Immunohistochemical Evaluation of NOTCH1 Signaling Pathway in Oral Squamous Cell Carcinoma: Clinical and Prognostic Significance
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
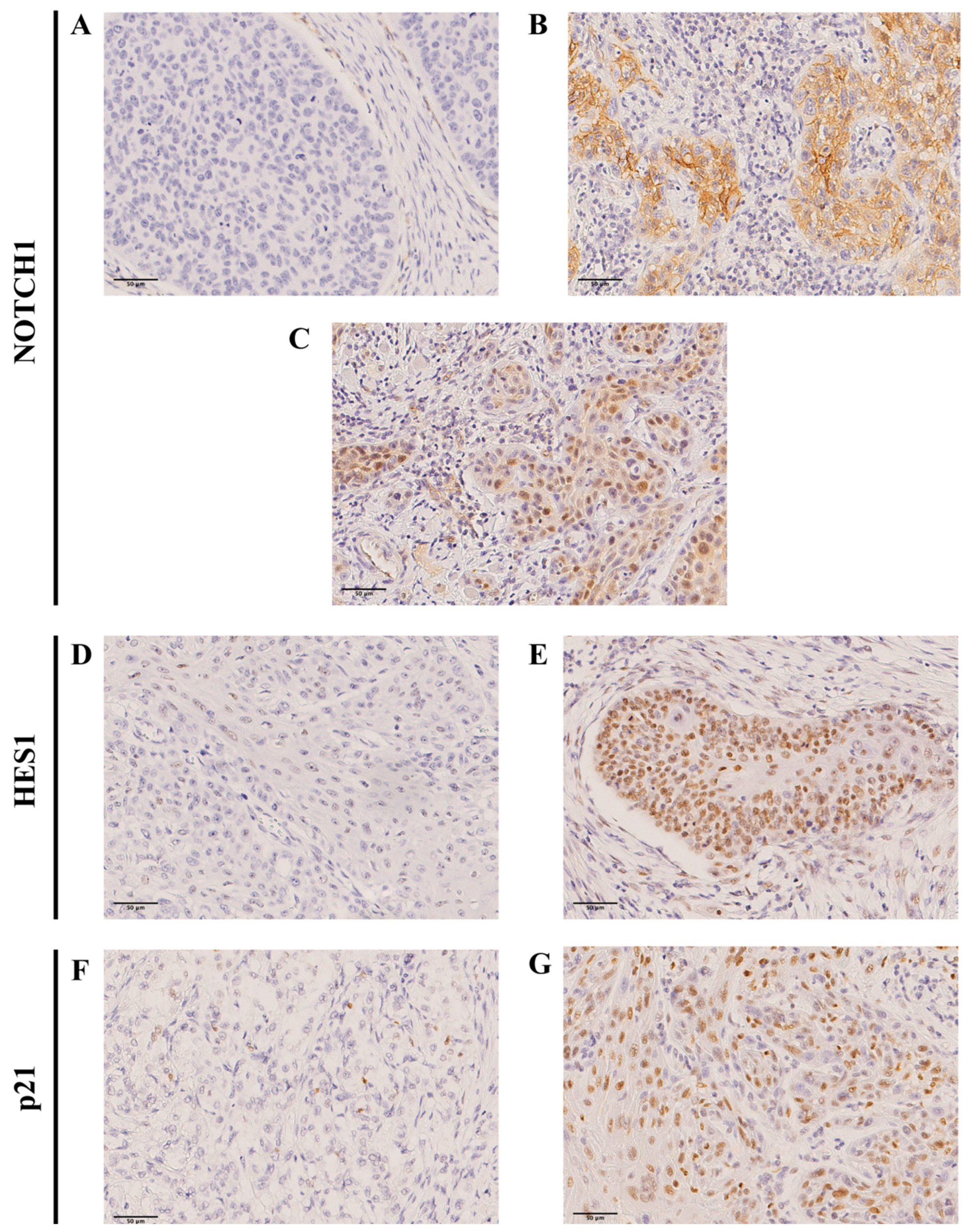
2.2. Immunohistochemical Evaluation of NOTCH1 Expression and Downstream Targets in OSCC Specimens
2.3. Associations of NOTCH1 Expression and Downstream Targets with Clinicopathological Variables
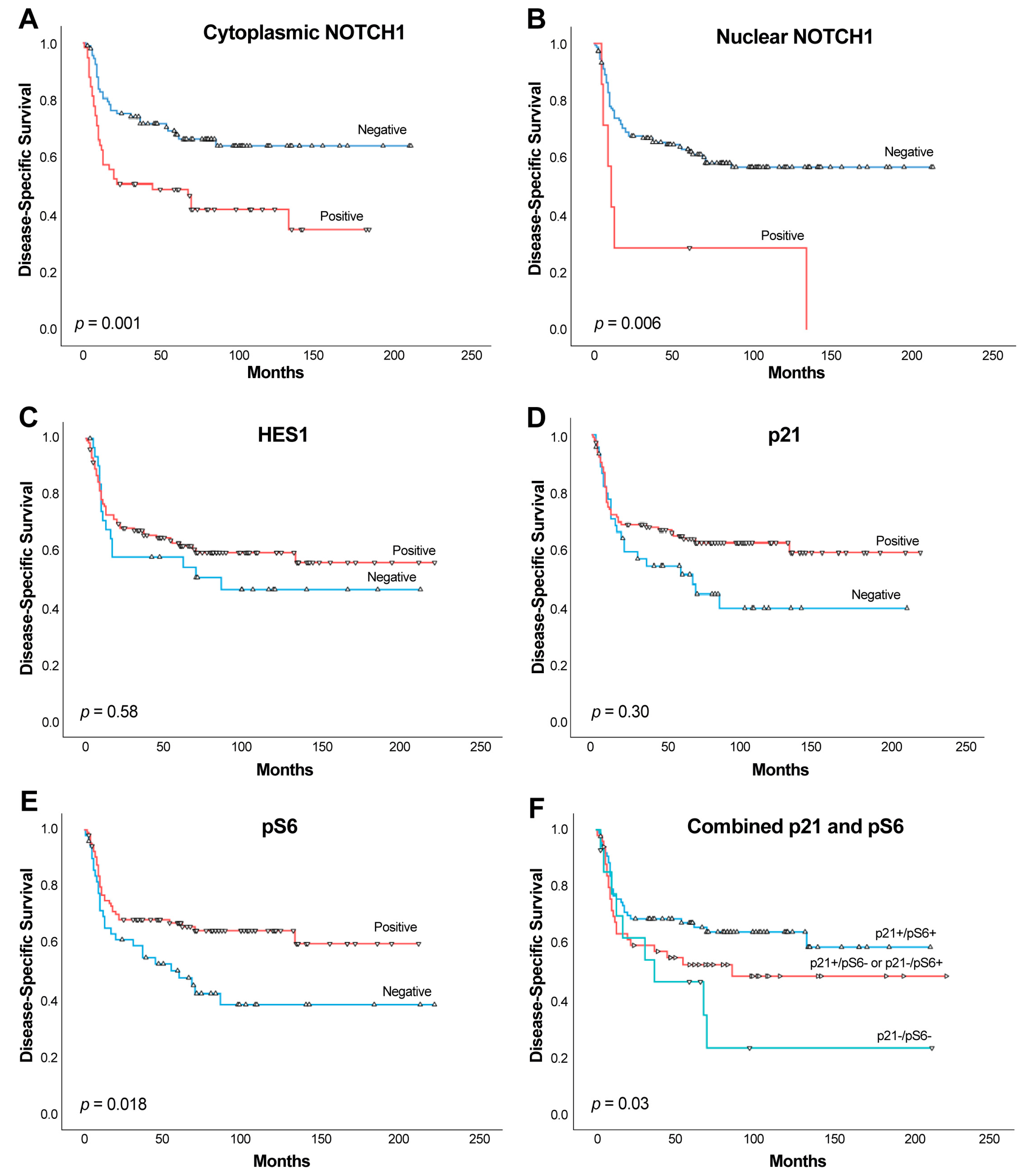
2.4. Impact of NOTCH1, HES1, and p21 on Patient Survival
2.5. Relationship Between the Expression of NOTCH1, HES1, and p21 and the EMT Status
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Specimens
4.2. Immunohistochemistry (IHC)
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hsu, P.J.; Yan, K.; Shi, H.; Izumchenko, E.; Agrawal, N. Molecular biology of oral cavity squamous cell carcinoma. Oral Oncol. 2020, 102, 104552. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, F.; Bittolo, T.; Tissino, E.; Zucchetto, A.; Bomben, R.; Polcik, L.; Dannewitz Prosseda, S.; Hartmann, T.N.; Gattei, V. Multiple mechanisms of NOTCH1 activation in chronic lymphocytic leukemia: NOTCH1 mutations and beyond. Cancers 2022, 14, 2997. [Google Scholar] [CrossRef] [PubMed]
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma-clinical implications. Cell. Oncol. 2021, 44, 473–494. [Google Scholar] [CrossRef]
- Shah, P.A.; Huang, C.; Li, Q.; Kazi, S.A.; Byers, L.A.; Wang, J.; Johnson, F.M.; Frederick, M.J. NOTCH1 signaling in head and neck squamous cell carcinoma. Cells 2020, 9, 2677. [Google Scholar] [CrossRef]
- Brou, C.; Logeat, F.; Gupta, N.; Bessia, C.; LeBail, O.; Doedens, J.R.; Cumano, A.; Roux, P.; Black, R.A.; Israël, A. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000, 5, 207–216. [Google Scholar] [CrossRef]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef]
- Devgan, V.; Mammucari, C.; Millar, S.E.; Brisken, C.; Dotto, G.P. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005, 19, 1485–1495. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, X.; Zhang, X.; Luo, M.; Zuo, X.; Huang, S.; Wang, Y.; Gu, S.; Zhao, X. Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner. Mol. Cancer 2015, 14, 28. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhang, J.; Yoo, S.Y.; Bengtsson, L.; Moorthy, S.; Neskey, D.M.; Zhao, M.; Ortega Alves, M.V.; Chang, K.; Drummond, J.; et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013, 3, 770–781. [Google Scholar] [CrossRef]
