Purified Native Collagen Extracellular Matrix Plus Polyhexamethylene Biguanide Functions as a Barrier to Protect Complex Wounds in an In Vivo Model
Abstract
1. Introduction
2. Results
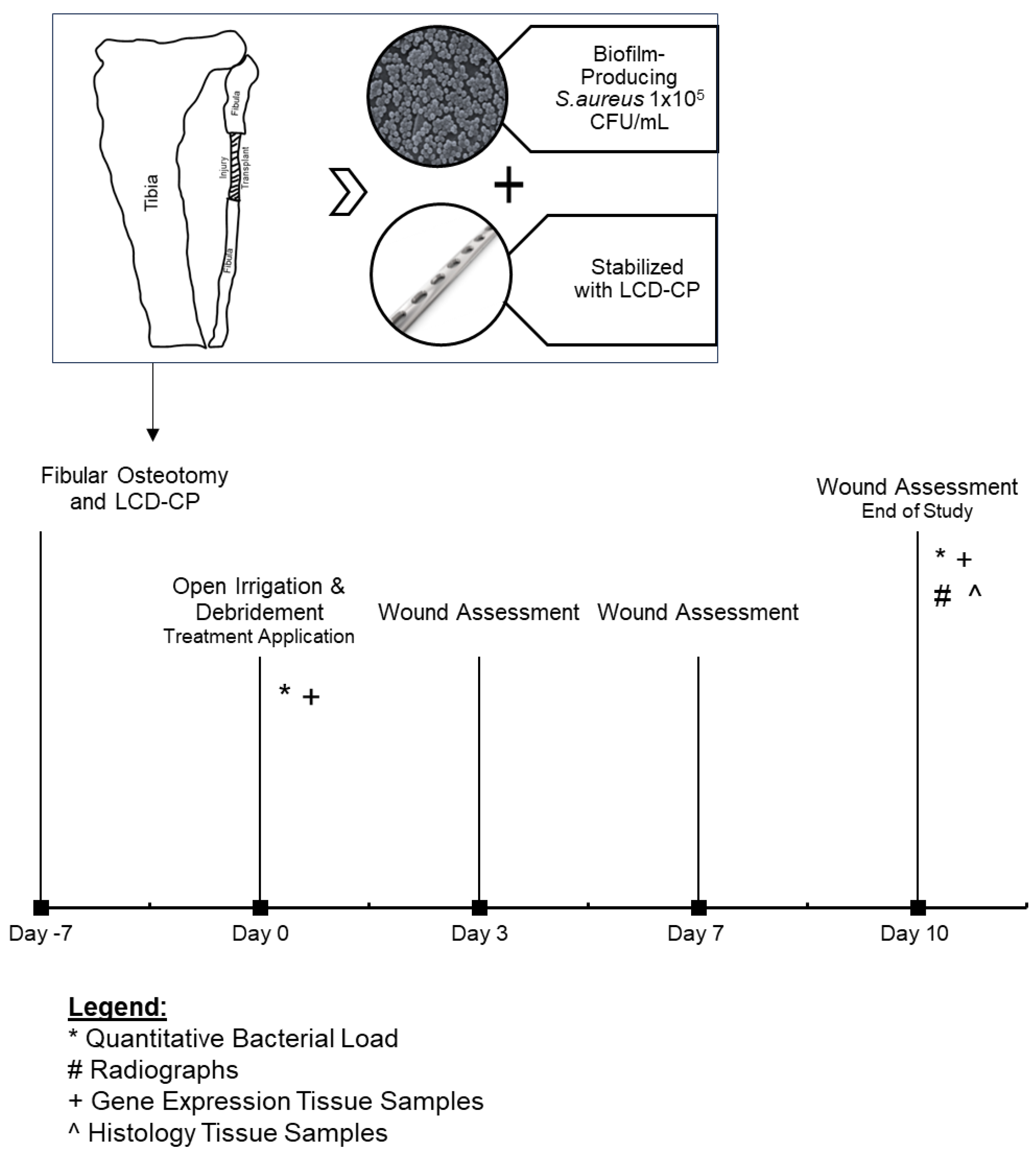
2.1. Model Overview, Generation, and Study Timeline
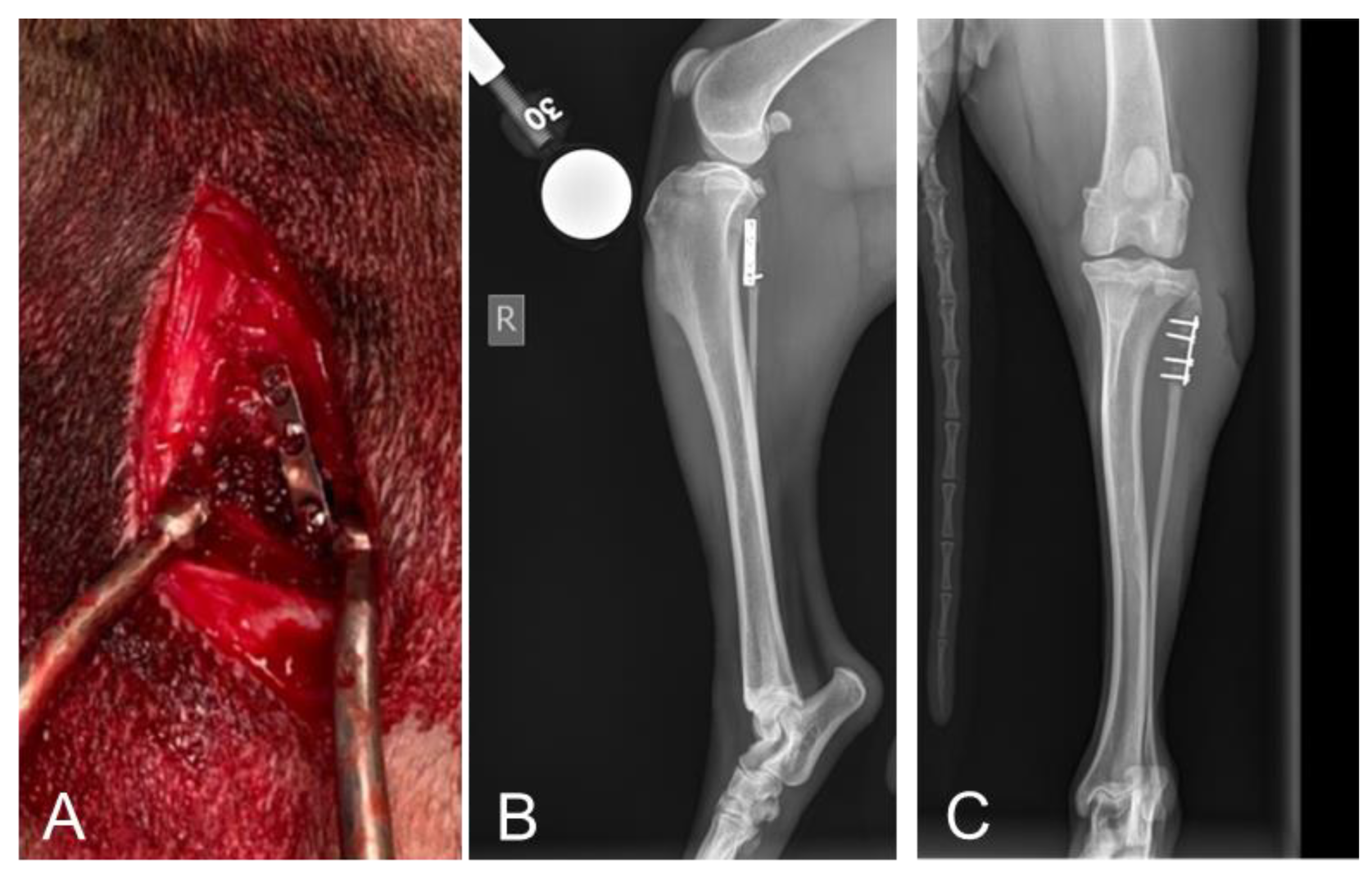
2.2. Radiographic Assessments Revealed No Implant Failures Following 10 Days of Treatment
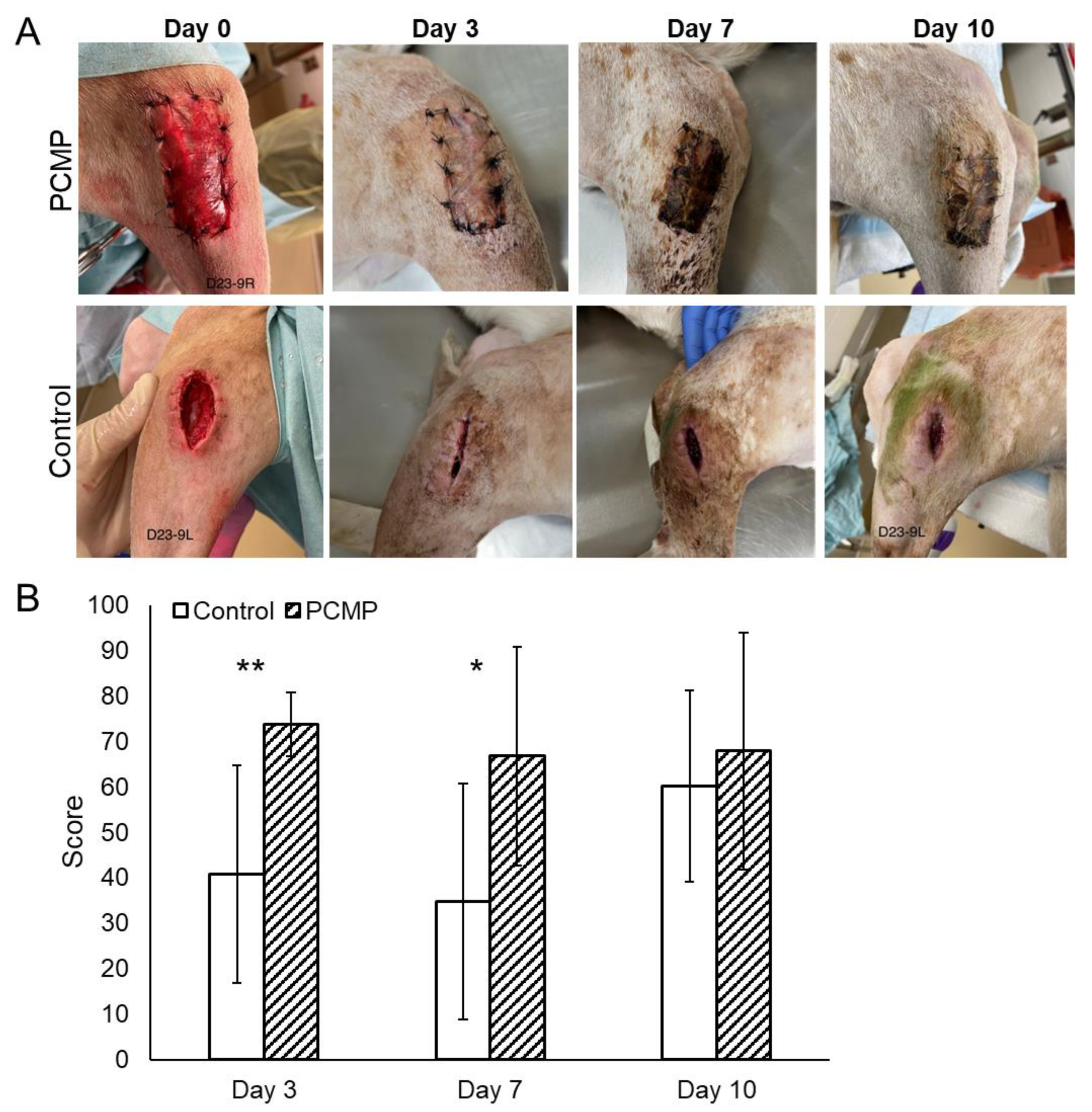
2.3. Clinical Assessments Showed Significantly Improved Wound Healing at 3 and 7 Days with Purified Native Collagen Extracellular Matrix Plus Polyhexamethylene Biguanide (PCMP)-Treated Wounds
2.4. Histological Assessments Showed Improved Wound Healing in PCMP-Treated Wounds
2.5. Quantitative Bacterial Analysis Showed a Reduction in Bacterial Load in the PCMP Group After 10 Days
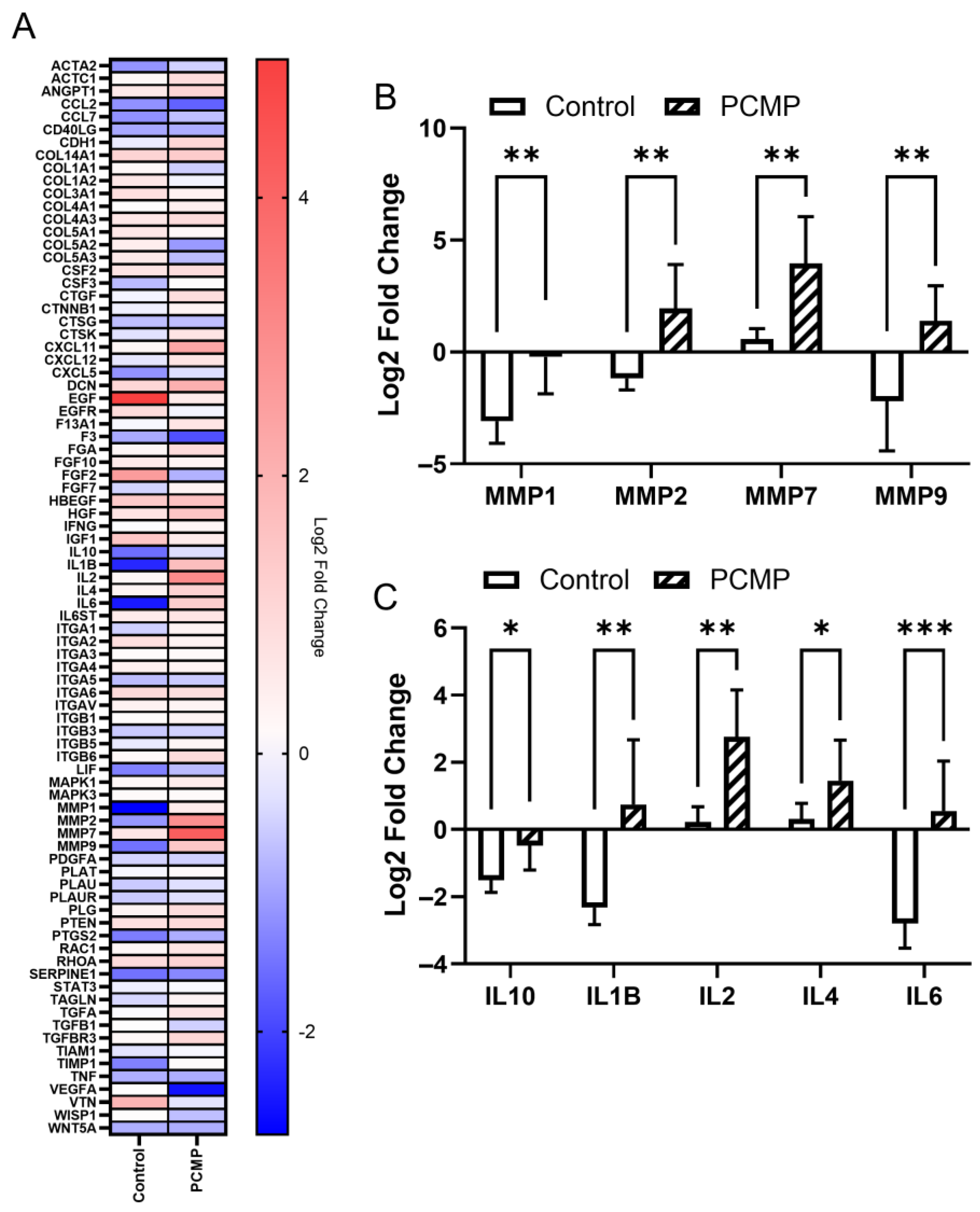
2.6. Comparative Gene Expression Analysis Showed Significant Differences in Matrix Metalloproteinase (MMP) and Inflammatory Targets in PCMP-Treated Wounds
3. Discussion
4. Materials and Methods
4.1. Animal Model Design and Justification
4.2. Surgical Procedures
4.3. Post-Surgical Monitoring
4.4. Wound Management Cohorts
4.5. Outcome Measures
4.5.1. Radiographic Evaluation
4.5.2. Wound Assessment Scoring
4.5.3. Histological Assessments
4.5.4. Microbial Culture
4.5.5. Gene Expression Analysis
