An Integrated Transcriptomic and Proteomic Approach Uncovers the Molecular Mechanisms of Hypoosmotic Adaptation in Scylla paramamosain Megalopa
Abstract
1. Introduction
2. Results
2.1. Overview of Transcriptome and Proteome Sequencing and Assembly
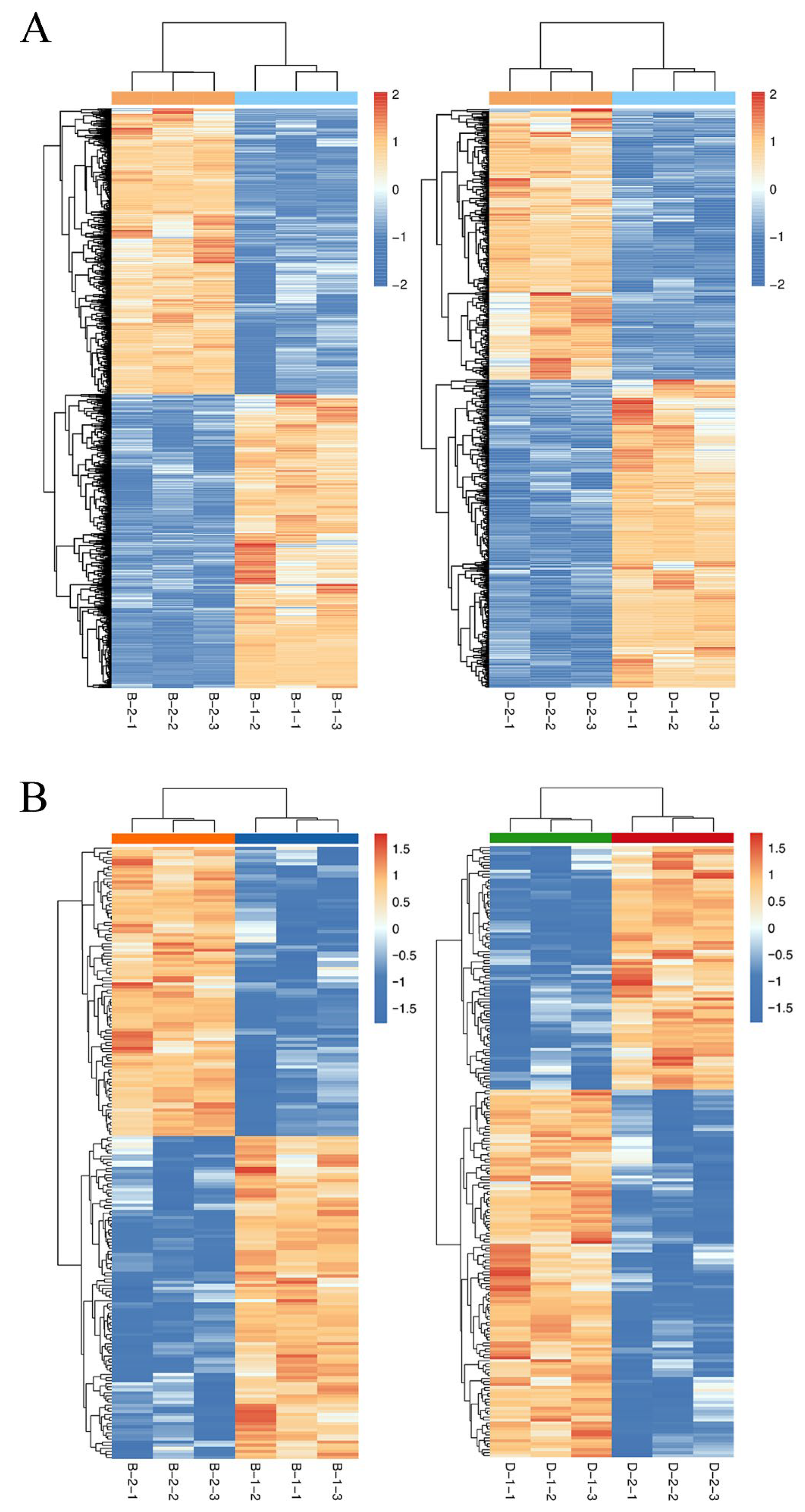
2.2. Principal Component Analysis (PCA)
2.3. Differential Expression Analysis of Transcriptomic and Proteomic Data
2.4. GO Enrichment of DEGs and DEPs
2.5. KEGG Pathway Enrichment of DEGs and DEPs
2.6. Integrated Analysis of Transcriptome-Proteome
3. Discussion
4. Materials and Methods
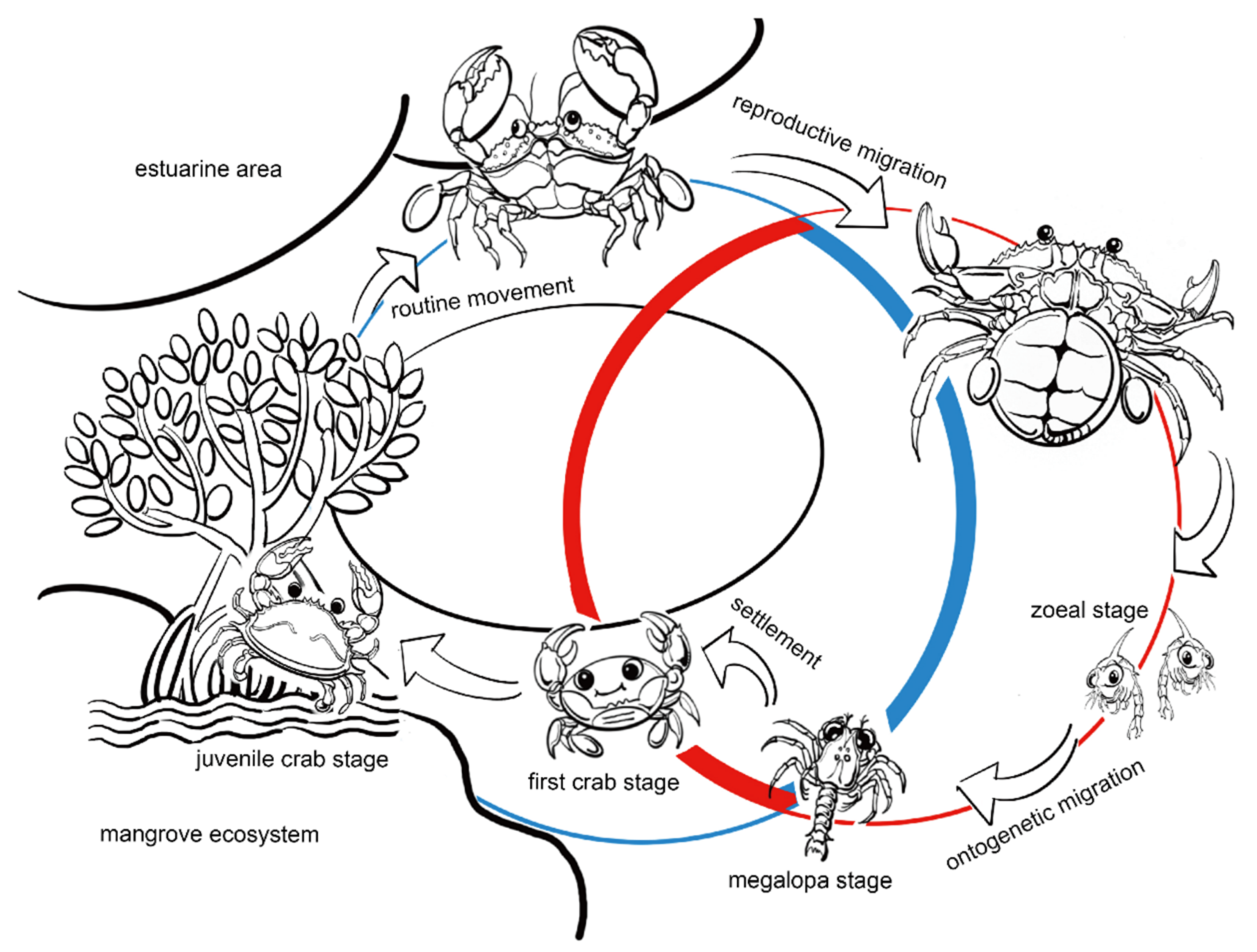
4.1. Salinity Challenge and Samples Collection
4.2. Transcriptomic Analysis
4.3. Proteomic Analysis
4.4. Functional Annotation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anger, K. Salinity as a key parameter in the larval biology of decapod crustaceans. Invertebr. Reprod. Dev. 2003, 43, 29–45. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 2012, 334, 12–23. [Google Scholar] [CrossRef]
- Mo, N.; Feng, T.; Zhu, D.; Liu, J.; Shao, S.; Han, R.; Lu, W.; Zhan, P.; Cui, Z. Analysis of adaptive molecular mechanisms in response to low salinity in antennal gland of mud crab, Scylla paramamosain. Heliyon 2024, 10, e25556. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.P.; Lucu, C.; Onken, H.; Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 2012, 3, 431. [Google Scholar] [CrossRef] [PubMed]
- Lucu, C.; Towle, D.W. Na+/K+-ATPase in gills of aquatic crustacea. Comp. Biochem. Physiol. Part A-Mol. Integr. Physiol. 2003, 135, 195–214. [Google Scholar] [CrossRef]
- Freire, C.A.; Onken, H.; McNamara, J.C. A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. Part A-Mol. Integr. Physiol. 2008, 151, 272–304. [Google Scholar] [CrossRef]
- Tsai, J.; Lin, H. V-type H+-ATPase and Na+,K+-ATPase in the gills of 13 euryhaline crabs during salinity acclimation. J. Exp. Biol. 2007, 210, 620–627. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.; Wei, H.; Lu, J.; Mu, C.; Wang, C. Transcriptomic analysis of adaptive mechanisms in response to sudden salinity drop in the mud crab, Scylla paramamosain. BMC Genom. 2018, 19, 421. [Google Scholar] [CrossRef]
- Niu, J.; Hu, X.L.; Ip, J.C.H.; Ma, K.Y.; Tang, Y.; Wang, Y.; Qin, J.; Qiu, J.-W.; Chan, T.F.; Chu, K.H. Multi-omic approach provides insights into osmoregulation and osmoconformation of the crab Scylla paramamosain. Sci. Rep. 2020, 10, 21771. [Google Scholar] [CrossRef]
