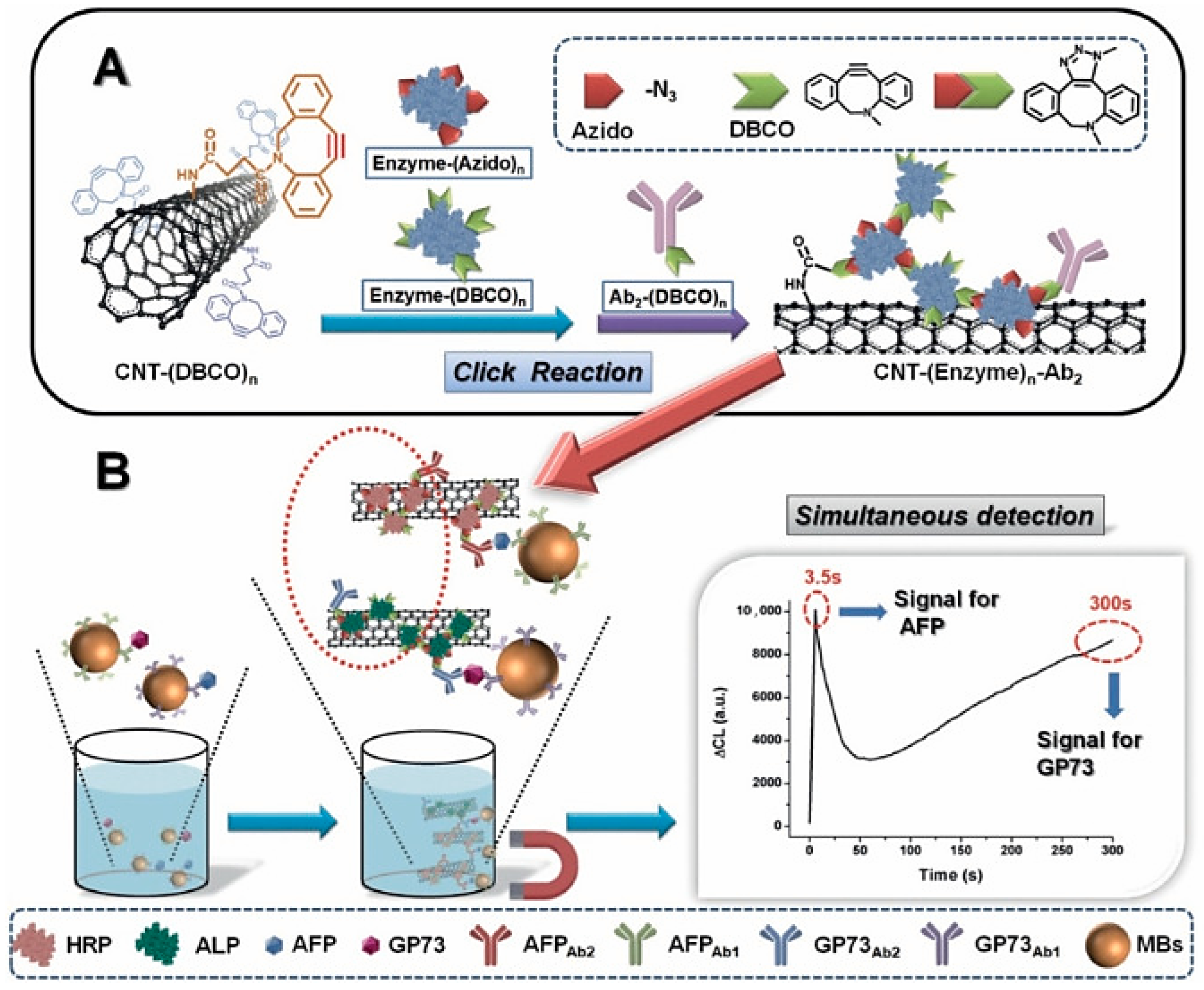
4.1.2. Carbon Nanotubes for Liver Cancer Diagnosis
4.1.3. Discussion on the Case Studies Previously Reported
4.2. Pancreatic Cancer Therapy
4.3. Liver Cancer Therapy
4.4. Discussion on the Case Studies Previously Reported
5. Carbon Nanotubes for Gastric and Colorectal Cancer Therapy and Diagnosis
5.1. Carbon Nanotubes for Gastric and Colorectal Cancer Diagnosis
5.2. Carbon Nanotubes for Gastric and Colorectal Cancer Therapy
5.3. Discussion on the Case Studies Previously Reported
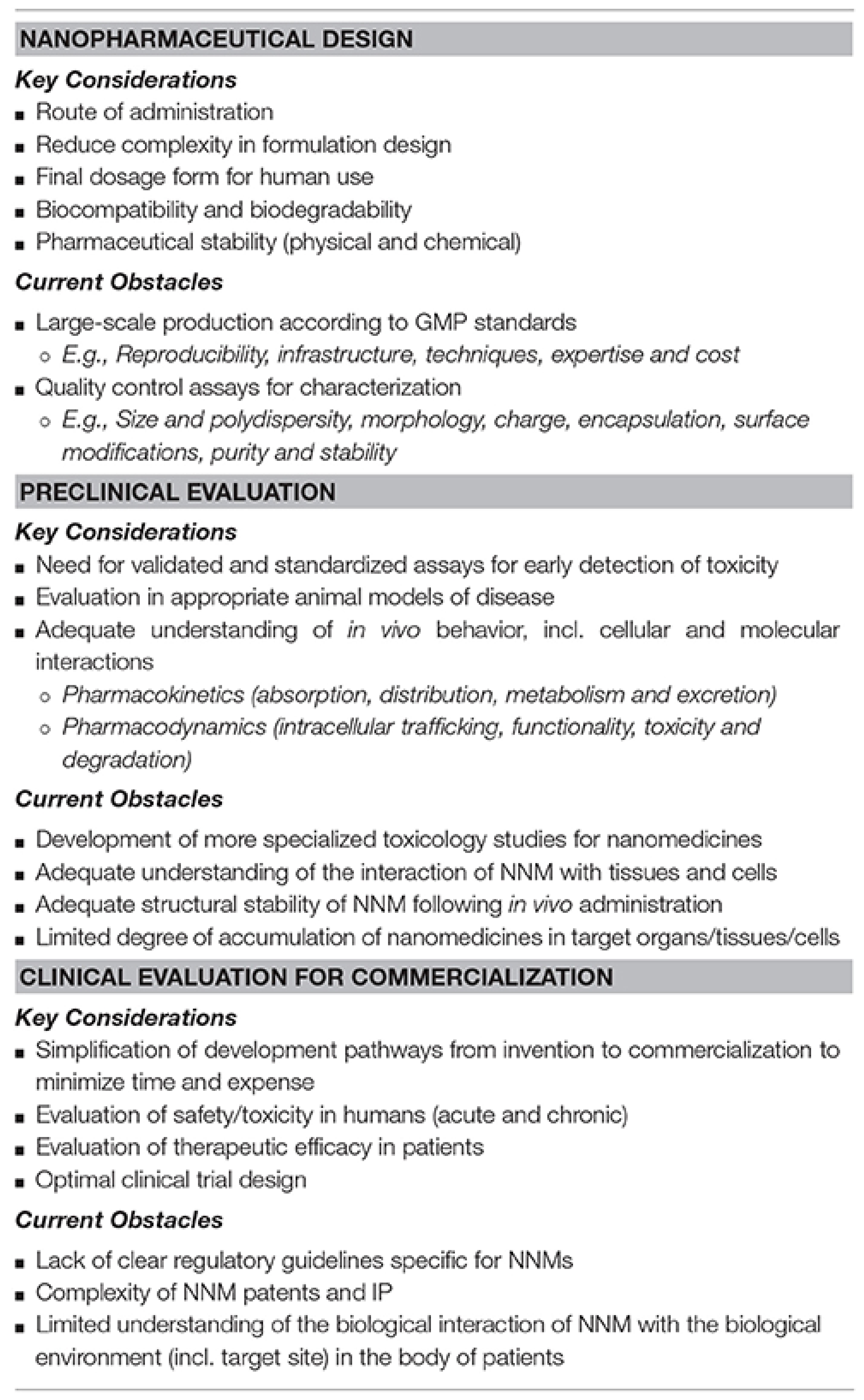
6. Main Factors Which Hamper CNTs Translation in Clinical Practice
6.1. Approaches to Reduce Possible CNT Toxicity
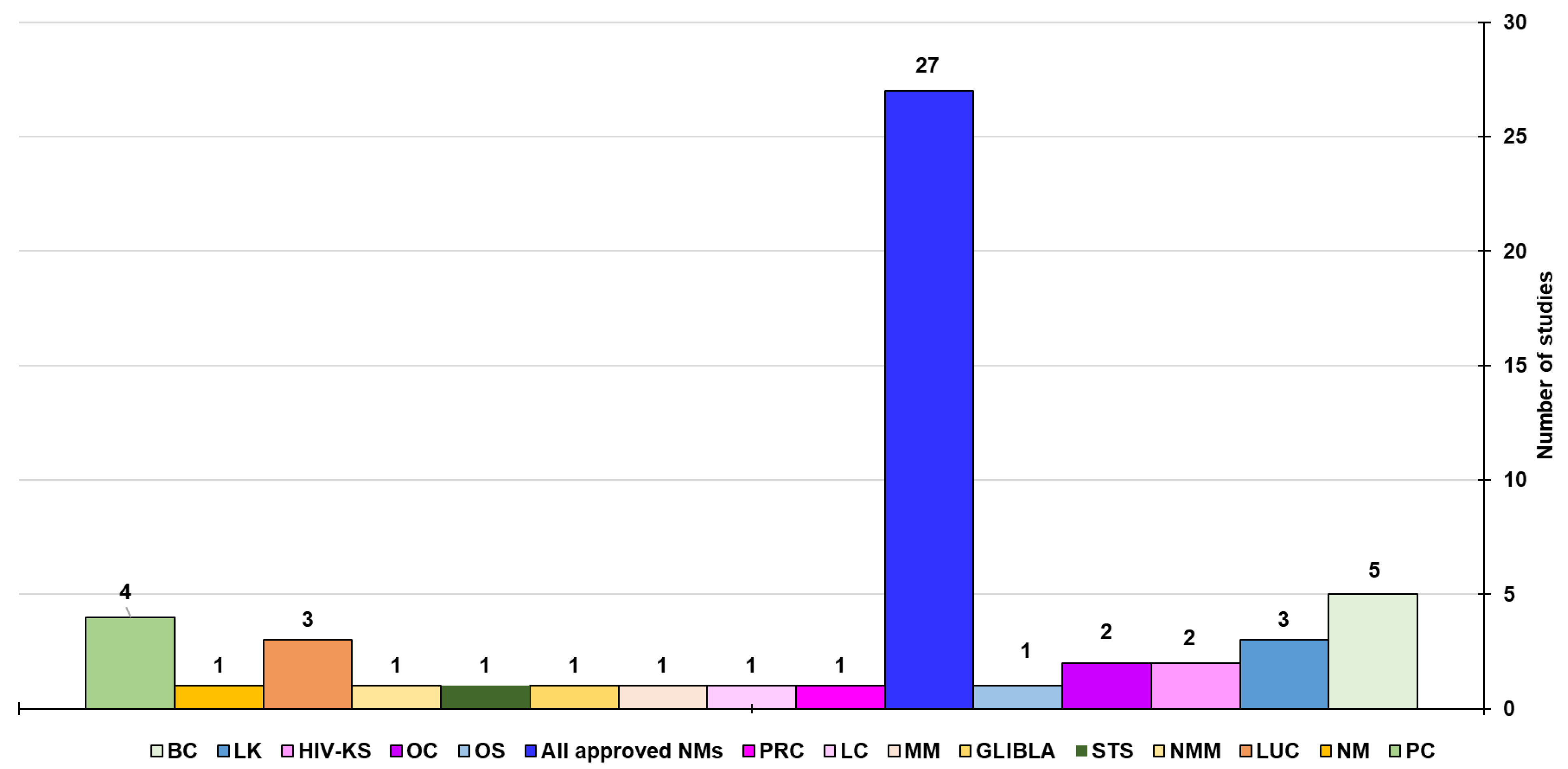
6.2. CNTs Technologies Closest to the Clinical Application
7. Authors Considerations
8. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Kumar, S. Cancer Targeting and Diagnosis: Recent Trends with Carbon Nanotubes. Nanomaterials 2022, 12, 2283. [Google Scholar] [CrossRef] [PubMed]
- Klochkov, S.G.; Neganova, M.E.; Nikolenko, V.N.; Chen, K.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Implications of Nanotechnology for the Treatment of Cancer: Recent Advances. Semin. Cancer Biol. 2021, 69, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cao, X.; Li, M.; Su, Y.; Li, H.; Xie, M.; Zhang, Z.; Gao, H.; Xu, X.; Han, Y.; et al. A TRAIL-Delivered Lipoprotein-Bioinspired Nanovector Engineering Stem Cell-Based Platform for Inhibition of Lung Metastasis of Melanoma. Theranostics 2019, 9, 2984–2998. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Alfei, S.; Reggio, C.; Zuccari, G. Carbon Nanotubes as Excellent Adjuvants for Anticancer Therapeutics and Cancer Diagnosis: A Plethora of Laboratory Studies Versus Few Clinical Trials. Cells 2025, 14, 1052. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer Potential of Alkaloids: A Key Emphasis to Colchicine, Vinblastine, Vincristine, Vindesine, Vinorelbine and Vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Soper, S.A.; Brown, K.; Ellington, A.; Frazier, B.; Garcia-Manero, G.; Gau, V.; Gutman, S.I.; Hayes, D.F.; Korte, B.; Landers, J.L.; et al. Point-of-Care Biosensor Systems for Cancer Diagnostics/Prognostics. Biosens. Bioelectron. 2006, 21, 1932–1942. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- WHO. Global Burden of Gastrointestinal Cancer. Available online: https://gco.iarc.who.int/stories/gastro-intestinal/en#slide1 (accessed on 28 May 2025).