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owens, C.D.; Stoessel, K. Surgical Site Infections: Epidemiology, Microbiology and Prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of Infected Wounds: Overcoming Clinical Challenges by Advanced Drug Delivery Systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. The Hospital Infection Control Practices Advisory Committee Guideline for Prevention of Surgical Site Infection, 1999. Infect. Control Hosp. Epidemiol. 1999, 20, 250–280. [Google Scholar] [CrossRef]
- Onyekwelu, I.; Yakkanti, R.; Protzer, L.; Pinkston, C.M.; Tucker, C.; Seligson, D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2017, 1, e022. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative Normothermia to Reduce the Incidence of Surgical-Wound Infection and Shorten Hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and Chronic Wound Inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Davis, S.; Fitzgerald, R.; Gehling, M.; Kapp, D.; Karr, J.; Samies, J.; Shultz, G.; Teichman, A.; Weir, D. Expert recommendations for optimizing outcomes in the management of biofilm to promote healing of chronic wounds. Wounds 2016, 28 (Suppl. 6), S1–S20. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef]
- Chindera, K.; Mahato, M.; Kumar Sharma, A.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The Antimicrobial Polymer PHMB Enters Cells and Selectively Condenses Bacterial Chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef]
- Andrée, B.; Bär, A.; Haverich, A.; Hilfiker, A. Small Intestinal Submucosa Segments as Matrix for Tissue Engineering: Review. Tissue Eng. Part B Rev. 2013, 19, 279–291. [Google Scholar] [CrossRef]
