WW Domain-Containing E3 Ubiquitin Protein Ligase 1 (WWP1) as a Factor in Obesity-Related Metabolic Disorders: Emerging Molecular Mechanisms in Metabolic Tissues
Abstract
1. Introduction
2. The Regulation of WWP1 Expression in Mammals
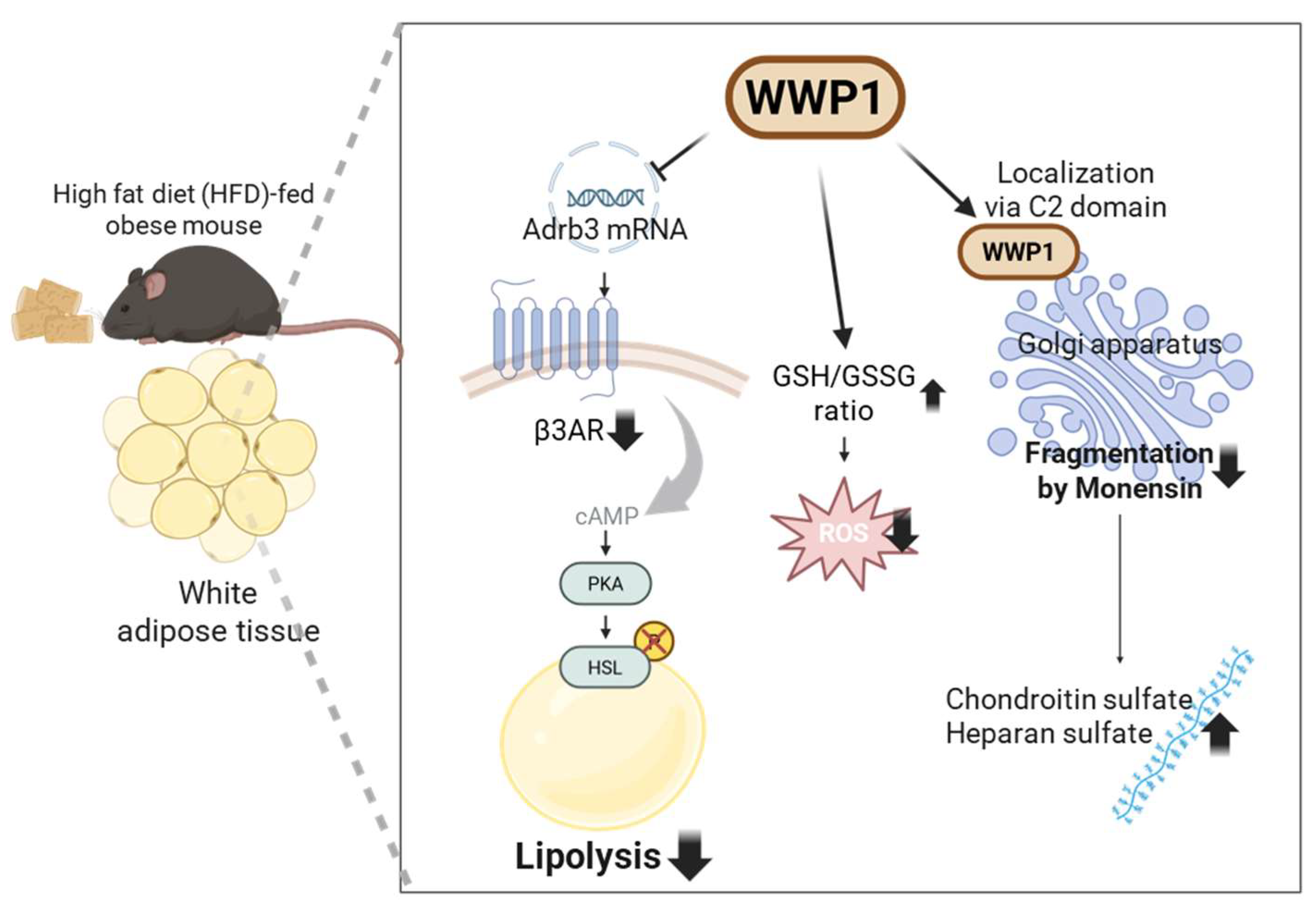
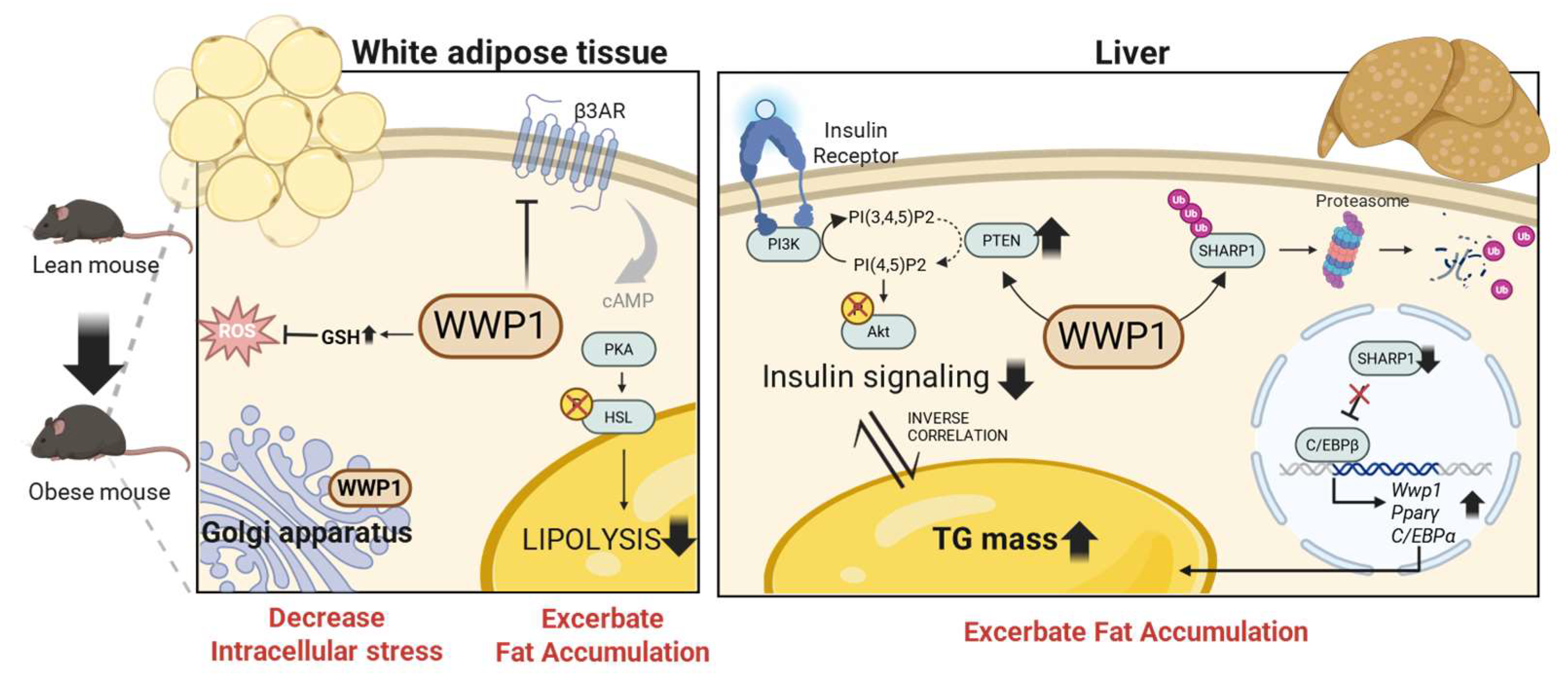
3. Lipid Homeostasis Regulation by WWP1 in Adipocytes
4. The Role of WWP1 in Protecting Against Golgi Apparatus Disruption and Oxidative Stress in Adipocytes
4.1. WWP1 Localizes to the Golgi Apparatus via Its C2 Domain and Protects Golgi Morphology
4.2. WWP1 Modulates Oxidative Stress in WAT During Obesity
5. WWP1 Decreases Insulin Sensitivity and Exacerbates Hepatic Steatosis
5.1. Systemic Depletion of WWP1 Improves Insulin Sensitivity in the Obese Liver
5.2. Systemic Depletion of WWP1 Improves Hepatic Fat Accumulation in Obese Mice
5.3. Systemic Depletion of WWP1 Improves Systemic Insulin Sensitivity in Obese Mice
6. Comparison of NEDD-Family-Deficient Mice and Obesity-Related Phenotypes
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 June 2025).
