Novel Polycaprolactone Based Coating for Catheters: Sustained Antibiotic Release for Enhanced Infection Prevention
Abstract
1. Introduction
2. Results
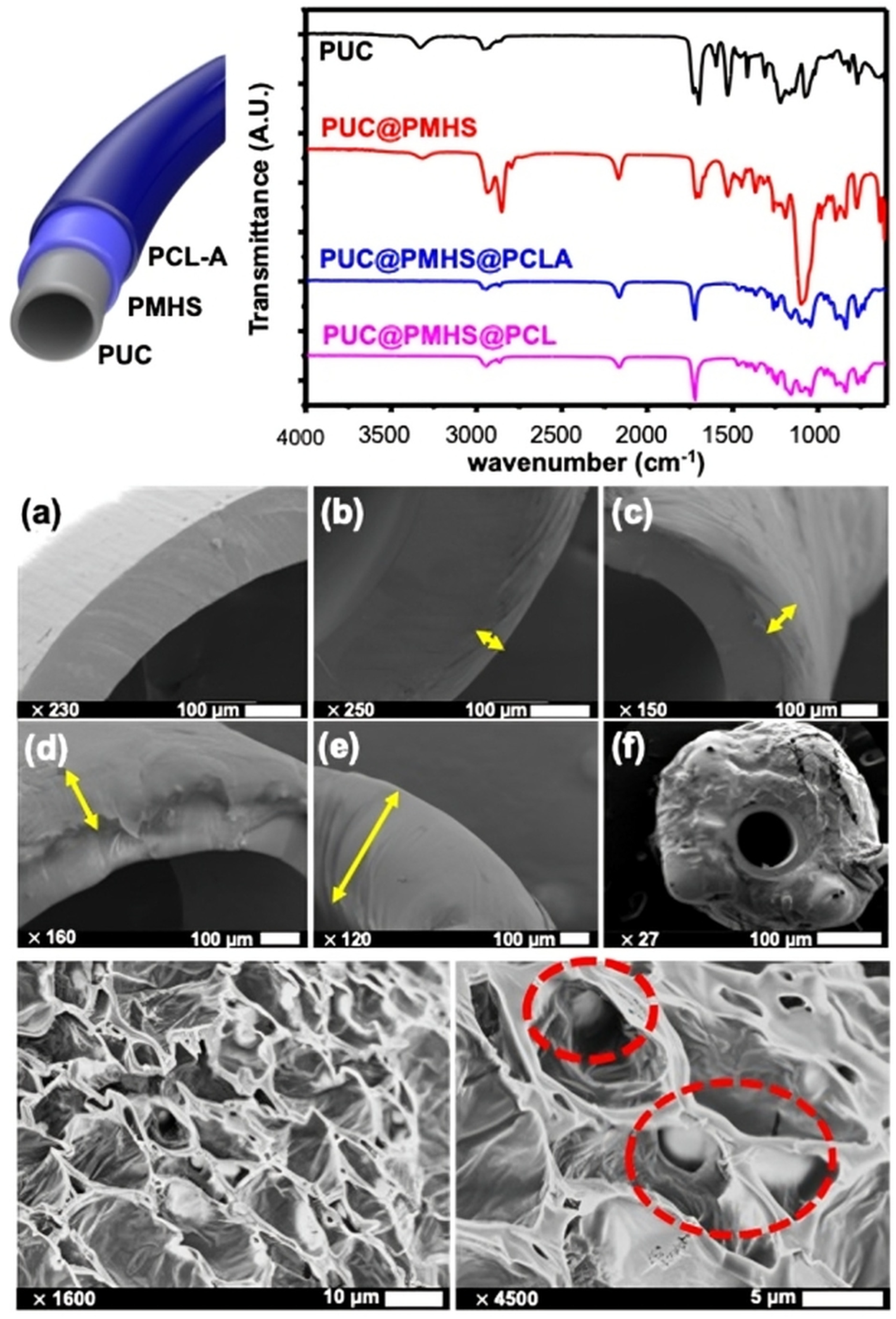
2.1. Chemical Characteristics of PCL-Coated Catheter
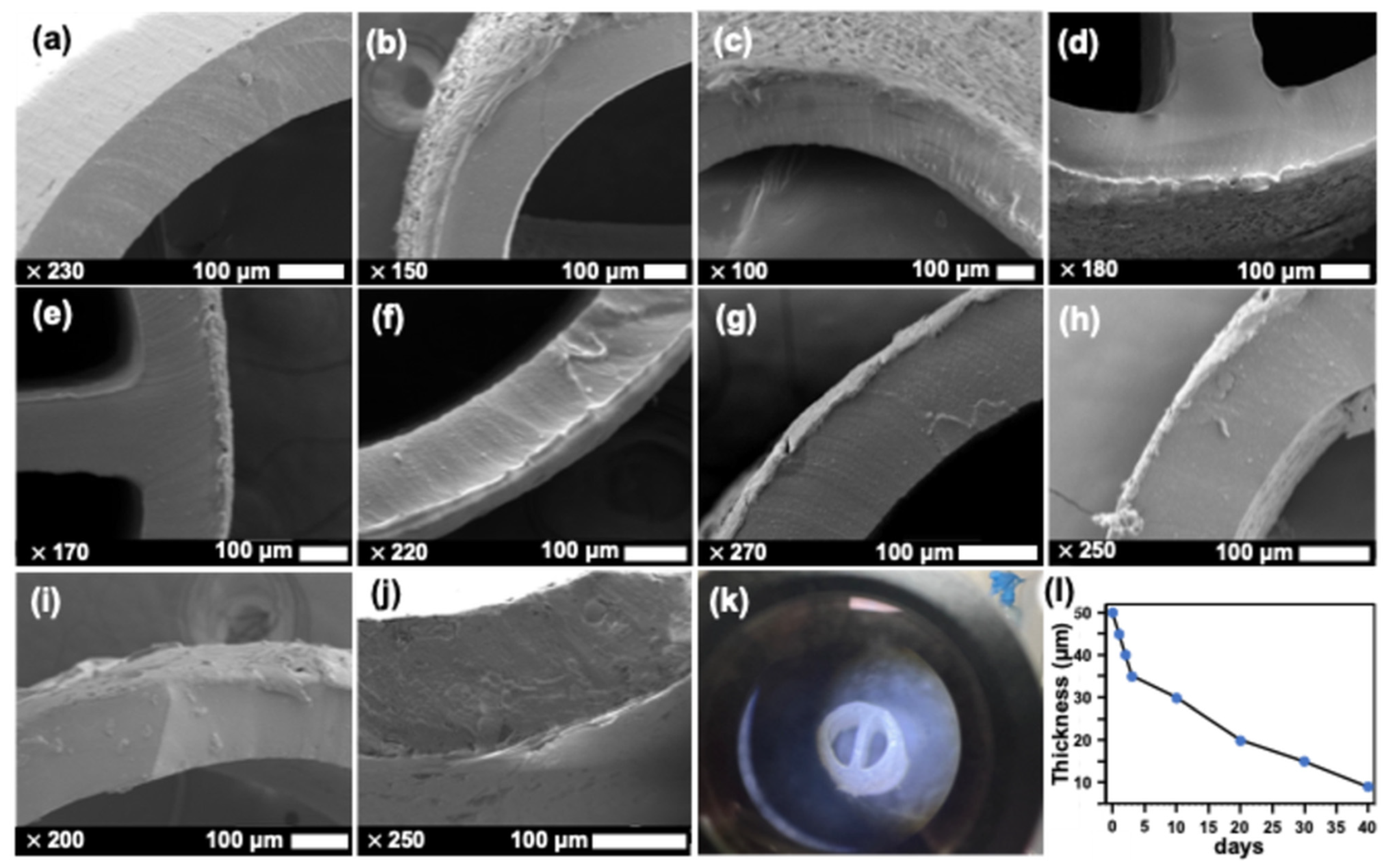
2.2. Physical Characteristics of PCL-Coated Catheter
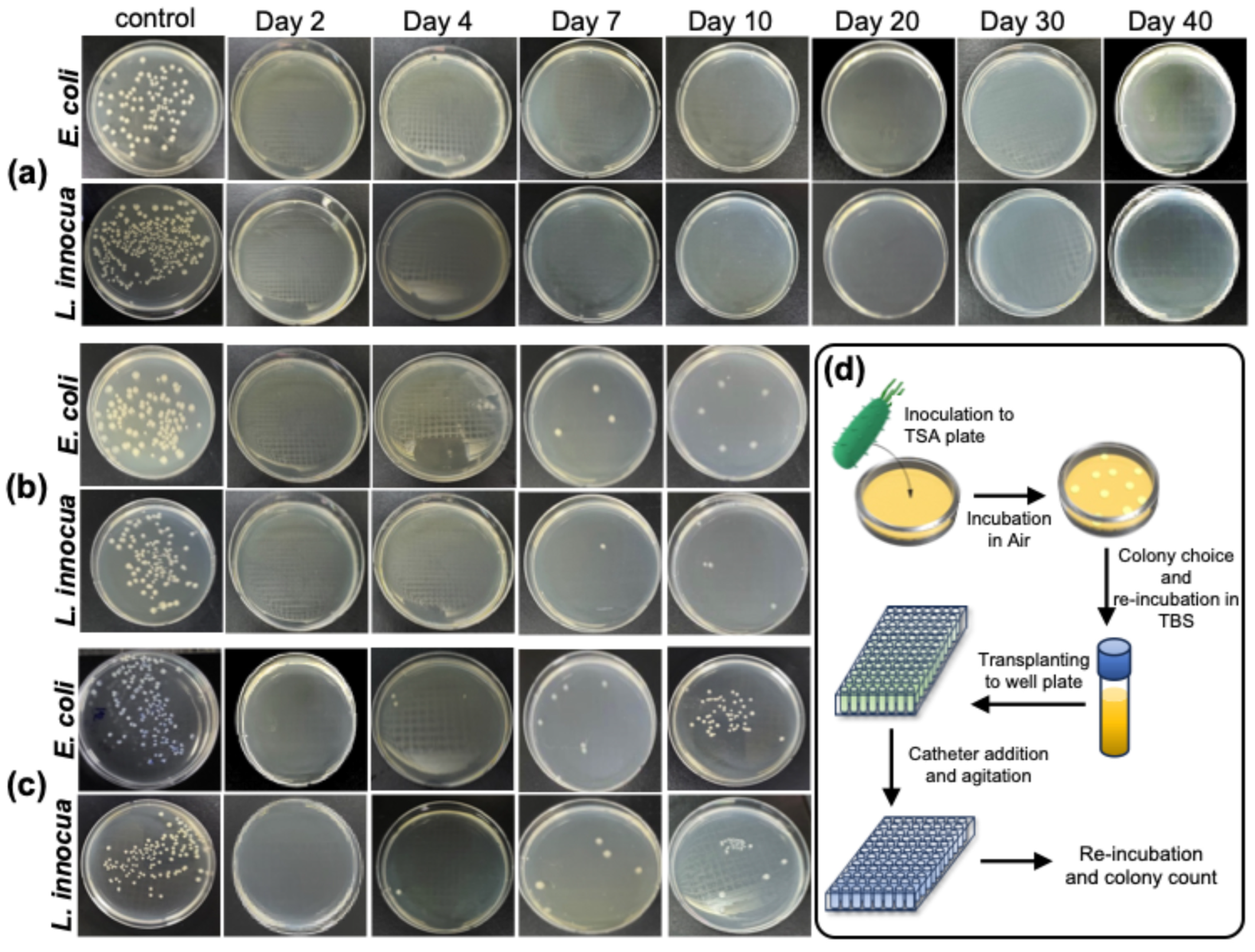
2.3. Antibacterial Activity
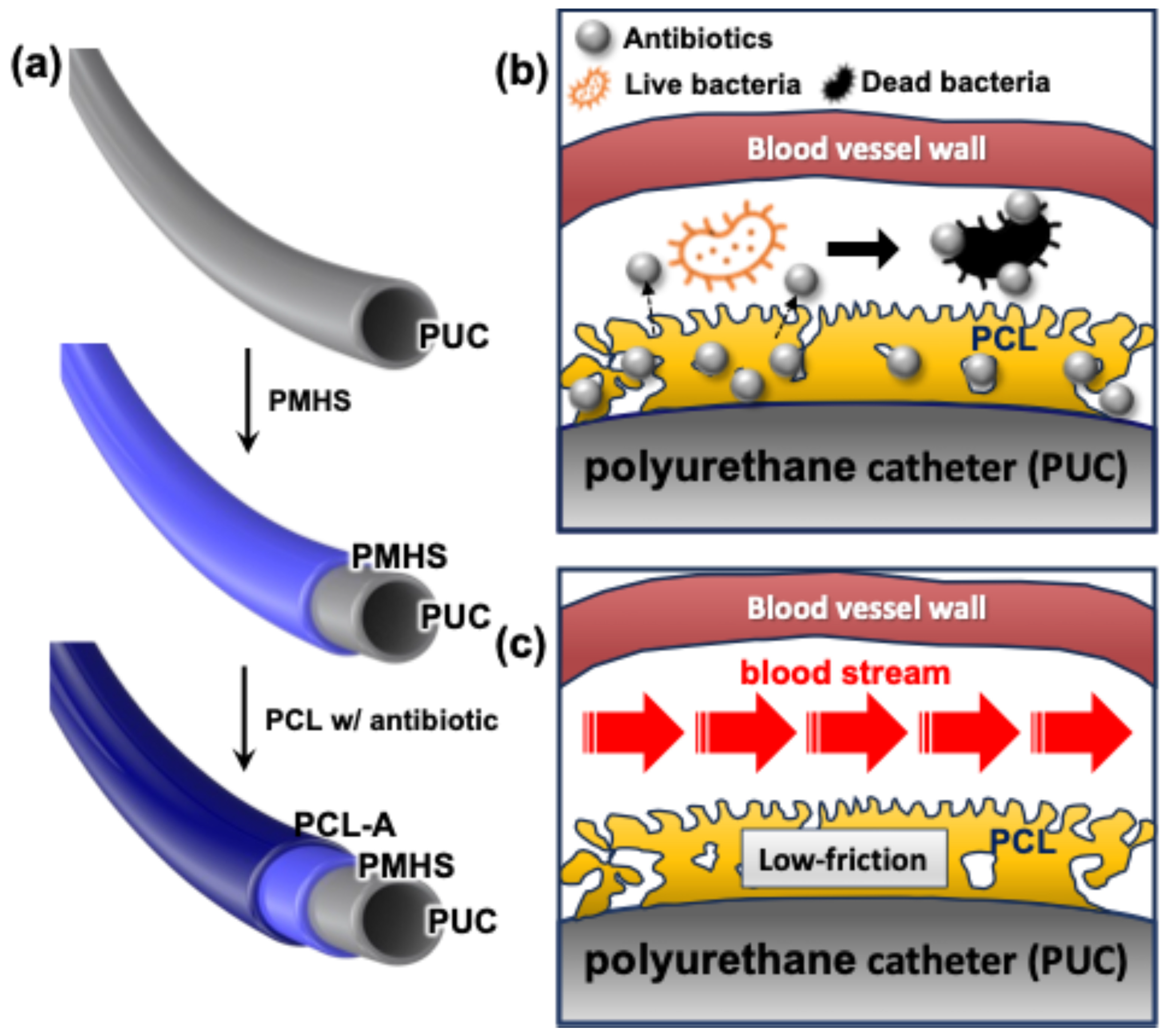
3. Discussion
4. Materials and Methods
4.1. Preparation of PCL-Coated Catheter
4.2. Characteristics of PCL-Coated Catheter
4.3. Antibacterial Activity Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IC | intravascular catheters |
| FT-IR | Fourier-transform infrared |
| PCL | polycaprolactone |
| PCL-A | a solution of PCL with ampicillin |
| PMHS | polymethylhydrosiloxane |
| PUC | polyurethane catheter |
| SEM | scanning electron microscopy |
References
- Al-Tawfiq, J.A.; Tambyah, P.A. Healthcare associated infections (HAI) perspectives. J. Infect. Public Health 2014, 7, 339–344. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, G.T. Catheter-associated urinary tract infections: Current challenges and future prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef]
- Rubi, H.; Mudey, G.; Kunjalwar, R. Catheter-associated urinary tract infection (CAUTI). Cureus 2022, 14, e30385. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.M.; Kim, J.-s.; Park, M.G.; Hwang, J.; Kim, W.Y.; Seo, D.