- Araghi, M.; Arnold, M.; Rutherford, M.J.; Guren, M.G.; Cabasag, C.J.; Bardot, A.; Ferlay, J.; Tervonen, H.; Shack, L.; Woods, R.R.; et al. Colon and Rectal Cancer Survival in Seven High-Income Countries 2010-2014: Variation by Age and Stage at Diagnosis (the ICBP SURVMARK-2 Project). Gut 2021, 70, 114–126. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Chowdhury, S.; Zhang, M.; Bajaj, V.; Dhir, M.; Are, C. Trends in the Global Incidence of Pancreatic Cancer and a Brief Review of Its Histologic and Molecular Subtypes. J. Gastrointest. Cancer 2025, 56, 71. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and Survival Analysis of Patients with Pancreatic Cancer: Retrospective Experience of a Single Institution. World J. Surg. Oncol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49, Erratum in CA Cancer J. Clin. 2024, 74, 203. [Google Scholar] [CrossRef]
- Ferlay, J.; Laversanne, M.; Ervik, M. Global Cancer Observatory: Cancer Tomorrow (Version 1.1); International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/tomorrow (accessed on 28 May 2025).
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global Trends in Hepatocellular Carcinoma Epidemiology: Implications for Screening, Prevention and Therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Toh, M.R.; Wong, E.Y.T.; Wong, S.H.; Ng, A.W.T.; Loo, L.H.; Chow, P.K.H.; Ngeow, J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology 2023, 164, 766–782. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Wang, J.J.; Luu, M.; Noureddin, M.; Kosari, K.; Agopian, V.G.; Rich, N.E.; Lu, S.C.; Nissen, N.N.; Singal, A.G.; et al. The Mortality and Overall Survival Trends of Primary Liver Cancer in the United States. J. Natl. Cancer Inst. 2021, 113, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and Limitations in Traditional Anti-Cancer Therapies: A Comprehensive Review of Surgery, Chemotherapy, Radiation Therapy, and Hormonal Therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Sidoti, A. Quali Sono i 5 Tumori Più Mortali? Available online: https://www.salutelab.it/quali-sono-i-5-tumori-piu-mortali/ (accessed on 21 July 2025).
- Ganesh, K.; Massagué, J. Targeting Metastatic Cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 1782. [Google Scholar] [CrossRef]
- Suzuki, H.; Fujiwara, N.; Singal, A.G.; Baumert, T.F.; Chung, R.T.; Kawaguchi, T.; Hoshida, Y. Prevention of Liver Cancer in the Era of Next-Generation Antivirals and Obesity Epidemic. Hepatology 2025. [Google Scholar] [CrossRef]
- Alharbi, W. Advancement and Recent Trends in Seeking Less Toxic and More Active Anti-Cancer Drugs: Insights into Thiourea Based Molecules. Main Group. Chem. 2022, 21, 885–901. [Google Scholar] [CrossRef]
- Teng, L.; Bi, Y.; Xing, X.; Yao, G. Nano-Oncology Revisited: Insights on Precise Therapeutic Advances and Challenges in Tumor. Fundam. Res. 2025; in press. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y.; et al. Nanoparticle-Based Medicines in Clinical Cancer Therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Rajeshkumar, S. Eco-Friendly Bio-Nanocomposites: Pioneering Sustainable Biomedical Advancements in Engineering. Discov. Nano 2024, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Draviana, H.T.; Fitriannisa, I.; Khafid, M.; Krisnawati, D.I.; Widodo; Lai, C.H.; Fan, Y.J.; Kuo, T.R. Size and Charge Effects of Metal Nanoclusters on Antibacterial Mechanisms. J. Nanobiotechnol. 2023, 21, 428. [Google Scholar] [CrossRef]
- Ajith, S.; Almomani, F.; Elhissi, A.; Husseini, G.A. Nanoparticle-Based Materials in Anticancer Drug Delivery: Current and Future Prospects. Heliyon 2023, 9, e21227. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The Applications of Carbon Nanotubes in the Diagnosis and Treatment of Lung Cancer: A Critical Review. Int. J. Nanomed. 2020, 15, 7063–7078. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Shakibaie, M.; Hajighasemi, E.; Adeli-Sardou, M.; Doostmohammadi, M.; Forootanfar, H. Antimicrobial and Anti-Biofilm Activities of Bi Subnitrate and BiNPs Produced by Delftia Sp. SFG against Clinical Isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. IET Nanobiotechnol. 2019, 13, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.N.; Al-Rawi, K.F.; Mohammed, A.M. Kinetic Evaluation and Study of Gold-Based Nanoparticles and Multi-Walled Carbon Nanotubes as an Alkaline Phosphatase Inhibitor in Serum and Pure Form. Carbon Lett. 2023, 33, 1217–1229. [Google Scholar] [CrossRef]
- Faiad Naief, M.; Mishaal Mohammed, A.; Khalaf, Y.H. Kinetic and Thermodynamic Study of ALP Enzyme in the Presence and Absence MWCNTs and Pt-NPs Nanocomposites. Results Chem. 2023, 5, 100844. [Google Scholar] [CrossRef]
- Naief, M.F.; Khalaf, Y.H.; Mohammed, A.M. Novel Photothermal Therapy Using Multi-Walled Carbon Nanotubes and Platinum Nanocomposite for Human Prostate Cancer PC3 Cell Line. J. Organomet. Chem. 2022, 975, 122422. [Google Scholar] [CrossRef]
- Naief, M.F.; Mohammed, S.N.; Ahmed, Y.N.; Mohammed, A.M. Synthesis and Characterisation of MWCNTCOOH and Investigation of Its Potential as Gas Sensor. Inorg. Chem. Commun. 2023, 157, 111338. [Google Scholar] [CrossRef]
- Abdullah, O.H.; Mohammed, A.M. Green Synthesis of Aunps from the Leaf Extract of Prosopis Farcta for Antibacterial and Anti-Cancer Applications. Dig. J. Nanomater. Biostruct. 2020, 15, 943–951. [Google Scholar] [CrossRef]
- Nasir, G.A.; Najm, M.A.; Hussein, A.L. The Effect of Silver Nanoparticles on Braf Gene Expression. Syst. Rev. Pharm. 2020, 11, 570–575. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Saud, W.M.; Ali, M.M. Green Synthesis of Fe2o3 Nanoparticles Using Olea Europaea Leaf Extract and Their Antibacterial Activity. Dig. J. Nanomater. Biostruct. 2020, 15, 175–183. [Google Scholar] [CrossRef]
- Jwied, D.H.; Nayef, U.M.; Mutlak, F.A.H. Synthesis of C:Se Nanoparticles Ablated on Porous Silicon for Sensing NO2 and NH3 Gases. Optik 2021, 241, 167013. [Google Scholar] [CrossRef]
- Latif, Z.A.A.; Mohammed, A.M.; Abbass, N.M. Synthesis and Characterization of Polymer Nanocomposites from Methyl Acrylate and Metal Chloride and Their Application. Polym. Bull. 2020, 77, 5879–5898. [Google Scholar] [CrossRef]
- Altaee, M.F.; Yaaqoob, L.A.; Kamona, Z.K. Evaluation of the Biological Activity of Nickel Oxide Nanoparticles as Antibacterial and Anticancer Agents. Iraqi J. Sci. 2020, 61, 2888–2896. [Google Scholar] [CrossRef]
- Ghdeeb, N.J.; AbdulMajeed, A.M.; Mohammed, A.H. Role of Extracted Nano-Metal Oxides from Factory Wastes in Medical Applications. Iraqi J. Sci. 2023, 64, 1704–1716. [Google Scholar] [CrossRef]
- Al-Ali, A.A.A.; Alsalami, K.A.S.; Athbi, A.M. Cytotoxic Effects of CeO2 NPs and β-Carotene and Their Ability to Induce Apoptosis in Human Breast Normal and Cancer Cell Lines. Iraqi J. Sci. 2022, 63, 923–937. [Google Scholar] [CrossRef]
- Salih, A.M.A.; Abdo, G.M. Water Conservation in the Arab Region. In Water, Energy and Food Sustainability in the Middle East: The Sustainability Triangle; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Azzazy, H.M.E.; Mansour, M.M.H.; Kazmierczak, S.C. Nanodiagnostics: A New Frontier for Clinical Laboratory Medicine. Clin. Chem. 2006, 52, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, G.C. Antimicrobial Nanotubes: From Synthesis and Promising Antimicrobial Upshots to Unanticipated Toxicities, Strategies to Limit Them, and Regulatory Issues. Nanomaterials 2025, 15, 633. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Jiang, Q.; Zhu, P.; Leu, K.; Zhang, R. Controlled Synthesis, Properties, and Applications of Ultralong Carbon Nanotubes. Nanoscale Adv. 2024, 6, 4504–4521. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, G.C. Nanotubes: Carbon-Based Fibers and Bacterial Nano-Conduits Both Arousing a Global Interest and Conflicting Opinions. Fibers 2022, 10, 75. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects. J. Xenobiot. 2025, 15, 76. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706, Erratum in Int. J. Nanomed. 2021, 16, 7283–7284. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.J.; Xie, Z.B.; Gao, Y.; Zhang, Y.F.; Yao, L.; Fu, D.L. LyP-1-FMWNTs Enhanced Targeted Delivery of MBD1siRNA to Pancreatic Cancer Cells. J. Cell Mol. Med. 2020, 24, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Zaboli, M.; Raissi, H.; Zaboli, M. Investigation of Nanotubes as the Smart Carriers for Targeted Delivery of Mercaptopurine Anticancer Drug. J. Biomol. Struct. Dyn. 2022, 40, 4579–4592. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; ER, S.; Mobashar, A.; Gelen, S.S.; Rahdar, A.; Ebrahimi, N.; Hosseinikhah, S.M.; Bilal, M.; Kyzas, G.Z. Aptamer-Conjugated Carbon-Based Nanomaterials for Cancer and Bacteria Theranostics: A Review. Chem. Biol. Interact. 2022, 361, 109964. [Google Scholar] [CrossRef]
- Asadipour, K.; Banerjee, N.; Cuffee, J.; Perry, K.; Brown, S.; Banerjee, A.; Armstrong, E.; Beebe, S.; Banerjee, H. Studying the Role of Novel Carbon Nano Tubes as a Therapeutic Agent to Treat Triple Negative Breast Cancer (TNBC)—An In Vitro and In Vivo Study. J. Cancer Res. Updates 2024, 13, 37–41. [Google Scholar] [CrossRef]
- Luo, X.; Wang, H.; Ji, D. Carbon Nanotubes (CNT)-Loaded Ginsenosides Rb3 Suppresses the PD-1/PD-L1 Pathway in Triple-Negative Breast Cancer. Aging 2021, 13, 17177–17189. [Google Scholar] [CrossRef]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2021, 19, 229–237. [Google Scholar] [CrossRef]