- Thabet, R.; Ayadi, H.; Koken, M.; Leignel, V. Homeostatic responses of crustaceans to salinity changes. Hydrobiologia 2017, 799, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Fang, S.; Li, S.; Zheng, H.; Zhang, Y.; Ikhwanuddin, M.; Ma, H. mRNA profile provides novel insights into stress adaptation in mud crab megalopa, Scylla paramamosain after salinity stress. BMC Genom. 2020, 21, 559. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Wang, Y.; Gao, B.; Chen, P.; Li, J. Transcriptome analysis of Portunus trituberculatus in response to salinity stress provides insights into the molecular basis of osmoregulation. PLoS ONE 2013, 8, e82155. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X.; Fu, Y.; Fang, W.; Wang, C. Whole-body transcriptome analysis provides insights into the cascade of sequential expression events involved in growth, immunity, and metabolism during the molting cycle in Scylla paramamosain. Sci. Rep. 2022, 12, 11395. [Google Scholar] [CrossRef] [PubMed]
- Le Vay, L.; Ut, V.N.; Walton, M. Population ecology of the mud crab Scylla paramamosain (Estampador) in an estuarine mangrove system: A mark-recapture study. Mar. Biol. 2007, 151, 1127–1135. [Google Scholar] [CrossRef]
- Ma, K.; Liu, Z.; Qiao, G.; Ma, L.; Zhang, F.; Zhao, M.; Ma, C.; Wang, W. Effects of four diets on the metabolism of megalopa metamorphosis of the mud crab, Scylla paramamosain. Front. Mar. Sci. 2023, 10, 1276717. [Google Scholar] [CrossRef]
- Xu, L.; Ma, K.; Zhang, F.; Wang, W.; Ma, L.; Jin, Z.; Zhao, M.; Chen, W.; Fu, Y.; Ma, C.; et al. Observations on the embryonic development of the mud crab, Scylla paramamosain. Front. Mar. Sci. 2023, 10, 1296509. [Google Scholar] [CrossRef]
- Li, Y.; Ai, C.; Liu, L. Mud crab, Scylla paramamosain China’s leading maricultured crab. In Aquaculture in China: Success Stories and Modern Trends; Gui, J.F., Tang, Q.S., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 226–233. [Google Scholar]
- Ye, H.; Tao, Y.; Wang, G.; Lin, Q.; Chen, X.; Li, S. Experimental nursery culture of the mud crab Scylla paramamosain (Estampador) in China. Aquac. Int. 2011, 19, 313–321. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Wang, X.; Zhang, F.; Ma, C.; Zhao, M.; Ma, K.; Ma, L. Feeding rhythm of the zoea larvae of Scylla paramamosain: The dynamic feeding rhythm is not completely synchronized with photoperiod. Heliyon 2024, 10, e29826. [Google Scholar] [CrossRef]