-W. Incidence and short-term outcomes of central line-related bloodstream infection in patients admitted to the emergency department: A single-center retrospective study. Sci. Rep. 2023, 13, 3867. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Bijie, H.; Maki, D.G.; Mehta, Y.; Apisarnthanarak, A.; Medeiros, E.A.; Leblebicioglu, H.; Fisher, D.; Álvarez-Moreno, C.; Khader, I.A. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am. J. Infect. Control 2012, 40, 396–407. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care; WHO Press: Geneva, Switzerland, 2009; ISBN 978-92-4-159790-6. [Google Scholar]
- McFee, R.B. Nosocomial or hospital-acquired infections: An overview. Dis.-A-Mon. 2009, 55, 422. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, Q.; Wang, L.; Shi, H.; Luan, S. Recent Progress in Antibacterial Surfaces for Implant Catheters. BME Front. 2025, 6, 0063. [Google Scholar] [CrossRef]
- Jaffer, I.; Fredenburgh, J.; Hirsh, J.; Weitz, J. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef]
- Stillman, R.M.; Soliman, F.; Garcia, L.; Sawyer, P.N. Etiology of catheter-associated sepsis: Correlation with thrombogenicity. Arch. Surg. 1977, 112, 1497–1499. [Google Scholar] [CrossRef]
- Thornburg, C.D.; Smith, P.B.; Smithwick, M.L.; Cotten, C.M.; Benjamin, D.K., Jr. Association between thrombosis and bloodstream infection in neonates with peripherally inserted catheters. Thromb. Res. 2008, 122, 782–785. [Google Scholar] [CrossRef]
- Mehta, R.I.; Mehta, R.I. Hydrophilic polymer embolism: An update for physicians. Am. J. Med. 2017, 130, e287–e290. [Google Scholar] [CrossRef]