- Bura, C.; Mocan, T.; Grapa, C.; Mocan, L. Carbon Nanotubes-Based Assays for Cancer Detection and Screening. Pharmaceutics 2022, 14, 781. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Carbon Nanomaterials: 30 Years of Research in Agroecosystems. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- de Almeida Barcelos, K.; Garg, J.; Ferreira Soares, D.C.; de Barros, A.L.B.; Zhao, Y.; Alisaraie, L. Recent Advances in the Applications of CNT-Based Nanomaterials in Pharmaceutical Nanotechnology and Biomedical Engineering. J. Drug Deliv. Sci. Technol. 2023, 87, 104834. [Google Scholar] [CrossRef]
- Su, Q.; Gan, L.; Liu, J.; Yang, X. Carbon Dots Derived from Pea for Specifically Binding with Cryptococcus neoformans. Anal. Biochem. 2020, 589, 113476. [Google Scholar] [CrossRef]
- Salari, S.; Jafari, S.M. Application of Nanofluids for Thermal Processing of Food Products. Trends Food Sci. Technol. 2020, 97, 100–113. [Google Scholar] [CrossRef]
- Creative Commons CC BY 4.0 License. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 21 July 2025).
- Joshi, M.; Kumar, P.; Kumar, R.; Sharma, G.; Singh, B.; Katare, O.P.; Raza, K. Aminated Carbon-Based “Cargo Vehicles” for Improved Delivery of Methotrexate to Breast Cancer Cells. Mater. Sci. Eng. C 2017, 75, 1376–1388. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Phototherapy Combined with Carbon Nanomaterials (1d and 2d) and Their Applications in Cancer Therapy. Materials 2020, 13, 4830. [Google Scholar] [CrossRef]
- Alfei, S. Cationic Materials for Gene Therapy: A Look Back to the Birth and Development of 2,2-Bis-(Hydroxymethyl)Propanoic Acid-Based Dendrimer Scaffolds. Int. J. Mol. Sci. 2023, 24, 16006. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S. Synthesis and Characterization of Polyester-Based Dendrimers Containing Peripheral Arginine or Mixed Amino Acids as Potential Vectors for Gene and Drug Delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and Characterization of Versatile Amphiphilic Dendrimers Peripherally Decorated with Positively Charged Amino Acids. Polym. Int. 2018, 67, 1572–1584. [Google Scholar] [CrossRef]
- Faraji Dizaji, B.; Khoshbakht, S.; Farboudi, A.; Azarbaijan, M.H.; Irani, M. Far-Reaching Advances in the Role of Carbon Nanotubes in Cancer Therapy. Life Sci. 2020, 257, 118059. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological Responses Triggered by Photothermal Therapy with Carbon Nanotubes in Combination with Anti-CTLA-4 Therapy to Inhibit Cancer Metastasis. Adv. Mater. 2014, 26, 8154–8162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Qi, Z.; Huang, X.; Li, M.; Liu, S.; Yan, Y.; Gao, M. Application of Carbon Nanomaterials to Enhancing Tumor Immunotherapy: Current Advances and Prospects. Int. J. Nanomed. 2024, 19, 10899–10915. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; Di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Patients at Risk of Gastric Adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Javed, Z.; Herrera-Bravo, J.; Sadia, H.; Anum, F.; Raza, S.; Tahir, A.; Shahwani, M.N.; Sharifi-Rad, J.; Calina, D.; et al. Biosensing Chips for Cancer Diagnosis and Treatment: A New Wave towards Clinical Innovation. Cancer Cell Int. 2022, 22, 354. [Google Scholar] [CrossRef]
- Chaudhry, G.e.S.; Akim, A.M.; Safdar, N.; Yasmin, A.; Begum, S.; Sung, Y.Y.; Muhammad, T.S.T. Cancer and Disease Diagnosis—Biosensor as Potential Diagnostic Tool for Biomarker Detection. J. Adv. Pharm. Technol. Res. 2022, 13, 243–247. [Google Scholar] [CrossRef]
- Ji, D.; Xu, N.; Liu, Z.; Shi, Z.; Low, S.S.; Liu, J.; Cheng, C.; Zhu, J.; Zhang, T.; Xu, H.; et al. Smartphone-Based Differential Pulse Amperometry System for Real-Time Monitoring of Levodopa with Carbon Nanotubes and Gold Nanoparticles Modified Screen-Printing Electrodes. Biosens. Bioelectron. 2019, 129, 216–223. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-Based Electrochemical System with Multi-Walled Carbon Nanotubes/Thionine/Gold Nanoparticles Modified Screen-Printed Immunosensor for Cancer Antigen 125 Detection. Microchem. J. 2022, 174, 107044. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Feng, J.; Wang, P.; Dong, Y. The Label-Free Immunosensor Based on Rhodium@palladium Nanodendrites/Sulfo Group Functionalized Multi-Walled Carbon Nanotubes for the Sensitive Analysis of Carcino Embryonic Antigen. Anal. Chim. Acta 2018, 1007, 61–70. [Google Scholar] [CrossRef]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Kumar Jana, S. Bio-Nanocomposite Based Highly Sensitive and Label-Free Electrochemical Immunosensor for Endometriosis Diagnostics Application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Malik, S.; Paladiya, R.; Ahsan, S.; Wasim, H.; Bharadwaj, H.R.; Goel, A.; Jaan, A.; Hayat, U.; Hasan, F.; et al. Advances in Non-Invasive Screening Methods for Gastrointestinal Cancers: How Continued Innovation Has Revolutionized Early Cancer Detection. Cancers 2025, 17, 1085. [Google Scholar] [CrossRef] [PubMed]
- de la Zerda, A.; Gambhir, S.S. Keeping Tabs on Nanocarriers. Nat. Nanotechnol. 2007, 2, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Sandini, M.; Negreros-Osuna, A.A.; Qadan, M.; Hank, T.; Patino, M.; Ferrone, C.R.; Warshaw, A.L.; Lillemoe, K.D.; Sahani, D.; Castillo, C.F. del Main Pancreatic Duct to Parenchymal Thickness Ratio at Preoperative Imaging Is Associated with Overall Survival in Upfront Resected Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M. The Role of Endoscopic Ultrasound and Associated Methods (Elastography, Contrast Enhancement) in the Diagnosis and Assessment of Resectability of Pancreatic Cancer. In Clinical Pancreatology for Practising Gastroenterologists and Surgeons, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Capula, M.; Mantini, G.; Funel, N.; Giovannetti, E. New Avenues in Pancreatic Cancer: Exploiting MicroRNAs as Predictive Biomarkers and New Approaches to Target Aberrant Metabolism. Expert Rev. Clin. Pharmacol. 2019, 12, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic Developments in Pancreatic Cancer: Current and Future Perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Fabbri, C.; Luigiano, C.; Lisotti, A.; Cennamo, V.; Virgilio, C.; Caletti, G.; Fusaroli, P. Endoscopic Ultrasound-Guided Treatments: Are We Getting Evidence Based—A Systematic Review. World J. Gastroenterol. 2014, 20, 8424–8448. [Google Scholar] [CrossRef]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A New Era of Cancer Treatment: Carbon Nanotubes as Drug Delivery Tools. Int. J. Nanomed. 2011, 6, 2963–2979. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, R.; Dong, C.; Jiang, T.; Tian, Y.; Yang, Q.; Yi, W.; Hou, J. A Simple MWCNTs@paper Biosensor for CA19-9 Detection and Its Long-Term Preservation by Vacuum Freeze Drying. Int. J. Biol. Macromol. 2020, 144, 995–1003. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F. CA 19-9 Pancreatic Tumor Marker Fluorescence Immunosensing Detection via Immobilized Carbon Quantum Dots Conjugated Gold Nanocomposite. Int. J. Mol. Sci. 2018, 19, 1162, Erratum in Int. J. Mol. Sci. 2025, 26, 1406. [Google Scholar] [CrossRef]
- Murakami, M.; Nagai, Y.; Tenjin, A.; Tanaka, Y. Proposed Cut-off Value of CA19-9 for Detecting Pancreatic Cancer in Patients with Diabetes: A Case-Control Study. Endocr. J. 2018, 65, 639–643. [Google Scholar] [CrossRef]
- Thapa, A.; Soares, A.C.; Soares, J.C.; Awan, I.T.; Volpati, D.; Melendez, M.E.; Fregnani, J.H.T.G.; Carvalho, A.L.; Oliveira, O.N. Carbon Nanotube Matrix for Highly Sensitive Biosensors to Detect Pancreatic Cancer Biomarker CA19-9. ACS Appl. Mater. Interfaces 2017, 9, 25878–25886. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, A.; Gao, F.; Weng, S.; Zhong, G.; Liu, J.; Lin, X.; Lin, J.-h.; Chen, X. Coupling Technique of Random Amplified Polymorphic DNA and Nanoelectrochemical Sensor for Mapping Pancreatic Cancer Genetic Fingerprint. Int. J. Nanomed. 2011, 6, 2933–2939. [Google Scholar] [CrossRef][Green Version]
- Lu, G.H.; Shang, W.T.; Deng, H.; Han, Z.Y.; Hu, M.; Liang, X.Y.; Fang, C.H.; Zhu, X.H.; Fan, Y.F.; Tian, J. Targeting Carbon Nanotubes Based on IGF-1R for Photothermal Therapy of Orthotopic Pancreatic Cancer Guided by Optical Imaging. Biomaterials 2019, 195, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Janas, D.; Eftekhari, A.; Zare, N. Application of Carbon Nanotubes in Sensing/Monitoring of Pancreas and Liver Cancer. Chemosphere 2022, 302, 134826. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Z.; Chai, Y.; Song, Z.; Zhuo, Y.; Su, H.; Liu, S.; Wang, D.; Yuan, R. Simultaneous Electrochemical Immunoassay of Three Liver Cancer Biomarkers Using Distinguishable Redox Probes as Signal Tags and Gold Nanoparticles Coated Carbon Nanotubes as Signal Enhancers. Chem. Commun. 2012, 48, 537–539. [Google Scholar] [CrossRef]
- Hu, D.; Yang, L.; Deng, S.; Hao, Y.; Zhang, K.; Wang, X.; Liu, Y.; Liu, H.; Chen, Y.; Xie, M. Development of Nanosensor by Bioorthogonal Reaction for Multi-Detection of the Biomarkers of Hepatocellular Carcinoma. Sens. Actuators B Chem. 2021, 334, 129653. [Google Scholar] [CrossRef]
- Elsevier License Number 6040780373949. Available online: https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=5ffeb2de-69b2-41de-9545-9dad79a8076d (accessed on 21 July 2025).