- Izumchenko, E.; Sun, K.; Jones, S.; Brait, M.; Agrawal, N.; Koch, W.; McCord, C.L.; Riley, D.R.; Angiuoli, S.V.; Velculescu, V.E.; et al. Notch1 mutations are drivers of oral tumorigenesis. Cancer Prev. Res. 2015, 8, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xia, R.; Li, J.; Long, Z.; Ren, H.; Chen, W.; Mao, L. Common and complex Notch1 mutations in Chinese oral squamous cell carcinoma. Clin. Cancer Res. 2014, 20, 701–710. [Google Scholar] [CrossRef]
- Sun, W.; Gaykalova, D.A.; Ochs, M.F.; Mambo, E.; Arnaoutakis, D.; Liu, Y.; Loyo, M.; Agrawal, N.; Howard, J.; Li, R.; et al. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 2014, 74, 1091–1104. [Google Scholar] [CrossRef]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef]
- Zhang, T.H.; Liu, H.C.; Zhu, L.J.; Chu, M.; Liang, Y.J.; Liang, L.Z.; Liao, G.Q. Activation of Notch signaling in human tongue carcinoma. J. Oral Pathol. Med. 2011, 40, 37–45. [Google Scholar] [CrossRef]
- Osathanon, T.; Nowwarote, N.; Pavasant, P. Expression and influence of Notch signaling in oral squamous cell carcinoma. J. Oral Sci. 2016, 58, 283–294. [Google Scholar] [CrossRef]
- Hijioka, H.; Setoguchi, T.; Miyawaki, A.; Gao, H.; Ishida, T.; Komiya, S.; Nakamura, N. Upregulation of Notch pathway molecules in oral squamous cell carcinoma. Int. J. Oncol. 2010, 36, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; Chung, C.H.; Bishop, J.A.; Howard, J.D.; Sharma, R.; Li, R.J.; Douville, C.; Karchin, R.; Izumchenko, E.; Sidransky, D.; et al. Cleaved NOTCH1 expression pattern in head and neck squamous cell carcinoma is associated with NOTCH1 mutation, HPV status, and high-risk features. Cancer Prev. Res. 2015, 8, 287–295. [Google Scholar] [CrossRef]
- Kujan, O.; Huang, G.; Ravindran, A.; Vijayan, M.; Farah, C.S. CDK4, CDK6, cyclin D1 and Notch1 immunocytochemical expression of oral brush liquid-based cytology for the diagnosis of oral leukoplakia and oral cancer. J. Oral Pathol. Med. 2019, 48, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T. Multifactorial contribution of Notch signaling in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2019, 20, 1520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Yu, G.T.; Xiao, T.; Hu, J. The Notch signaling pathway in head and neck squamous cell carcinoma: A meta-analysis. Adv. Clin. Exp. Med. 2017, 26, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol. Ther. 2015, 150, 33–46. [Google Scholar] [CrossRef]