- Harmon, K.A.; Burnette, M.D.; Avery, J.T.; Kimmerling, K.A.; Mowry, K.C. Varying Properties of Extracellular Matrix Grafts Impact Their Durability and Cell Attachment and Proliferation in an in Vitro Chronic Wound Model. J. Tissue Eng. Regen. Med. 2024, 2024, 6632276. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Rigden, B.W.; Stoker, A.M.; Bozynski, C.C.; Gull, T.; Cook, C.R.; Kuroki, K.; Stannard, J.P.; Cook, J.L. Development and Validation of a Preclinical Canine Model for Early Onset Fracture-Related Infections. Injury 2024, 55, 111957. [Google Scholar] [CrossRef] [PubMed]
- Hellwinkel, J.E.; Working, Z.M.; Certain, L.; García, A.J.; Wenke, J.C.; Bahney, C.S. The Intersection of Fracture Healing and Infection: Orthopaedics Research Society Workshop 2021. J. Orthop. Res. 2022, 40, 541–552. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Klopper, J.H.; Rayamajhi, S.; Venter, J.J.; De Villiers, D.J.; Almgla, N.; Kloppers, J.C. Provision of Acute and Elective General Surgical Care at a Tertiary Facility in the Era of Subspecialisation. South Afr. Med. J. 2017, 107, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.C.; Murray, C.K.; Nelson, K.J.; Bosse, M.J. Infection after Orthopaedic Trauma: Prevention and Treatment. J. Orthop. Trauma 2016, 30, S21–S26. [Google Scholar] [CrossRef]