- Kucukayan-Dogu, G.; Gozen, D.; Bitirim, V.; Akcali, K.C.; Bengu, E. A New Tool for Differentiating Hepatocellular Cancer Cells: Patterned Carbon Nanotube Arrays. Appl. Surf. Sci. 2015, 351, 27–32. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Akhond, M.; Ghorbankhani, G.A.; Absalan, G. Electrochemical Sensing of Hydrogen Peroxide Using a Glassy Carbon Electrode Modified with Multiwalled Carbon Nanotubes and Zein Nanoparticle Composites: Application to HepG2 Cancer Cell Detection. Microchim. Acta 2020, 187, 105. [Google Scholar] [CrossRef]
- Rawashdeh1, I.; Al-Fandi, M.G.; Makableh, Y.; Harahsha, T. Developing a Nano-Biosensor for Early Detection of Pancreatic Cancer. Sens. Rev. 2021, 41, 93–100. [Google Scholar] [CrossRef]
- Bi, S.; Zhou, H.; Zhang, S. Multilayers Enzyme-Coated Carbon Nanotubes as Biolabel for Ultrasensitive Chemiluminescence Immunoassay of Cancer Biomarker. Biosens. Bioelectron. 2009, 24, 2961–2966. [Google Scholar] [CrossRef]
- Ou, C.; Yuan, R.; Chai, Y.; Tang, M.; Chai, R.; He, X. A Novel Amperometric Immunosensor Based on Layer-by-Layer Assembly of Gold Nanoparticles-Multi-Walled Carbon Nanotubes-Thionine Multilayer Films on Polyelectrolyte Surface. Anal. Chim. Acta 2007, 603, 205–213. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Jin, X.; Lu, H.; Shen, G.; Yu, R. Poly-L-Lysine/Hydroxyapatite/Carbon Nanotube Hybrid Nanocomposite Applied for Piezoelectric Immunoassay of Carbohydrate Antigen 19-9. Analyst 2008, 133, 184–190. [Google Scholar] [CrossRef]
- Lin, J.; He, C.; Zhang, L.; Zhang, S. Sensitive Amperometric Immunosensor for α-Fetoprotein Based on Carbon Nanotube/Gold Nanoparticle Doped Chitosan Film. Anal. Biochem. 2009, 384, 130–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, F.; Dan, W.; Fu, Y.; Liu, S. Construction of Carbon Nanotube Based Nanoarchitectures for Selective Impedimetric Detection of Cancer Cells in Whole Blood. Analyst 2014, 139, 5086–5092. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M.; Golbabaie, A.; Kasaeian, A. Carbon Nanotubes: A Review of Novel Strategies for Cancer Diagnosis and Treatment. Mater. Sci. Eng. C 2017, 76, 1289–1304. [Google Scholar] [CrossRef]
- Erkan, M.; Hausmann, S.; Michalski, C.W.; Fingerle, A.A.; Dobritz, M.; Kleeff, J.; Friess, H. The Role of Stroma in Pancreatic Cancer: Diagnostic and Therapeutic Implications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 454–467. [Google Scholar] [CrossRef]
- Erkan, M.; Michalski, C.W.; Rieder, S.; Reiser-Erkan, C.; Abiatari, I.; Kolb, A.; Giese, N.A.; Esposito, I.; Friess, H.; Kleeff, J. The Activated Stroma Index Is a Novel and Independent Prognostic Marker in Pancreatic Ductal Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2008, 6, 1155–1161. [Google Scholar] [CrossRef]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef]
- Mccarroll, P.R. The End of Hope—The Beginning; Fortress Press: Minneapolis, MN, USA, 2014. [Google Scholar]
- Vonlaufen, A.; Phillips, P.A.; Xu, Z.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Pancreatic Stellate Cells and Pancreatic Cancer Cells: An Unholy Alliance. Cancer Res. 2008, 68, 7707–7710. [Google Scholar] [CrossRef]
- Xu, Z.; Vonlaufen, A.; Phillips, P.A.; Fiala-Beer, E.; Zhang, X.; Yang, L.; Biankin, A.V.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; et al. Role of Pancreatic Stellate Cells in Pancreatic Cancer Metastasis. Am. J. Pathol. 2010, 177, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res. 2008, 68, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Zare, H. Carbon-Based Nanomaterials in Gene Therapy. OpenNano 2022, 7, 100062. [Google Scholar] [CrossRef]
- McCarroll, J.; Teo, J.; Boyer, C.; Goldstein, D.; Kavallaris, M.; Phillips, P.A. Potential Applications of Nanotechnology for the Diagnosis and Treatment of Pancreatic Cancer. Front. Physiol. 2014, 5 JAN, 2. [Google Scholar] [CrossRef]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Bele, C.; Orza, A.I.; Lucan, C.; Stiufiuc, R.; Manaila, I.; Iulia, F.; Dana, I.; et al. Selective Ex-Vivo Photothermal Ablation of Human Pancreatic Cancer with Albumin Functionalized Multiwalled Carbon Nanotubes. Int. J. Nanomed. 2011, 6, 915–928. [Google Scholar] [CrossRef]
- Karmakar, A.; Iancu, C.; Bartos, D.M.; Mahmood, M.W.; Ghosh, A.; Xu, Y.; Dervishi, E.; Collom, S.L.; Khodakovskaya, M.; Mustafa, T.; et al. Raman Spectroscopy as a Detection and Analysis Tool for in Vitro Specific Targeting of Pancreatic Cancer Cells by EGF-Conjugated, Single-Walled Carbon Nanotubes. J. Appl. Toxicol. 2012, 32, 365–375. [Google Scholar] [CrossRef]
- Mahmood, R.; Keeling, T.; Foster, S.A.; Hubbard, K.G. Did Irrigation Impact 20th Century Air Temperature in the High Plains Aquifer Region? Appl. Geogr. 2013, 38, 11–21, Erratum in Appl. Geogr. 2013, 40, 51. [Google Scholar] [CrossRef]
- Andreoli, E.; Suzuki, R.; Orbaek, A.W.; Bhutani, M.S.; Hauge, R.H.; Adams, W.; Fleming, J.B.; Barron, A.R. Preparation and Evaluation of Polyethyleneimine-Single Walled Carbon Nanotube Conjugates as Vectors for Pancreatic Cancer Treatment. J. Mater. Chem. B 2014, 2, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Matea, C.T.; Cojocaru, I.; Ilie, I.; Tabaran, F.A.; Zaharie, F.; Iancu, C.; Bartos, D.; Mocan, L. Photothermal Treatment of Human Pancreatic Cancer Using PEGylated Multi-Walled Carbon Nanotubes Induces Apoptosis by Triggering Mitochondrial Membrane Depolarization Mechanism. J. Cancer 2014, 5, 679–688. [Google Scholar] [CrossRef]
- Saeed, L.M.; Mahmood, M.; Pyrek, S.J.; Fahmi, T.; Xu, Y.; Mustafa, T.; Nima, Z.A.; Bratton, S.M.; Casciano, D.; Dervishi, E.; et al. Single-Walled Carbon Nanotube and Graphene Nanodelivery of Gambogic Acid Increases Its Cytotoxicity in Breast and Pancreatic Cancer Cells. J. Appl. Toxicol. 2014, 34, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Hu, R.; Yang, C.; Yoon, H.S.; Yong, K.T. Pancreatic Cancer Gene Therapy Using an SiRNA-Functionalized Single Walled Carbon Nanotubes (SWNTs) Nanoplex. Biomater. Sci. 2014, 2, 1244–1253. [Google Scholar] [CrossRef]
- Sobhani, Z.; Behnam, M.A.; Emami, F.; Dehghanian, A.; Jamhiri, I. Photothermal Therapy of Melanoma Tumor Using Multiwalled Carbon Nanotubes. Int. J. Nanomed. 2017, 12, 4509–4517. [Google Scholar] [CrossRef]
- Razzazan, A.; Atyabi, F.; Kazemi, B.; Dinarvand, R. In Vivo Drug Delivery of Gemcitabine with PEGylated Single-Walled Carbon Nanotubes. Mater. Sci. Eng. C 2016, 62, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, G.; Cherukuri, P.; Kingston, J.; Cognet, L.; Lemos, R.; Leeuw, T.K.; Gumbiner-Russo, L.; Weisman, R.B.; Powis, G. In Vivo Therapeutic Silencing of Hypoxia-Inducible Factor 1 Alpha (HIF-1α) Using Single-Walled Carbon Nanotubes Noncovalently Coated with SiRNA. Nano Res. 2009, 2, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Torazza, C.; Bacchetti, F.; Milanese, M.; Passalaqua, M.; Khaledizadeh, E.; Vernazza, S.; Domenicotti, C.; Marengo, B. TPP-Based Nanovesicles Kill MDR Neuroblastoma Cells and Induce Moderate ROS Increase, While Exert Low Toxicity To-Wards Primary Cell Cultures: An in Vitro Study. IJMS 2025, 26, 4991. [Google Scholar] [CrossRef]
- Marengo, B.; Monti, P.; Miele, M.; Menichini, P.; Ottaggio, L.; Foggetti, G.; Pulliero, A.; Izzotti, A.; Speciale, A.; Garbarino, O.; et al. Etoposide-Resistance in a Neuroblastoma Model Cell Line Is Associated with 13q14.3 Mono-Allelic Deletion and MiRNA-15a/16-1 down-Regulation. Sci. Rep. 2018, 8, 13762. [Google Scholar] [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-Mediated Antioxidant Response and Aerobic Metabolism: Two Crucial Factors Involved in Determining the Multi-Drug Resistance of High-Risk Neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef]
- Pan, B.; Cui, D.; Xu, P.; Ozkan, C.; Feng, G.; Ozkan, M.; Huang, T.; Chu, B.; Li, Q.; He, R.; et al. Synthesis and Characterization of Polyamidoamine Dendrimer-Coated Multi-Walled Carbon Nanotubes and Their Application in Gene Delivery Systems. Nanotechnology 2009, 20, 125101. [Google Scholar] [CrossRef]
- Iancu, C.; Mocan, L.; Bele, C.; Orza, A.I.; Tabaran, F.A.; Catoi, C.; Stiufiuc, R.; Stir, A.; Matea, C.; Iancu, D.; et al. Enhanced Laser Thermal Ablation for the in Vitro Treatment of Liver Cancer by Specific Delivery of Multiwalled Carbon Nanotubes Functionalized with Human Serum Albumin. Int. J. Nanomed. 2011, 6, 129–141. [Google Scholar] [CrossRef]