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Madeo, F.; Kratky, D. Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Large, V.; Reynisdottir, S.; Langin, D.; Fredby, K.; Klannemark, M.; Holm, C.; Arner, P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J. Lipid Res. 1999, 40, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Bougnères, P.; Stunff, C.L.; Pecqueur, C.; Pinglier, E.; Adnot, P.; Ricquier, D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J. Clin. Investig. 1997, 99, 2568–2573. [Google Scholar] [CrossRef]
- Valentine, J.M.; Ahmadian, M.; Keinan, O.; Abu-Odeh, M.; Zhao, P.; Zhou, X.; Keller, M.P.; Gao, H.; Yu, R.T.; Liddle, C.; et al. β3-Adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J. Clin. Investig. 2022, 132, e153357. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Lim, S.; Meigs, J.B. Links between ectopic fat and vascular disease in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1820–1826. [Google Scholar] [CrossRef]
- Byrne, C.D.; Olufadi, R.; Bruce, K.D.; Cagampang, F.R.; Ahmed, M.H. Metabolic disturbances in non-alcoholic fatty liver disease. Clin. Sci. 2009, 116, 539–564. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D. Dorothy Hodgkin Lecture 2012: Non-alcoholic fatty liver disease, insulin resistance and ectopic fat: A new problem in diabetes management. Diabet. Med. 2012, 29, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.R.; Lallemand, F.; Ferrand, N.; Pessah, M.; L’Hoste, S.; Camonis, J.; Atfi, A. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J. 2004, 23, 3780–3792. [Google Scholar] [CrossRef]
- Bernassola, F.; Karin, M.; Ciechanover, A.; Melino, G. The HECT family of E3 ubiquitin ligases: Multiple players in cancer development. Cancer Cell 2008, 14, 10–21. [Google Scholar] [CrossRef]
- Bernassola, F.; Chillemi, G.; Melino, G. HECT-Type E3 Ubiquitin Ligases in Cancer. Trends Biochem. Sci. 2019, 44, 1057–1075. [Google Scholar] [CrossRef]
- Singh, S.; Ng, J.; Sivaraman, J. Exploring the “Other” subfamily of HECT E3-ligases for therapeutic intervention. Pharmacol. Ther. 2021, 224, 107809. [Google Scholar] [CrossRef]
- Boase, N.A.; Kumar, S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene 2015, 557, 113–122. [Google Scholar] [CrossRef]
- Ye, X.; Wang, L.; Shang, B.; Wang, Z.; Wei, W. NEDD4: A promising target for cancer therapy. Curr. Cancer Drug Targets 2014, 14, 549–556. [Google Scholar] [CrossRef]
- Wang, Z.W.; Hu, X.; Ye, M.; Lin, M.; Chu, M.; Shen, X. NEDD4 E3 ligase: Functions and mechanism in human cancer. Semin. Cancer Biol. 2020, 67, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Chen, C. WWP1: A versatile ubiquitin E3 ligase in signaling and diseases. Cell. Mol. Life Sci. 2012, 69, 1425–1434. [Google Scholar] [CrossRef]
- Behera, A.; Reddy, A.B.M. WWP1 E3 ligase at the crossroads of health and disease. Cell Death Dis. 2023, 14, 853. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, J.; Lin, Z.; Feng, R.; Wang, Z.W.; Chen, G. The emerging role of WWP1 in cancer development and progression. Cell Death Discov. 2021, 7, 163. [Google Scholar] [CrossRef]