- Chug, M.K.; Griffin, L.; Garren, M.; Tharp, E.; Nguyen, G.H.; Handa, H.; Brisbois, E.J. Antimicrobial efficacy of a nitric oxide-releasing ampicillin conjugate catheter lock solution on clinically-isolated antibiotic-resistant bacteria. Biomater. Sci. 2023, 11, 6561–6572. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Mendoza-Mendoza, E.; Peralta-Rodriguez, R.D.; Pérez-Díaz, M.A.; Portales-Pérez, D.; Magaña-Aquino, M.; Aragón-Piña, A.; Infante-Martínez, R.; Barriga-Castro, E.D.; Sánchez-Sánchez, R.; et al. Characterization, antibiofilm and biocompatibility properties of chitosan hydrogels loaded with silver nanoparticles and ampicillin: An alternative protection to central venous catheters. Colloids Surf. B Biointerfaces 2020, 196, 111292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhao, Y.-Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.-J. Antimicrobial peptide-conjugated hierarchical antifouling polymer brushes for functionalized catheter surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yu, M.; Su, Y.; Xie, M.; Zhao, X.; Li, P.; Ma, P.X. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 2017, 51, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Basu, A.; Chua, R.R.Y.; Saravanan, R.; Tambyah, P.A.; Ho, B.; Chang, M.W.; Leong, S.S.J. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: Evaluation against urinary tract infection pathogens. J. Mater. Chem. B 2014, 2, 1706–1716. [Google Scholar] [CrossRef]