- Davis, S.C.; Gil, J.; Solis, M.; Higa, A.; Mills, A.; Simms, C.; Pena, P.V.; Li, J.; Raut, V. Antimicrobial Effectiveness of Wound Matrices Containing Native Extracellular Matrix with Polyhexamethylene Biguanide. Int. Wound J. 2022, 19, 86–99. [Google Scholar] [CrossRef]
- Nocini, P.F.; Menchini Fabris, G.B.; Gelpi, F.; Lotti, J.; Favero, V.; Zanotti, G.; Jurlaro, A.; Rosskopf, I.; Lotti, T.; Barone, A.; et al. Treatment of Skin Defects with Growth Factors and Biodegradable Collagen Carrier: Histological Evaluation in Animal Model. J. Biol. Regul. Homeost. Agents 2017, 31, 1–13. [Google Scholar] [PubMed]
- Cosola, S.; Di Dino, B.; Traini, T.; Kim, Y.S.; Park, Y.M.; Marconcini, S.; Covani, U.; Vinci, R. Radiographic and Histomorphologic Evaluation of the Maxillary Bone after Crestal Mini Sinus Lift Using Absorbable Collagen—Retrospective Evaluation. Dent. J. 2022, 10, 58. [Google Scholar] [CrossRef]
- Kim, Y.W.; Cosola, S.; Kim, Y.S.; Park, Y.M.; Covani, U.; Fabbri, A.; Menchini-Fabris, G.B. Clinical Application of RhBMP-2 and Three-Dimensinal Preformed Titanium Mesh with Allograft and Xenograft for Peri-Implant Horizontal and Vertical Bone Augmentation–A Narrative Review with Technical Report. J. Clin. Med. 2025, 14, 4788. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Kampf, G.; Cooper, R. Antimicrobial Stewardship of Antiseptics That Are Pertinent to Wounds: The Need for a United Approach. JAC Antimicrob. Resist. 2021, 3, dlab027. [Google Scholar] [CrossRef]
- Hess, C.T. Comprehensive Patient and Wound Assessments. Adv. Skin. Wound Care 2019, 32, 287–288. [Google Scholar] [CrossRef]
