Synthesis of α,ε-N,N′-Di-stearoyl Lysine-Derived Amide Lipids and Their Application to Liposome Formulation: Incorporation of Lipid A-Ligand for Bacterial Targeting and Sialic Acid for Phagocytosis Resistance
Abstract
1. Introduction
2. Results and Discussion
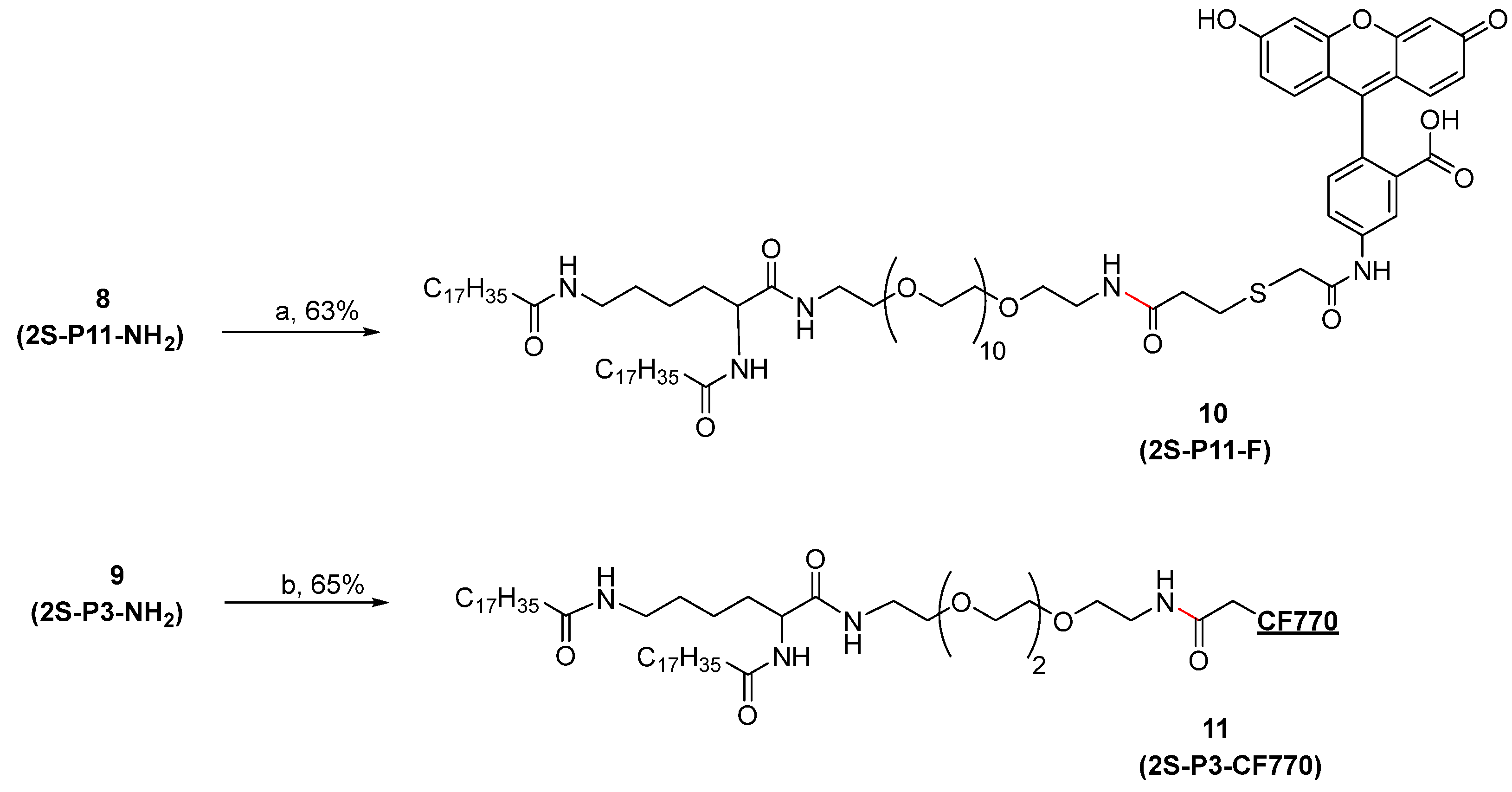
2.1. Synthesis of Amide Lipids
2.2. Liposome Formulations
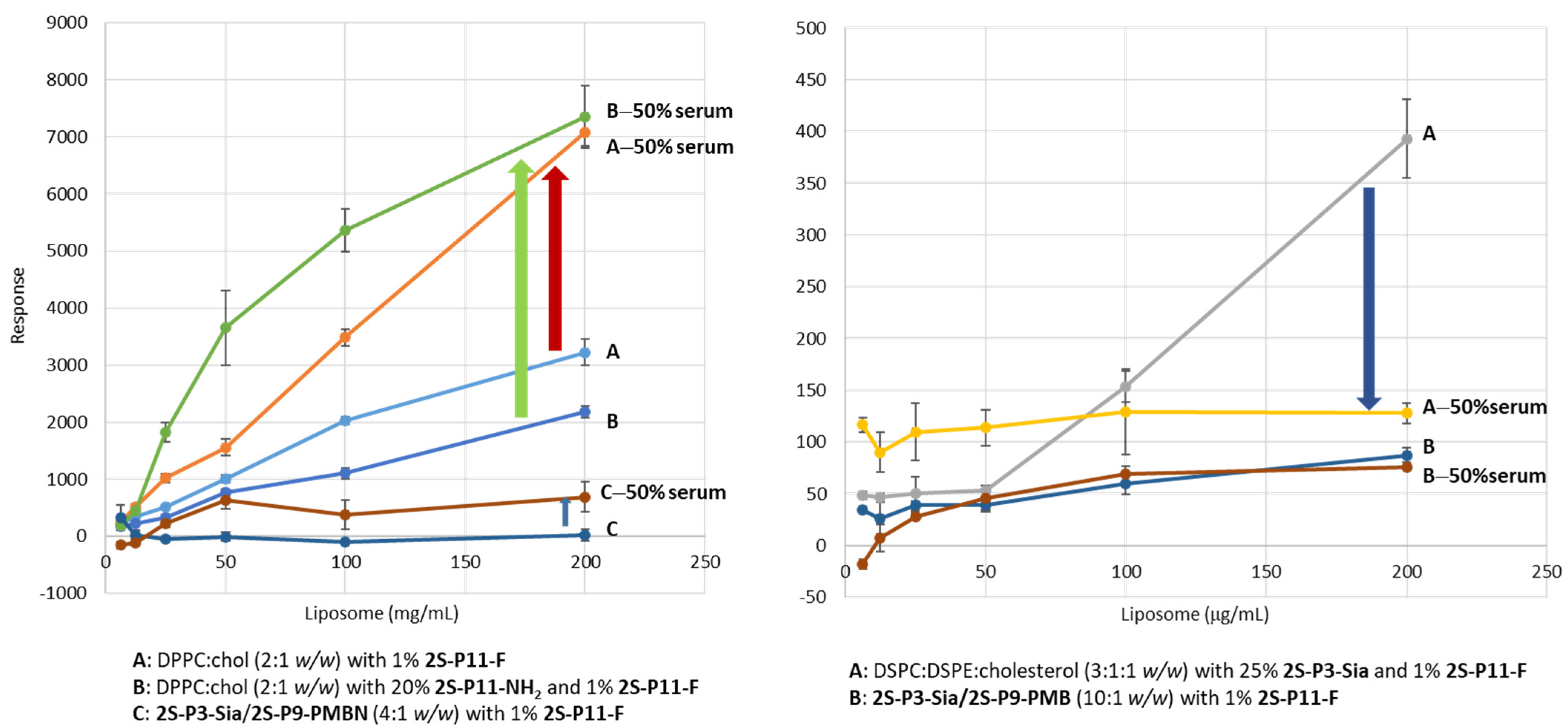
2.3. Ligand-Directed Binding of Liposomes to Bacteria
2.4. Liposome Uptake by THP-1 Cells
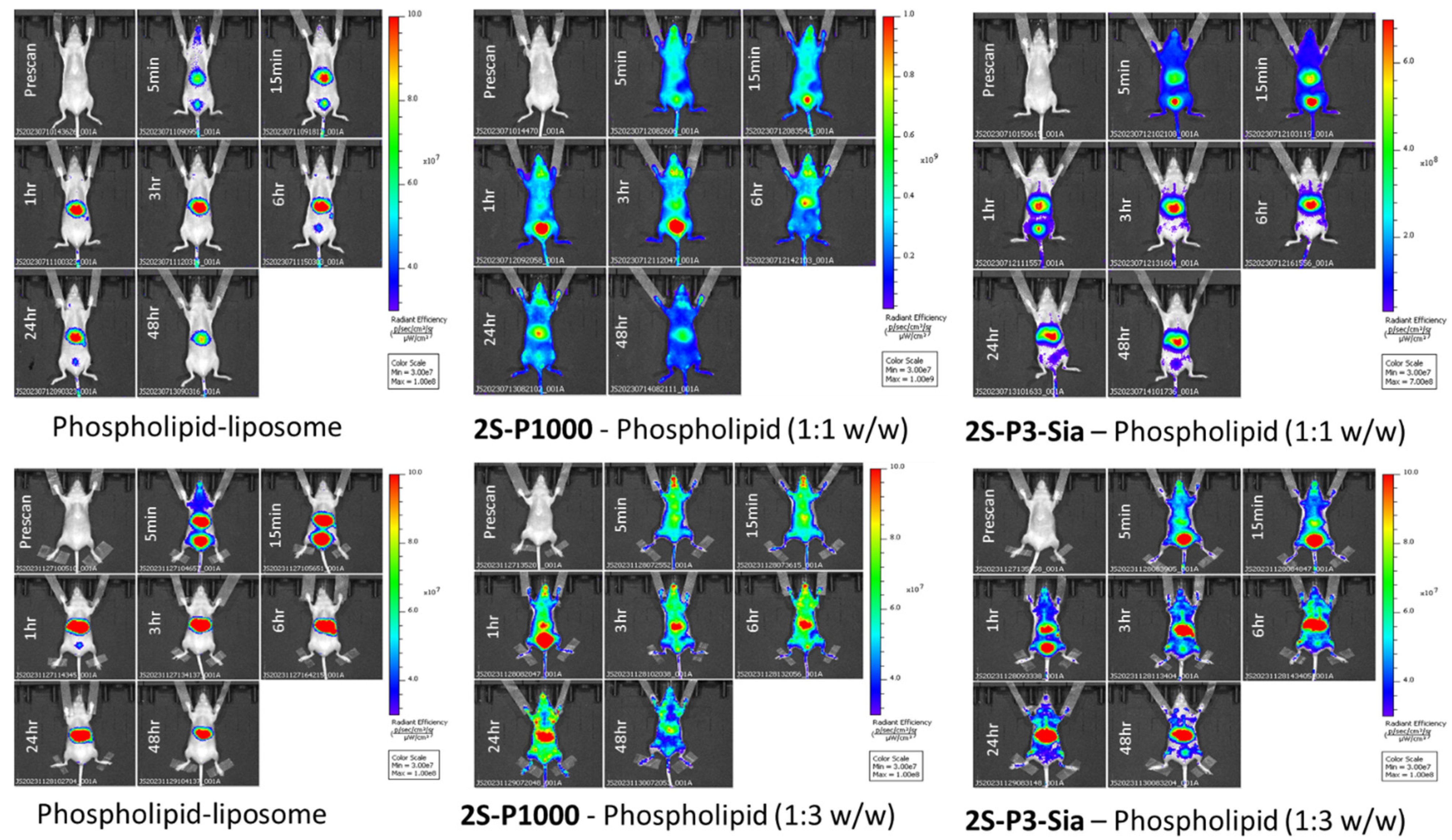
2.5. Liposome Biodistribution and Retention in Mice
3. Experimental and Methods
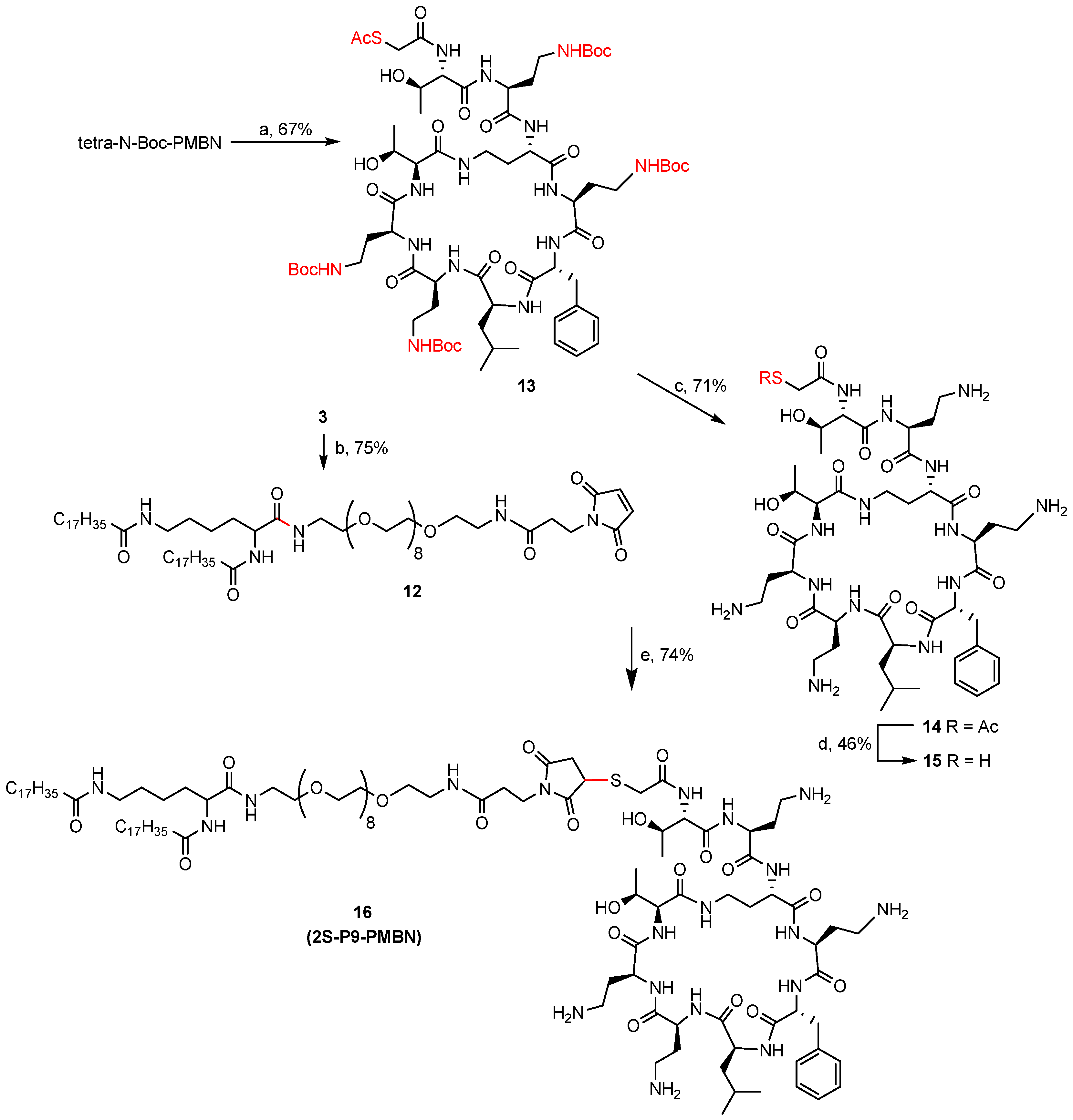
3.1. Synthesis of Amide Lipids
3.1.1. Stearic Acid N-Hydroxysuccinimide Ester (1)
3.1.2. α,ε-N,N′-Di-stearoyl Lysine (2)
3.1.3. α,ε-N,N′-Di-stearoyl Lysine N-Hydroxysuccinimide Ester (3)
3.1.4. α,ε-N,N′-Di-stearoyl Lysine (α-Sialo-PEG3-amine)-amide (4, 2S-P3-Sia)
3.1.5. α,ε-N,N′-Di-stearoyl Lysine (PEG1000-Amine)-amide (5, 2S-P1000)
3.1.6. α,ε-N,N′-Di-stearoyl Lysine (Boc-N-PEG11-amine)-amide (6, 2S-P11-NHBoc)
3.1.7. α,ε-N,N′-Di-stearoyl Lysine (Boc-N-PEG3-amine)-amide (7, 2S-P3-NHBoc)
3.1.8. α,ε-N,N′-Di-stearoyl Lysine (PEG11-Amine)-amide (8, 2S-P11-NH2)
3.1.9. α,ε-N,N′-Di-stearoyl Lysine (PEG3-Amine)-amide (9, 2S-P3-NH2)
3.1.10. α,ε-N,N′-Di-stearoyl Lysine (Fluorescein-5-EX-N-amido-PEG11 amine)-amide (10, 2S-P11-F)
3.1.11. α,ε-N,N′-Di-stearoyl Lysine (CF770-N-Amido-PEG3-amine)-amide (11, 2S-P3-CF770)
3.1.12. α,ε-N,N′-Di-stearoyl Lysine (N-Maleimido-PEG9 amine)-amide (12, 2S-P9-Mal)
