Bone Marrow Mononuclear Cells Administration Restore Lysophosphatidic Acid (LPA) Levels and Cellular Signaling Axis in Rats Submitted to Renal Ischemia–Reperfusion
Abstract
1. Introduction
2. Results
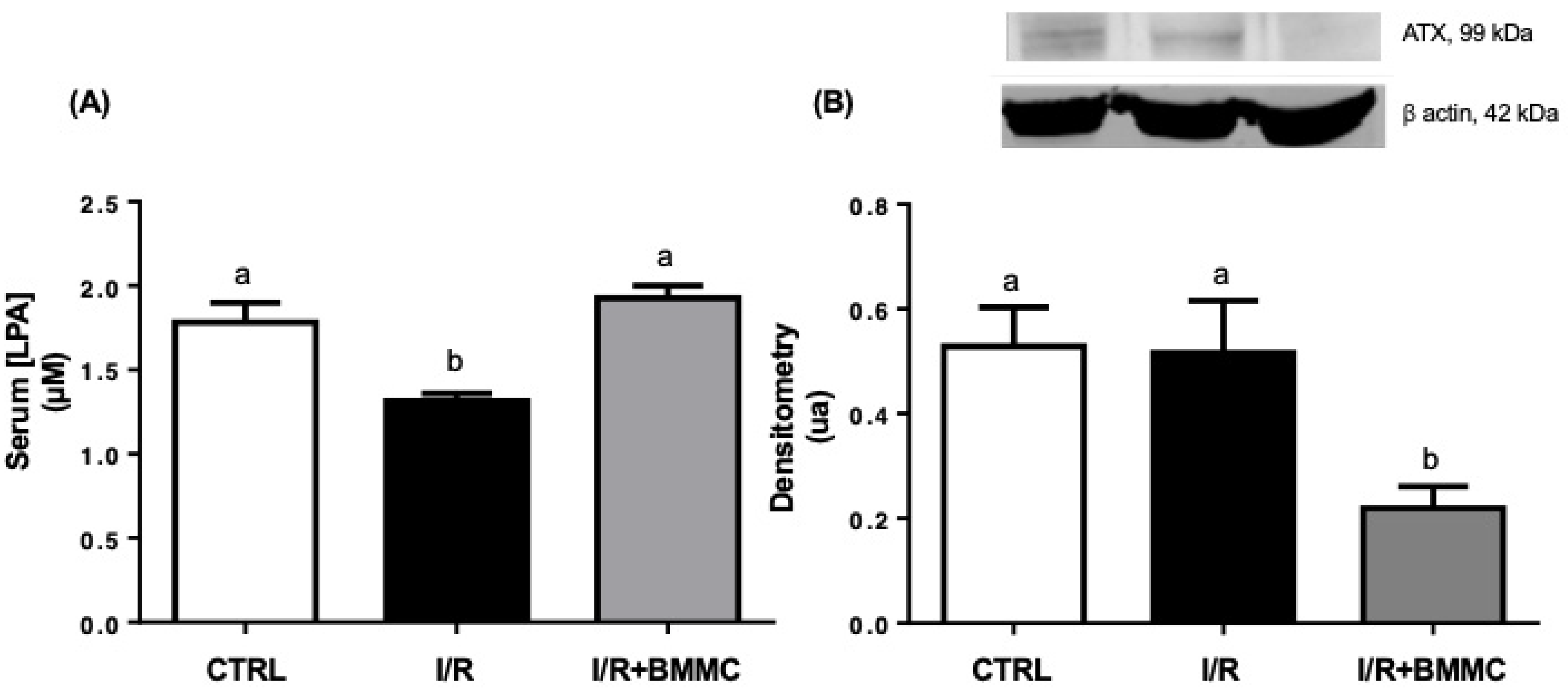
2.1. BMMC Treatment Restores Circulating LPA Levels and Modulates Renal LPA Signaling After I/R
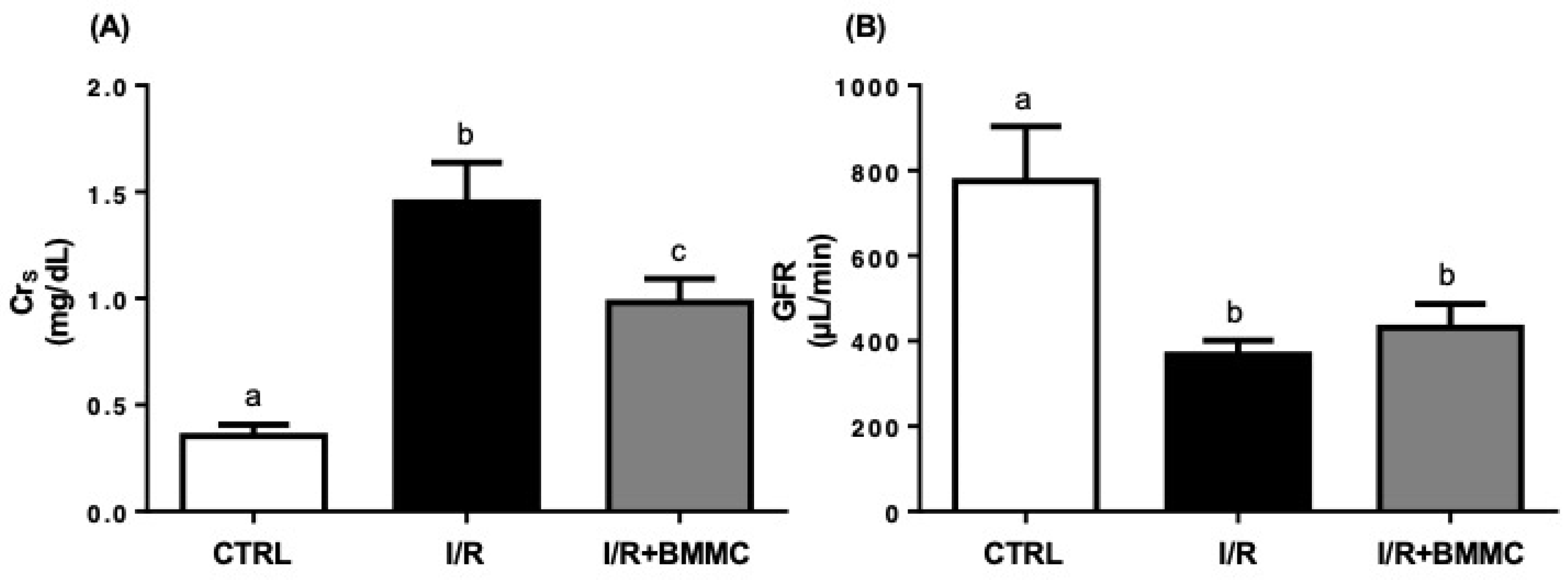
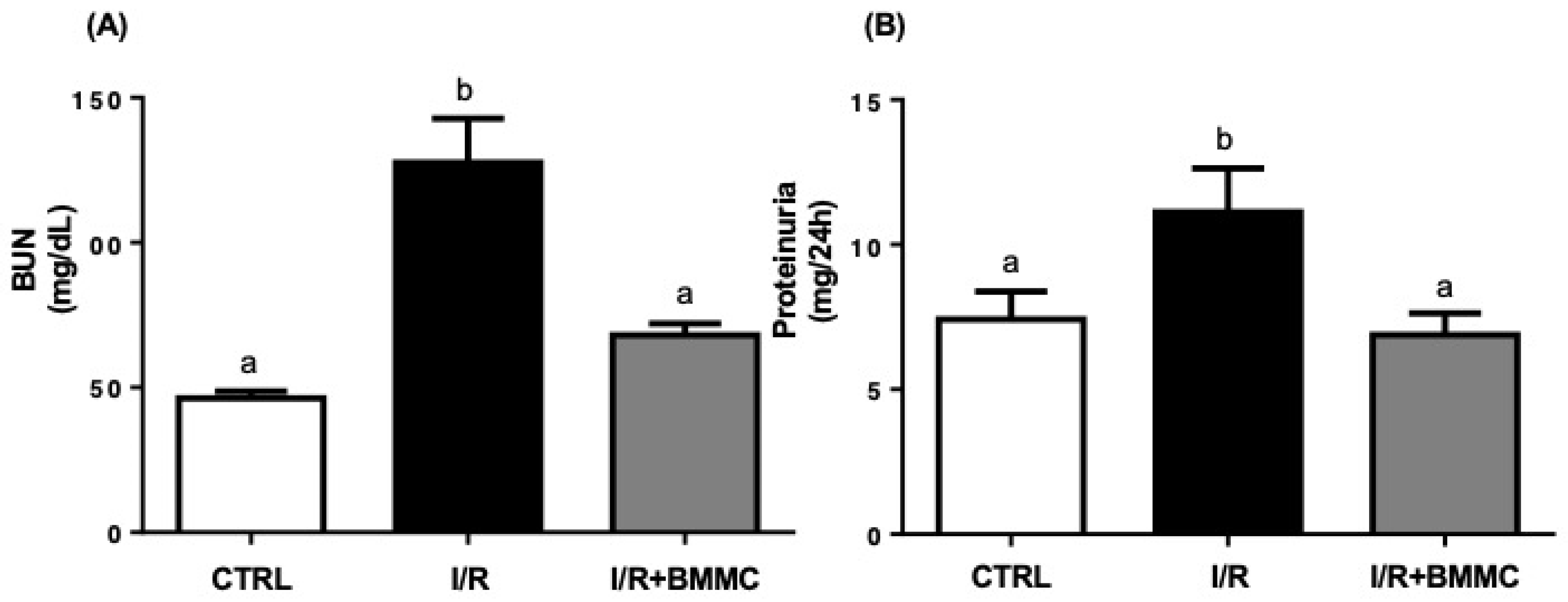
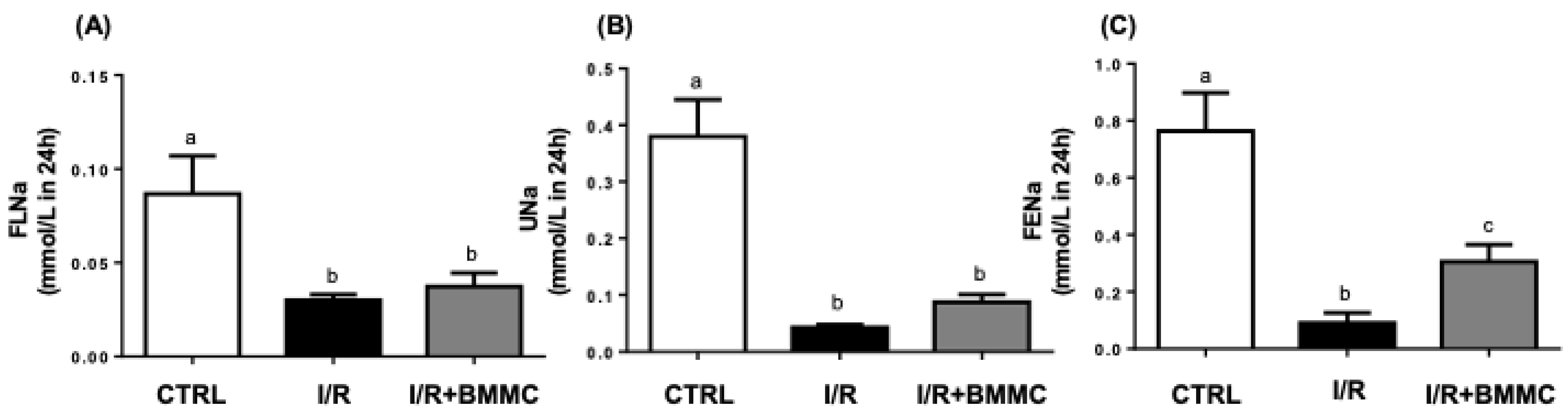
2.2. BMMC Treatment Reduces Accumulation of Nitrogenous Metabolites and Proteinuria Induced by I/R
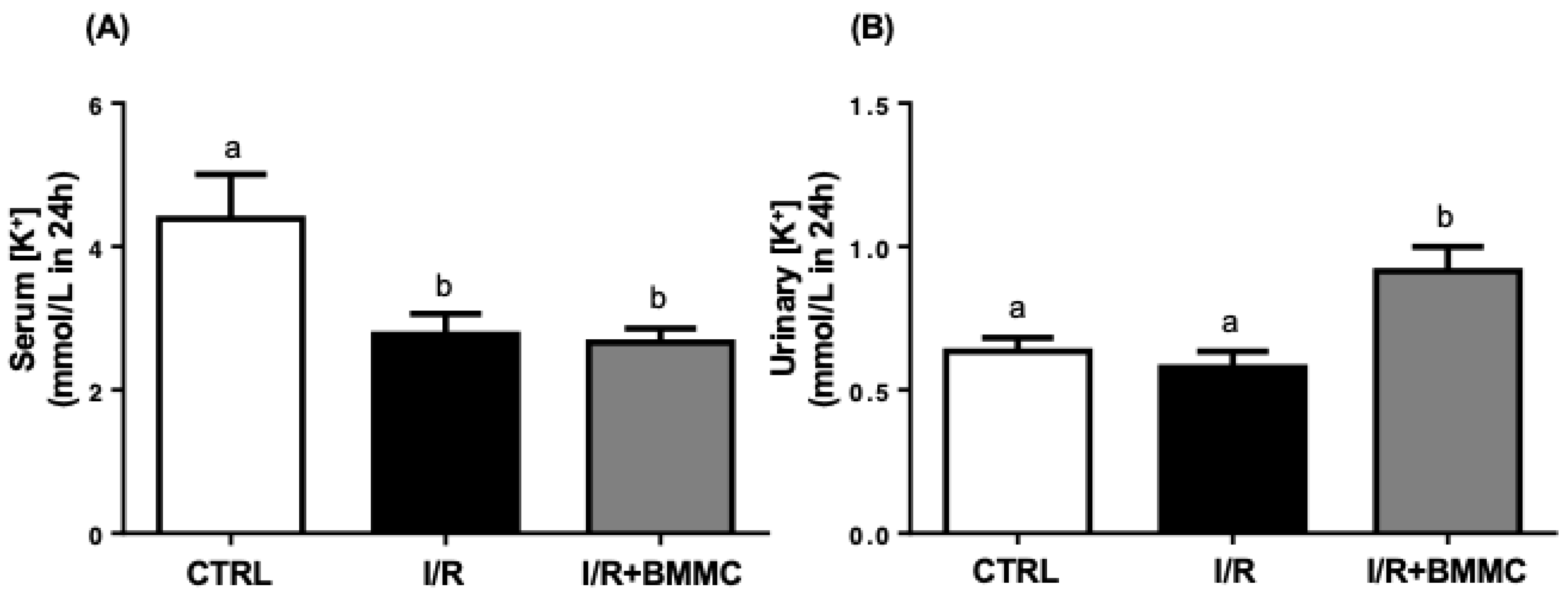
2.3. BMMC Partially Restores Electrolyte Handling Following I/R
3. Discussion
4. Material and Methods
4.1. Animal Care and Ethics
4.2. Kidney Ischemia/Reperfusion (I/R) Model
4.3. BMMC Isolation and Administration
4.4. Western Blotting
4.5. Plasma LPA Quantification
4.6. Assessment of Renal Function
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E. Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef]
- Tumlin, J.; Wali, R.; Williams, W.; Murray, P.; Tolwani, A.J.; Vinnikova, A.K.; Szerlip, H.M.; Ye, J.; Paganini, E.P.; Dworkin, L.; et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J. Am. Soc. Nephrol. 2008, 19, 1034–1040. [Google Scholar] [CrossRef]
- Roushani, J.; Thomas, D.; Oliver, M.J.; Ip, J.; Tang, Y.; Yeung, A.; Taji, L.; Cooper, R.; Magner, P.O.; Garg, A.X.; et al. Acute kidney injury requiring renal replacement therapy in people with COVID-19 disease in Ontario, Canada: A prospective analysis of risk factors and outcomes. Clin. Kidney J. 2021, 15, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.S.; Silva, J.S.; Couto, R.D.; Barreto, E.P., Jr.; Ribeiro-dos-Santos, R.; dos Santos, W.L.; Soares, M.B. Transplantation of bone marrow mononuclear cells reduces mortality and improves renal function on mercury-induced kidney injury in mice. Ren. Fail. 2013, 35, 776–781. [Google Scholar] [CrossRef][Green Version]