- Chen, C.; Matesic, L.E. The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev. 2007, 26, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, J.; Zhang, H.; Wang, Y.; Matesic, L.E.; Takahata, M.; Awad, H.; Chen, D.; Xing, L. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells 2011, 29, 1601–1610. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.Y.; Fu, T.M.; Chen, H.; Ishikawa, T.; Chiang, S.Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Laine, A.; Ronai, Z. Regulation of p53 localization and transcription by the HECT domain E3 ligase WWP1. Oncogene 2007, 26, 1477–1483. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Chen, C. WW domain-containing E3 ubiquitin protein ligase 1 targets p63 transcription factor for ubiquitin-mediated proteasomal degradation and regulates apoptosis. Cell Death Differ. 2008, 15, 1941–1951. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, M.; An, J.; Sun, H.; Li, T.; Li, D. A positive feedback loop of miR-30a-5p-WWP1-NF-κB in the regulation of glioma development. Int. J. Biochem. Cell Biol. 2019, 112, 39–49. [Google Scholar] [CrossRef]
- Ma, L.; Chen, X.; Li, C.; Cheng, R.; Gao, Z.; Meng, X.; Sun, C.; Liang, C.; Liu, Y. miR-129-5p and -3p co-target WWP1 to suppress gastric cancer proliferation and migration. J. Cell. Biochem. 2019, 120, 7527–7538. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kojima, S.; Kurozumi, A.; Kato, M.; Okato, A.; Matsushita, R.; Ichikawa, T.; Seki, N. Regulation of E3 ubiquitin ligase-1 (WWP1) by microRNA-452 inhibits cancer cell migration and invasion in prostate cancer. Br. J. Cancer 2016, 114, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Wei, S.; Wang, W.; Chen, Z.; Zhang, L.; Chen, L.; Li, B.; Sun, G.; Xu, J.; et al. Overexpression of miR-584-5p inhibits proliferation and induces apoptosis by targeting WW domain-containing E3 ubiquitin protein ligase 1 in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 59. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hoshino, S.; Abe, T.; Okita, N.; Tagawa, R.; Nagai, W.; Konno, R.; Suzuki, Y.; Furuya, K.; Ishikawa, N.; et al. Identification of WWP1 as an obesity-associated E3 ubiquitin ligase with a protective role against oxidative stress in adipocytes. Biochem. Biophys. Res. Commun. 2019, 508, 117–122. [Google Scholar] [CrossRef]
- Reynisdottir, S.; Langin, D.; Carlström, K.; Holm, C.; Rössner, S.; Arner, P. Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin. Sci. 1995, 89, 421–429. [Google Scholar] [CrossRef]
- Collins, S.; Daniel, K.W.; Rohlfs, E.M. Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 669–677. [Google Scholar] [CrossRef][Green Version]
- Taosis, M.; Valet, P.; Estan, L.; Lafontan, M.; Montastruc, P.; Berlan, M. Obesity modifies the adrenergic status of dog adipose tissue. J. Pharmacol. Exp. Ther. 1989, 250, 1061–1066. [Google Scholar] [CrossRef]
- Muzzin, P.; Revelli, J.P.; Kuhne, F.; Gocayne, J.D.; McCombie, W.R.; Venter, J.C.; Giacobino, J.P.; Fraser, C.M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J. Biol. Chem. 1991, 266, 24053–24058. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1144–E1152. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Peters, E.J.; Klein, S.; Holland, O.B.; Rosenblatt, J.; Gary, H., Jr. Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am. J. Physiol. Endocrinol. Metab. 1987, 252 Pt 1, E189–E196. [Google Scholar] [CrossRef]
- Nozaki, Y.; Ose, Y.; Ohmori, C.; Mizunoe, Y.; Kobayashi, M.; Saitoh, A.; Higami, Y. Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice. Int. J. Mol. Sci. 2025, 26, 4219. [Google Scholar] [CrossRef]