- Xu, L.; Hu, X.; Ma, C.; Wang, W.; Zhang, F.; Zhao, M.; Xu, J.; Lv, X.; Su, Z.; Ma, L.; et al. Integration of metagenome and full-length 16S rRNA gene sequencing of the mariculture environmental bacterial community reveal potential pathogenic bacteria in broodstock breeding of the mud crab, Scylla paramamosain. Aquac. Rep. 2025, 43, 102880. [Google Scholar] [CrossRef]
- Farella, I.; D’Amato, G.; Orellana-Manzano, A.; Segura, Y.; Vitale, R.; Clodoveo, M.L.; Corbo, F.; Faienza, M.F. “OMICS” in human milk: Focus on biological effects on bone homeostasis. Nutrients 2024, 16, 3921. [Google Scholar] [CrossRef]
- Yang, M.; Han, Y.; Chang, Y.; Li, C.; Niu, D. Transcriptomic and metabolomic analyses reveal response mechanisms of Sinonovacula constricta to saline-alkalinity stresses. Mar. Biotechnol. 2025, 27, 68. [Google Scholar] [CrossRef]
- Gao, B.; Sun, D.; Lv, J.; Ren, X.; Liu, P.; Li, J. Transcriptomic analysis provides insight into the mechanism of salinity adjustment in swimming crab Portunus trituberculatus. Genes Genom. 2019, 41, 961–971. [Google Scholar] [CrossRef]
- Moore, J.M.; Bell, E.L.; Hughes, R.O.; Garfield, A.S. ABC transporters: Human disease and pharmacotherapeutic potential. Trends Mol. Med. 2023, 29, 152–172. [Google Scholar] [CrossRef]
- Gagnon, É.; Forbush, B.; Caron, L.; Isenring, P. Functional comparison of renal Na-K-Cl cotransporters between distant species. Am. J. Physiol.-Cell Physiol. 2003, 284, C365–C370. [Google Scholar] [CrossRef]
- Luquet, C.M.; Weihrauch, D.; Senek, M.; Towle, D.W. Induction of branchial ion transporter mRNA expression during acclimation to salinity change in the euryhaline crab Chasmagnathus granulatus. J. Exp. Biol. 2005, 208, 3627–3636. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Si, L.; Hu, D. Ion transport signal pathways mediated by neurotransmitter (biogenic amines) of Litopenaeus vannamei under low salinity challenge. J. Ocean. Univ. China 2019, 18, 210–218. [Google Scholar] [CrossRef]
- Kaeodee, M.; Pongsomboon, S.; Tassanakajon, A. Expression analysis and response of Penaeus monodon 14-3-3 genes to salinity stress. Comp. Biochem. Physiol. Part B-Biochem. Mol. Biol. 2011, 159, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Mo, N.; Shao, S.C.; Cui, Z.X.; Bao, C.C. Roles of eyestalk in salinity acclimatization of mud crab (Scylla paramamosain) by transcriptomic analysis. Comp. Biochem. Physiol. Part D-Genom. Proteom. 2024, 52, 101276. [Google Scholar] [CrossRef]