- Volk, C.F.; Burgdorf, S.; Edwardson, G.; Nizet, V.; Sakoulas, G.; Rose, W.E. Interleukin (IL)-1β and IL-10 Host Responses in Patients with Staphylococcus Aureus Bacteremia Determined by Antimicrobial Therapy. Clin. Infect. Dis. 2020, 70, 2634–2640. [Google Scholar] [CrossRef]
- Elkington, P.T.G.; O’Kane, C.M.; Friedland, J.S. The Paradox of Matrix Metalloproteinases in Infectious Disease. Clin. Exp. Immunol. 2005, 142, 12–20. [Google Scholar] [CrossRef]
- Kanangat, S.; Postlethwaite, A.; Hasty, K.; Kang, A.; Smeltzer, M.; Appling, W.; Schaberg, D. Induction of Multiple Matrix Metalloproteinases in Human Dermal and Synovial Fibroblasts by Staphylococcus Aureus: Implications in the Pathogenesis of Septic Arthritis and Other Soft Tissue Infections. Arthritis Res. Ther. 2006, 8, R176. [Google Scholar] [CrossRef] [PubMed]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of Matrix Metalloproteinase in Wound Healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar] [PubMed]
- Muller, M.; Trocme, C.; Lardy, B.; Morel, F.; Halimi, S.; Benhamou, P.Y. Matrix Metalloproteinases and Diabetic Foot Ulcers: The Ratio of MMP-1 to TIMP-1 Is a Predictor of Wound Healing. Diabet. Med. 2008, 25, 419–426. [Google Scholar] [CrossRef]
- Nwomeh, B.C.; Liang, H.X.; Cohen, I.K.; Yager, D.R. MMP-8 Is the Predominant Collagenase in Healing Wounds and Nonhealing Ulcers. J. Surg. Res. 1999, 81, 189–195. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix Metalloproteinases: The Sculptors of Chronic Cutaneous Wounds. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef]