3.1.13. Tetra-N-Boc-PMBN-SAc (13)
3.1.14. PMBN-SAc (14)
3.1.15. PMBN-SH (15)
3.1.16. α,ε-N,N′-Di-stearoyl Lysine (PMBN-N-Maleimido-PEG9 amine)-amide (16, 2S-P9-PMBN)
3.2. Liposome Formulation and Characterization
3.2.1. Microscopy Images
3.2.2. Cryo-TEM Images
3.2.3. Liposome Size
3.2.4. Measurement Parameters for Zeta Potential (ZP)
3.3. In Vitro PMBN-Mediated Liposome Binding to Bacteria
3.4. The Effect of Serum Treatment of Liposomes on Bacteria-Binding
3.5. Liposome Uptake by THP-1 Cells
3.6. Biodistribution and Retention in Mice (In Vivo Imaging)
3.7. Animal Care
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMBN | polymyxin B nonapeptide |
| DCC | N,N′-dicyclohexylcarbodiimide |
| NHS | N-hydroxysuccinimide |
| SUV | Small unilamellar vesicle |
| LUV | Large unilamellar vesicle |
| MLV | Multilamellar vesicle |
| DSPC | Distearoylphosphatidylcholine |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
| DPPC | Dipalmitoyl phosphatidylcholine |
| Chol | Cholesterol |
| DLS | Dynamic Light Scattering |
| Cryo-TEM | Cryogenic transmission electron microscopy |
References
- Jia, Y.J.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved nanomedicine against diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Pande, S. Liposomes for drug delivery: Review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A. Development of pharmaceutical nanomedicines: From the bench to the market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene glycol immunogenicity: Theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef]