- Li, J.J.; Ferry, R.J., Jr.; Diao, S.; Xue, B.; Bahouth, S.W.; Liao, F.F. Nedd4 haploinsufficient mice display moderate insulin resistance, enhanced lipolysis, and protection against high-fat diet-induced obesity. Endocrinology 2015, 156, 1283–1291. [Google Scholar] [CrossRef]
- Jaworski, K.; Sarkadi-Nagy, E.; Duncan, R.E.; Ahmadian, M.; Sul, H.S. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1–G4. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y. Glycosylation Quality Control by the Golgi Structure. J. Mol. Biol. 2016, 428, 3183–3193. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, X.; Nix, D.B.; Katoh, T.; Aoki, K.; Tiemeyer, M.; Wang, Y. Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65. Nat. Commun. 2013, 4, 1659. [Google Scholar] [CrossRef]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef]
- Wilsie, L.C.; Chanchani, S.; Navaratna, D.; Orlando, R.A. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Pessentheiner, A.R.; Ducasa, G.M.; Gordts, P. Proteoglycans in Obesity-Associated Metabolic Dysfunction and Meta-Inflammation. Front. Immunol. 2020, 11, 769. [Google Scholar] [CrossRef]
- Buzuk, L.; Hellerschmied, D. Ubiquitin-mediated degradation at the Golgi apparatus. Front. Mol. Biosci. 2023, 10, 1197921. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Nozaki, Y.; Suwa, F.; Furuya, K.; Komeno, M.; Hoshino, S.; Mizunoe, Y.; Higashi, K.; Kobayashi, M.; Higami, Y. The effects of WWP1 overexpression on the golgi apparatus stress response and proteoglycan production in adipocytes. Sci. Rep. 2024, 14, 29004. [Google Scholar] [CrossRef]
- Dinter, A.; Berger, E.G. Golgi-disturbing agents. Histochem. Cell Biol. 1998, 109, 571–590. [Google Scholar] [CrossRef]
- Viettri, M.; Zambrano, J.L.; Rosales, R.; Caraballo, G.I.; Gutiérrez-Escolano, A.L.; Ludert, J.E. Flavivirus infections induce a Golgi stress response in vertebrate and mosquito cells. Sci. Rep. 2021, 11, 23489. [Google Scholar] [CrossRef]
- Mollenhauer, H.H.; Morré, D.J.; Minnifield, N. Swelling response of Golgi apparatus cisternae in cells treated with monensin is reduced by cell injury. Cell Biol. Int. Rep. 1992, 16, 217–220. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.; Chan, E.C.; Liu, G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013, 22, 878–888. [Google Scholar] [CrossRef]
- Hoshino, S.; Kobayashi, M.; Tagawa, R.; Konno, R.; Abe, T.; Furuya, K.; Miura, K.; Wakasawa, H.; Okita, N.; Sudo, Y.; et al. WWP1 knockout in mice exacerbates obesity-related phenotypes in white adipose tissue but improves whole-body glucose metabolism. FEBS Open Bio 2020, 10, 306–315. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, Z.; Zeng, L.; Lu, W.; Zhang, H.; Li, T.; Xiao, H. The role of the Golgi apparatus in oxidative stress: Is this organelle less significant than mitochondria? Free Radic. Biol. Med. 2011, 50, 907–917. [Google Scholar] [CrossRef]
- Xie, L.; Boyle, D.; Sanford, D.; Scherer, P.E.; Pessin, J.E.; Mora, S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J. Biol. Chem. 2006, 281, 7253–7259. [Google Scholar] [CrossRef]
- Ding, L.; Huwyler, F.; Long, F.; Yang, W.; Binz, J.; Wernlé, K.; Pfister, M.; Klug, M.; Balaz, M.; Ukropcova, B.; et al. Glucose controls lipolysis through Golgi PtdIns4P-mediated regulation of ATGL. Nat. Cell Biol. 2024, 26, 552–566. [Google Scholar] [CrossRef]