- Nijkamp, M.M.; Span, P.N.; Hoogsteen, I.J.; van der Kogel, A.J.; Kaanders, J.H.; Bussink, J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother. Oncol. 2011, 99, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Wangmo, C.; Charoen, N.; Jantharapattana, K.; Dechaphunkul, A.; Thongsuksai, P. Epithelial-mesenchymal transition predicts survival in oral squamous cell carcinoma. Pathol. Oncol. Res. 2020, 26, 1511–1518. [Google Scholar] [CrossRef]
- Llanos, S.; García-Pedrero, J.; Morgado-Palacin, L.; Rodrigo, J.P.; Serrano, M. Stabilization of p21 by mTORC1/4E-BP1 predicts clinical outcome of head and neck cancers. Nat. Commun. 2016, 7, 10438. [Google Scholar] [CrossRef]
- Cierpikowski, P.; Lis-Nawara, A.; Bar, J. Prognostic value of WNT1, NOTCH1, PDGFRβ, and CXCR4 in oral squamous cell carcinoma. Anticancer Res. 2023, 43, 591–602. [Google Scholar] [CrossRef]
- Yoshida, R.; Nagata, M.; Nakayama, H.; Niimori-Kita, K.; Hassan, W.; Tanaka, T.; Shinohara, M.; Ito, T. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab. Investig. 2013, 93, 1068–1081. [Google Scholar] [CrossRef]
- Wu-Chou, Y.H.; Hsieh, C.H.; Liao, C.T.; Lin, Y.T.; Fan, W.L.; Yang, C.H. NOTCH1 mutations as prognostic marker in oral squamous cell carcinoma. Pathol. Res. Pract. 2021, 223, 153474. [Google Scholar] [CrossRef]
- Kaka, A.S.; Nowacki, N.B.; Kumar, B.; Zhao, S.; Old, M.O.; Agrawal, A.; Ozer, E.; Carrau, R.L.; Schuller, D.E.; Kumar, P.; et al. Notch1 overexpression correlates to improved survival in cancer of the oropharynx. Otolaryngol. Head Neck Surg. 2017, 156, 652–659. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, M.K.; Yeh, K.T.; Chang, C.S.; Chang, T.H.; Lin, C.Y.; Wu, Y.C.; Su, B.W.; Lee, K.D.; Chang, P.J. Association of high levels of Jagged-1 and Notch-1 expression with poor prognosis in head and neck cancer. Ann. Surg. Oncol. 2010, 17, 2976–2983. [Google Scholar] [CrossRef]
- Li, D.; Dong, P.; Wu, C.; Cao, P.; Zhou, L. Notch1 overexpression associates with poor prognosis in human laryngeal squamous cell carcinoma. Ann. Otol. Rhinol. Laryngol. 2014, 123, 705–710. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, Y.; Wang, Z.; Zhang, W.; Dong, Y.; Chen, W.; Li, J.; Chu, W.; Zhang, W.; Zhong, Y.; et al. Expression and oncogenic properties of membranous Notch1 in oral leukoplakia and oral squamous cell carcinoma. Oncol. Rep. 2018, 39, 2584–2594. [Google Scholar] [CrossRef]
