Molecular Breeding for Abiotic Stress Tolerance in Crops: Recent Developments and Future Prospectives
Abstract
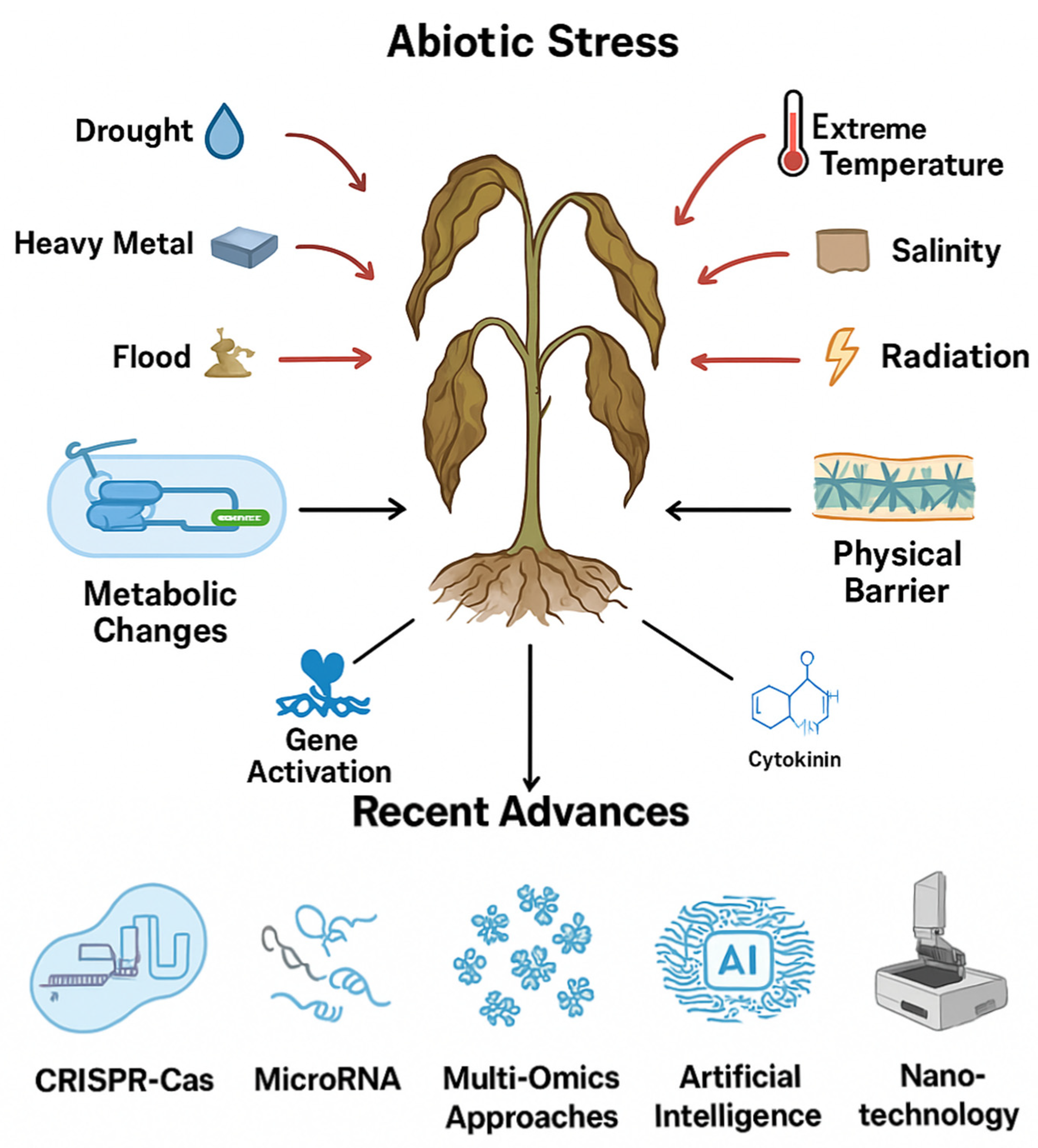
1. Plants and Abiotic Stresses
1.1. Hormonal Regulation
1.2. Antioxidant Defence
1.3. Osmotic Adjustment
1.4. Gene Expression Regulation
2. Advanced Tools to Improve Tolerance
2.1. Genome Editing
2.2. AI and Machine Learning
2.3. Nature-Based Solutions
2.4. Nano-Thecnologies
| Application | Nanomaterial(s) | Main Effects | Example Crops | Ref. |
|---|---|---|---|---|
| Nanofertilizers | Nano-hydroxyapatite, ZnO, urea nanoparticles | Controlled nutrient release, higher uptake efficiency, reduced leaching | Maize, wheat, rice | [149] |
| Nanopesticides | Silver nanoparticles (AgNPs), CuO NPs, polymeric nano-carriers | Targeted delivery of active ingredients, suppression of pathogens, reduced toxicity | Tomato, rice, wheat | [150,151] |
| Stress protectants | ZnO, TiO2, SiO2 nanoparticles | Enhanced tolerance to drought, salinity, heavy metals via antioxidant defense and ion homeostasis | Wheat, rice, soybean | [152] |
| Nanocarriers for gene delivery | Carbon nanotubes, mesoporous silica nanoparticles | DNA/RNA/CRISPR delivery, improved transformation efficiency, transient expression | Spinach, arugula, tobacco | [153] |
| Nanobiosensors | Gold nanoparticles, quantum dots, carbon nanotubes | Early detection of pathogens, nutrient deficiencies, and stress markers | Various (e.g., virus detection in crops) | [154] |
| Postharvest nanocoatings | Chitosan nanoparticles, AgNP-chitosan films | Prolonged shelf life, reduced microbial spoilage, maintained fruit quality | Strawberry, tomato, banana | [155] |
3. SI: Molecular Breeding for Abiotic Stress Tolerance in Crops
3.1. Gibberellin
3.2. Polyamine Seed Priming
3.3. Cadmium Stress
3.4. Genome-Wide Association (GWA)
3.5. Transcription Factors and Gene Silencing
3.6. Salinity Tolerance
3.7. Gene Editing
4. Future Perspectives and Concluding Remarks
4.1. Breeding Approaches to Improve Plant Tolerance to Abiotic Stresses
4.2. Concluding Remarks
Funding
Conflicts of Interest
References
- Ray, M.; Burman, S.; Meshram, S. A Mini Review on Plant Immune System Dynamics: Modern Insights into Biotic and Abiotic Stress. Phyton-Int. J. Exp. Bot. 2025, 94, 2285–2312. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Swapna, T.S. Abiotic stress in the production of food grains and methods to alleviate the impact of stress. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 215–240. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses. Plants Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Villalobos-López, M.A.; Arroyo-Becerra, A.; Quintero-Jiménez, A.; Iturriaga, G. Biotechnological Advances to Improve Abiotic Stress Tolerance in Crops. Int. J. Mol. Sci 2022, 23, 12053. [Google Scholar] [CrossRef]
- Han, Y.; Lou, X.; Zhang, W.; Xu, T.; Tang, M. Arbuscular mycorrhizal fungi enhanced drought resistance of Populus cathayana by regulating the 14-3-3 family protein genes. Microbiol. Spectr. 2022, 10, e02456-21. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, P.; Gao, Z.; Long, T.; Xing, C.; Li, J.; Xue, J.; Chen, G.; Xie, Q.; Hu, Z. Silencing of SlMYB78-like Reduces the Tolerance to Drought and Salt Stress via the ABA Pathway in Tomato. Int. J. Mol. Sci. 2024, 25, 11449. [Google Scholar] [CrossRef]
- Gu, L.; Cao, Y.; Chen, X.; Wang, H.; Zhu, B.; Du, X.; Sun, Y. The Genome-Wide Identification, Characterization, and Expression Analysis of the Strictosidine Synthase-like Family in Maize (Zea mays L.). Int. J. Mol. Sci. 2023, 24, 14733. [Google Scholar] [CrossRef]
- Takahashi, Y.; Zhang, J.; Hsu, P.K.; Ceciliato, P.H.; Zhang, L.; Dubeaux, G.; Munemasa, S.; Ge, C.; Zhao, Y.; Hauser, F.; et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat. Commun. 2020, 11, 12. [Google Scholar] [CrossRef]
