Copper, Cuproptosis, and Neurodegenerative Diseases
Abstract
1. Introduction
2. Chemical Forms and Properties of Copper
3. Effects of Copper on Mitochondria
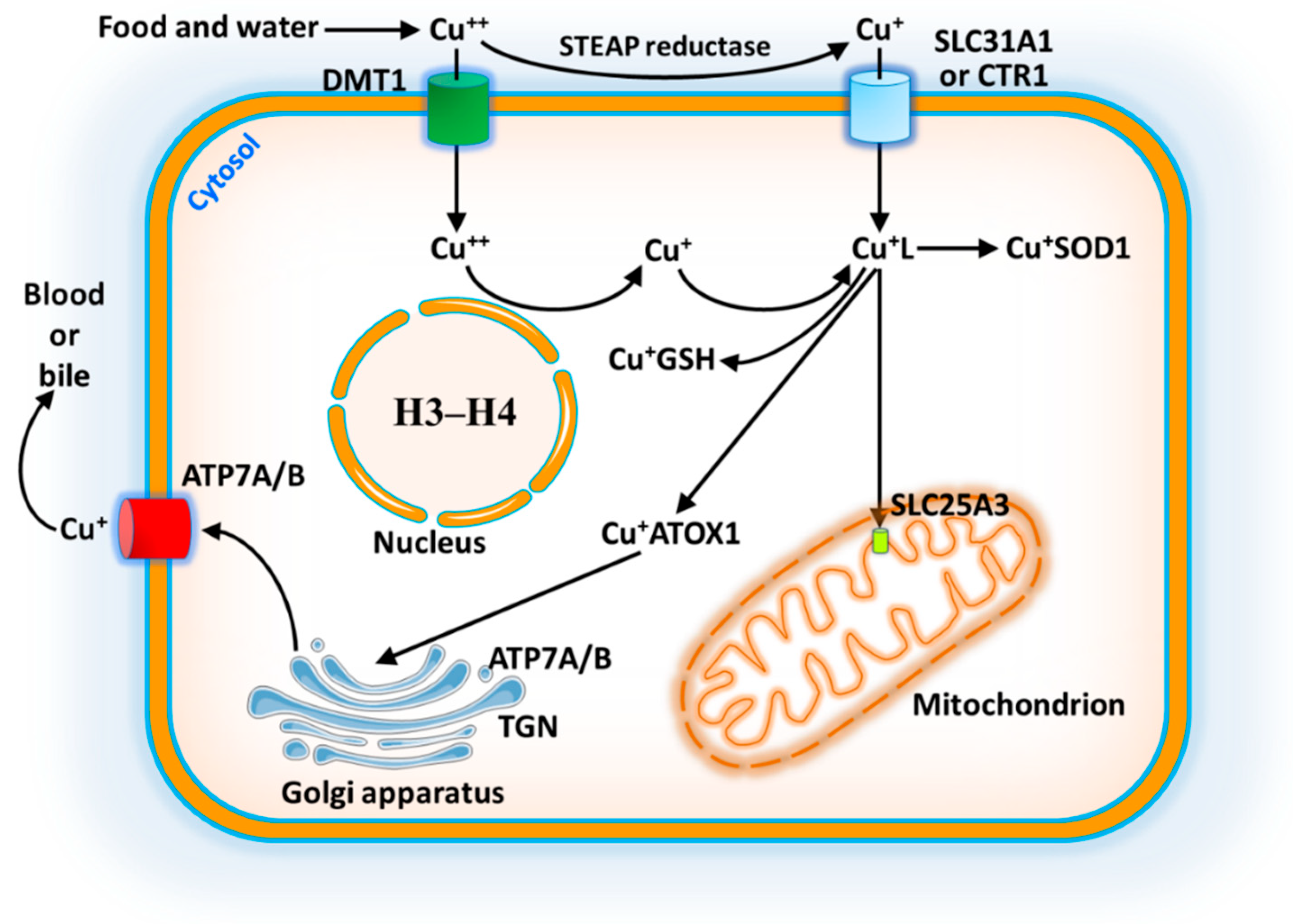
4. Absorption and Metabolism of Copper
5. Copper Trafficking in Mitochondria
6. Cuproptosis Mechanism in Mitochondria
7. Copper and Pathological Conditions
7.1. Menkes Disease (MD)
7.2. Wilson Disease (WD)
7.3. Alzheimer Disease (AD)
7.4. Parkinson Disease (PD)
7.5. Huntington Disease (HD)
7.6. Amyotrophic Lateral Sclerosis (ALS)
8. Phytoremediation of Copper
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lutsenko, S.; Roy, S.; Tsvetkov, P. Mammalian copper homeostasis: Physiological roles and molecular mechanisms. Physiol. Rev. 2025, 105, 441–491, Erratum in Physiol. Rev. 2025, 105, 2231. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E. Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements? Curr. Issues Mol. Biol. 2024, 46, 8441–8463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Xu, K.; Wang, G.; Zhang, F. Copper homeostasis and neurodegenerative diseases. Neural Regen. Res. 2025, 20, 3124–3143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol. Ther. 2012, 133, 177–188. [Google Scholar] [CrossRef]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper dyshomeostasis in neurodegenerative diseases—Therapeutic implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Pascual, J.M.; Menkes, J.H. Menkes disease and other ATP7A disorders. In Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease; Academic Press: Cambridge, MA, USA, 2025; Chapter 40; pp. 757–764. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261, Erratum in Science 2022, 376, eabq4855. [Google Scholar] [CrossRef]
- McHenry, R. The New Enciclopedia Britannica, 15th ed.; Encyclopaedia Britannica, Inc.: Chicago, IL, USA, 1992; Volume 3, 612p. [Google Scholar]
- Okazawa, H.; Yonekura, Y.; Fujibayashi, Y.; Nishizawa, S.; Magata, Y.; Ishizu, K.; Tanaka, F.; Tsuchida, T.; Tamaki, N.; Konishi, J. Clinical application and quantitative evaluation of generator-produced copper-62-PTSM as a brain perfusion tracer for PET. J. Nucl. Med. 1994, 35, 1910–1915. [Google Scholar] [PubMed]
- Bernhard, D.; Rossmann, A.; Wick, G. Metals in cigarette smoke. IUBMB Life 2005, 57, 805–809. [Google Scholar] [CrossRef]
- Coates, C.J.; Costa-Paiva, E.M. Multifunctional Roles of Hemocyanins. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins: Subcellular Biochemistry; Springer: Cham, Switzerland, 2020; Volume 94, pp. 233–250. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, X.; Li, J.; Xie, Z.; Wu, Q.; Chen, J.; Wang, Y.; Chen, Z.; Cao, X.; Li, T.; et al. Insights into the role of copper in neurodegenerative diseases and the therapeutic potential of natural compounds. Curr. Neuropharmacol. 2024, 22, 1650–1671. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, A.Q. Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front. Immunol. 2023, 14, 1236063. [Google Scholar] [CrossRef]
- Oh-hama, T. Evolutionary consideration on 5-aminolevulinate synthase in nature. Orig. Life Evol. Biosph. 1997, 27, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341 Pt 2, 233–249. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Molkenting, J.D. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef]