- Wang, H.; Wei, H.; Tang, L.; Lu, J.; Mu, C.; Wang, C. A proteomics of gills approach to understanding salinity adaptation of Scylla paramamosain. Gene 2018, 677, 119–131. [Google Scholar] [CrossRef]
- Gao, W.; Tan, B.; Mai, K.; Chi, S.; Liu, H.; Dong, X.; Yang, Q. Profiling of differentially expressed genes in hepatopancreas of white shrimp (Litopenaeus vannamei) exposed to long-term low salinity stress. Aquaculture 2012, 364, 186–191. [Google Scholar] [CrossRef]
- Guo, Z.; Zuo, J.; Feng, J.; Li, J.; Zhang, S.; Ma, K. Impact of titanium dioxide-graphene oxide (TiO2-GO) composite nanoparticle on the juveniles of the giant river prawn, Macrobrachium rosenbergii: Physio-biochemistry and transcriptional response. Mar. Biotechnol. 2023, 25, 45–56. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, M.; Li, Y.; Wu, D.; Liu, Z.; Jiang, Q.; Zhao, Y. Effects of salinity acclimation on the growth performance, osmoregulation and energy metabolism of the oriental river prawn, Macrobrachium nipponense (De Haan). Aquac. Res. 2019, 50, 685–693. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish Shellfish Immunol. 2017, 62, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, E.; Liu, Y.; Wang, X.; Qin, J.G.; Chen, L. Comparative proteome analysis of the hepatopancreas from the Pacific white shrimp Litopenaeus vannamei under long-term low salinity stress. J. Proteom. 2017, 162, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Choi, C.Y. Temporal changes in physiological responses of bay scallop: Performance of antioxidant mechanism in Argopecten irradians in response to sudden changes in habitat salinity. Antioxidants 2021, 10, 1673. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I. Growth retardation and suppression of ubiquitin-dependent catabolic processes in the brackish water clam Corbicula japonica in response to salinity changes and bioaccumulation of toxic heavy metals. Environ. Pollut. 2023, 337, 122554. [Google Scholar] [CrossRef]
- Santos, J.L.; Nick, F.; Adhitama, N.; Fields, P.D.; Stillman, J.H.; Kato, Y.; Watanabe, H.; Ebert, D. Trehalose mediates salinity-stress tolerance in natural populations of a freshwater crustacean. Curr. Biol. 2024, 34, 4160–4169. [Google Scholar] [CrossRef]