- Virág, E.; Nagy, Á.; Tóth, B.B.; Kutasy, B.; Pallos, J.P.; Szigeti, Z.M.; Máthé, C.; Kardos, G.; Hegedűs, G. Master Regulatory Transcription Factors in β-Aminobutyric Acid-Induced Resistance (BABA-IR): A Perspective on Phytohormone Biosynthesis and Signaling in Arabidopsis thaliana and Hordeum vulgare. Int. J. Mol. Sci. 2024, 25, 9179. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bullock, D.A., Jr.; Alonso, J.M.; Stepanova, A.N. To Fight or to Grow: The Balancing Role of Ethylene in Plant Abiotic Stress Responses. Plants 2022, 11, 33. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, K.X.; Ma, B.; Yin, C.C.; Hu, Y.; Tao, J.J.; Huang, Y.H.; Cao, W.Q.; Chen, H.; Yang, C.; et al. Histidine kinase MHZ1/OsHK1 interacts with ethylene receptors to regulate root growth in rice. Nat. Commun. 2020, 11, 518. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity F. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Rehman, M.; Saeed, M.S.; Fan, X.; Salam, A.; Munir, R.; Yasin, M.U.; Khan, A.R.; Muhammad, S.; Ali, B.; Ali, I.; et al. The Multifaceted Role of Jasmonic Acid in Plant Stress Mitigation: An Overview. Plants 2023, 12, 3982. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Bhavanam, S.; Stout, M. Seed Treatment With Jasmonic Acid and Methyl Jasmonate Induces Resistance to Insects but Reduces Plant Growth and Yield in Rice, Oryza sativa. Front. Plant Sci. 2021, 12, 691768. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Cui, X.; Wang, K.; Wang, Y.; He, Y. Phytohormones regulate the abiotic stress: An overview of physiological, biochemical, and molecular responses in horticultural crops. Front. Plant Sci. 2023, 13, 1095363. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Cheng, J.; Hill, C.B.; Shabala, S.; Li, C.; Zhou, M. Manipulating GA-Related Genes for Cereal Crop Improvement. Int. J. Mol. Sci. 2022, 23, 14046. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Yu, Q.; Hua, X.; Yao, H.; Zhang, Q.; He, J.; Peng, L.; Li, D.; Yang, Y.; Li, X. Abscisic acid receptors are involves in the Jasmonate signaling in Arabidopsis. Plant Signal. Behav. 2021, 16, 1948243. [Google Scholar] [CrossRef]
- Virág, E.; Hegedűs, G.; Nagy, Á.; Pallos, J.P.; Kutasy, B. Temporal Shifts in Hormone Signaling Networks Orchestrate Soybean Floral Development Under Field Conditions: An RNA-Seq Study. Int. J. Mol. Sci. 2025, 26, 6455. [Google Scholar] [CrossRef]
- Graham, J.; Marshall, B.; Squire, G. Genetic differentiation over a spatial environmental gradient in wild Rubus ideaus populations. New Phytol. 2003, 157, 667–675. [Google Scholar] [CrossRef]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant Enzyme Activities and Abiotic Stress Tolerance Relationship in Vegetable Crops; InTech: Vienna, Austria, 2016. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. J. Xenobiot. 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.U.; Fujita, M.; Nahar, K.; Rahman, A.; Anee, T.I.; Masud, A.A.C.; Amin, A.R.; Hasanuzzaman, M. Seed priming upregulates antioxidant defense and glyoxalase systems to conferring simulated drought tolerance in wheat seedlings. Plant Stress 2022, 6, 100120. [Google Scholar] [CrossRef]
- Wang, R.R.-C.; Xu, S.S.; Monaco, T.A.; Robbins, M.D. Dual Mechanisms of Salinity Tolerance in Wheat Germplasm Lines W4909 and W4910. Int. J. Mol. Sci. 2024, 25, 12892. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, S.; Giorgi, D.; Kuzmanovic, L.; Jrad, O.; Farina, A.; Capoccioni, A.; Ben, A.R.; Brini, F.; Ceoloni, C. Coping with salinity stress: Segmental group 7 chromosome introgressions from halophytic Thinopyrum species greatly enhance tolerance of recipient durum wheat. Front. Plant Sci. 2024, 15, 1378186. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and physiological stress responses of lettuce to different intensities of continuous light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef]