- Grilli, G.; Hermida-Prado, F.; Álvarez-Fernández, M.; Allonca, E.; .Álvarez-González, M.; Astudillo, A.; Moreno-Bueno, G.; Cano, A.; García-Pedrero, J.M.; Rodrigo, J.P. Impact of notch signaling on the prognosis of patients with head and neck squamous cell carcinoma. Oral Oncol. 2020, 110, 105003. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.M.; Schmidt, B.L.; Fridlyand, J.; Dekker, N.; Pinkel, D.; Jordan, R.C.; Albertson, D.G. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 2005, 24, 4232–4242. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yao, J.; Wu, X.; Fan, M. Growth suppression induced by Notch1 activation involves Wnt-beta-catenin down-regulation in human tongue carcinoma cells. Biol. Cell. 2006, 98, 479–490. [Google Scholar] [CrossRef]
- Sakamoto, K.; Fujii, T.; Kawachi, H.; Miki, Y.; Omura, K.; Morita, K.; Kayamori, K.; Katsube, K.; Yamaguchi, A. Reduction of NOTCH1 expression pertains to maturation abnormalities of keratinocytes in squamous neoplasms. Lab. Investig. 2012, 92, 688–702. [Google Scholar] [CrossRef]
- Egloff, A.M.; Grandis, J.R. Molecular pathways: Context-dependent approaches to Notch targeting as cancer therapy. Clin. Cancer Res. 2012, 18, 5188–5195. [Google Scholar] [CrossRef] [PubMed]
- Talora, C.; Sgroi, D.C.; Crum, C.P.; Dotto, G.P. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002, 16, 2252–2263. [Google Scholar] [CrossRef]
- Nyman, P.E.; Buehler, D.; Lambert, P.F. Loss of function of canonical Notch signaling drives head and neck carcinogenesis. Clin. Cancer Res. 2018, 24, 6308–6318. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Peña, I.; Rodrigo, J.P.; Rodríguez-Santamarta, T.; Lequerica-Fernández, P.; Suárez-Fernández, L.; Allonca, E.; García-Pedrero, J.M. Phosphorylated ribosomal protein S6 correlation with p21 expression and inverse association with tumor size in oral squamous cell carcinoma. Head Neck 2017, 39, 1876–1887. [Google Scholar] [CrossRef]
- Bennani-Baiti, I.M.; Aryee, D.N.; Ban, J.; Machado, I.; Kauer, M.; Mühlbacher, K.; Amann, G.; Llombart-Bosch, A.; Kovar, H. Notch signalling is off and is uncoupled from HES1 expression in Ewing's sarcoma. J. Pathol. 2011, 225, 353–363. [Google Scholar] [CrossRef]