- Oliveira, M.; Lira, R.; Freire, T.; Luna, C.; Martins, M.; Almeida, A.; Carvalho, S.; Cortez, E.; Stumbo, A.C.; Thole, A.; et al. Bone marrow mononuclear cell transplantation rescues the glomerular filtration barrier and epithelial cellular junctions in a renovascular hypertension model. Exp. Physiol. 2019, 104, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.E.; Roemeling-van Rhijn, M.; Khairoun, M.; Lievers, E.; de Vries, D.K.; Schaapherder, A.F.; Wong, S.W.; Zwaginga, J.J.; Duijs, J.M.; van Zonneveld, A.J.; et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy 2013, 15, 663–672. [Google Scholar] [CrossRef]
- Lim, C.Y.; Han, J.I.; Kim, S.G.; Lee, C.M.; Park, H.M. Evaluation of autologous bone marrow-derived mesenchymal stem cells on renal regeneration after experimentally induced acute kidney injury in dogs. Am. J. Vet. Res. 2016, 77, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y. Current advances of stem cell-based therapy for kidney diseases. World J. Stem Cells 2021, 13, 914–933. [Google Scholar] [CrossRef]
- Torrico, S.; Hotter, G.; Játiva, S. Development of cell therapies for renal disease and regenerative medicine. Int. J. Mol. Sci. 2022, 23, 15943. [Google Scholar] [CrossRef]
- Lindoso, R.S.; Araujo, D.S.; Adão-Novaes, J.; Mariante, R.M.; Verdoorn, K.S.; Fragel-Madeira, L.; Caruso-Neves, C.; Linden, R.; Vieyra, A.; Einicker-Lamas, M. Paracrine interaction between bone marrow-derived stem cells and renal epithelial cells. Cell. Physiol. Biochem. 2011, 28, 267–278. [Google Scholar] [CrossRef]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, C.; Yang, C.; Zhang, J.; Dong, Q.; Wang, W. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018, 215, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Hasanpour, A.; Nazeri, N.; Razavi, H.; Salehi, M.; Shafei, S.; Nooshabadi, V.T.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; et al. Extracellular micro/nanovesicles rescue kidney from ischemia-reperfusion injury. J. Cell Physiol. 2019, 234, 12290–12300. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Medina, J.P.; Dodia, C.; Weng, L.; Mesaros, C.; Blair, I.A.