- Bai, F.; Gusbeth, C.; Frey, W.; Nick, P. Nanosecond pulsed electric fields modulate the expression of the astaxanthin biosynthesis genes psy, crtR-b and bkt 1 in Haematococcus pluvialis. Sci. Rep. 2020, 10, 15508. [Google Scholar] [CrossRef]
- Singh, V.K.; Upadhyay, R.S. Fusaric acid induced cell death and changes in oxidative metabolism of Solanum lycopersicum L. Bot. Stud. 2014, 55, 66. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Wleklik, K.; Borek, S.; Garnczarska, M. Polyamine Seed Priming: A Way to Enhance Stress Tolerance in Plants. Int. J. Mol. Sci. 2024, 25, 12588. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Huang, Y.; Mei, G.; Zhang, S.; Wu, H.; Zhao, T. Spermidine Enhances Chilling Tolerance of Kale Seeds by Modulating ROS and Phytohormone Metabolism. PLoS ONE 2023, 18, e0289563. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Plitta-Michalak, B.P.; Małecka, A.; Ciszewska, L.; Sikorski, Ł.; Staszak, A.M.; Michalak, M.; Ratajczak, E. The Chances in the Redox Priming of Nondormant Recalcitrant Seeds by Spermidine. Tree Physiol. 2023, 43, 1142–1158. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.-L.; Zhang, R.-X.; Yuan, H.-Y.; Wang, M.-M.; Yang, H.-Y.; Ma, H.-Y.; Liu, D.; Jiang, C.-J.; Liang, Z.-W. Root Damage under Alkaline Stress Is Associated with Reactive Oxygen Species Accumulation in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef]
- Gorgues, L.; Li, X.; Maurel, C.; Martinière, A.; Nacry, P. Root osmotic sensing from local perception to systemic responses. Stress Biol. 2022, 2, 36. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Brini, F.; Masmoudi, K. Ion transporters and abiotic stress tolerance in plants. Int. Sch. Res. Not. 2012, 1, 927436. [Google Scholar] [CrossRef]
- Silva Lobato, A.K.; Silveira, J.A.G.; Costa, R.C.L.; Oliveira Neto, C.F. Tolerance to Drought in Leguminous Plants Mediated by Rhizobium and Bradyrhizobium; InTech: Vienna, Austria, 2013. [Google Scholar] [CrossRef][Green Version]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological analysis of common bean (Phaseolus vulgaris L.) cultivars uncovers characteristics related to terminal drought resistance. Plant Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Al Hinai, M.S.; Rehman, A.; Siddique, K.H.; Farooq, M. The Role of Trehalose in Improving Drought Tolerance in Wheat. J. Agron. Crop Sci. 2025, 211, e70053. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Nemeskeri, E.; Molnar, K.; Vg, R.; Dobos, A.; Nagy, J. Defence Strategies of Annual Plants Against Drought; InTech: Vienna, Austria, 2012. [Google Scholar] [CrossRef]
- Rosa, V.D.R.; Silva, A.A.D.; Brito, D.S.; Pereira, J.D.; Silva, C.O.; Dal-Bianco, M.; de Oliveira, J.A.; Ribeiro, C. Drought stress during the reproductive stage of two soybean lines. Pesqui. Agropecuária Bras. 2020, 55, e01736. [Google Scholar] [CrossRef]
- Lo, S.F.; Ho, T.D.; Liu, Y.L.; Jiang, M.J.; Hsieh, K.T.; Chen, K.T.; Yu, L.C.; Lee, M.H.; Chen, C.Y.; Huang, T.P.; et al. Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice. Plant Biotechnol. J. 2017, 15, 850–864. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Wang, J.; Li, Y.; Zhang, Y.; Zhao, X.; Zheng, T.; Li, Z.; Xu, J.; Wang, W.; et al. Molecular dissection of the gene OsGA2ox8 conferring osmotic stress tolerance in rice. Int. J. Mol. Sci. 2021, 22, 9107. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A.; Kamal, A. Osmoprotective Role of Sugar in Mitigating Abiotic Stress in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Wiley: Hoboken, NJ, USA, 2020; pp. 53–70. [Google Scholar]