- Carreño, J.M.; Singh, G.; Tcheou, J.; Srivastava, K.; Gleason, C.; Muramatsu, H.; Desai, P.; Aberg, J.A.; Miller, R.L.; PARIS study group; et al. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine 2022, 40, 6114–6124. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.; Gioria, S.; Sauer, A.V.; Lucchesi, S.; Montagnani, F.; Pastore, G.; Ciabattini, A.; Medaglini, D.; Calzolai, L. Monitoring anti-PEG antibodies level upon repeated lipid nanoparticle-based COVID-19 vaccine administration. Int. J. Mol. Sci. 2022, 23, 8838. [Google Scholar] [CrossRef]
- Crisafulli, S.; Cutroneo, P.M.; Luxi, N.; Fontana, A.; Ferrajolo, C.; Marchione, P.; Sottosanti, L.; Zanoni, G.; Moretti, U.; Franzè, S.; et al. Is PEGylation of drugs associated with hypersensitivity reactions? An analysis of the Italian national spontaneous adverse drug reaction reporting system. Drug Saf. 2023, 46, 343–355. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Adler, A.; Inoue, Y.; Sato, Y.; Ishihara, K.; Ekdahl, K.N.; Nilsson, B.; Teramura, Y. Synthesis of poly(2-methacryloyloxyethyl phosphorylcholine)-conjugated lipids and their characterization and surface properties of modified liposomes for protein interactions. Biomater. Sci. 2021, 9, 5854–5867. [Google Scholar] [CrossRef]
- Singla, A.; Simbassa, S.B.; Chirra, B.; Gairola, A.; Southerland, M.R.; Shah, K.N.; Rose, R.E.; Chen, Q.; Basharat, A.; Baeza, J.; et al. Hetero-multivalent targeted liposomal drug delivery to treat Pseudomonas aeruginosa infections. ACS Appl. Mater. Interfaces 2022, 14, 40724–40737. [Google Scholar] [CrossRef]