- Attar, N.; Campos, O.A.; Vogelauer, M.; Cheng, C.; Xue, Y.; Schmollinger, S.; Salwinski, L.; Mallipeddi, N.V.; Boone, B.A.; Yen, L.; et al. The histone H3-H4 tetramer is a copper reductase enzyme. Science 2020, 369, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-R.; Bu, L.-L.; Cai, L. Cuproptosis: Lipoylated TCA Cycle Proteins-Mediated Novel Cell Death Pathway. Signal Transduct. Target. Ther. 2022, 7, 158. [Google Scholar] [CrossRef]
- Tian, Z.; Jiang, S.; Zhou, J.; Zhang, W. Copper homeostasis and cuproptosis in mitochondria. Life Sci. 2023, 334, 122223. [Google Scholar] [CrossRef]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018, 293, 1887–1896. [Google Scholar] [CrossRef]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118867. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Hadjiloi, T.; Martinelli, M.; Palumaa, P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6803–6808. [Google Scholar] [CrossRef]
- Singh, R.P.; Jeyaraju, D.V.; Voisin, V.; Hurren, R.; Xu, C.; Hawley, J.R.; Barghout, S.H.; Khan, D.H.; Gronda, M.; Wang, X.; et al. Disrupting Mitochondrial Copper Distribution Inhibits Leukemic Stem Cell Self-Renewal. Cell Stem Cell 2020, 26, 926–937.e10. [Google Scholar] [CrossRef]
- Rowland, E.A.; Snowden, C.K.; Cristea, I.M. Protein lipoylation: An Evolutionarily Conserved Metabolic Regulator of Health and Disease. Curr. Opin. Chem. Biol. 2018, 42, 76–85. [Google Scholar] [CrossRef]
- Lu, K.; Wijaya, C.S.; Yao, Q.; Jin, H.; Feng, L. Cuproplasia and cuproptosis, two sides of the coin. Cancer Commun. 2025, 45, 505–524. [Google Scholar] [CrossRef]
- Tümer, Z.; Møller, L.B. Menkes disease. Eur. J. Hum. Genet. 2010, 18, 511–518. [Google Scholar] [CrossRef]
- Hicks, J.D.; Donsante, A.; Pierson, T.M.; Gillespie, M.J.; Chou, D.E.; Kaler, S.G. Increased frequency of congenital heart defects in Menkes disease. Clin. Dysmorphol. 2012, 21, 59–63. [Google Scholar] [CrossRef]
- Kaler, S.G. Inborn errors of copper metabolism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1745–1754. [Google Scholar] [CrossRef]
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.F.; Cox, M.; Tambo, W.; Eskander, N.; Wirth, M.; Valdez, M.; Niño, M. Neurological manifestations of Wilson’s disease: Pathophysiology and localization of each component. Cureus 2020, 12, 11509. [Google Scholar] [CrossRef] [PubMed]
- Low, Q.J.; Siaw, C.; Lee, R.A.; Cheo, S.W. Kayser–Fleischer Rings and Wilson’s Disease. QJM Int. J. Med. 2020, 113, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Przybyłkowski, A.; Gromadzka, G.; Chabik, G.; Wierzchowska, A.; Litwin, T.; Członkowska, A. Liver cirrhosis in patients newly diagnosed with neurological phenotype of Wilson’s disease. Funct. Neurol. 2014, 29, 23–29. [Google Scholar] [PubMed]
- Zischka, H.; Lichtmannegger, J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann. N. Y. Acad. Sci. 2014, 1315, 6–15. [Google Scholar] [CrossRef]