- Li, Y.; Ding, L.; Zhou, M.; Chen, Z.; Ding, Y.; Zhu, C. Transcriptional Regulatory Network of Plant Cadmium Stress Response. Int. J. Mol. Sci 2023, 24, 4378. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Xie, C.; Yang, L.; Jia, G.; Yan, K.; Zhang, S.; Yang, G.; Wu, C.; Gai, Y.; Zheng, C.; Huang, J. Maize HEAT UP-REGULATED GENE 1 plays vital roles in heat stress tolerance. J. Exp. Bot. 2022, 73, 6417–6433. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Gust, A.A.; Nurnberger, T. Plant immunity unified. Nat. Plants. 2021, 7, 382–383. [Google Scholar] [CrossRef]
- Tang, D.; Wang, G.; Zhou, J.M. Receptor Kinases in Plant-Pathogen Interactions: More Than Pattern Recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef]
- de Nadal, E.; Ammerer, G.; Posas, F. Controlling gene expression in response to stress. Nat. Rev. Genet. 2011, 12, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Roychoudhury, A. Seed Priming with Spermine and Spermidine Regulates the Expression of Diverse Groups of Abiotic Stress-Responsive Genes during Salinity Stress in the Seedlings of Indica Rice Varieties. Plant Gene 2017, 11, 124–132. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Zhang, J.; Li, Y.; Xu, A.; Huang, Z. Genome-Wide Association Studies of Salt Tolerance at the Seed Germination Stage and Yield-Related Traits in Brassica napus L. Int. J. Mol. Sci. 2022, 23, 15892. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamaji, N.; Costa, A.; Okuma, E.; Kobayashi, N.I.; Kashiwagi, T.; Katsuhara, M.; Wang, C.; Tanoi, K.; Murata, Y.; et al. OsHKT1;4 mediated Na+ transport in stems contributes to Na+ exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biol. 2016, 16, 22. [Google Scholar] [CrossRef]
- Chang, Y.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.; Duan, C. Epigenetic Regulation in Plant Abiotic Stress Responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Harris, C.J.; Amtmann, A.; Ton, J. Epigenetic Processes in Plant Stress Priming: Open Questions and New Approaches. Curr. Opin. Plant Biol. 2023, 75, 102432. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Zafar, S.A.; Zaidi, S.S.; Gaba, Y.; Singla-Pareek, S.L.; Dhankher, O.P.; Li, X.; Mansoor, S.; Pareek, A. Engineering abiotic stress tolerance via CRISPR/ Cas-mediated genome editing. J. Exp. Bot. 2020, 71, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, N.; Shen, G.; Zhang, H. Genetic manipulation for abiotic stress resistance traits in crops. Front. Plant Sci. 2022, 13, 1011985. [Google Scholar] [CrossRef]
- Nerkar, G.; Devarumath, S.; Purankar, M.; Kumar, A.; Valarmathi, R.; Devarumath, R.; Appunu, C. Advances in Crop Breeding Through Precision Genome Editing. Front. Genet. 2022, 13, 880195. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Khan, A.I.; Negm, M.A.; Iqbal, R.; Azhar, M.T.; Khan, S.H.; Rana, I.A. Enhancing cotton resilience to challenging climates through genetic modifications. J. Cotton Res. 2024, 7, 10. [Google Scholar] [CrossRef]
- Munaweera, T.I.K.; Jayawardana, N.U.; Rajaratnam, R.; Dissanayake, N. Modern plant biotechnology as a strategy in addressing climate change and attaining food security. Agric. Food Secur. 2022, 11, 26. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Ai, Z.; Tan, Y.; Hu, Y.; Guo, X.; Liu, X.; Sun, Z.; Yu, D.; Chen, J.; Tang, N.; et al. Novel Salinity-Tolerant Third-Generation Hybrid Rice Developed via CRISPR/Cas9-Mediated Gene Editing. Int. J. Mol. Sci. 2023, 24, 8025. [Google Scholar] [CrossRef]
- Santosh Kumar, V.V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.V.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol. Mol. Biol. Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef]
- Zhang, H.; Gou, F.; Zhang, J.; Liu, W.; Li, Q.; Mao, Y.; Botella, J.R.; Zhu, J.K. TALEN-Mediated Targeted Mutagenesis Produces a Large Variety of Heritable Mutations in Rice. Plant Biotechnol. J. 2016, 14, 186–194. [Google Scholar] [CrossRef]