- Riya, P.A.; Basu, B.; Surya, S.; Parvathy, S.; Lalitha, S.; Jyothi, N.P.; Meera, V.; Jaikumar, V.S.; Sunitha, P.; Shahina, A.; et al. HES1 promoter activation dynamics reveal the plasticity, stemness and heterogeneity in neuroblastoma cancer stem cells. J. Cell Sci. 2022, 135, jcs260157. [Google Scholar] [CrossRef]
- Curry, C.L.; Reed, L.L.; Nickoloff, B.J.; Miele, L.; Foreman, K.E. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab. Investig. 2006, 86, 842–852. [Google Scholar] [CrossRef]
- Zheng, X.; Narayanan, S.; Zheng, X.; Luecke-Johansson, S.; Gradin, K.; Catrina, S.B.; Poellinger, L.; Pereira, T.S. A Notch-independent mechanism contributes to the induction of Hes1 gene expression in response to hypoxia in P19 cells. Exp. Cell Res. 2017, 358, 129–139. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dai, X.M.; Du, B. Hes1: A key role in stemness, metastasis and multidrug resistance. Cancer Biol. Ther. 2015, 16, 353–359. [Google Scholar] [CrossRef]
- Leong, K.G.; Niessen, K.; Kulic, I.; Raouf, A.; Eaves, C.; Pollet, I.; Karsan, A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J. Exp. Med. 2007, 204, 2935–2948. [Google Scholar] [CrossRef]
- Wang, S.C.; Lin, X.L.; Wang, H.Y.; Qin, Y.J.; Chen, L.; Li, J.; Jia, J.S.; Shen, H.F.; Yang, S.; Xie, R.Y.; et al. Hes1 triggers epithelial-mesenchymal transition (EMT)-like cellular marker alterations and promotes invasion and metastasis of nasopharyngeal carcinoma by activating the PTEN/AKT pathway. Oncotarget 2015, 6, 36713–36730. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Gu, W.; Kalady, M.; Xin, W.; Zhou, L. Loss of HES1 expression is associated with extracellular matrix remodeling and tumor immune suppression in KRAS mutant colon adenocarcinomas. Sci. Rep. 2023, 13, 15999. [Google Scholar] [CrossRef] [PubMed]
- Müller, S. Update from the 4th edition of the World Health Organization of Head and Neck Tumours: Tumours of the oral cavity and mobile tongue. Head Neck Pathol. 2017, 11, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B. AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017; pp. 79–94. [Google Scholar]
- García-Pedrero, J.M.; García-Cabo, P.; Ángeles-Villaronga, M.; Hermida-Prado, F.; Granda-Díaz, R.; Allonca, E.; Rodrigo, J.P. Prognostic significance of E-cadherin and β-catenin expression in HPV-negative oropharyngeal squamous cell carcinomas. Head Neck 2017, 39, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number (%) |