- Lu, H.; Chen, W.; Peng, K.; Huang, M.; Zhao, J.; Chen, X.; Sun, Y.; Ruan, Z.; Li, C.; Liu, D.; et al. Rapid adaptive and acute stimulatory responses to low salinity stress in Pacific white shrimp (Litopenaeus vannamei): Insights from integrative transcriptomic and proteomic analysis. Comp. Biochem. Physiol. Part D-Genom. Proteom. 2023, 48, 101149. [Google Scholar] [CrossRef]
- Li, Y.; Si, M.; Jiang, S.; Yang, Q.; Jiang, S.; Yang, L.; Huang, J.; Chen, X.; Zhou, F.; Li, E. Transcriptome and molecular regulatory mechanisms analysis of gills in the black tiger shrimp Penaeus monodon under chronic low-salinity stress. Front. Physiol. 2023, 14, 1118341. [Google Scholar] [CrossRef]
- Giomi, F.; Beltramini, M. The molecular heterogeneity of hemocyanin: Its role in the adaptive plasticity of Crustacea. Gene 2007, 398, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Jaenicke, E. Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev. Comp. Immunol. 2004, 28, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Ahmed, H.; Du, S.J.; Henrikson, D. Galectins in teleost fish: Zebrafish (Danio rerio) as a model species to address their biological roles in development and innate immunity. Glycoconj. J. 2004, 21, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Y.; Guo, X.; Xu, L.; Lei, P.; Luo, Q.; Liu, J.; Li, W.; Tao, L.; Meng, F. The lectin gene TRpL1 of tetraploid Robinia pseudoacacia L. response to salt stress. J. For. Res. 2023, 34, 497–505. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, A.; Xia, D.; Wang, X.; Sun, Z.; Shang, X.; Yang, Z.; Qu, J. Immunological characterization and expression of lily-type lectin in response to environmental stress in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2016, 58, 323–331. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Somboonwiwat, K.; Amparyup, P. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev. Comp. Immunol. 2015, 48, 324–341. [Google Scholar] [CrossRef]
- Shekhar, M.S.; Kiruthika, J.; Ponniah, A.G. Identification and expression analysis of differentially expressed genes from shrimp (Penaeus monodon) in response to low salinity stress. Fish Shellfish Immunol. 2013, 35, 1957–1968. [Google Scholar] [CrossRef]
- Wang, H.; Wei, H.; Tang, L.; Lu, J.; Mu, C.; Wang, C. Gene identification and characterization of correlations for DEPs_DEGs same trend responding to salinity adaptation in Scylla paramamosain. Int. J. Genom. 2019, 2019, 7940405. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]