- Karauzum, H.; Datta, S.K. Adaptive Immunity against Staphylococcus Aureus. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 409, pp. 419–439. [Google Scholar]
- Infante-Duarte, C.; Kamradt, T. Th1/Th2 Balance in Infection. Springer Semin. Immunopathol. 1999, 21, 317–338. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Avery, J.T.; Gil, J.; Solis, M.R.; Jozic, I.; Kimmerling, K.A.; Mowry, K.C. Protection with a Collagen Wound Matrix Containing Polyhexamethylene Biguanide Supports Innate Wound Healing in Biofilm-infected Porcine Wounds. Wound Repair Regen. 2025, 33, e70025. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; De Biasi, M.G.; De Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus Aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Bottagisio, M.; D’Arrigo, D.; Talò, G.; Bongio, M.; Ferroni, M.; Boschetti, F.; Moretti, M.; Lovati, A.B. Achilles Tendon Repair by Decellularized and Engineered Xenografts in a Rabbit Model. Stem Cells Int. 2019, 2019, 5267479. [Google Scholar] [CrossRef]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A Systematic Review of Animal Models for Staphylococcus Aureus Osteomyelitis. Eur. Cells Mater. 2014, 27, 196–212. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Smeets, B.; Nijs, S.; Hoekstra, H. Infection after Fracture Fixation of the Tibia: Analysis of Healthcare Utilization and Related Costs. Injury 2017, 48, 1204–1210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrallah, R.A.; Kimmerling, K.A.; Cook, J.L.; Bozynski, C.C.; Stoker, A.M.; Stannard, J.P.; Mowry, K.C. Purified Native Collagen Extracellular Matrix Plus Polyhexamethylene Biguanide Functions as a Barrier to Protect Complex Wounds in an In Vivo Model. Int. J. Mol. Sci. 2025, 26, 9195. https://doi.org/10.3390/ijms26189195
Nasrallah RA, Kimmerling KA, Cook JL, Bozynski CC, Stoker AM, Stannard JP, Mowry KC. Purified Native Collagen Extracellular Matrix Plus Polyhexamethylene Biguanide Functions as a Barrier to Protect Complex Wounds in an In Vivo Model. International Journal of Molecular Sciences. 2025; 26(18):9195. https://doi.org/10.3390/ijms26189195
Chicago/Turabian StyleNasrallah, Rami A., Kelly A. Kimmerling, James L. Cook, Chantelle C. Bozynski, Aaron M. Stoker, James P. Stannard, and Katie C. Mowry. 2025. "Purified Native Collagen Extracellular Matrix Plus Polyhexamethylene Biguanide Functions as a Barrier to Protect Complex Wounds in an In Vivo Model" International Journal of Molecular Sciences 26, no. 18: 9195. https://doi.org/10.3390/ijms26189195
APA StyleNasrallah, R. A., Kimmerling, K. A., Cook, J. L., Bozynski, C. C., Stoker, A. M., Stannard, J. P., & Mowry, K. C. (2025). Purified Native Collagen Extracellular Matrix Plus Polyhexamethylene Biguanide Functions as a Barrier to Protect Complex Wounds in an In Vivo Model. International Journal of Molecular Sciences, 26(18), 9195. https://doi.org/10.3390/ijms26189195