- Einer, C.; Munk, D.E.; Park, E.; Akdogan, B.; Nagel, J.; Lichtmannegger, J.; Eberhagen, C.; Rieder, T.; Vendelbo, M.H.; Michalke, B.; et al. ARBM101 (methanobactin SB2) drains excess liver copper via biliary excretion in Wilson’s disease rats. Gastroenterology 2023, 165, 187–200.e7. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid beta in aging and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective effects of curcumin in neurodegenerative diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Kitazawa, M.; Cheng, D.; Laferla, F.M. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 2009, 108, 1550–1560. [Google Scholar] [CrossRef]
- Sparks, D.L.; Schreurs, B.G. Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 11065–11069. [Google Scholar] [CrossRef]
- Yao, D.; Jing, T.; Niu, L.; Huang, X.; Wang, Y.; Deng, X.; Wang, M. Amyloidogenesis induced by diet cholesterol and copper in a model mouse for Alzheimer’s disease and protection effects of zinc and fluvastatin. Brain Res. Bull. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Atwood, C.S.; Perry, G.; Zeng, H.; Kato, Y.; Jones, W.D.; Ling, K.Q.; Huang, X.; Moir, R.D.; Wang, D.; Sayre, L.M.; et al. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-β. Biochemistry 2004, 43, 560–568. [Google Scholar] [CrossRef]
- Noda, Y.; Asada, M.; Kubota, M.; Maesako, M.; Watanabe, K.; Uemura, M.; Kihara, T.; Shimohama, S.; Takahashi, R.; Kinoshita, A.; et al. Copper enhances APP dimerization and promotes Aβ production. Neurosci. Lett. 2013, 547, 10–15. [Google Scholar] [CrossRef]
- Cherny, R.A.; Atwood, C.S.; Xilinas, M.E.; Gray, D.N.; Jones, W.D.; McLean, C.A.; Barnham, K.J.; Volitakis, I.; Fraser, F.W.; Kim, Y.; et al. Treatment with a copper–zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 2001, 30, 665–676. [Google Scholar] [CrossRef]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786, Erratum in Lancet Neurol. 2009, 8, 981. [Google Scholar] [CrossRef]
- Squitti, R.; Rossini, P.M.; Cassetta, E.; Moffa, F.; Pasqualetti, P.; Cortesi, M.; Colloca, A.; Rossi, L.; Finazzi-Agró, A. D-penicillamine reduces serum oxidative stress in Alzheimer’s disease patients. Eur. J. Clin. Investig. 2002, 32, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Lockman, P.R.; Atwood, C.S.; Hsu, C.-H.; Gupte, A.; Allen, D.D.; Mumper, R.J. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Eur. J. Pharm. Biopharm. 2005, 59, 263–272. [Google Scholar] [CrossRef]
- Zhong, M.; Kou, H.; Zhao, P.; Zheng, W.; Xu, H.; Zhang, X.; Lan, W.; Guo, C.; Wang, T.; Guo, F.; et al. Nasal delivery of D-penicillamine hydrogel upregulates a disintegrin and metalloprotease 10 expression via melatonin receptor 1 in Alzheimer’s disease models. Front. Aging Neurosci. 2021, 13, 660249. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Rovito, N.; Carocci, A.; Genchi, G. Acetyl-L-carnitine in Parkinson’s disease. In Mechanisms in Parkinson’s Disease—Models and Treatments; Dushanova, J., Ed.; InTech: London, UK, 2012; Chapter 19; pp. 367–392. ISBN 978-953-307-876-2. [Google Scholar]