- Yeop Han, C.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O’Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Hirabara, S.M.; dos Reis Silveira, L.; Levada-Pires, A.C.; Curi, R.; Pithon-Curi, T.C. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J. Cell. Physiol. 2008, 216, 796–804. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Stambolic, V.; Suzuki, A.; de la Pompa, J.L.; Brothers, G.M.; Mirtsos, C.; Sasaki, T.; Ruland, J.; Penninger, J.M.; Siderovski, D.P.; Mak, T.W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998, 95, 29–39. [Google Scholar] [CrossRef]
- Sun, H.; Lesche, R.; Li, D.M.; Liliental, J.; Zhang, H.; Gao, J.; Gavrilova, N.; Mueller, B.; Liu, X.; Wu, H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 6199–6204. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Matsumoto, M.; Pocai, A.; Rossetti, L.; Depinho, R.A.; Accili, D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007, 6, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wan, M.; Leavens, K.F.; Chu, Q.; Monks, B.R.; Fernandez, S.; Ahima, R.S.; Ueki, K.; Kahn, C.R.; Birnbaum, M.J. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 2012, 18, 388–395. [Google Scholar] [CrossRef]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Kobayashi, M.; Wakasawa, H.; Hoshino, S.; Suwa, F.; Ose, Y.; Tagawa, R.; Higami, Y. Systemic depletion of WWP1 improves insulin sensitivity and lowers triglyceride content in the liver of obese mice. FEBS Open Bio 2023, 13, 1086–1094. [Google Scholar] [CrossRef]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef]
- Lee, Y.R.; Yehia, L.; Kishikawa, T.; Ni, Y.; Leach, B.; Zhang, J.; Panch, N.; Liu, J.; Wei, W.; Eng, C.; et al. WWP1 Gain-of-Function Inactivation of PTEN in Cancer Predisposition. N. Engl. J. Med. 2020, 382, 2103–2116. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Zhou, S.F.; Lu, N. Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors. Drug Des. Dev. Ther. 2014, 8, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gong, Y.; Wu, F.; Wu, M.; Li, S.; Chen, B.; Wang, J.; Qiu, M.; Xu, Y.; Zhao, W.; et al. WWP1-SHARP1-C/EBPβ positive feedback loop modulates development of metabolic dysfunction-associated steatotic liver disease. Metabolism 2025, 169, 156271. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Ingham, R.J.; Gish, G.; Pawson, T. The Nedd4 family of E3 ubiquitin ligases: Functional diversity within a common modular architecture. Oncogene 2004, 23, 1972–1984. [Google Scholar] [CrossRef]
- Dodson, E.J.; Fishbain-Yoskovitz, V.; Rotem-Bamberger, S.; Schueler-Furman, O. Versatile communication strategies among tandem WW domain repeats. Exp. Biol. Med. 2015, 240, 351–360. [Google Scholar] [CrossRef]
- Marino, A.; Menghini, R.; Fabrizi, M.; Casagrande, V.; Mavilio, M.; Stoehr, R.; Candi, E.; Mauriello, A.; Moreno-Navarrete, J.M.; Gómez-Serrano, M.; et al. ITCH deficiency protects from diet-induced obesity. Diabetes 2014, 63, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Abou-Rjeileh, U.; Contreras, G.A. Redox Regulation of Lipid Mobilization in Adipose Tissues. Antioxidants 2021, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
| Knockout Mice | Feed Composition and Diet | Parameter | Knockout Phenotypes | Notes | Reference | |
|---|---|---|---|---|---|---|