- Hsieh, S.A.; Allen, P.M. Immunomodulatory roles of polysaccharide capsules in the intestine. Front. Immunol. 2020, 11, 690. [Google Scholar] [CrossRef]
- Ogawara, K.; Furumoto, K.; Nagayama, S.; Minato, K.; Higaki, K.; Kai, T.; Kimura, T. Pre-coating with serum albumin reduces receptor-mediated hepatic disposition of polystyrene nanosphere: Implications for rational design of nanoparticles. J. Control. Release 2004, 100, 451–455. [Google Scholar] [CrossRef]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, T.; Zhao, L.; Liu, M.; You, Y.; Zeng, Y.; Chen, D.; Xie, T.; Zhang, L.; Fu, C.; et al. Recent advancements in liposome-targeting strategies for the treatment of gliomas: A systematic review. ACS Appl. Bio Mater. 2020, 3, 5500–5528. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for tumor targeted therapy: A review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Grit, M.; Crommelin, D.J.A. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Vial, H.J.; Wengelnik, K. Phospholipid metabolism. In Encyclopedia of Malaria; Hommel, M., Kremsner, P.G., Eds.; Springer: New York, NY, USA, 2021; pp. 1–23. [Google Scholar]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Safavi-Sohi, R.; Sharifi, S.; Mahmoud, N.; Ashkarran, A.A.; Voke, E.; Serpooshan, V.; Ramezankhani, M.; Milani, A.S.; Landry, M.P.; et al. An overview of nanoparticle protein corona literature. Small 2023, 19, e2301838. [Google Scholar] [CrossRef]
- Nishizuka, Y. Membrane phospholipid degradation and protein kinase C for cell signalling. Neurosci. Res. 1992, 15, 3–5. [Google Scholar] [CrossRef]