- Ogata, T.; Ishizaki, T.; Fujita, M.; Fujita, Y. CRISPR/Cas9-targeted mutagenesis of OsERA1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE 2020, 15, e0243376. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.; Nawaz, G.; Zhao, N.; Liao, S.; Liu, Y.; Li, R. Precise Editing of the OsPYL9 Gene by RNA-Guided Cas9 Nuclease Confers Enhanced Drought Tolerance and Grain Yield in Rice (Oryza sativa L.) by Regulating Circadian Rhythm and Abiotic Stress Responsive Proteins. Int. J. Mol. Sci. 2020, 21, 7854. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef]
- Du, L.; Ding, L.; Huang, X.; Tang, D.; Chen, B.; Tian, H.; Kang, Z.; Mao, H. Natural variation in a K+-preferring HKT transporter contributes to wheat shoot K+ accumulation and salt tolerance. Plant Cell Environ. 2024, 47, 540–556. [Google Scholar] [CrossRef]
- Yang, Y.; Al-Baidhani, H.H.J.; Harris, J.; Riboni, M.; Li, Y.; Mazonka, I.; Bazanova, N.; Chirkova, L.; Hussain, S.S.; Hrmova, M.; et al. DREB/CBF expression in wheat and barley using the stress-inducible promoters of HD-Zip I genes: Impact on plant development, stress tolerance and yield. Plant Biotechnol. J. 2020, 18, 829–844. [Google Scholar] [CrossRef]
- Vlčko, T.; Ohnoutková, L. Allelic Variants of CRISPR/Cas9 Induced Mutation in an Inositol Trisphosphate 5/6 Kinase Gene Manifest Different Phenotypes in Barley. Plants 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 Variants Generated by CRISPR-Cas9 Improve Maize Grain Yield Under Field Drought Stress Conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Si, X.; Tian, Y.; Chen, K.; Liu, J.; Chen, H.; Gao, C. Generation of Thermosensitive Male-Sterile Maize by Targeted Knockout of the ZmTMS5. Gene J. Genet. Genom. 2017, 44, 465–468. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Lv, P.; Antwi-Boasiako, A.; Begum, N.; Zhao, T.; Zhao, J. NAC Transcription Factor GmNAC12 Improved Drought Stress Tolerance in Soybean. Int. J. Mol. Sci. 2022, 23, 12029. [Google Scholar] [CrossRef]
- Carrijo, J.; Illa-Berenguer, E.; LaFayette, P.; Torres, N.; Aragão, F.J.; Parrott, W.; Vianna, G.R. Two efficient CRISPR/Cas9 systems for gene editing in soybean. Transgenic Res. 2021, 30, 239–249. [Google Scholar] [CrossRef]
- Mustafa, M.D.; Mazhar, S.; Syed, A.; Ali, U. A mini review on genome editing via CRISPR-CAS system to enhance agronomic traits of wheat and tomato against environmental stresses. Pure Appl. Biol. (PAB) 2025, 14, 468–478. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Y.; Liu, D.; Zhang, X.; Guo, J.; Zhang, T.; Lai, L.; Li, Y.; Chen, Q.; Yu, L. Genome-Wide Analysis of the Membrane Attack Complex and Perforin Genes and Their Expression Pattern under Stress in the Solanaceae. Int. J. Mol. Sci. 2023, 24, 13193. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Wang, L.; Chen, L.; Zhao, R.; Sheng, J.; Shen, L. Reduction of Tomato-Plant Chilling Tolerance by CRISPR–Cas9- Mediated SlCBF1 Mutagenesis. J. Agric. Food Chem. 2018, 66, 9042–9051. [Google Scholar] [CrossRef]
- Li, Y.; Yue, N.; Basit, A.; Li, Y.; Zhang, D.; Qin, L.; Crabbe, M.J.C.; Xu, W.; Wang, Y.; Yan, J. Targeted editing of SlMAPK6 using CRISPR/Cas9 technology to promote the development of axillary buds in tomato plants. J. Agric. Sci. 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Feng, F.; Liang, D.; Cheng, L.; Ma, F.; Shi, S. Overexpression of a malus vacuolar Na+/H+ antiporter gene (MdNHX1) in apple rootstock m. 26 and its influence on salt tolerance. Plant Cell Tissue Organ Cult. 2010, 102, 337–345. [Google Scholar] [CrossRef]
- He, X.; Luo, X.; Wang, T.; Liu, S.; Zhang, X.; Zhu, L. GhHB12 Negatively Regulates Abiotic Stress Tolerance in Arabidopsis and Cotton. Environ. Exp. Bot. 2020, 176, 10408. [Google Scholar] [CrossRef]