|---|---|
| Age (year) (mean ± SD; median; range) | 63.8 ± 12.65; 64; 30–92 |
| Gender | |
| Men | 113 (68.5) |
| Women | 52 (31.5) |
| Tobacco use | |
| Smoker | 107 (65) |
| Non-smoker | 58 (35) |
| Alcohol use | |
| Drinker | 89 (54) |
| Non-drinker | 76 (46) |
| Location of oral squamous cell carcinoma | |
| Tongue | 75 (45) |
| Floor of the mouth | 34 (21) |
| Other sites within the oral cavity | 56 (34) |
| Tumor status | |
| pT1 | 39 (25) |
| pT2 | 70 (44) |
| pT3 | 22 (14) |
| pT4 | 19 (12) |
| Unknown | 8 (5) |
| Nodal status | |
| pN0 | 95 (57.6) |
| pN1-3 | 63 (38.2) |
| No neck dissection | 7 (4.2) |
| Clinical stage | |
| Stage I | 37 (22.4) |
| Stage II | 51 (30.9) |
| Stage III | 30 (18.2) |
| Stage IV | 47 (28.5) |
| G status | |
| G1 | 105 (63.6) |
| G2 | 51 (30.9) |
| G3 | 9 (5.5) |
| Clinical status at the end of the follow-up | |
| Alive and without recurrence | 77 (46.7) |
| Dead of index cancer | 67 (40.6) |
| Lost or died of other causes (censored) | 18 (10.9) |
| Second primary carcinoma | 3 (1.8) |
| Variable | Cytoplasmic NOTCH1 (%) | p | Nuclear NOTCH1 (%) | p | Nuclear HES1 (%) | p | Nuclear p21 (%) | p |
|---|---|---|---|---|---|---|---|---|
| Negative Positive | Negative Positive | Negative Positive | Negative Positive | |||||
| Gender | 0.29 | 0.67 | 0.20 | 0.76 | ||||
| Men | 64 (59) 44 (41) | 102 (95) 6 (5) | 19 (17) 93 (83) | 21 (20) 84 (80) | ||||
| Women | 32 (68) 15 (32) | 46 (98) 1 (2) | 13 (26) 38 (74) | 9 (18) 41 (82) | ||||
| Tobacco use | 0.88 | 0.016 | 0.62 | |||||
| Smoker | 64 (62) 40 (38) | 97 (93) 7 (7) | 0.06 | 15 (14) 91 (86) | 18 (18) 81 (82) | |||
| Non-smoker | 32 (63) 19 (37) | 51 (100) 0 (0) | 17 (30) 40 (70) | 12 (21) 44 (79) | ||||
| Alcohol use | 0.61 | 0.69 | 0.07 | 0.85 | ||||
| Drinker | 53 (60) 35 (40) | 83 (94) 5 (6) | 13 (15) 76 (85) | 16 (19) 69 (81) | ||||
| Non-drinker | 43 (64) 24 (36) | 65 (97) 2 (3) | 19 (26) 55 (74) | 14 (20) 56 (80) | ||||
| pT | 0.004 | <0.001 | 0.05 | |||||
| pT1 + T2 | 73 (68) 34 (32) | 104 (97) 3 (3) | 0.34 | 13 (12) 99 (88) | 16 (15) 90 (85) | |||
| pT3 + T4 | 17 (43) 23 (57) | 37 (93) 3 (7) | 15 (35) 28 (65) | 12 (29) 29 (71) | ||||
| pN | 0.013 | 0.64 | ||||||