- Lapidot, Y.; Rappoport, S.; Wolman, Y. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J. Lipid Res. 1967, 8, 142–145. [Google Scholar]
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [CrossRef]
- Refaat, A.; Yap, M.L.; Pietersz, G.; Walsh, A.P.G.; Zeller, J.; del Rosal, B.; Wang, X.; Peter, K. In vivo fluorescence imaging: Success in preclinical imaging paves the way for clinical applications. J. Nanobiotechnol. 2022, 20, 450. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Duwe, A.K.; Rupar, C.A.; Horsman, G.B.; Vas, S.I. In vitro cytotoxicity and antibiotic activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 1986, 30, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Feigman, M.S.; Kim, S.; Pidgeon, S.E.; Yu, Y.; Ongwae, G.M.; Patel, D.S.; Regen, S.; Im, W.; Pires, M.M. Synthetic immunotherapeutics against Gram-negative pathogens. Cell Chem. Biol. 2018, 25, 1185–1194.e5. [Google Scholar] [CrossRef]
- Huskens, J. Multivalent interactions at interfaces. Curr. Opin. Chem. Biol. 2006, 10, 537–543. [Google Scholar] [CrossRef]
- Kane, R.S. Thermodynamics of multivalent interactions: Influence of the linker. Langmuir 2010, 26, 8636–8640. [Google Scholar] [CrossRef] [PubMed]
- Danner, R.L.; A Joiner, K.; Rubin, M.; Patterson, W.H.; Johnson, N.; Ayers, K.M.; E Parrillo, J. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 1989, 33, 1428–1434. [Google Scholar] [CrossRef]