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation via lysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. FASEB J. 2016, 30, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.J.; Humphreys, I.S.; Rudge, S.A.; Wilson, G.K.; Bhattacharya, B.; Ciaccia, M.; Hu, K.; Zhang, Q.; Mailly, L.; Reynolds, G.M.; et al. Autotaxin-lysophosphatidic acid receptor signalling regulates hepatitis C virus replication. J. Hepatol. 2017, 66, 919–929. [Google Scholar] [CrossRef]
- Hemmings, D.G.; Brindley, D.N. Signalling by lysophosphatidate and its health implications. Essays Biochem. 2020, 64, 547–563. [Google Scholar] [CrossRef]
- Park, F.; Miller, D.D. Role of lysophosphatidic acid and its receptors in the kidney. Physiol. Genomics 2017, 49, 659–666. [Google Scholar] [CrossRef]
- Pradère, J.P.; Gonzalez, J.; Klein, J.; Valet, P.; Grès, S.; Salant, D.; Bascands, J.L.; Saulnier-Blache, J.S.; Schanstra, J.P. Lysophosphatidic acid and renal fibrosis. Biochim. Biophys. Acta 2008, 1781, 582–587. [Google Scholar] [CrossRef]
- Geng, H.; Lan, R.; Singha, P.K.; Gilchrist, A.; Weinreb, P.H.; Violette, S.M.; Weinberg, J.M.; Saikumar, P.; Venkatachalam, M.A. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF Through LPA2- and Gαq-mediated Rho and αvβ6 integrin-dependent activation of TGF-β. Am. J. Pathol. 2012, 181, 1236–1249. [Google Scholar] [CrossRef]
- Prade, J.P.; Klein, J.; Gre, S.; Guigne, C.; Neau, E.; Valet, P.; Calise, D.; Chun, J.; Bascands, J.L.; Schanstra, J.P. LPA1 receptor activation promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2007, 18, 3110–3118. [Google Scholar] [CrossRef]
- de Vries, B.; Matthijsen, R.A.; van Bijnen, A.A.; Wolfs, T.G.; Buurman, W.A. Lysophosphatidic acid prevents renal ischemia-reperfusion injury by inhibition of apoptosis and complement activation. Am. J. Pathol. 2003, 163, 47–56. [Google Scholar] [CrossRef]
- Chen, J.; Baydoun, A.R.; Xu, R.; Deng, L.; Liu, X.; Zhu, W.; Shi, L.; Cong, X.; Hu, S.; Chen, X. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells 2008, 26, 135–145. [Google Scholar] [CrossRef]