- Carocci, A.; Sinicropi, M.S.; Catalano, A.; Lauria, G.; Genchi, G. Melatonin in Parkinson’s disease. In A Synopsis of Parkinson’s Disease; InTech: London, UK, 2014; Chapter 3; pp. 71–99. ISBN 978-953-51-1229-7. [Google Scholar]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Perry, G.; Smith, M.A.; Robertson, D.; Olson, S.J.; Graham, D.G.; Montine, T.J. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999, 154, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.; Ives, N.; Clarke, C.E.; Van Hilten, J.; Ferreira, J.; Hawker, R.J.; Shah, L.; Wheatley, K.; Gray, R. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst. Rev. 2008, 16, CD006564. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. S3), S26–S36; Discussion S36–S38. [Google Scholar] [CrossRef]
- Dikiy, I.; Eliezer, D. N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound α-synuclein and increases its affinity for physiological membranes. J. Biol. Chem. 2014, 289, 3652–3665. [Google Scholar] [CrossRef]
- McDowall, J.S.; Brown, D.R. Alpha-synuclein: Relating metals to structure, function and inhibition. Metallomics 2016, 8, 385–397. [Google Scholar] [CrossRef]
- Hung, L.W.; Villemagne, V.L.; Cheng, L.; Sherratt, N.A.; Ayton, S.; White, A.R.; Crouch, P.J.; Lim, S.; Leong, S.L.; Wilkins, S.; et al. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J. Exp. Med. 2012, 209, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Okazawa, H.; Kudo, T.; Kuriyama, M.; Fujibayashi, Y.; Yoneda, M. Evaluation of striatal oxidative stress in patients with Parkinson’s disease using [62Cu]ATSM PET. Nucl. Med. Biol. 2011, 38, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Dayalu, P.; Albin, R.L. Huntington disease: Pathogenesis and treatment. Neurol. Clin. 2015, 33, 101–114. [Google Scholar] [CrossRef]
- Fox, J.H.; Kama, J.A.; Lieberman, G.; Chopra, R.; Dorsey, K.; Chopra, V.; Volitakis, I.; Cherny, R.A.; Bush, A.I.; Hersch, S. Mechanisms of copper ion mediated Huntington’s disease progression. PLoS ONE 2007, 2, e334. [Google Scholar] [CrossRef] [PubMed]
- Hands, S.L.; Mason, R.; Sajjad, M.U.; Giorgini, F.; Wyttenbach, A. Metallothioneins and copper metabolism are candidate therapeutic targets in Huntington’s disease. Biochem. Soc. Trans. 2010, 38, 552–558. [Google Scholar] [CrossRef]
- Sheline, C.T.; Choi, D.W. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann. Neurol. 2004, 55, 645–653. [Google Scholar] [CrossRef]
- Kasischke, K.A.; Vishwasrao, H.D.; Fisher, P.J.; Zipfel, W.R.; Webb, W.W. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 2004, 305, 99–103. [Google Scholar] [CrossRef]
- Tallaksen-Greene, S.J.; Janiszewska, A.; Benton, K.; Hou, G.; Dick, R.; Brewer, G.J.; Albin, R.L. Evaluation of tetrathiomolybdate in the R6/2 model of Huntington disease. Neurosci. Lett. 2009, 452, 60–62. [Google Scholar] [CrossRef]
- Cherny, R.A.; Ayton, S.; Finkelstein, D.I.; Bush, A.I.; McColl, G.; Massa, S.M. PBT2 Reduces toxicity in a C. elegans model of polyQ aggregation and extends lifespan, reduces striatal atrophy and improves motor Performance in the R6/2 mouse model of Huntington’s disease. J. Huntington’s Dis. 2012, 1, 211–219. [Google Scholar] [CrossRef]
- Lobato, A.G.; Ortiz-Vega, N.; Zhu, Y.; Neupane, D.; Meier, K.K.; Zhai, R.G. Copper enhances aggregational toxicity of mutant huntingtin in a Drosophila model of Huntington’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166928. [Google Scholar] [CrossRef]