- O’Dowd, H.; Kim, B.; Margolis, P.; Wang, W.; Wu, C.; Lopez, S.L.; Blais, J. Preparation of tetra-Boc-protected polymyxin B nonapeptide. Tetrahedron Lett. 2007, 48, 2003–2005. [Google Scholar] [CrossRef]
- D’Souza, G. Liposomes: Methods and Protocols, 2nd ed.; Humana Press: Totowa, NJ, USA, 2017; Volume 1522. [Google Scholar]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Crepin, S.; Ottosen, E.N.; Chandler, C.E.; Sintsova, A.; Ernst, R.K.; Mobley, H.L.T. The UDP-GalNAcA biosynthesis genes gna-gne2 are required to maintain cell envelope integrity and in vivo fitness in multi-drug resistant Acinetobacter baumannii. Mol. Microbiol. 2020, 113, 153–172. [Google Scholar] [CrossRef]
- Poursoleiman, A.; Karimi-Jafari, M.; Zolmajd-Haghighi, Z.; Bagheri, M.; Haertlé, T.; Behbehani, G.R.; Ghasemi, A.; Stroylova, Y.; Muronetz, V.; Saboury, A. Polymyxins interaction to the human serum albumin: A thermodynamic and computational study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 155–163. [Google Scholar] [CrossRef]
- Zou, W.; McAdorey, A.; Yan, H.; Chen, W. Nanomedicine to overcome antimicrobial resistance: Challenges and prospects. Nanomedicine 2023, 18, 471–484. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]

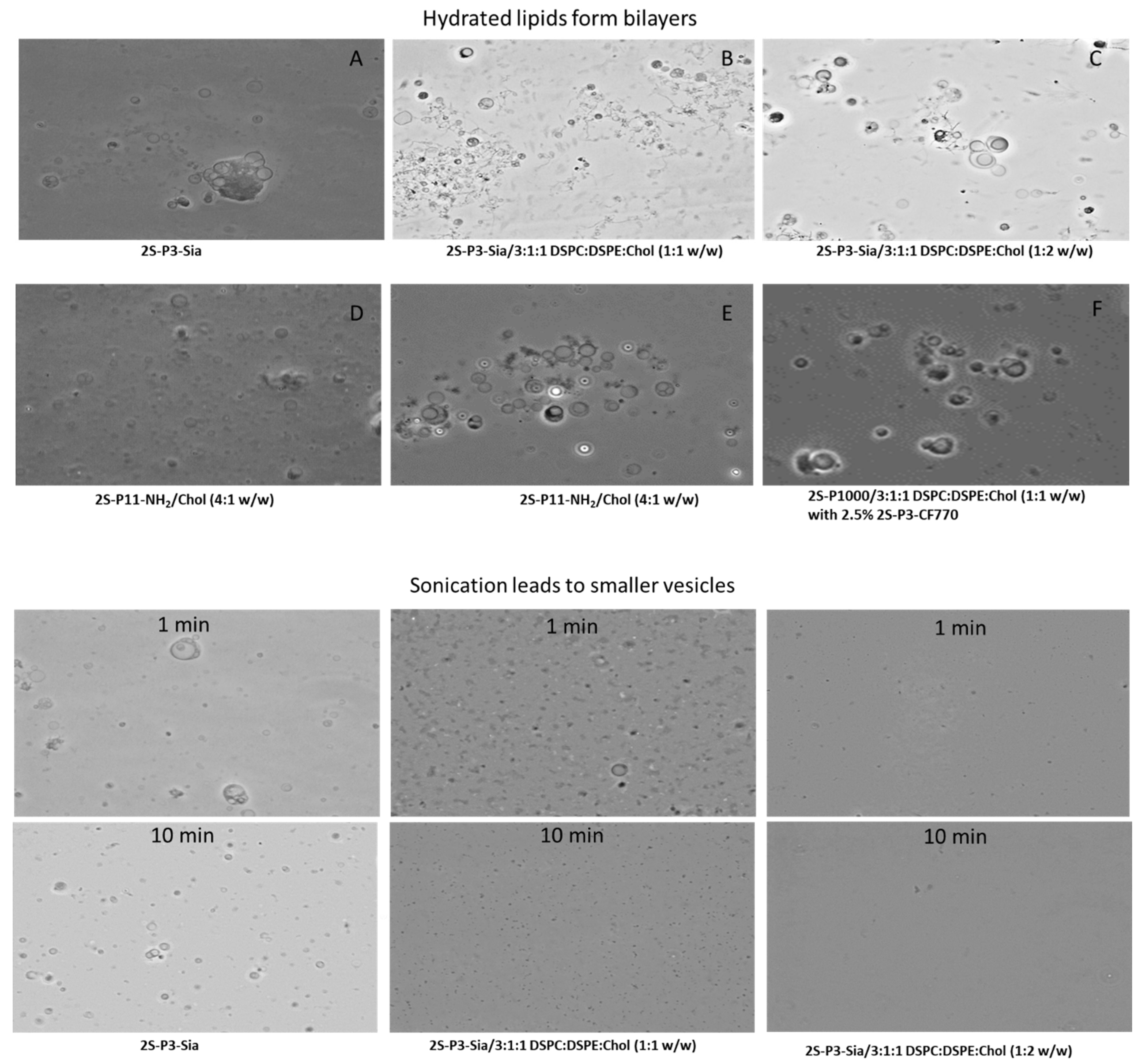
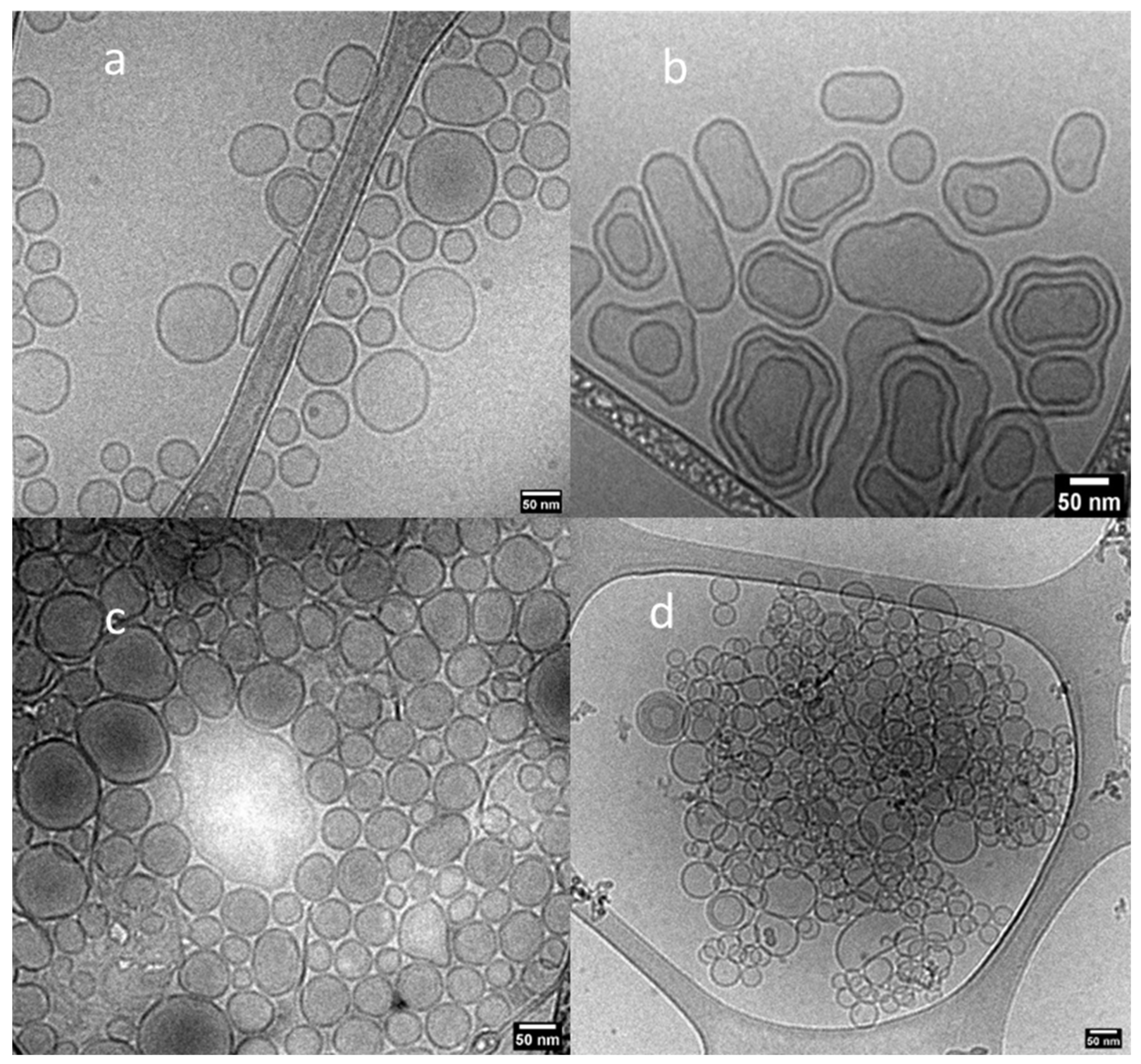
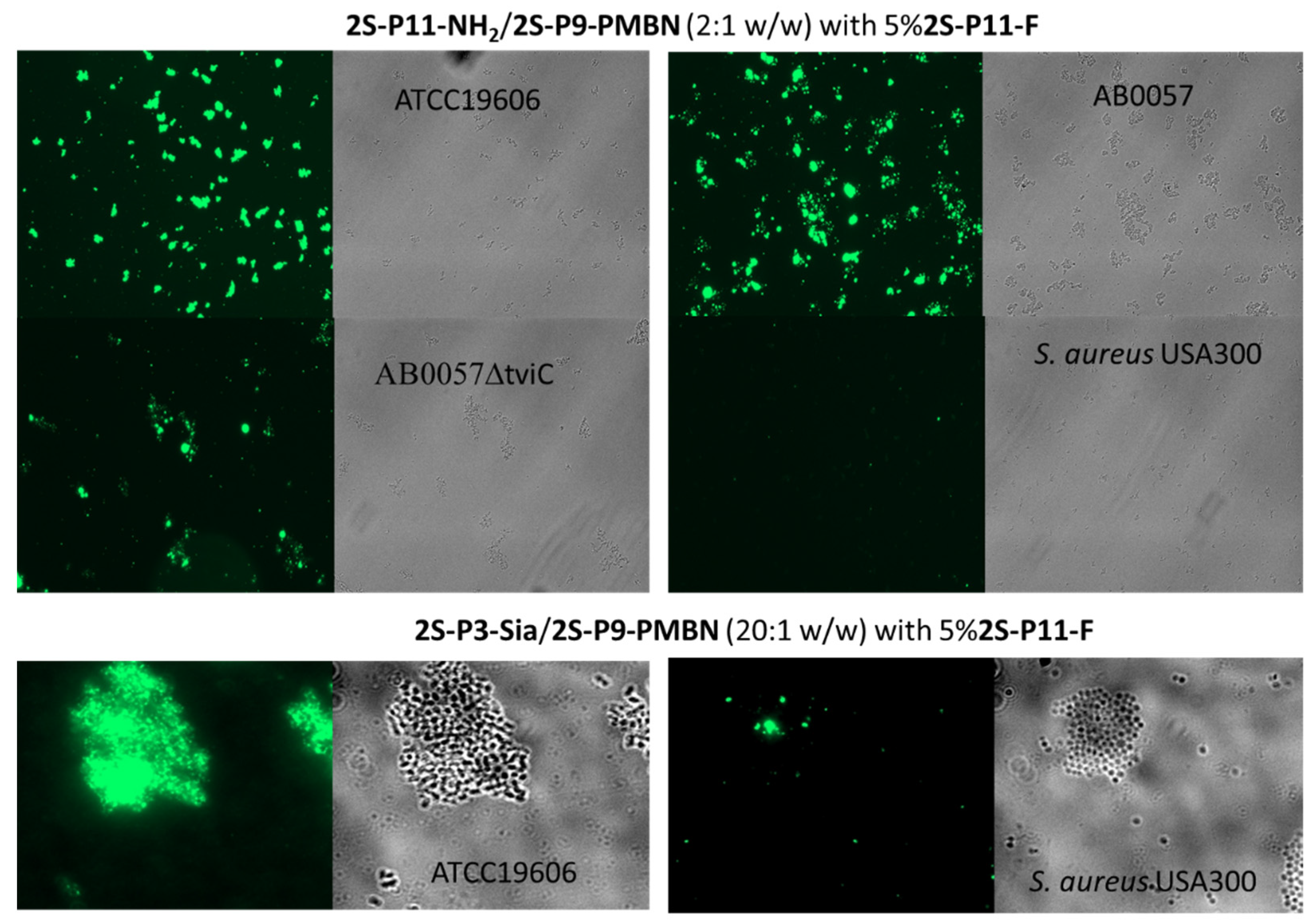
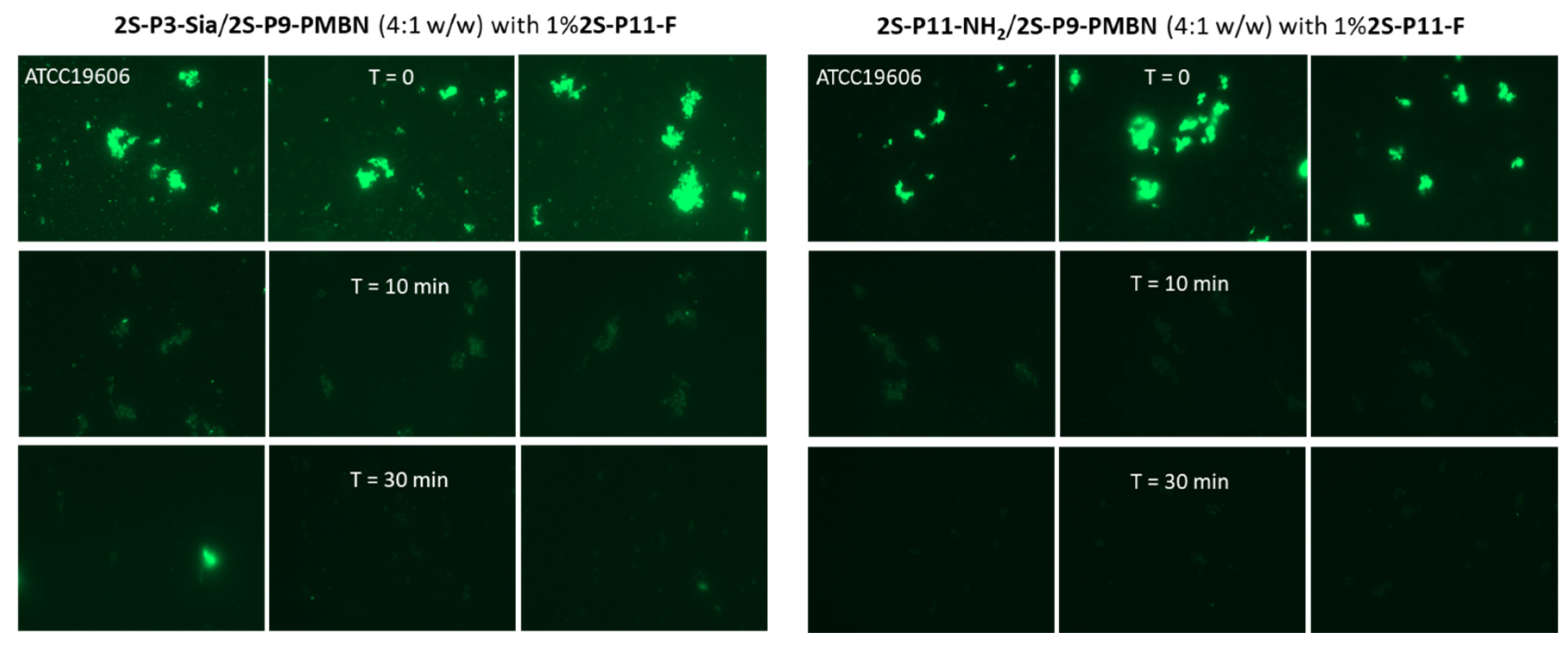
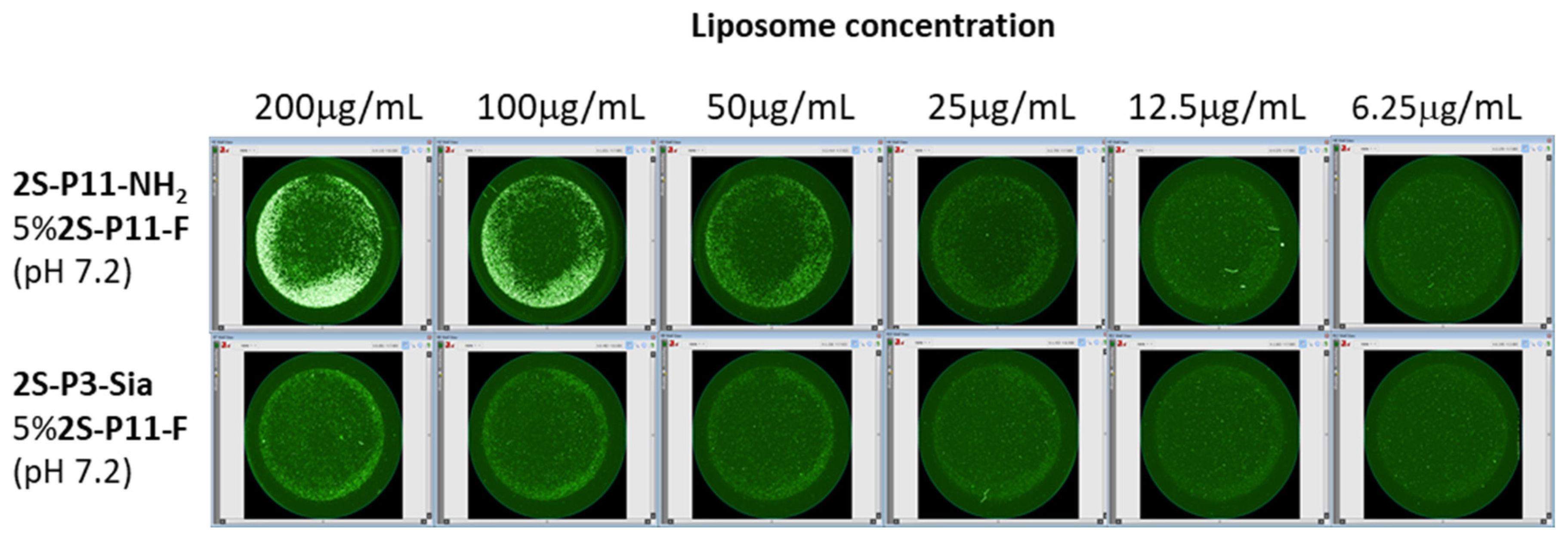
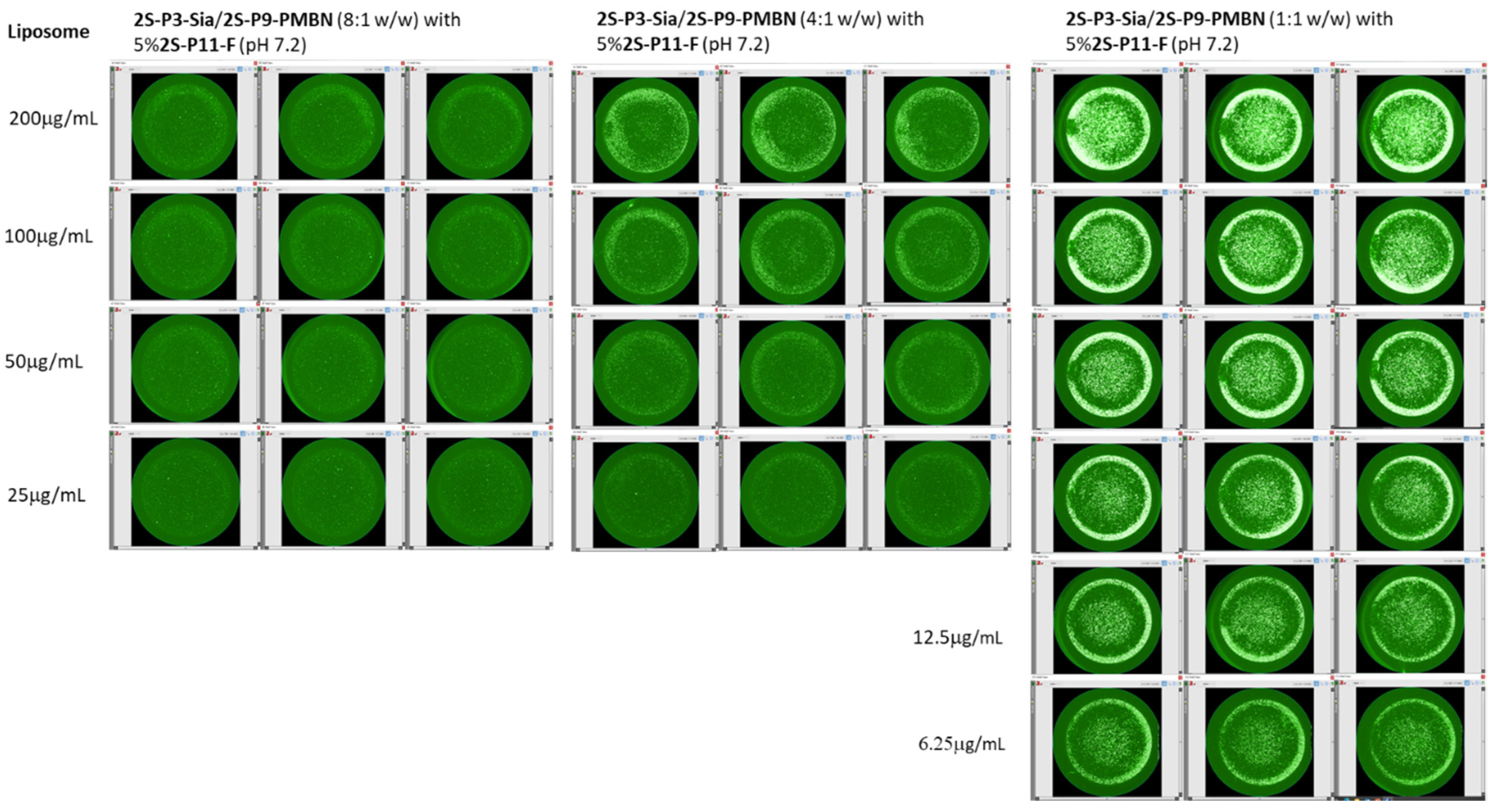
| Sample | Lipids | Buffer | Nanoparticle |
|---|---|---|---|
| A | 2S-P3-Sia | 20 mM phosphate (pH 7.2) | Bilayer vesicles |