- An, X.; Zhong, C.; Han, B.; Chen, E.; Zhu, Q.; Yang, Y.; Li, R.; Yang, R.; Zha, D.; Han, Y. Lysophosphatidic acid exerts protective effects on HEI-OC1 cells against cytotoxicity of cisplatin by decreasing apoptosis, excessive autophagy, and accumulation of ROS. Cell Death Discov. 2023, 9, 415. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, H.; Li, M. Emerging roles of lysophosphatidic acid in macrophages and inflammatory diseases. Int. J. Mol. Sci. 2023, 24, 12524. [Google Scholar] [CrossRef]
- Valdés Rives, S.A.; González Arenas, A. Autotaxin–lysophosphatidic acid: From inflammation to cancer development. Mediators Inflamm. 2017, 2017, 9173090. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; Spohr, T.C.L.D.S.; Amaral, R.F.D.; Fonseca, A.C.C.D.; Garcia, C.; Mendes, F.D.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef]
- van Leeuwen, F.N.; Giepmans, B.N.; van Meeteren, L.A.; Moolenaar, W.H. Lysophosphatidic acid: Mitogen and motility factor. Biochem. Soc. Trans. 2003, 31, 1209–1212. [Google Scholar] [CrossRef]
- Gardell, S.E.; Dubin, A.E.; Chun, J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol. Med. 2006, 12, 65–75. [Google Scholar] [CrossRef]
- Meyer Zu Heringdorf, D.; Jakobs, K.H. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta 2007, 1768, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Kidney disease modeling in rodents: Ischemia-reperfusion injury model. Pathophysiology 2018, 25, 213–221. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. Immune cells in experimental acute kidney injury. Nat. Rev. Nephrol. 2015, 11, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Cullen-McEwen, L.A.; Armitage, J.A.; Nyengaard, J.R.; Moritz, K.M.; Bertram, J.F. Estimating nephron number in the developing rat kidney. Am. J. Physiol. Renal Physiol. 2011, 300, F139–F146. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Le Clef, N.; Ver-Hulst, A.; D’Haese, P.C.; Vervaet, B.A. Unilateral ureteral obstruction: A model of renal fibrosis in mice and rats. Nephrol. Dial. Transplant. 2016, 31, 325–332. [Google Scholar] [CrossRef][Green Version]
- Zuk, A.; Bonventre, J.V. Acute kidney injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Multivariate Data Analysis, 17th ed.; Bookman, Ed.; Kennesaw State University: Kennesaw, GA, USA, 2009. [Google Scholar]