| Sample | Raw Reads (M) | Raw Bases (G) | Clean Reads (M) | Clean Bases (G) | Valid Bases (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|---|
| B-1-2 | 48.37 | 7.13 | 47 | 6.92 | 97.17 | 97 | 53.31 |
| B-1-2 | 44.78 | 6.59 | 43.42 | 6.39 | 96.95 | 96.95 | 53.89 |
| B-1-3 | 48.28 | 7.1 | 46.79 | 6.88 | 96.92 | 96.94 | 53.33 |
| B-2-1 | 47.41 | 7 | 46.19 | 6.82 | 97.42 | 97 | 52.74 |
| B-2-2 | 48.07 | 7.08 | 46.66 | 6.87 | 97.07 | 97.01 | 52.44 |
| B-2-3 | 48.23 | 7.13 | 47.05 | 6.96 | 97.55 | 97.13 | 52.58 |
| D-1-1 | 43.75 | 6.45 | 42.52 | 6.27 | 97.19 | 96.92 | 52.27 |
| D-1-2 | 49.57 | 7.28 | 47.94 | 7.04 | 96.72 | 96.82 | 52.75 |
| D-1-3 | 50.61 | 7.34 | 48.41 | 7.02 | 95.65 | 96.91 | 54.07 |
| D-2-1 | 46.18 | 6.79 | 44.72 | 6.57 | 96.83 | 96.91 | 53.67 |
| D-2-2 | 48.66 | 7.16 | 47.19 | 6.94 | 96.97 | 97.02 | 52.89 |
| D-2-3 | 49.34 | 7.26 | 47.78 | 7.03 | 96.84 | 96.83 | 52.36 |
| Comparison Group | Upregulated DEGs | Downregulated DEGs | Upregulated DEPs | Downregulated DEPs |
|---|---|---|---|---|
| B-1 vs. B-2 | 1332 | 1295 | 105 | 94 |
| D-1 vs. D-2 | 390 | 343 | 124 | 82 |
| UniProt ID | DEGs | DEPs | Annotation | |||||
|---|---|---|---|---|---|---|---|---|
| log2FC | p-Value | Regulation | log2FC | p-Value | Regulation | |||
| B-1 vs. B-2 | Q2V6T8 | −2.55 | 4.94 × 10−170 | Down | 0.24 | 0.0056 | Up | cuticle protein AMP16.5 |
| D2DST4 | 1.10 | 5.64 × 10−70 | Up | 0.62 | 0.0053 | Up | serine protease | |
| D6N3A2 | 1.38 | 1.07 × 10−95 | Up | 0.52 | 0.00089 | Up | serine proteinase | |
| F8UN04 | −1.11 | 1.91 × 10−78 | Down | −0.34 | 0.0044 | Down | chymotrypsin | |
| H6ACV4 | 1.29 | 7.72 × 10−77 | Up | 0.43 | 0.0073 | Up | clip domain serine proteinase 1 | |
| I1VGP3 | 1.70 | 1.99 × 10−110 | Up | 1.64 | 0.00029 | Up | anti-lipopolysaccharide factor 1 | |
| A0A0U1ZZP8 | −1.03 | 5.06 × 10−47 | Down | −0.67 | 0.00065 | Down | Hemocyanin subunit 4 | |
| A0A3S5XFQ9 | −1.16 | 2.00 × 10−8 | Down | −0.44 | 0.00091 | Down | carboxylic ester hydrolase 4 | |
| A0A3S5WLH8 | 1.20 | 7.40 × 10−17 | Up | 0.46 | 0.0012 | Up | carboxylic ester hydrolase 6 | |
| A0A343T7I5 | 1.17 | 1.92 × 10−44 | Up | 1.045 | 0.0032 | Up | type I crustin 6 | |
| A0A2K9UW22 | 1.36 | 1.65 × 10−40 | Up | 1.97 | 0.0040 | Up | type I crustin 3 | |
| G0M6G3 | 1.39 | 4.41 × 10−55 | Up | 0.24 | 0.037 | Up | nitric oxide synthase | |