- Wu, J.; Yan, G.; Duan, Z.; Wang, Z.; Kang, C.; Guo, L.; Liu, K.; Tu, J.; Shen, J.; Yi, B. Roles of the Brassica napus Della Protein BnaA6. RGA, in modulating drought tolerance by interacting with the ABA signalling component BnaA10.ABF2. Front. Plant Sci. 2020, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Badhan, S.; Ball, A.S.; Mantri, N. First Report of CRISPR/Cas9 Mediated DNA-Free Editing of 4CL and RVE7 Genes in Chickpea Protoplasts. Int. J. Mol. Sci. 2021, 22, 396. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bulley, S.M.; Varkonyi-Gasic, E.; Zhong, C.; Li, D. Kiwifruit bZIP transcription factor AcePosF21 elicits ascorbic acid biosynthesis during cold stress. Plant Physiol. 2023, 192, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, R.; Bulley, S.M.; Zhong, C.; Li, D. Kiwifruit MYBS1-like and GBF3 tran-scription factors influence L-Ascorbic acid biosynthesis by activating transcription of GDP-L-galactose phosphorylase 3. New Phytol. 2022, 234, 1782–1800. [Google Scholar] [CrossRef]
- Qin, H.; Gu, Q.; Kuppu, S.; Sun, L.; Zhu, X.; Mishra, N.; Hu, R.; Shen, G.; Zhang, J.; Zhang, Y.; et al. Expression of the Arabidopsis vacuolar h+-pyrophosphatase gene AVP1 in peanut to improve drought and salt tolerance. Plant Biotechnol. Rep. 2013, 7, 345–355. [Google Scholar] [CrossRef]
- Bastet, A.; Robaglia, C.; Gallois, J.L. eIF4E resistance: Natural variation should guide gene editing. Trends Plant Sci. 2017, 22, 411–419. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker—Resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene Cs LOB 1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Maioli, A.; De Marchi, F.; Valentino, D.; Gianoglio, S.; Patono, D.L.; Miloro, F.; Bai, Y.; Comino, C.; Lanteri, S.; Lovisolo, C.; et al. Knock-out of SlDMR6-1 in tomato pro-motes a drought-avoidance strategy and increases tolerance to Late Blight. Plant Stress 2024, 13, 100541. [Google Scholar] [CrossRef]
- Movahedi, A.; Zhang, J.; Gao, P.; Yang, Y.; Wang, L.; Yin, T.; Zhuge, Q. Expression of the chickpea CarNAC3 gene enhances salinity and drought tolerance in transgenic poplars. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 120, 141–154. [Google Scholar] [CrossRef]
- Han, Y.P. Application of CRISPR/Cas9 technology in editing poplar drought resistance genes. Mol. Plant Breed. 2024, 5, 81–89. [Google Scholar] [CrossRef]
- Liu, J.; Ye, M.; Fan, Z.; Wang, Y.; Chen, J.; Li, H.; Deng, X.; Kim, H.S.; Kwak, S.-S.; Ke, Q. PagHCF106 negatively regulates drought stress tolerance in poplar (Populus alba× Populus glandulosa) by modulating stomatal aperture. Tree Physiol. 2025, 45, tpaf012. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, R.; Lu, T.; Jiang, S.; You, K.; Xia, X.; Du, K.; Kang, X. PagHB7/PagABF4–PagEPFL9 Module Regulates Stomatal Density and Drought Tolerance in Poplar. Plant Biotechnol. J. 2025, 1–15. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Ding, H.; Zou, Q. Machine learning and its applications in plant molecular studies. Brief. Funct. Genom. 2020, 19, 40–48. [Google Scholar] [CrossRef]
- Pearson, S. Artificial intelligence in horticulture. J. Hortic. Sci. Biotechnol. 2025, 100, 564–570. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gao, S.; Hassan, M.A.; Huang, Z.; Rasheed, A.; Hearne, S.; Prasanna, B.; Li, X.; Li, H. Artificial intelligence in plant breeding. Trends Genet. 2024, 40, 891–908. [Google Scholar] [CrossRef]
- Ahmed, B.; Haque, M.A.; Iquebal, M.A.; Jaiswal, S.; Angadi, U.B.; Kumar, D.; Rai, A. DeepAProt: Deep learning based abiotic stress protein sequence classification and identification tool in cereals. Front. Plant Sci. 2023, 13, 1008756. [Google Scholar] [CrossRef]