- Beiral, H.J.; Rodrigues-Ferreira, C.; Fernandes, A.M.; Gonsalez, S.R.; Mortari, N.C.; Takiya, C.M.; Sorenson, M.M.; Freitas, C.F.; Galina, A.; Vieyra, A. The impact of stem cells on electron fluxes, proton translocation, and ATP synthesis in kidney mitochondria after ischemia/reperfusion. Cell Transplant. 2014, 23, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Barreira, A.L.; Takiya, C.M.; Castiglione, R.C.; Maron-Gutierrez, T.; Barbosa, C.M.; Ornellas, D.S.; Verdoorn, K.S.; Pascarelli, B.M.L.; Borojevic, R.; Lamas, M.R.; et al. Bone marrow mononuclear cells attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells after unilateral ureteral obstruction. Cell. Physiol. Biochem. 2009, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Verdoorn, K.S.; Lindoso, R.S.; Lowe, J.; Lara, L.S.; Vieyra, A.; Einicker-Lamas, M. Bone marrow mononuclear cells shift bioactive lipid pattern in injured kidney towards tissue repair in rats with unilateral ureteral obstruction. Nephrol. Dial. Transplant. 2010, 25, 3867–3874. [Google Scholar] [CrossRef]
- Morigi, M.; De Coppi, P. Cell therapy for kidney injury: Different options and mechanisms—Mesenchymal and amniotic fluid stem cells. Nephron Exp. Nephrol. 2014, 126, 59. [Google Scholar] [CrossRef] [PubMed]
- Bochon, B.; Kozubska, M.; Surygała, G.; Witkowska, A.; Kuźniewicz, R.; Grzeszczak, W.; Wystrychowski, G. Mesenchymal stem cells—Potential applications in kidney diseases. Int. J. Mol. Sci. 2019, 20, 2462. [Google Scholar] [CrossRef]
- Song, J.J.; Guyette, J.P.; Gilpin, S.E.; Gonzalez, G.; Vacanti, J.P.; Ott, H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013, 19, 646–651. [Google Scholar] [CrossRef]
- Ornellas, F.M.; Ornellas, D.S.; Martini, S.V.; Castiglione, R.C.; Ventura, G.M.; Rocco, P.R.; Gutfilen, B.; Souza, S.A.; Takiya, C.M.; Morales, M.M. Bone marrow-derived mononuclear cell therapy accelerates renal ischemia-reperfusion injury recovery by modulating inflammatory, antioxidant and apoptotic related molecules. Cell. Physiol. Biochem. 2017, 41, 1736–1752. [Google Scholar] [CrossRef]