| pN0 | 61 (69) 27 (31) | 84 (96) 4 (4) | 1.0 | 13 (14) 80 (86) | 0.11 | 15 (17) 73 (83) | ||
| pN1-3 | 30 (49) 31 (51) | 58 (95) 3 (5) | 15 (24) 48 (76) | 12 (20) 48 (80) | ||||
| Clinical stage | 0.003 | <0.001 | 0.04 | |||||
| I + II | 59 (73) 22 (27) | 78 (96) 3 (4) | 0.71 | 7 (8) 79 (92) | 11 (14) 70 (86) | |||
| III + IV | 37 (50) 37 (50) | 70 (95) 4 (5) | 25 (32) 52 (68) | 19 (26) 55 (74) | ||||
| G status | 0.001 | 0.62 | 0.89 | |||||
| Well | 70 (73) 26 (27) | 91 (95) 5 (5) | 1.0 | 18 (18) 85 (82) | 19 (19) 79 (81) | |||
| Moderate | 22 (44) 28 (56) | 48 (96) 2 (4) | 12 (24) 39 (76) | 10 (21) 38 (79) | ||||
| Poor | 4 (44) 5 (56) | 9 (100) 0 (0) | 2 (22) 7 (78) | 1 (11) 8 (89) | ||||
| Perineural invasion | 0.15 | 1.0 | 0.69 | 0.68 | ||||
| No | 89 (60) 58 (40) | 140 (95) 7 (5) | 30 (20) 124 (80) | 28 (19) 118 (81) | ||||
| Yes | 7 (88) 1 (12) | 8 (100) 0 (0) | 2 (22) 7 (78) | 2 (22) 7 (78) | ||||
| Vascular invasion | 0.67 | 0.24 | 0.34 | 0.62 | ||||
| No | 93 (62) 56 (38) | 143 (96) 6 (4) | 32 (21) 124 (79) | 28 (19) 120 (81) | ||||
| Yes | 3 (50) 3 (50) | 5 (83) 1 (17) | 0 (0) 7 (100) | 2 (29) 5 (71) | ||||
| Clinical status at the end of the follow-up | ||||||||
| Alive without recurrence | 52 (73) 19 (27) | 0.002 | 71 (100) 0 (0) | 0.03 | 12 (16) 63 (84) | 0.57 | 12 (17) 58 (83) | 0.81 |
| Dead of index cancer | 31 (48) 34 (52) | 59 (91) 6 (9) | 16 (24) 51 (76) | 15 (23) 51 (77) | ||||
| Censored | 13 (77) 4 (23) | 16 (94) 1 (6) | 4 (22) 14 (78) | 3 (18) 14 (82) | ||||
| Second primary cancer | 0 (0) 2 (100) | 2 (100) 0 (0) | 0 (0) 3 (100) | 0 (0) 2 (100) |
| EMT Status | Cytoplasmic NOTCH1 | p | Nuclear NOTCH1 | p | Nuclear HES1 | p | Nuclear p21 | p |
|---|---|---|---|---|---|---|---|---|
| Negative Positive | Negative Positive | Negative Positive | Negative Positive | |||||
| No EMT * Partial EMT ** Complete EMT *** | 41 (67%) 20 (33%) 34 (57%) 26 (43%) 21 (62%) 13 (38%) | 0.49 | 57 (93%) 4 (7%) 58 (97%) 2 (3%) 33 (97%) 1 (3%) | 0.69 | 5 (8%) 57 (92%) 13 (20%) 51 (80%) 14 (38%) 23 (62%) | 0.001 | 16 (27%) 43 (73%) 9 (15%) 51 (85%) 5 (14%) 31 (86%) | 0.15 |
| Survival | Variable | Number of Cases | Survival Number (%) | Survival Time (Months) Mean (95% CI) | HR (95% CI) | p |
|---|---|---|---|---|---|---|