- Barros, A.N.A.B.; Dourado, M.E.T.; Pedrosa, L.d.F.C.; Leite-Lais, L. Association of copper status with lipid profile and functional status in patients with amyotrophic lateral sclerosis. J. Nutr. Metab. 2018, 2018, 5678698. [Google Scholar] [CrossRef]
- Ellerby, L.M.; Cabelli, D.E.; Graden, J.A.; Valentine, J.S. Copper−zinc superoxide dismutase: Why not pH-dependent? J. Am. Chem. Soc. 1996, 118, 6556–6561. [Google Scholar] [CrossRef]
- Hilton, J.B.; White, A.R.; Crouch, P.J. Metal-deficient SOD1 in amyotrophic lateral sclerosis. J. Mol. Med. 2015, 93, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Enge, T.G.; Ecroyd, H.; Jolley, D.F.; Yerbury, J.J.; Kalmar, B.; Dosseto, A. Assessment of metal concentrations in the SOD1G93A mouse model of amyotrophic lateral sclerosis and its potential role in muscular denervation, with particular focus on muscle tissue. Mol. Cell. Neurosci. 2018, 88, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Brown, H.H.; Borchelt, D.R.; Vogt, S.; Miller, L.M. Metal-deficient aggregates and diminished copper found in cells expressing SOD1 mutations that cause ALS. Front. Aging Neurosci. 2014, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.V.; Winkler, D.D.; Taylor, A.B.; Cao, X.; Whitson, L.J.; Doucette, P.A.; Valentine, J.S.; Schirf, V.; Demeler, B.; Carroll, M.C.; et al. Disrupted zinc-binding sites in structures of pathogenic SOD1 variants D124V and H80R. Biochemistry 2010, 49, 5714–5725. [Google Scholar] [CrossRef]
- Gil-Bea, F.J.; Aldanondo, G.; Lasa-Fernández, H.; de Munain, A.L.; Vallejo-Illarramendi, A. Insights into the mechanisms of copper dyshomeostasis in amyotrophic lateral sclerosis. Expert Rev. Mol. Med. 2017, 19, e7. [Google Scholar] [CrossRef]
- Hottinger, A.F.; Fine, E.G.; Gurney, M.E.; Zurn, A.D.; Aebischer, P. The copper chelator D-penicillamine delays onset of disease and extends survival in a transgenic mouse model of familial amyotrophic lateral sclerosis. Eur. J. Neurosci. 1997, 9, 1548–1551. [Google Scholar] [CrossRef]
- Tokuda, E.; Ono, S.; Ishige, K.; Watanabe, S.; Okawa, E.; Ito, Y.; Suzuki, T. Ammonium tetrathiomolybdate delays onset, prolongs survival, and slows progression of disease in a mouse model for amyotrophic lateral sclerosis. Exp. Neurol. 2008, 213, 122–128. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Das, S. Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotoxicol. Environ. Saf. 2016, 126, 211–218. [Google Scholar] [CrossRef]
- Zand, A.D.; Mühling, K.H. Phytoremediation capability and copper uptake of maize (Zea mays L.) in copper contaminated soils. Pollutants 2022, 2, 53–65. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Díaz-Raviña, M.; Arias-Estévez, M. Copper accumulation and fractionation in vineyard soils from temperate humid zone (NW Iberian Peninsula). Geoderma 2009, 153, 119–129. [Google Scholar] [CrossRef]
- Mirlean, N.; Roisenberg, A.; Chies, J.O. Metal contamination of vineyard soils in wet subtropics (southern Brazil). Environ. Pollut. 2007, 149, 10–17. [Google Scholar] [CrossRef]