- Molinas, S.M.; Trumper, L.; Serra, E.; Elías, M.M. Evolution of renal function and Na+,K+-ATPase expression during ischaemia-reperfusion injury in rat kidney. Mol. Cell. Biochem. 2006, 287, 33–42. [Google Scholar] [CrossRef]
- Takeda, R.; Nishimatsu, H.; Suzuki, E.; Satonaka, H.; Nagata, D.; Oba, S.; Sata, M.; Takahashi, M.; Yamamoto, Y.; Kadowaki, T.; et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 113–121. [Google Scholar] [CrossRef]
- Kusch, A.; Hoff, U.; Bubalo, G.; Zhu, Y.; Fechner, M.; Schmidt-Ullrich, R.; Müller, D.M.; Schmidt-Ott, K.M.; Gürgen, D.; Blum, M.; et al. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury. Acta Physiol. 2013, 208, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Liu, X.; Hu, S.; Cong, X.; Chen, X. LPA rescues ER stress-associated apoptosis in hypoxia and serum deprivation-stimulated mesenchymal stem cells. J. Cell. Biochem. 2010, 111, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cohen, A.; Hudson, T.E.; Motlagh, D.; Amrani, D.L.; Duffield, J.S. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 2010, 121, 2211–2220. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wang, X.; Yang, H.; Fogo, A.B.; Murphy, B.J.; Kaltenbach, R.; Cheng, P.; Zinker, B.; Harris, R. Lysophosphatidic acid receptor antagonism protects against diabetic nephropathy in a type 2 diabetic model. J. Am. Soc. Nephrol. 2017, 28, 3300–3311. [Google Scholar] [CrossRef]
- Sakai, N.; Chun, J.; Duffield, J.S.; Lagares, D.; Wada, T.; Luster, A.D.; Tager, A.M. Lysophosphatidic acid signaling Through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor. Kidney Int. 2017, 91, 628–641. [Google Scholar] [CrossRef]
- Li, H.Y.; Oh, Y.S.; Choi, J.W.; Jung, J.Y.; Jun, H.S. Blocking lysophosphatidic acid receptor 1 signaling inhibits diabetic nephropathy in db/db mice. Kidney Int. 2017, 91, 1362–1373. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Y.; Bao, X.; Li, G.; Zhou, W. Lysophosphatidic acid signaling contributes to kidney fibrosis in diabetic nephropathy. Front. Pharmacol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Oh, Y.S.; Jun, H.S. Lysophosphatidic acid signaling in diabetic nephropathy. Int. J. Mol. Sci. 2019, 20, 2850. [Google Scholar] [CrossRef]