- Ji, Z.; Lin, G.; Lu, Q.; Meng, L.; Shen, X.; Dong, L.; Fu, C.; Zhang, X. Targeted Therapy of SMMC-7721 Liver Cancer in Vitro and in Vivo with Carbon Nanotubes Based Drug Delivery System. J. Colloid Interface Sci. 2012, 365, 143–149. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zeng, L.; Zhao, Z.; Chen, T. Functionalized Multiwalled Carbon Nanotubes as Carriers of Ruthenium Complexes to Antagonize Cancer Multidrug Resistance and Radioresistance. ACS Appl. Mater. Interfaces 2015, 7, 14933–14945. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Ding, W.; Yang, S.; Xing, D. Microwave Pumped High-Efficient Thermoacoustic Tumor Therapy with Single Wall Carbon Nanotubes. Biomaterials 2016, 75, 163–173, Erratum in Biomaterials 2019, 219, 119392. [Google Scholar] [CrossRef]
- Gu, Y.J.; Cheng, J.; Jin, J.; Cheng, S.H.; Wong, W.T. Development and Evaluation of PH-Responsive Single-Walled Carbon Nanotube-Doxorubicin Complexes in Cancer Cells. Int. J. Nanomed. 2011, 6, 2889–2898. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Mostafa, M.E.; Alaaeldin, E.; Sarhan, H.A.A.; Shaykoon, M.S.; Allam, S.; Ahmed, A.R.H.; Elsadek, B.E.M. Design and Characterisation of Novel Sorafenib-Loaded Carbon Nanotubes with Distinct Tumour-Suppressive Activity in Hepatocellular Carcinoma. Int. J. Nanomed. 2019, 14, 8445–8467. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Wang, J.; Du, J.; Gao, Y.; Zhang, L.; Chen, L.; Yang, Y.; Liu, X. A Targeted Drug Delivery System Based on Carbon Nanotubes Loaded with Lobaplatin toward Liver Cancer Cells. J. Mater. Res. 2018, 33, 2565–2575. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Kong, H.; Zhang, W.; Wang, C.; Xing, J.; Xie, S.; Xu, H. Subcutaneous Injection of Water-Soluble Multi-Walled Carbon Nanotubes in Tumor-Bearing Mice Boosts the Host Immune Activity. Nanotechnology 2010, 21, 145104. [Google Scholar] [CrossRef]
- Das, M.; Nariya, P.; Joshi, A.; Vohra, A.; Devkar, R.; Seshadri, S.; Thakore, S. Carbon Nanotube Embedded Cyclodextrin Polymer Derived Injectable Nanocarrier: A Multiple Faceted Platform for Stimulation of Multi-Drug Resistance Reversal. Carbohydr. Polym. 2020, 247, 116751. [Google Scholar] [CrossRef]
- Abdolahad, M.; Taghinejad, M.; Taghinejad, H.; Janmaleki, M.; Mohajerzadeh, S. A Vertically Aligned Carbon Nanotube-Based Impedance Sensing Biosensor for Rapid and High Sensitive Detection of Cancer Cells. Lab Chip 2012, 12, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Abdolahad, M.; Janmaleki, M.; Taghinejad, M.; Taghnejad, H.; Salehi, F.; Mohajerzadeh, S. Single-Cell Resolution Diagnosis of Cancer Cells by Carbon Nanotube Electrical Spectroscopy. Nanoscale 2013, 5, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Villa, C.H.; Holland, J.P.; Sprinkle, S.R.; May, C.; Lewis, J.S.; Scheinberg, D.A.; McDevitt, M.R. Imaging and Treating Tumor Vasculature with Targeted Radiolabeled Carbon Nanotubes. Int. J. Nanomed. 2010, 5, 783–802. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, S.; Umezawa, M.; Iizumi, Y.; Ube, T.; Okazaki, T.; Kamimura, M.; Soga, K. Delayed Increase in Near-Infrared Fluorescence in Cultured Murine Cancer Cells Labeled with Oxygen-Doped Single-Walled Carbon Nanotubes. Langmuir 2019, 35, 831–837. [Google Scholar] [CrossRef]
- Wang, X.; Shu, G.; Gao, C.; Yang, Y.; Xu, Q.; Tang, M. Electrochemical Biosensor Based on Functional Composite Nanofibers for Detection of K-Ras Gene via Multiple Signal Amplification Strategy. Anal. Biochem. 2014, 466, 51–58. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wang, H.; Xie, Q.; Xu, Y. Notch1-Nuclear Factor ΚB Involves in Oxidative Stress-Induced Alcoholic Steatohepatitis. Alcohol Alcohol. 2014, 49, 10–16. [Google Scholar] [CrossRef]
- Lee, P.C.; Lin, C.Y.; Peng, C.L.; Shieh, M.J. Development of a Controlled-Release Drug Delivery System by Encapsulating Oxaliplatin into SPIO/MWNT Nanoparticles for Effective Colon Cancer Therapy and Magnetic Resonance Imaging. Biomater. Sci. 2016, 4, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tabakman, S.; Sherlock, S.; Li, X.; Chen, Z.; Jiang, K.; Fan, S.; Dai, H. Multiplexed Five-Color Molecular Imaging of Cancer Cells and Tumor Tissues with Carbon Nanotube Raman Tags in the near-Infrared. Nano Res. 2010, 3, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Bian, X.; Zhu, Z.; Ding, D.; Li, X.; Jia, Z.; Jiang, X.; Hu, Y. Covalently Combining Carbon Nanotubes with Anticancer Agent: Preparation and Antitumor Activity. ACS Nano 2009, 3, 2740–2750. [Google Scholar] [CrossRef] [PubMed]
- Tahermansouri, H.; Chitgar, F. Synthesis of Isatin Derivative on the Short Multiwalled Carbon Nanotubes and Their Effect on the MKN-45 and SW742 Cancer Cells. J. Chem. 2013, 2013, 697839. [Google Scholar] [CrossRef]
- Tahermansouri, H.; Aryanfar, Y.; Biazar, E. Synthesis, Characterization, and the Influence of Functionalized Multi-Walled Carbon Nanotubes with Creatinine and 2-Aminobenzophenone on the Gastric Cancer Cells. Bull. Korean Chem. Soc. 2013, 34, 149–153. [Google Scholar] [CrossRef]
- Tahermansouri, H.; Ghobadinejad, H. Functionalization of Short Multi-Walled Carbon Nanotubes with Creatinine and Aromatic Aldehydes via Microwave and Thermal Methods and Their Influence on the MKN45 and MCF7 Cancer Cells. Comptes Rendus Chim. 2013, 16, 838–844. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Sun, L.; Liu, Y. The Effect of Hyaluronic Acid Functionalized Carbon Nanotubes Loaded with Salinomycin on Gastric Cancer Stem Cells. Biomaterials 2014, 35, 9208–9223. [Google Scholar] [CrossRef]
- Buyana, B.; Naki, T.; Alven, S.; Aderibigbe, B.A. Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 11261. [Google Scholar] [CrossRef]
- Wu, L.; Man, C.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y.; Ma, W. PEGylated Multi-Walled Carbon Nanotubes for Encapsulation and Sustained Release of Oxaliplatin. Pharm. Res. 2013, 30, 412–423. [Google Scholar] [CrossRef]
- Taghavi, S.; Nia, A.H.; Abnous, K.; Ramezani, M. Polyethylenimine-Functionalized Carbon Nanotubes Tagged with AS1411 Aptamer for Combination Gene and Drug Delivery into Human Gastric Cancer Cells. Int. J. Pharm. 2017, 516, 301–312. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Wei, X.; Yang, Z.; Liu, D.; Pu, X.; He, S.; Zhang, Y. Construction of Aptamer-SiRNA Chimera/PEI/5-FU/Carbon Nanotube/Collagen Membranes for the Treatment of Peritoneal Dissemination of Drug-Resistant Gastric Cancer. Adv. Healthc. Mater. 2020, 9, e2001153. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Grasa, L.; Frontiñán-Rubio, J.; Abás, E.; Domínguez-Alfaro, A.; Mesonero, J.E.; Criado, A.; Ansón-Casaos, A. Intrinsic and Selective Activity of Functionalized Carbon Nanotube/Nanocellulose Platforms against Colon Cancer Cells. Colloids Surf. B Biointerfaces 2022, 212, 112363. [Google Scholar] [CrossRef]
- Jin, H.; Gao, S.; Song, D.; Liu, Y.; Chen, X. Intratumorally CpG Immunotherapy with Carbon Nanotubes Inhibits Local Tumor Growth and Liver Metastasis by Suppressing the Epithelial-Mesenchymal Transition of Colon Cancer Cells. Anticancer Drugs 2021, 32, 278–285. [Google Scholar] [CrossRef]
- Lee, P.C.; Chiou, Y.C.; Wong, J.M.; Peng, C.L.; Shieh, M.J. Targeting Colorectal Cancer Cells with Single-Walled Carbon Nanotubes Conjugated to Anticancer Agent SN-38 and EGFR Antibody. Biomaterials 2013, 34, 8756–8765. [Google Scholar] [CrossRef]
- Ramezani Farani, M.; Lak, M.; C. Cho, W.; Kang, H.; Azarian, M.; Yazdian, F.; Harirchi, S.; Khoshmaram, K.; Alipourfard, I.; Hushmandi, K.; et al. Carbon Nanomaterials: A Promising Avenue in Colorectal Cancer Treatment. Carbon Lett. 2024, 34, 2035–2053. [Google Scholar] [CrossRef]
- Keum, N.N.; Giovannucci, E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J.; Pan, T.; Yin, Y.; Mei, Y.; Xiao, Q.; Wang, R.; Yan, Z.; Wang, W. Versatile Carbon Nanoplatforms for Cancer Treatment and Diagnosis: Strategies, Applications and Future Perspectives. Theranostics 2022, 12, 2290–2321. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zarghami, N.; Abasi, M.; Ertas, Y.N.; Pilehvar, Y. Implantable Magnetic Nanofibers with ON–OFF Switchable Release of Curcumin for Possible Local Hyperthermic Chemotherapy of Melanoma. J. Biomed. Mater. Res. A 2022, 110, 851–860. [Google Scholar] [CrossRef]