- Bartas, M. Abiotic Stresses in Plants: From Molecules to Environment. Int. J. Mol. Sci. 2024, 25, 8072. [Google Scholar] [CrossRef]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, E.; Andolfo, G.; Di Matteo, A.; Ercolano, M.R. Empowering crop resilience to environmental multiple stress through the modulation of key response components. J. Plant Physiol. 2020, 246, 153134. [Google Scholar] [CrossRef] [PubMed]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.-G.; Yun, B.-W. Abiotic Stress in Plants; Stress Perception to Molecular Response and Role of Biotechnological Tools in Stress Resistance. Agronomy 2021, 11, 1579. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahim, Z.; Khaskheli, M.B.; Raza, H.; Zhou, F.; Shamsi, I.H. Integrative Approaches to Abiotic Stress Management in Crops: Combining Bioinformatics Educational Tools and Artificial Intelligence Applications. Sustainability 2024, 16, 7651. [Google Scholar] [CrossRef]
- Sakeef, N.; Scandola, S.; Kennedy, C.; Lummer, C.; Chang, J.; Uhrig, R.G.; Lin, G. Machine learning classification of plant genotypes grown under different light conditions through the integration of multi-scale time-series data. Comput. Struct. Biotechnol. J. 2023, 21, 3183–3195. [Google Scholar] [CrossRef]
- Kempelis, A.; Polaka, I.; Romanovs, A.; Patlins, A. Computer Vision and Machine Learning-Based Predictive Analysis for Urban Agricultural Systems. Future Internet 2024, 16, 44. [Google Scholar] [CrossRef]
- Aldakheel, E.A.; Zakariah, M.; Alabdalall, A.H. Detection and identification of plant leaf diseases using YOLOv4. Front. Plant Sci. 2024, 15, 1355941. [Google Scholar] [CrossRef]
- Bhagwat, R.; Dandawate, Y. Comprehensive multilayer convolutional neural network for plant disease detection. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 204–211. [Google Scholar] [CrossRef]
- Nautiyal, M.; Joshi, S.; Hussain, I.; Rawat, H.; Joshi, A.; Saini, A.; Kapoor, R.; Verma, H.; Nautiyal, A.; Chikara, A.; et al. Revolutionizing agriculture: A comprehensive review on artificial intelligence applications in enhancing properties of agricultural produce. Food Chem. 2025, 29, 102748. [Google Scholar] [CrossRef] [PubMed]
- Branstad-Spates, E.H.; Castano-Duque, L.; Mosher, G.A.; Hurburgh, C.R., Jr.; Owens, P.; Winzeler, E.; Bowers, E.L. Gradient boosting machine learning model to predict aflatoxins in Iowa corn. Front. Microbiol. 2023, 14, 1248772. [Google Scholar] [CrossRef]
- Niedbała, G.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Nawracała, J. Application of Artificial Neural Networks to Analyze the Concentration of Ferulic Acid, Deoxynivalenol, and Nivalenol in Winter Wheat Grain. Agriculture 2020, 10, 127. [Google Scholar] [CrossRef]
- Navarro, A.; Nicastro, N.; Costa, C.; Pentangelo, A.; Cardarelli, M.; Ortenzi, L.; Pallottino, F.; Cardi, T.; Pane, C. Sorting biotic and abiotic stresses on wild rocket by leaf-image hyperspectral data mining with an artificial intelligence model. Plant Methods 2022, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Chellaswamy, C.; Geetha, T.S.; Venkatachalam, K.; Mulla, M.A. Soil test based smart agriculture management system. In Proceedings of the 2020 7th International Conference on Smart Structures and Systems (ICSSS), Chennai, India, 23–24 July 2020; IEEE: Toulouse, France, 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Singh, M.; Subahan, G.M.; Sharma, S.; Singh, G.; Sharma, N.; Sharma, U.; Kumar, V. Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops. Stresses 2025, 5, 23. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Kutasy, B.; Hegedűs, G.; Kiniczky, M.; Pallos, J.P.; Nagy, Á.; Pócsi, I.; Pákozdi, K.; Kállai, M.; Weingart, C.; Andor, K.; et al. Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium oxysporum f. sp. pisi Infecting Pisum sativum. Agronomy 2025, 15, 991. [Google Scholar] [CrossRef]
- Di Sario, L.; Boeri, P.; Matus, J.T.; Pizzio, G.A. Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops. Int. J. Mol. Sci. 2025, 26, 1129. [Google Scholar] [CrossRef]