- Zhao, J.; He, D.; Su, Y.; Berdyshev, E.; Chun, J.; Natarajan, V.; Zhao, Y. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L547–L556. [Google Scholar] [CrossRef]
- Hirata, T.; Smith, S.V.; Takahashi, T.; Miyata, N.; Roman, R.J. Increased levels of renal lysophosphatidic acid in rodent models with renal disease. J. Pharmacol. Exp. Ther. 2021, 376, 240–249. [Google Scholar] [CrossRef]
- Kanehira, M.; Fujiwara, T.; Nakajima, S.; Okitsu, Y.; Ohnishi, Y.; Fukuhara, N.; Ichinohasama, R.; Harigae, H. Impaired Lysophosphatidic Acid Receptor 3 Signaling in Mesenchymal Stromal Cells Promotes Multiple Myeloma Progression Through Cellular Senescence and Transdifferentiation into Tumor-Associated Fibroblasts. Blood 2015, 126, 1764. [Google Scholar] [CrossRef]
- Mirzoyan, K.; Dennis, C.; Casemayou, A.; Gilet, M.; Marsal, D.; Goudounéche, D.; Faguer, S.; Bascands, J.-L.; Schanstra, J.P.; Blache, J.-S.S. Lysophosphatidic acid protects Against endotoxin-induced acute kidney injury. Inflammation 2017, 40, 1707–1716. [Google Scholar] [CrossRef]
- Fan, H.; Liu, J.; Sun, J.; Feng, G.; Li, J. Advances in the study of B cells in renal ischemia-reperfusion injury. Front. Immunol. 2023, 14, 1216094. [Google Scholar] [CrossRef] [PubMed]
- Gonsalez, S.R.; Cortes, A.L.; Romanelli, M.A.; Mattos-Silva, P.; Curnow, A.C.; Prieto, M.C.; Einicker-Lamas, M.; Lara, L.S. Lysophosphatidic acid prevents ischemia reperfusion injury but does not prevent tubular dysfunction. J. Nephrol. Sci. 2020, 2, 5–19. [Google Scholar] [CrossRef]
- Benítez-Bribiesca, L.; Gómez-Camarillo, M.; Castellanos-Juárez, E.; Mravko, E.; Sánchez-Suárez, P. Morphologic, biochemical and molecular mitochondrial changes During reperfusion phase following brief renal ischemia. Ann. N. Y. Acad. Sci. 2000, 926, 165–179. [Google Scholar] [CrossRef]