- Soyhan, I.; Polat, T.; Mozioglu, E.; Ozal Ildenız, T.A.; Acikel Elmas, M.; Cebeci, S.; Unubol, N.; Gok, O. Effective Immobilization of Novel Antimicrobial Peptides via Conjugation onto Activated Silicon Catheter Surfaces. Pharmaceutics 2024, 16, 1045. [Google Scholar] [CrossRef]
- Salgado, C.L.; Sanchez, E.M.; Zavaglia, C.A.; Granja, P.L. Biocompatibility and biodegradation of polycaprolactone-sebacic acid blended gels. J. Biomed. Mater. Res. Part A 2012, 100, 243–251. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun polycaprolactone (PCL) degradation: An in vitro and in vivo study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Conde, G.; De Carvalho, J.R.G.; do Patrocínio Dias, P.; Moranza, H.G.; Montanhim, G.L.; de Oliveira Ribeiro, J.; Chinelatto, M.A.; Moraes, P.C.; Taboga, S.R.; Bertolo, P.H.L. In vivo biocompatibility and biodegradability of poly (lactic acid)/poly (ε-caprolactone) blend compatibilized with poly (ε-caprolactone-b-tetrahydrofuran) in Wistar rats. Biomed. Phys. Eng. Express 2021, 7, 035005. [Google Scholar] [CrossRef]
- Castro, J.I.; Araujo-Rodríguez, D.G.; Valencia-Llano, C.H.; López Tenorio, D.; Saavedra, M.; Zapata, P.A.; Grande-Tovar, C.D. Biocompatibility Assessment of Polycaprolactone/Polylactic Acid/Zinc Oxide Nanoparticle Composites under In Vivo Conditions for Biomedical Applications. Pharmaceutics 2023, 15, 2196, Correction in Pharmaceutics 2025, 17, 1113. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.R.; Conde, G.; Antonioli, M.L.; Dias, P.P.; Vasconcelos, R.O.; Taboga, S.R.; Canola, P.A.; Chinelatto, M.A.; Pereira, G.T.; Ferraz, G.C. Biocompatibility and biodegradation of poly (lactic acid)(PLA) and an immiscible PLA/poly (ε-caprolactone)(PCL) blend compatibilized by poly (ε-caprolactone-b-tetrahydrofuran) implanted in horses. Polym. J. 2020, 52, 629–643. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.J.; Lee, S.S.; Lyubovitsky, J.; Bent, S.F. Infrared spectroscopy of methyl groups on silicon. Chem. Phys. Lett. 1996, 263, 1–7. [Google Scholar] [CrossRef]
- Holešová, S.; Čech Barabaszová, K.; Hundáková, M.; Ščuková, M.; Hrabovská, K.; Joszko, K.; Antonowicz, M.; Gzik-Zroska, B. Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties. Polymers 2021, 13, 3193. [Google Scholar] [CrossRef]
- Bisson-Boutelliez, C.; Fontanay, S.; Finance, C.; Kedzierewicz, F. Preparation and physicochemical characterization of amoxicillin beta-cyclodextrin complexes. AAPS PharmSciTech 2010, 11, 574–581. [Google Scholar] [CrossRef]
- Naz, N.; Khatoon, S.; Ajaz, H.; Sadiq, Z.; Iqbal, M.Z. Synthesis, spectral characterization and biological evaluation of Schiff base transition metal complexes derived from ampicillin with D-glucose. Asian J. Chem. 2013, 25, 2239. [Google Scholar] [CrossRef]
- Kahraman, A.; Sakar, D.; Altikatoglu Yapaoz, M. Maleic Anhydride-Derived Copolymers Conjugated with Beta-Lactam Antibiotics: Synthesis, Characterization, In Vitro Activity/Stability Tests with Antibacterial Studies. Appl. Sci. 2024, 14, 6112. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, K.-B.; Kim, S.-Y.; Jang, Y.-S.; Kim, J.H.; Lee, M.-H. Improvement of osteogenesis by a uniform PCL coating on a magnesium screw for biodegradable applications. Sci. Rep. 2018, 8, 13264. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lim, J.K.; Park, J.J.; Huh, H.; Kim, D.J.; Gong, C.H.; Yoon, S.Z. Chlorhexidine and silver sulfadiazine coating on central venous catheters is not sufficient for protection against catheter-related infection: Simulation-based laboratory research with clinical validation. J. Int. Med. Res. 2017, 45, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Bonne, S.; Mazuski, J.E.; Sona, C.; Schallom, M.; Boyle, W.; Buchman, T.G.; Bochicchio, G.V.; Coopersmith, C.M.; Schuerer, D.J. Effectiveness of Minocycline and Rifampin vs Chlorhexidine and Silver Sulfadiazine-Impregnated Central Venous Catheters in Preventing Central Line-Associated Bloodstream Infection in a High-Volume Academic Intensive Care Unit: A Before and after Trial. J. Am. Coll. Surg. 2015, 221, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lai, X.; Li, H.; Pan, K.; Liu, Z.; Zeng, X. Synthesis and application of adhesion promoter containing phenolic hydroxyl/acrylate groups for addition-cure liquid silicone rubber. Int. J. Adhes. Adhes. 2022, 118, 103220. [Google Scholar] [CrossRef]
- Wu, J.-K.; Zheng, K.-W.; Nie, X.-C.; Ge, H.-R.; Wang, Q.-Y.; Xu, J.-T. Promoters for improved adhesion strength between Addition-Cured liquid silicone rubber and Low-Melting-Point thermoplastic polyurethanes. Materials 2022, 15, 991. [Google Scholar] [CrossRef]