| B | 2S-P3-Sia/Phospholipids (1:1 w/w) | ||
| C | 2S-P3-Sia/Phospholipids (1:2 w/w) | ||
| D | 2S-P11-NH2/Cholesterol (4:1 w/w) | 10 mM NaH2PO4 (pH 4.5) | Bilayer vesicles |
| E | 10 mM NaOAc-HOAc (pH 5.5) | ||
| F | 2S-P1000/phospholipids (1:1 w/w) with 2.5% 2S-P3-CF770 | 20 mM phosphate (pH 7.2) | Bilayer vesicles/LNP |
| Liposome | Average Size (d.nm) | |||
|---|---|---|---|---|
| pH 4.5 | pH 7.0 | |||
| 0 h | 2 h | 5 h | ||
| 2S-P11-NH2/Chol (4:1 w/w) | 170 | 164 | 173 | 221 |
| 2S-P11-NH2/DPPC-Chol (0.8:3.2:1 w/w) | 317 | 291 | 702 | - |
| DPPC-Chol (4:1 w/w) | 211 | 225 | - | 224 |
| Exp. | Liposome | Size (id-nm) b | Original Particles/mL | Fluorescent Intensity | Original Concentration (mg/mL) | Adjusted Concentration (mg/mL) c |
|---|---|---|---|---|---|---|
| No. 1 | Phospholipids | 114.0 | 2.9 × 1012 | 2.08 × 109 | 4 | 2.56 |
| Phospholipids/2S-P1000 (1:1 w/w) | 161.8 | 1.7 × 1012 | 1.33 × 109 | 4 | 4.00 | |
| Phospholipids/2S-P3-Sia (1:1 w/w) | 148 | 3.3 × 1012 | 6.29 × 109 | 4 | 0.85 | |
| No. 2 | Phospholipids | 245.8 | 4.2 × 1013 | 5.73 × 109 | 5 | 3.35 |
| Phospholipids/2S-P1000 (3:1 w/w) | 241.9 | 6.3 × 1011 | 7.61 × 109 | 5 | 2.53 | |
| Phospholipids/2S-P3-Sia (3:1 w/w) | 203.8 | 5.7 × 1011 | 3.84 × 109 | 5 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, D.; McAdorey, A.; Lei, E.; Beaudoin, G.; Ling, B.; Callaghan, D.; Fatehi, D.; Verner, A.; Slinn, J.; Moreno, M.; et al. Synthesis of α,ε-N,N′-Di-stearoyl Lysine-Derived Amide Lipids and Their Application to Liposome Formulation: Incorporation of Lipid A-Ligand for Bacterial Targeting and Sialic Acid for Phagocytosis Resistance. Int. J. Mol. Sci. 2025, 26, 9176. https://doi.org/10.3390/ijms26189176