- Jiao, J.; Li, C.; Yu, G.; Zhang, L.; Shi, X.; Yan, J.; Zhang, H.; Guo, P. Efficacy of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Management of Malignant Ascites. World J. Surg. Oncol. 2020, 18, 180. [Google Scholar] [CrossRef]
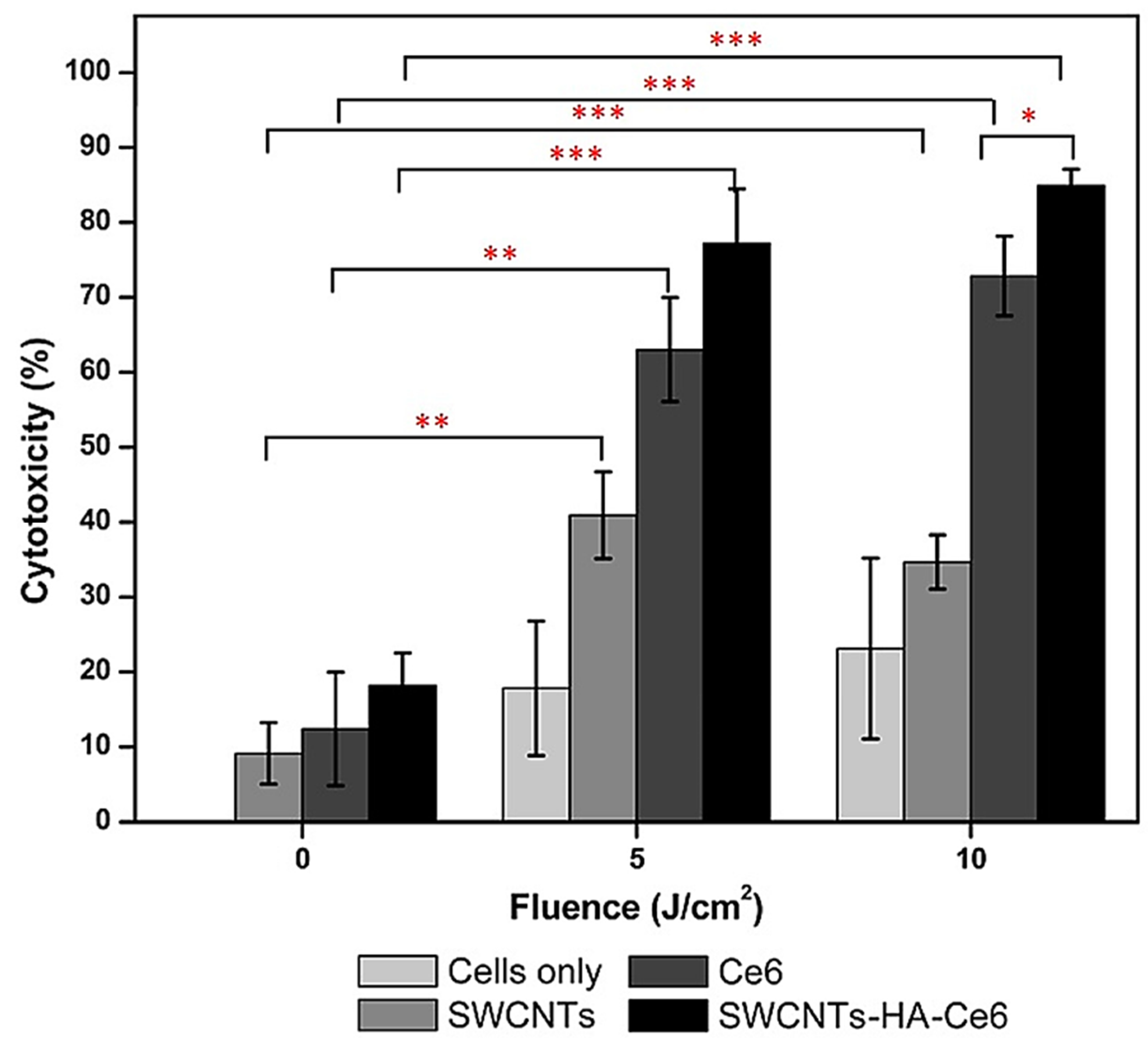
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin E6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef]
- Tripisciano, C.; Rümmeli, M.H.; Chen, X.; Borowiak-Palen, E. Multi-Wall Carbon Nanotubes—A Vehicle for Targeted Irinotecan Drug Delivery. Phys. Status Solidi B Basic Res. 2010, 247, 2673–2677. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biocompatibility and Carcinogenicity of Carbon Nanotubes as Biomaterials. Nanomaterials 2020, 10, 264. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized Carbon Nanotubes: Synthesis, Properties and Applications in Water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Zhang, S.; Hu, Y.; Li, H.; Zhang, T.; Xue, Y.; Pu, Y. Systemic and Immunotoxicity of Pristine and PEGylated Multi-Walled Carbon Nanotubes in an Intravenous 28 Days Repeated Dose Toxicity Study. Int. J. Nanomed. 2017, 12, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of Carbon Nanotube Toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-Based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Rasras, S.; Kalantari, H.; Rezaei, M.; Dehghani, M.A.; Zeidooni, L.; Alikarami, K.; Dehghani, F.; Alboghobeish, S. Single-Walled and Multiwalled Carbon Nanotubes Induce Oxidative Stress in Isolated Rat Brain Mitochondria. Toxicol. Ind. Health 2019, 35, 497–506. [Google Scholar] [CrossRef]
- Meschini, R.; D’Eliseo, D.; Filippi, S.; Bertini, L.; Bizzarri, B.M.; Botta, L.; Saladino, R.; Velotti, F. Tyrosinase-Treated Hydroxytyrosol-Enriched Olive Vegetation Waste with Increased Antioxidant Activity Promotes Autophagy and Inhibits the Inflammatory Response in Human THP-1 Monocytes. J. Agric. Food Chem. 2018, 66, 12274–12284. [Google Scholar] [CrossRef] [PubMed]
- Emerce, E.; Ghosh, M.; Öner, D.; Duca, R.C.; Vanoirbeek, J.; Bekaert, B.; Hoet, P.H.M.; Godderis, L. Carbon Nanotube- and Asbestos-Induced DNA and RNA Methylation Changes in Bronchial Epithelial Cells. Chem. Res. Toxicol. 2019, 32, 850–860. [Google Scholar] [CrossRef]
- Keshavan, S.; Gupta, G.; Martin, S.; Fadeel, B. Multi-Walled Carbon Nanotubes Trigger Lysosome-Dependent Cell Death (Pyroptosis) in Macrophages but Not in Neutrophils. Nanotoxicology 2021, 15, 1125–1150. [Google Scholar] [CrossRef]
- Sun, Y.; Gong, J.; Cao, Y. Multi-Walled Carbon Nanotubes (MWCNTs) Activate Apoptotic Pathway through Er Stress: Does Surface Chemistry Matter? Int. J. Nanomed. 2019, 14, 9285–9294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Forman, H.J.; Ge, Y.; Lunec, J. Multi-Walled Carbon Nanotubes: A Cytotoxicity Study in Relation to Functionalization, Dose and Dispersion. Toxicol. Vitr. 2017, 42, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of Functionalized Multiwalled Carbon Nanotubes and Comparison of Their Cellular Toxicity between HEK 293 Cells and Zebra Fish in Vivo. Heliyon 2019, 5, e02605. [Google Scholar] [CrossRef] [PubMed]
- Galassi, T.V.; Antman-Passig, M.; Yaari, Z.; Jessurun, J.; Schwartz, R.E.; Heller, D.A. Long-Term in Vivo Biocompatibility of Single-Walled Carbon Nanotubes. PLoS ONE 2020, 15, e0226791. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Lv, Z.; Wang, Z.; Zhao, Y.; Xie, Y.; Xu, Y.; Qian, L.; Yang, Y.; Zhao, Z.; et al. Transforming the Synthesis of Carbon Nanotubes with Machine Learning Models and Automation. Matter 2025, 8, 101913. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of Carbon Nanotubes, Graphene, and Their Derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef]
- Lettiero, B.; Andersen, A.J.; Hunter, A.C.; Moghimi, S.M. Complement System and the Brain: Selected Pathologies and Avenues toward Engineering of Neurological Nanomedicines. J. Control. Release 2012, 161, 283–289. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Peer, D.; Langer, R. Reshaping the Future of Nanopharmaceuticals: Ad Iudicium. ACS Nano 2011, 5, 8454–8458. [Google Scholar] [CrossRef]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and Long-Term Fate of Functionalized, Biocompatible Single-Walled Carbon Nanotubes in Mice Probed by Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef]
- Yang, S.; Fernando, K.A.S.; Liu, J.; Wang, J.; Sun, H.; Liu, Y.; Chen, M.; Huang, Y.; Wang, X.; Wang, H.; et al. Covalently PEGylated Carbon Nanotubes with Stealth Character In Vivo. Small 2008, 4, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Pondman, K.M.; Pednekar, L.; Paudyal, B.; Tsolaki, A.G.; Kouser, L.; Khan, H.A.; Shamji, M.H.; ten Haken, B.; Stenbeck, G.; Sim, R.B.; et al. Innate Immune Humoral Factors, C1q and Factor H, with Differential Pattern Recognition Properties, Alter Macrophage Response to Carbon Nanotubes. Nanomedicine 2015, 11, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization Density Dependence of Single-Walled Carbon Nanotubes Cytotoxicity In Vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin Improves Nonalcoholic Fatty Liver by Ameliorating Inflammation, Oxidative Stress, and Lipid Metabolism in Db/Db Mice. Phytother. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Sallam, A.A.; Ahmed, M.M.; El-Magd, M.A.; Magdy, A.; Ghamry, H.I.; Alshahrani, M.Y.; Abou El-Fotoh, M.F. Quercetin-Ameliorated, Multi-Walled Carbon Nanotubes-Induced Immunotoxic, Inflammatory, and Oxidative Effects in Mice. Molecules 2022, 27, 2117. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, X.; Ji, Z.; Shen, X.; Dong, L.; Wu, M.; Gu, T.; Liu, Y. Long-Term Hepatotoxicity of Polyethylene-Glycol Functionalized Multi-Walled Carbon Nanotubes in Mice. Nanotechnology 2010, 21, 175101. [Google Scholar] [CrossRef]
- Pérez-Luna, V.; Moreno-Aguilar, C.; Arauz-Lara, J.L.; Aranda-Espinoza, S.; Quintana, M. Interactions of Functionalized Multi-Wall Carbon Nanotubes with Giant Phospholipid Vesicles as Model Cellular Membrane System. Sci. Rep. 2018, 8, 17998. [Google Scholar] [CrossRef]
- Awasthi, S.; De, S.; Pandey, S.K. Surface Grafting of Carbon Nanostructures. In Handbook of Functionalized Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–45. [Google Scholar]
- Yang, M.; Zhang, M. Biodegradation of Carbon Nanotubes by Macrophages. Front. Mater. 2019, 6, 225. [Google Scholar] [CrossRef]
- Li, J.; Deng, X.; Wang, L.; Liu, J.; Xu, K. Clinical Application of Carbon Nanoparticles in Lymphatic Mapping during Colorectal Cancer Surgeries: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2020, 52, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Gu, J. The Application of Carbon Nanoparticles in the Lymph Node Biopsy of CN0 Papillary Thyroid Carcinoma: A Randomized Controlled Clinical Trial. Asian J. Surg. 2017, 40, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ao, S.; Bu, Z.; Wu, A.; Wu, X.; Shan, F.; Ji, X.; Zhang, Y.; Xing, Z.; Ji, J. Clinical Study of Harvesting Lymph Nodes with Carbon Nanoparticles in Advanced Gastric Cancer: A Prospective Randomized Trial. World J. Surg. Oncol. 2016, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Tucker, A.; Gidcumb, E.; Shan, J.; Yang, G.; Calderon-Colon, X.; Sultana, S.; Lu, J.; Zhou, O.; Spronk, D.; et al. High Resolution Stationary Digital Breast Tomosynthesis Using Distributed Carbon Nanotube X-Ray Source Array. Med. Phys. 2012, 39, 2090–2099. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of Precancerous Gastric Lesions and Gastric Cancer through Exhaled Breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; et al. A Nanomaterial-Based Breath Test for Distinguishing Gastric Cancer from Benign Gastric Conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef]
- Conventional Bite Wing Radiography Versus Stationary Intraoral Tomosynthesis, a Comparison Study. 2018. Available online: https://inclinicaltrials.com/dental-caries/NCT02873585/ (accessed on 12 September 2025).