- Widmer, J.; Norgrove, L. Identifying candidates for the phytoremediation of copper in viticultural soils: A systematic review. Environ. Res. 2023, 216, 114518. [Google Scholar] [CrossRef]
- Malagoli, M.; Rossignolo, V.; Salvalaggio, N.; Schiavon, M. Potential for phytoextraction of copper by Sinapis alba and Festuca rubra cv. Merlin grown hydroponically and in vineyard soils. Environ. Sci. Pollut. Res. 2014, 21, 3294–3303. [Google Scholar] [CrossRef] [PubMed]
- Devi, Y.; Surendran, A.; Thatheyus, A.J. Phytoremediation of copper using the tomato plant, Lycopersicon esculentum. Res. Biotechnol. Environ. Sci. 2024, 3, 23–28. [Google Scholar] [CrossRef]


| Atomic number | 29 |
| Atomic weight | 63.55 g/mol |
| Electronic configuration | [Ar] 3d104s1 |
| Melting point | 1084.6 °C |
| Boiling point | 2562 °C |
| Van der Walls radius | 140 pm |
| Density at 20 °C | 8.94 g/cm3 |
| Heat of fusion | 13.26 KJ/mol |
| Heat of vaporization | 300.4 KJ/mol |
| Molar heat capacity | 24.5 J/(mol⋅K) |
| Pauling electronegativity number | 1.90 |
| Electrical conductivity | 59.6 × 106 S/m |
| First ionization energy | 745.5 KJ/mol |
| Second ionization energy | 1957.9 KJ/mol |
| Third ionization energy | 3555 KJ/mol |
| Mohs hardness | 3.0 |
| Cristal structure | Face-centered cubic |
| Oxidation states | −2, −1, 0, +1, +2, +3, +4 |
| Magnetic ordering | Diamagnetic |
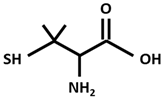 | DPA [D-Penicillamine (2-amino-3-mercapto-3-methyl butanoic acid)] |
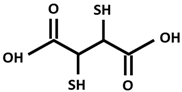 | DMSA [Dimercapto succinic acid] |
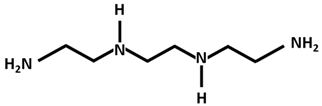 | Trientine |
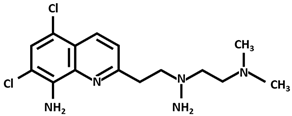 | DTMQ20 |
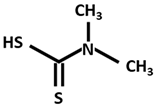 | DMDTC [Dimethyldithiocarbamate] |
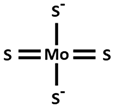 | TTM [Tetrathiomolybdate] |
 | Clioquinol [5-Chloro-7-iodo-8-quinolinol] |
 | PBT2 [5,7-Dichloro-2-(dimethylamino)methyl-8-quinolinol] |
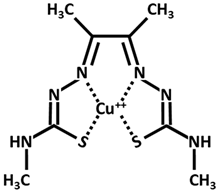
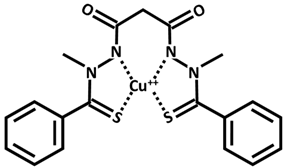 | Cu++-elesclomol |
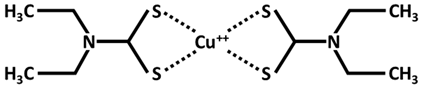 | Cu++ disulfiram Tetraethyldisulfamdicarbothioamide |
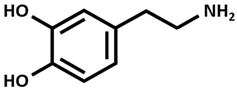 | Dopamine |
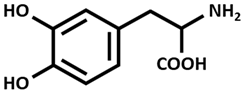 | L-DOPA [Levodopa] |
 | Cu-ATSM [Cu diacetyl bis(N4-methylthiosemicarbazone)] |
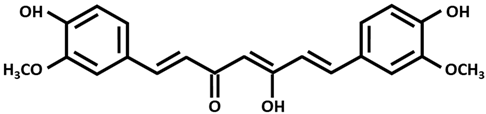 | Curcumin (enol form) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genchi, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S.; Lauria, G. Copper, Cuproptosis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 9173. https://doi.org/10.3390/ijms26189173
Genchi G, Catalano A, Carocci A, Sinicropi MS, Lauria G. Copper, Cuproptosis, and Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(18):9173. https://doi.org/10.3390/ijms26189173
Chicago/Turabian StyleGenchi, Giuseppe, Alessia Catalano, Alessia Carocci, Maria Stefania Sinicropi, and Graziantonio Lauria. 2025. "Copper, Cuproptosis, and Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 18: 9173. https://doi.org/10.3390/ijms26189173
APA StyleGenchi, G., Catalano, A., Carocci, A., Sinicropi, M. S., & Lauria, G. (2025). Copper, Cuproptosis, and Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(18), 9173. https://doi.org/10.3390/ijms26189173