- Raad, I.I.; Darouiche, R.O.; Hachem, R.; Abi-Said, D.; Safar, H.; Darnule, T.; Mansouri, M.; Morck, D. Antimicrobial durability and rare ultrastructural colonization of indwelling central catheters coated with minocycline and rifampin. Crit. Care Med. 1998, 26, 219–224. [Google Scholar] [CrossRef]
- Cui, Y.H.; Choi, Y.J.; Kim, E.H.; Yu, J.H.; Seong, H.Y.; Choi, S.U.; Yoon, S.Z.; Huh, H. Effects of blood flow on the antibacterial efficacy of chlorhexidine and silver sulfadiazine coated central venous catheter: A simulation-based pilot study. Medicine 2020, 99, e22218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Kim, E.H.; Yoon, S.Z.; Lee, S.J. Novel Polycaprolactone Based Coating for Catheters: Sustained Antibiotic Release for Enhanced Infection Prevention. Int. J. Mol. Sci. 2025, 26, 9177. https://doi.org/10.3390/ijms26189177
Kim K, Kim EH, Yoon SZ, Lee SJ. Novel Polycaprolactone Based Coating for Catheters: Sustained Antibiotic Release for Enhanced Infection Prevention. International Journal of Molecular Sciences. 2025; 26(18):9177. https://doi.org/10.3390/ijms26189177
Chicago/Turabian StyleKim, Kyungmi, Eung Hwi Kim, Seung Zhoo Yoon, and Suk Joong Lee. 2025. "Novel Polycaprolactone Based Coating for Catheters: Sustained Antibiotic Release for Enhanced Infection Prevention" International Journal of Molecular Sciences 26, no. 18: 9177. https://doi.org/10.3390/ijms26189177
APA StyleKim, K., Kim, E. H., Yoon, S. Z., & Lee, S. J. (2025). Novel Polycaprolactone Based Coating for Catheters: Sustained Antibiotic Release for Enhanced Infection Prevention. International Journal of Molecular Sciences, 26(18), 9177. https://doi.org/10.3390/ijms26189177