| DSS | NOTCH1 negative NOTCH1 positive, complete EMT NOTCH1 positive, partial EMT NOTCH1 positive, no EMT | 95 13 26 20 | 64 (67.4) 4 (30.8) 11 (42.3) 10 (50.0) | 144.27 (124.87–163.67) 70.48 (27.28–113.68) 81.58 (48.42–114.73) 79.50 (52.37–106.62) | 1 (reference) 2.75 (1.30–5.79) 2.32 (1.25–4.30) 1.62 (0.79–3.30) | 0.01 0.008 0.008 0.18 |
| OS | NOTCH1 negative NOTCH1 positive, complete EMT NOTCH1 positive, partial EMT NOTCH1 positive, no EMT | 95 13 26 20 | 52 (54.7) 3 (23.1) 8 (30.8) 8 (40.0) | 118.66 (98.51–138.82) 65.20 (24.94–105.46) 69.06 (37.56–100.57) 70.23 (44.68–95.77) | 1 (reference) 2.01 (1.00–4.02) 2.02 (1.16–3.52) 1.46 (0.77–2.77) | 0.03 0.04 0.01 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vicente, J.C.; Lequerica-Fernández, P.; Rivas, H.T.; Blanco-Lorenzo, V.; López-Fernández, A.; Escalante-Narváez, S.A.; Herrera i Nogués, S.; Rodrigo, J.P.; Álvarez-Teijeiro, S.; García-Pedrero, J.M. Immunohistochemical Evaluation of NOTCH1 Signaling Pathway in Oral Squamous Cell Carcinoma: Clinical and Prognostic Significance. Int. J. Mol. Sci. 2025, 26, 9167. https://doi.org/10.3390/ijms26189167
de Vicente JC, Lequerica-Fernández P, Rivas HT, Blanco-Lorenzo V, López-Fernández A, Escalante-Narváez SA, Herrera i Nogués S, Rodrigo JP, Álvarez-Teijeiro S, García-Pedrero JM. Immunohistochemical Evaluation of NOTCH1 Signaling Pathway in Oral Squamous Cell Carcinoma: Clinical and Prognostic Significance. International Journal of Molecular Sciences. 2025; 26(18):9167. https://doi.org/10.3390/ijms26189167
Chicago/Turabian Stylede Vicente, Juan Carlos, Paloma Lequerica-Fernández, Héctor Torres Rivas, Verónica Blanco-Lorenzo, Ana López-Fernández, Samuel Andrés Escalante-Narváez, Sergi Herrera i Nogués, Juan P. Rodrigo, Saúl Álvarez-Teijeiro, and Juana M. García-Pedrero. 2025. "Immunohistochemical Evaluation of NOTCH1 Signaling Pathway in Oral Squamous Cell Carcinoma: Clinical and Prognostic Significance" International Journal of Molecular Sciences 26, no. 18: 9167. https://doi.org/10.3390/ijms26189167
APA Stylede Vicente, J. C., Lequerica-Fernández, P., Rivas, H. T., Blanco-Lorenzo, V., López-Fernández, A., Escalante-Narváez, S. A., Herrera i Nogués, S., Rodrigo, J. P., Álvarez-Teijeiro, S., & García-Pedrero, J. M. (2025). Immunohistochemical Evaluation of NOTCH1 Signaling Pathway in Oral Squamous Cell Carcinoma: Clinical and Prognostic Significance. International Journal of Molecular Sciences, 26(18), 9167. https://doi.org/10.3390/ijms26189167