- Cavaglieri, R.C.; Martini, D.; Sogayar, M.C.; Noronha, I.L. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant. Proc. 2009, 41, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.L.; Gonsalez, S.R.; Rioja, L.S.; Oliveira, S.S.C.; Santos, A.L.S.; Prieto, M.C.; Melo, P.A.; Lara, L.S. Protective outcomes of low-dose doxycycline on renal function of Wistar rats subjected to acute ischemia/reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 102–114. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins During the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]

| CONTROL n = 10 | I/R n = 10 | I/R + BMMC n = 10 | |
|---|---|---|---|
| Weight (g) | 185.4 ± 13.47 a | 171.1 ± 9.23 a | 165.0 ± 4.35 a |
| Water intake (mL/100 g) | 6.5 ± 1.62 a | 6.2 ± 0.72 a | 8.9 ± 0.97 a |
| Urinary pH | 7.4 ± 0.31 a | 7.2 ± 0.38 a | 7.6 ± 0.27 a |
| Urinary vol. (mL/100 g/24 h) | 3.7 ± 0.34 a | 7.7 ± 1.03 b | 7.4 ± 1.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattos-Silva, P.; Gonsalez, S.R.; Lara, L.S.; Einicker-Lamas, M. Bone Marrow Mononuclear Cells Administration Restore Lysophosphatidic Acid (LPA) Levels and Cellular Signaling Axis in Rats Submitted to Renal Ischemia–Reperfusion. Int. J. Mol. Sci. 2025, 26, 9186. https://doi.org/10.3390/ijms26189186
Mattos-Silva P, Gonsalez SR, Lara LS, Einicker-Lamas M. Bone Marrow Mononuclear Cells Administration Restore Lysophosphatidic Acid (LPA) Levels and Cellular Signaling Axis in Rats Submitted to Renal Ischemia–Reperfusion. International Journal of Molecular Sciences. 2025; 26(18):9186. https://doi.org/10.3390/ijms26189186
Chicago/Turabian StyleMattos-Silva, Paula, Sabrina Ribeiro Gonsalez, Lucienne S. Lara, and Marcelo Einicker-Lamas. 2025. "Bone Marrow Mononuclear Cells Administration Restore Lysophosphatidic Acid (LPA) Levels and Cellular Signaling Axis in Rats Submitted to Renal Ischemia–Reperfusion" International Journal of Molecular Sciences 26, no. 18: 9186. https://doi.org/10.3390/ijms26189186
APA StyleMattos-Silva, P., Gonsalez, S. R., Lara, L. S., & Einicker-Lamas, M. (2025). Bone Marrow Mononuclear Cells Administration Restore Lysophosphatidic Acid (LPA) Levels and Cellular Signaling Axis in Rats Submitted to Renal Ischemia–Reperfusion. International Journal of Molecular Sciences, 26(18), 9186. https://doi.org/10.3390/ijms26189186