Williams D, McAdorey A, Lei E, Beaudoin G, Ling B, Callaghan D, Fatehi D, Verner A, Slinn J, Moreno M, et al. Synthesis of α,ε-N,N′-Di-stearoyl Lysine-Derived Amide Lipids and Their Application to Liposome Formulation: Incorporation of Lipid A-Ligand for Bacterial Targeting and Sialic Acid for Phagocytosis Resistance. International Journal of Molecular Sciences. 2025; 26(18):9176. https://doi.org/10.3390/ijms26189176
Chicago/Turabian StyleWilliams, Dean, Alyssa McAdorey, Eric Lei, Greg Beaudoin, Binbing Ling, Debbie Callaghan, Dorothy Fatehi, Angie Verner, Jacqueline Slinn, Maria Moreno, and et al. 2025. "Synthesis of α,ε-N,N′-Di-stearoyl Lysine-Derived Amide Lipids and Their Application to Liposome Formulation: Incorporation of Lipid A-Ligand for Bacterial Targeting and Sialic Acid for Phagocytosis Resistance" International Journal of Molecular Sciences 26, no. 18: 9176. https://doi.org/10.3390/ijms26189176
APA StyleWilliams, D., McAdorey, A., Lei, E., Beaudoin, G., Ling, B., Callaghan, D., Fatehi, D., Verner, A., Slinn, J., Moreno, M., Iqbal, U., Qian, H., Yan, H., Chen, W., & Zou, W. (2025). Synthesis of α,ε-N,N′-Di-stearoyl Lysine-Derived Amide Lipids and Their Application to Liposome Formulation: Incorporation of Lipid A-Ligand for Bacterial Targeting and Sialic Acid for Phagocytosis Resistance. International Journal of Molecular Sciences, 26(18), 9176. https://doi.org/10.3390/ijms26189176