| A0A5B7CRZ2 | −1.36 | 3.32 × 10−27 | Down | −0.15 | 0.011 | Down | DNA replication licensing factor MCM4 | |
| A0A5B7DHC6 | −1.78 | 9.16 × 10−48 | Down | 0.14 | 0.012 | Up | cuticle protein 18.6 | |
| A0A5B7FPJ8 | −1.55 | 6.47 × 10−52 | Down | −0.38 | 0.044 | Down | chitin binding protein type-2 | |
| A0A5B7GC89 | 1.46 | 5.43 × 10−117 | Up | 0.23 | 0.029 | Up | histone H5 | |
| A0A6G9W3K2 | −1.66 | 1.45 × 10−106 | Down | −0.61 | 0.0055 | Down | carboxypeptidase B | |
| A0A858Z4L6 | 1.04 | 3.67 × 10−40 | Up | 1.98 | 3.63 × 10−6 | Up | type I crustin 7 | |
| D-1 vs. D-2 | K9LU24 | 1.32 | 1.16 × 10−11 | Up | 0.74 | 0.0036 | Up | duplex-specific nuclease |
| A0A068BC87 | 1.26 | 2.48 × 10−30 | Up | 0.71 | 0.0044 | Up | lectin 3 | |
| A0A2K9UW22 | −1.99 | 2.93 × 10−37 | Down | −0.92 | 0.00050 | Down | type I crustin 3 | |
| A0A5B7E7D2 | 1.41 | 2.01 × 10−37 | Up | 0.42 | 0.022 | Up | digestive cysteine proteinase 3 | |
| A0A6G9W3Z8 | 1.04 | 1.61 × 10−26 | Up | −1.09 | 4.76 × 10−5 | Down | trypsin | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, N.; Liu, Z.; Li, Y.; Zhang, F.; Ma, C.; Wang, X.; Xu, J.; Ma, L.; Ma, K.; Wang, W. An Integrated Transcriptomic and Proteomic Approach Uncovers the Molecular Mechanisms of Hypoosmotic Adaptation in Scylla paramamosain Megalopa. Int. J. Mol. Sci. 2025, 26, 9188. https://doi.org/10.3390/ijms26189188
Qiao N, Liu Z, Li Y, Zhang F, Ma C, Wang X, Xu J, Ma L, Ma K, Wang W. An Integrated Transcriptomic and Proteomic Approach Uncovers the Molecular Mechanisms of Hypoosmotic Adaptation in Scylla paramamosain Megalopa. International Journal of Molecular Sciences. 2025; 26(18):9188. https://doi.org/10.3390/ijms26189188
Chicago/Turabian StyleQiao, Ning, Zhiqiang Liu, Yuanyuan Li, Fengying Zhang, Chunyan Ma, Xueyang Wang, Jiayuan Xu, Lingbo Ma, Keyi Ma, and Wei Wang. 2025. "An Integrated Transcriptomic and Proteomic Approach Uncovers the Molecular Mechanisms of Hypoosmotic Adaptation in Scylla paramamosain Megalopa" International Journal of Molecular Sciences 26, no. 18: 9188. https://doi.org/10.3390/ijms26189188
APA StyleQiao, N., Liu, Z., Li, Y., Zhang, F., Ma, C., Wang, X., Xu, J., Ma, L., Ma, K., & Wang, W. (2025). An Integrated Transcriptomic and Proteomic Approach Uncovers the Molecular Mechanisms of Hypoosmotic Adaptation in Scylla paramamosain Megalopa. International Journal of Molecular Sciences, 26(18), 9188. https://doi.org/10.3390/ijms26189188