- Schlesinger, I. Exploratory Study Using Nanotechnology to Detect Biomarkers of Parkinson’s Disease from Exhaled Breath. 2009. Available online: https://ichgcp.net/clinical-trials-registry/NCT01246336 (accessed on 12 September 2025).
- Bittar, R.; Alves, N.G.P.; Bertoldo, C.; Brugnera, C.; Oiticica, J. Efficacy of Carbon Microcoils in Relieving Cervicogenic Dizziness. Int. Arch. Otorhinolaryngol. 2017, 21, 4–7. [Google Scholar] [CrossRef]

| NPs (nm) | Concentration/TE | Species/Cell Culture | Assay | Result |
|---|---|---|---|---|
| Al2O3 (8–12) | 1–10 µM/24 h | HBMVECs | MTT/DHE | ↓ Cell viability, ↓ mitochondrial function, ↑ OS Alter proteins expression of the BBB |
| Al2O3 (50–80) | 10–400 µg/mL/24 h | Mammalian cells | EZ4U | No significant toxic effect on cell viability |
| Al2O3 (160) | 25–40 µg/mL/12 h | HMSC | MTT | ↓ Cell viability |
| Al2O3 (30–40) | 500–2000 mg/kg/72 h | Rat blood cells | Comet, micronucleus | Dose-dependent genotoxicity |
| Al2O3 (50) | 0–5000 µg/mL/2 h | MLCL | Comet | DNA damage |
| CuO (50) | 10–50 µg/mL/24 h | HLECs | MTT/LDH | ↓ Cell viability, ↑ LDH, ↑ lipid peroxidation |
| MWCNTs (20) | 0.002–0.2 µg/mL/4 days | LCCs | MTT | ↓ Cell viability |
| SWCNT (800) | 0–400 µg/mL/10 days | HACECs, NHBECs | Clonogenic | Cell death |
| SWCNTs (10–30) | 40 and 200 µg/mouse, 1 mg/mouse, 90 days | in vivo | Commercial kits | ↑ LDH, ↑ AST, ↑ ALT |
| Fullerenes (178) | 1 ng/mL/80 days | CHO, HELA, HEK293 | Micronucleus test | DNA strand breakage, chromosomal damage |
| Silica (15–46) | 10–100 µg/mL/48 h | HBACCs | DCFH-DA/Commercial kit | ↑ ROS, ↑ LDH, ↑ Malondialdehyde |
| Silica (43) | 25–200 µg/mL/3–24 h | HepG2 | DCFH-DA/TEBCI | ↑ ROS, MD, OS |
| Ag (15–100) | 10–50 µg/mL/24 h | BRL 3A | LDH, MTT, Glutathione, DCFH-DA | ↓ Cell viability, ↑ LDH, ↑ ROS |
| Ag (30–50) | 0–20 µg/mL/24 h | HACs | MTT, DCFH-DA | ↓ Cell viability, ↑ ROS |
| Ag (20–40) | --- | HLKCs | WST-1, LDH | ↓ Cell viability, ↑ LDH |
| ZnO (50–70) | 11.5 µg/mL/24 h | HCCCs | ELISA, flow cytometry | ↑ OS, ↓ Cell viability, inflammatory biomarkers |
| ZnO (307–419) | 10–100 µg/mL/24–48 h | HEp-2 | Comet micronucleus test, MTT | DNA damage; ↓ cell viability |
| ZnO (30–70) | 14–20 µg/mL/12 h | in vivo | MTT, Comet, DCFH-DA | ↓ Cell viability; DNA damage; ROS, apoptosis |
| ZnO (50) | 0–100 µg/mL/24 h | HEK 293 | MTT; Comet | DNA damage, ↓ cell viability, OS, MD |
| ZnO (<20) | 100 µg/mL | HBECCs | - | ↓ Cell viability, ↑ OS, ↑ LDH release |
| Fe2O3 (30) | 25–200 µg/mL/2 h | MMCs | MTT | ↓ Cell viability |
| Fe2O3 (100–150) | 0.1 mg/mL/7 days | HMs | MTS | ↓ Cell viability |
| Fe2O3 (13.8) | 123.52 µg/mL/12 h | HPCCs | MTT | ↓ Cell viability |
| Fe2O3 (20) | 0.1 mg/mL/2 days | RMSCs | MTS | ↓ Cell viability |
| TiO2 (160) | 1800 µg/mouse/10 days | in vivo | Comet, micronucleus test | DNA damage, genotoxicity |
| TiO2 (<100) | 10–50 µg/mL/6–24 h | HLCs | ELISA, Trypan blue, DCFH-DA | ↑ OS, DNA adduct formation, ↑ cytotoxicity |
| CNT-Based Sensor | Biomarker/Effectiveness | Cancer | Refs. |
|---|---|---|---|
| MWCNTs | CA19-9 | PC | [94] |
| MWCNTs | GdGTP in DNA | PC | [98] |
| SWCNTs@immune sensor | CA19-9 | PC | [97] |
| Au@CNTs | AFP, AFP-L3, APT | LC | [101] |
| DBCO-PEG5-NHS ester@CNTs | GP73, α-FTP | HCC | [102] |
| Collagen@patterned CNTs | Detection of CCs versus NCs | LC | [104] |
| Zein NPs@MWCNTs | H2O2 monitoring HepG2 cells | HepG2 | [105] |
| MWCNTs (NBS) | ⬆ BA, WLRD miR-21, ⬇⬇⬇ LOD | PC | [106] |
| CNTs | Cell Line/Biomarkers | Linear Range | LOD | Techniques | Ref. |
|---|---|---|---|---|---|
| MWCNTs | AFP (LC) | 0.02–2.0 ng/mL | 8.0 pg/mL | Immune sensing | [107] |
| MWCNTs | AFP (LC) | 0.1–15.0 and 15.0–200.0 ng/mL | 0.08 ng/mL | Immune sensing | [108] |
| MWCNTs | CA 19-9 (PC) | 12.5–270.0 U/mL | 8.3 U/mL | Immune sensing | [109] |
| CNTs | AFP (LC) | 1–55 ng/mL | 0.6 ng/mL | Immune sensing | [110] |
| MWCNTs | CA19-9 (PC) | 0–1000 U/mL | N.R. | Electrochemical | [94] |
| CNTs | GP73 (HCC) | 0–80 ng/mL | 58.1 pg/mL | Immune sensing | [102] |
| CNTs | AFP (LC) | 0–64 ng/mL | 47.1 pg/mL | Immune sensing | [102] |
| CNTs | HepG2 (LC) | 10–105 cells/mL | 5 cells/mL | Electrochemical | [111] |
| CNTs-Based NC | Highlights | Refs. |
|---|---|---|
| f-HSA@MWCNTs | HAS-MWCNTs accumulates in PCCs for laser irradiation PTT | [123] |
| EGF@SWCNTs | ⬆ Rapid uptake of nanocomposite | [124] |
| f-ETO@ SWCNTs | Synergic effect of SWCNTs and ETO | [125] |
| f-PEI@SWCNTs | PEI@SWCNT pass both cytoplasmic and nuclear membranes | [126] |
| f-PEG@MWCNTs | Cause apoptosis pathways to activate through mitochondrial deficiency | [127] |
| f-GA@SWCNTs | ⬆ GA effects by usage of CNTs | [128] |
| siRNA@SWCNTs | ⬆ siRNA targeted delivery, ⬆ transfection, ⬆ gene therapy effects | [129] |
| f-PEG@oMWCNTs | ⬆ Cell toxicity, ⬆ PTT, ⬆ TVR in animal group (PEG-O-CNTs) | [130] |
| f-PEG@GEM@SWCNTs | ⬇ Metastatic lymph nodes in BxPC-3–B/c | [131] |
| f-HIF-1α/siRNA@SWCNTs | ⬆ Transfection in PCCs, ⬆ RNA response, ⬇⬇⬇ TG, ⬇ harm to NCs | [132] |
| CNTs-Based NC | Highlights | Refs. |
|---|---|---|
| HSA@MWCNTs | ⬆ Apoptosis by PTT in HepG2 cancer cells than in normal ones | [137] |
| CHI@FA@SWCNTs@DOX | ⬆ DL (%), ⬆ AE, ⬆ targeting capacity, ⬆ biocompatibility, ⬆ WS | [138] |
| RuPOP@MWCNTs | ⬆ Cellular uptake, ⬆ AE, ⬆ apoptosis induction under X-ray | [139] |
| CoMoCAT®-SWCNTs@PL@PEG-NH2 | ⬆ Tumour growth inhibition by apoptotic death under TAT | [140] |
| as-ODNs@PAMAM-NH2@f-MWCNTs | ⬆⬆⬆ Gene delivery efficiency | [136] |
| DOX@SWCNTs/DOX@HBA@SWCNTs | pH-dependent DR, max DR at pH = 5.5, ⬆ cytotoxic effects | [141] |
| PEG@o-MWCNTs | ⬇ cytotoxicity in HepG2, ⬇⬇⬇ tumour size under PTT in vivo | [130] |
| CNT-Based Formulation | Treatment Approach | Cancer Type | Refs. |
|---|---|---|---|
| LyP-1@siRNA@MWCNTs | Delivery of siRNA | Pancreatic cancer | [57] |
| f-CNTs | Sorafenib delivery | HepG2 cell line | [142] |
| PEG@CNTs | Lobaplatin delivery | HepG2 cell line | [143] |
| TT | DDS | TM | Model | Effectiveness | Refs. |
|---|---|---|---|---|---|
| Cytoplasm | SWCNT@CY7@IGF-1Ra | PTT, IT | ASPC-1, BXPC-3, PANC-1 SW1990 * | BS, LT, PTTT, ⬆ BW, ⬆ SR | [99] |
| Macrophages | ws-MWCNTs@COOH | IT | H22 HCCs | ⬆ WS, ⬆ CSA, ⬆ CP, ⬆ MA | [144] |
| 140TV | DOX/CD-CNT, CUR/CD-CNT | PTT + CT | Hepatocellular | ⬆ DEE and achieved sustained release of both drugs | [145] |