| Wwp1 knockout (Wwp1−/−) | High fat diet (HFD32, CREA: 32.0% crude lipid, 25.5% crude protein, and 2.9% crude fiber) | HFD-fed for 8 weeks from 5-week-age of male C57BL/6J mice | Glucose tolerance (GTT and ITT) | ↑ (Improved response to glucose and insulin) | [61] | |
| Liver steatosis | ↓ (Decreased in triglyceride contents) | [75] | ||||
| HFD-fed for 10 weeks from 5-week-age of male C57BL/6J mice | Lipolysis | ↑ (Increased Adrb3 expression and phosphorylation of HSL in WAT) | Insulin (1U/kg BW) i.p. | [42] | ||
| HFD-fed for 18 weeks from 5-week-age of male C57BL/6J mice | Insulin signaling | ↑ (Increased phosphorylation of Akt and decreased PTEN with significant in liver, and non-significant in WAT and muscle) | [75] | |||
| Heterogenous Nedd4 knockout (Nedd4+/−) | High-fat, high-cholesterol diet (TD.06414 from Teklad, Harlan Laboratories: 43.7% fat, 36.6% carbohydrate, 19.7% protein, and 0.203% cholesterol) | HFHS-fed for 24 weeks from 6-week-age of male and female C57/BL6J mice | Glucose tolerance (GTT and ITT) | GTT has no change in both ND and HFD, ITT was impaired in HFD | [43] | |
| Insulin signaling | ↓ (Decreased in phosphorylation of IRβ and Akt in WAT, liver and muscle) | |||||
| Lipolysis | ↑ (Increased serum glycerol) | 16 weeks HFD feeding, After 10 mg/kg BW isoproterenol i.p. | ||||
| ↑ (Increased B2AR expression in WAT and primary adipocyte) | 16 weeks HFD feeding | |||||
| Liver steatosis | Not measured | |||||
| Itch knockout (Itch−/−) | High fat diet (GLP Mucedola Srl, Settimo Milanese: 32.0% crude lipid, 25.5% crude protein, and 2.9% crude fiber) | HFD-fed for 12 weeks from 6 to 8-week-age of male C57/BL10 mice | Glucose metabolism (GTT and HOMA-IR) | ↑ (Improved response to glucose) | [85] | |
| Insulin signaling | ↑ (Increased in phosphorylation of Akt in muscle) | |||||
| Lipolysis | Not measured | |||||
| Liver steatosis | ↓ (Decreased triglyceride contents) | |||||
| ↓ (Decreased onset of steatosis in HE stain and lipid accumulation in Oil Red O stain) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozaki, Y.; Mizunoe, Y.; Kobayashi, M.; Higami, Y. WW Domain-Containing E3 Ubiquitin Protein Ligase 1 (WWP1) as a Factor in Obesity-Related Metabolic Disorders: Emerging Molecular Mechanisms in Metabolic Tissues. Int. J. Mol. Sci. 2025, 26, 9172. https://doi.org/10.3390/ijms26189172
Nozaki Y, Mizunoe Y, Kobayashi M, Higami Y. WW Domain-Containing E3 Ubiquitin Protein Ligase 1 (WWP1) as a Factor in Obesity-Related Metabolic Disorders: Emerging Molecular Mechanisms in Metabolic Tissues. International Journal of Molecular Sciences. 2025; 26(18):9172. https://doi.org/10.3390/ijms26189172
Chicago/Turabian StyleNozaki, Yuka, Yuhei Mizunoe, Masaki Kobayashi, and Yoshikazu Higami. 2025. "WW Domain-Containing E3 Ubiquitin Protein Ligase 1 (WWP1) as a Factor in Obesity-Related Metabolic Disorders: Emerging Molecular Mechanisms in Metabolic Tissues" International Journal of Molecular Sciences 26, no. 18: 9172. https://doi.org/10.3390/ijms26189172
APA StyleNozaki, Y., Mizunoe, Y., Kobayashi, M., & Higami, Y. (2025). WW Domain-Containing E3 Ubiquitin Protein Ligase 1 (WWP1) as a Factor in Obesity-Related Metabolic Disorders: Emerging Molecular Mechanisms in Metabolic Tissues. International Journal of Molecular Sciences, 26(18), 9172. https://doi.org/10.3390/ijms26189172