| ST | CNTs | Model | Results | Refs. |
|---|---|---|---|---|
| PET-RII * | [89Zr] DOTA@SWCNTs@antiE4G10 | LS174T ** | ⬆ STNR by only SA, WT | [148] |
| RI | f-SWCNTs@Erb@RGD@anti-CEA@RTX@HER | LS174T ** | Zero interfering, ⬆ EGFR, MCOI | [153] |
| ECIS-BS | VACNTs | SW48 CRCCs | ⬆ Adhesivity, ⬆ conductance, ⬆ cells interaction | [146] |
| EM | VANCTs | HT29, SW480 | ⬆ Efficiency, ⬆ sensitivity, ⬆ conductivity ⬆ CP | [147] |
| FI, RI | o-SWCNT@PEG | Colon-26 | DC, ⬆ RT in cells, ⬆ FS after 48 h | [149] |
| ECBS | PA6@MWCNT@SH@ | CRCCs | ⬆ K-ras GD, MSAS, ⬆ sensitivity (30 fm) | [150] |
| PAI | Si/Au@MWCNTs@RDG | GCCs | ⬆ WS, ⬇ toxicity, ⬆ TTC | [151] |
| MRI | SPIO@PEG@MWCNTs@OP | HCT116 | ⬆ T2-weighted MRI signal after IVA | [152] |
| CNTs-Based NC | Highlights | Refs. |
|---|---|---|
| HCPT@DATEG@f-MWCNTs | ⬆ Antitumor activity in MKN-28 cells * | [154] |
| [225Ac]/[89Zr] DOTA@SWCNTs@antiE4G10 | ⬇ TM, ⬆ MS, ⬆ TS accumulation, RC (CRC) | [148] |
| Sh-MWCNTs@Amide | ⬆ Toxicity to MKN-45 | [155] |
| Sh-MWCNTs@Quino | ⬆ Toxicity to MKN-45 | [156] |
| Sh-MWCNTs@Imidazole | MKN-45 cells viability was lowered by 71–77% | [157] |
| FITC-SAL@SWCNT@CHI@HA | Significant decrease in mammosphere (GC) | [158] |
| Ir@MWCNTs | ⬆ DL, ⬆ inner diameter tube, pH sensitive (CRC) | [172] |
| CNTs | FM | Effectiveness | Tumour Model | Biocompatibility Test | Refs. |
|---|---|---|---|---|---|
| MWCNTs | PEG600, OP | Delayed cytotoxic activity | Colorectal cancer ** | ⬇ Toxicity | [160] |
| SWCNTs | PEG-10–10%PEI/Bcl-xL-shRNA, DOX | ⬇ by 58-fold usual DOX IC50; ⬆ WS, ⬆ BFS, no PA | AGS GCCs/L929 ** | ⬇ Toxicity | [161] |
| SWCNTs | Chim/PEI/5-FU/CNT | ⬆⬆⬆ TP, ⬆⬆⬆ DL, ⬇ invasion/proliferation, apoptosis | Gastric cancer ** | ⬆ Biocompatibility | [162] |
| SWCNTs | II-NCC | ⬆⬆⬆ WD; ⬆ ACEs of CAP | Caco-2 colon ** | ⬆ Biocompatibility | [163] |
| SWCNTs | CpG | ACA in gliomas, ⬇ CCP, ⬇ invasion/migration | HCCsT116 ** | ⬆ Mice survival rate | [164] |
| SWCNTs | SN38, C225 | Specific binding, controlled release of SN38, ⬆ ACEs | HCT116, HT29, SW620 ** | ⬆ Biocompatibility | [165] |
| MWCNTs | OP, PEG, SPIO | SR (56%, 144 h), ⬆ ACEs in vitro (96 h) ⬆ MRI signal | HCT116 **, BALB/c ANM * | ⬇ Adverse effects | [152] |
| SWCNTs | HA, Ce6 | ⬆ ACEs, ⬆ WD, ⬆ early and late apoptosis | Caco-2 colon ** | Possible ⬇ toxicity due to ⬆ WS | [171] |
| Strategy | Goal | Modifying Agents/Methods | Results |
|---|---|---|---|
| CNTs surface modification with biocompatible materials or other molecules | ⬆ Dispersion in biological fluids Influenced CU, ⬆ Solubility ⬇ Toxicity | Proteins, surfactants | ⬆ TT, ⬆ TB, ⬇ Toxicity |
| FA | ⬆ In vivo tumour targeting, ⬆ Therapeutic benefits ⬇ Toxicity | ||
| PA hydrogels *, biomaterial, TiO2 | 100% survival of L929 mouse fibroblast | ||
| Coatings of CNTs | ⬆ CNTs biocompatibility ⬇ Potential toxicity Prevent direct contact with BS ⬆ CNTs solubility | Curcumin lysine ** | ⬇ IL-6, IL-8, IL-1β, TNFα, N-FκB ⬆ Antioxidant enzyme catalase, ⬇ ROS generation Recovery of MM, ⬇ Cell death |
| CNTs encapsulation CNTs to entrap BAM | ⬇ Direct cells exposure to CNTs Control of CNTs release ⬇ CNTs impact on tissues | PEG (entrapping agent) Oxaliplatin (entrapped agent) | PEGylation delayed oxaliplatin release rate ⬆ Drug’s anticancer effects on HT-29 cells |
| Tailor Ø size and L | ⬇ Toxicity | N.A. | ⬆ SSA, ⬆ TM, ⬇ Toxicity, ⬇ Harm to lysosomes *** |
| Optimized PP | Remove MI Remove RC | Chemical/electrochemical oxidation High chlorine partial pressure MA digestion Incandescent annealing | ⬇ Lower harmful effects |
| Engineering controls Suitable PPE | ⬇ Inhalation | Proper ventilation/respiratory protection | ⬇ Respiratory toxicity |
| CA with AO | ⬇ OS ⬇ Damage to cells | Quercetin | Prevention of the oxidative damage ⬇ Inflammatory effects, ⬇ Immuno-toxic effects |
| Name | NPIs | API | AI | TGs |
|---|---|---|---|---|
| Lipid-Based NPs | ||||
| Doxil/Caelyx | LIP, PEG | Doxorubicin | FDA (1995), EMA (1996) | OC, HIV-KS, MM |
| Onivyde | LIP, PEG | Irinotecan | FDA (2015) | MPC |
| DaunoXome | LIP | Daunorubicin | FDA (1996) | HIV-KS |
| Myocet | LIP | Doxorubicin | EMA (2000) | MBC |
| Marqibo | LIP | Vincristine | FDA (2012) | PCNALLK |
| Mepact | LIP | Mifamurtide | EMA (2009) | NMR-OS |
| Vyxeos | LIP | Cytarabine/daunorubicin (5/1 M) | FDA (2017), EMA (2018) | HR-AMLK |
| Lipusu | LIP | Paclitaxel | China (2006) | BC, LC, OC |
| DepoCyt | LIP | Cytarabine | FDA (1999) | NM |
| Polymer-based NPs | ||||
| Oncaspar | PEG-P | ASNase | FDA (1994) | ALLK |
| Genexol-PM | Micelle | Paclitaxel | South Korea (2007) | MBC, PC |
| Eligard | PLGA | Leuprolide acetate | FDA (2002) | PRC |
| Neulasta | PEG-P | G-CSF | FDA (2002), EMA (2002) | NMMs |
| Zinostatin Stimalamer | SMA | NCS | Japan (1994) | PUHCC |
| Albumin-based NPs | ||||
| Abraxane | Albumin | Paclitaxel | FDA (2005), EMA (2008) | LC, MBC, MPC |
| Pazenir | Albumin | Paclitaxel | EMA (2019) | MBC, MPAC, NSCs-LC |
| Other NPs | ||||
| NanoTherm | F2O3 | Not applicable | EMA (2011) | Glioblastoma |
| Hensify (NBTXR3) | HfO2 | Radiotherapeutic | EMA (2019) | STS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Reggio, C.; Zuccari, G. From Early Diagnoses to New Treatments for Liver, Pancreatic, Gastric, and Colorectal Cancers Using Carbon Nanotubes: New Chances Still Underexplored. Int. J. Mol. Sci. 2025, 26, 9201. https://doi.org/10.3390/ijms26189201
Alfei S, Reggio C, Zuccari G. From Early Diagnoses to New Treatments for Liver, Pancreatic, Gastric, and Colorectal Cancers Using Carbon Nanotubes: New Chances Still Underexplored. International Journal of Molecular Sciences. 2025; 26(18):9201. https://doi.org/10.3390/ijms26189201
Chicago/Turabian StyleAlfei, Silvana, Caterina Reggio, and Guendalina Zuccari. 2025. "From Early Diagnoses to New Treatments for Liver, Pancreatic, Gastric, and Colorectal Cancers Using Carbon Nanotubes: New Chances Still Underexplored" International Journal of Molecular Sciences 26, no. 18: 9201. https://doi.org/10.3390/ijms26189201
APA StyleAlfei, S., Reggio, C., & Zuccari, G. (2025). From Early Diagnoses to New Treatments for Liver, Pancreatic, Gastric, and Colorectal Cancers Using Carbon Nanotubes: New Chances Still Underexplored. International Journal of Molecular Sciences, 26(18), 9201. https://doi.org/10.3390/ijms26189201