- Asif, A.; Ali, M.; Qadir, M.; Karthikeyan, R.; Singh, Z.; Khangura, R.; Di Gioia, F.; Ahmed, Z.F. Enhancing crop resilience by harnessing the synergistic effects of biostimulants against abiotic stress. Front. Plant Sci. 2023, 14, 1276117. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial Inoculants as Plant Biostimulants: A Review on Risk Status. Life 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Akhtar, M.S.; Siraj, M.; Zaman, W. Molecular Communication of Microbial Plant Biostimulants in the Rhizosphere Under Abiotic Stress Conditions. Int. J. Mol. Sci. 2024, 25, 12424. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Himanshu, S.K.; García-Caparrós, P.; Tisarum, R.; Cha-um, S.; Datta, A. Ascophyllum nodosum seaweed extract and potassium alleviate drought damage in tomato by improving plant water relations, photosynthetic performance, and stomatal function. J. Appl. Phycol. 2024, 36, 2255–2268. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Zhang, M.; Chen, Q.; Zheng, L.; Li, Y.C.; Sun, L. The combined application of controlled-release urea and fulvic acid improved the soil nutrient supply and maize yield. Arch. Agron. Soil Sci. 2020, 67, 633–646. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous pig blood-derived protein hydrolysates as a promising method for alleviation of salt stress in tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- MeCarty, J.S.; Sharma, N.; Kumar, R.; Lalrammawii, C.; Dwivedi, N.; Srivastava, H.; Farooq, F.; Mehta, S. Impact of silicon fertilization on crop growth, productivity and nutrients enhancement in rice: A review. Plant Sci. Today 2024, 12, 1–10. [Google Scholar] [CrossRef]
- Al Hinai, M.S.; Ullah, A.; Al-Toubi, A.K.M.; Al Harrasi, I.R.; Alamri, A.A.; Farooq, M. Co-application of Biochar and Seed Priming with Nano-sized Chitosan-Proline Improves Salt Tolerance in Differentially Responding Bread Wheat Genotypes. J. Soil Sci. Plant Nutr. 2023, 23, 3058–3073. [Google Scholar] [CrossRef]
- Salem, D.; El-Garhy, H.A.S.; Ismail, I.A.; Dessoky, E.S.; Samra, B.N.; Shoala, T. Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform. Molecules 2022, 27, 5112. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-Formulations of Drugs: Recent Developments, Impact and Challenges. Biochimie 2016, 128, 99–112. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Nano-Enabled Agriculture: How Do Nanoparticles Cross Barriers in Plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.-S.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Bouhadi, M.; Javed, Q.; Jakubus, M.; Elkouali, M.; Fougrach, H.; Ansar, A.; Ban, S.G.; Ban, D.; Heath, D.; Černe, M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy 2025, 15, 1131. [Google Scholar] [CrossRef]
- Nawaz, A.; ur Rehman, H.; Usman, M.; Wakeel, A.; Shahid, M.S.; Alam, S.; Sanaullah, M.; Atiq, M.; Farooq, M. Nanobiotechnology in crop stress management: An overview of novel applications. Discov. Nano 2023, 18, 74. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar] [CrossRef]
- Abhiram, G. Contributions of Nano-Nitrogen Fertilizers to Sustainable Development Goals: A Comprehensive Review. Nitrogen 2023, 4, 397–415. [Google Scholar] [CrossRef]
- Ullah, Z.; Iqbal, J.; Abbasi, B.A.; Ijaz, S.; Ahmad, S.; Khan, S.; Sampath, S.; Murtaza, G.; Mehmood, Y.; Kanwal, S.; et al. Nano-formulation for Agriculture Applicability. In Revolutionizing Agriculture: A Comprehensive Exploration of Agri-Nanotechnology. Nanotechnology in the Life Sciences; Shahzad, R., Fiaz, S., Qayyum, A., Ul Islam, M., Lee, I.J., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 325–367. [Google Scholar] [CrossRef]
- Izuafa, A.; Chimbekujwo, K.I.; Raji, R.O.; Oyewole, O.A.; Oyewale, R.O.; Abioye, O.P. Application of Nanoparticles for Targeted Management of Pests, Pathogens and Disease of Plants. Plant Nano Biol. 2025, 13, 100177. [Google Scholar] [CrossRef]
- Jalil, A.; Munir, M.; Alqahtani, N.; Alyas, T.; Ahmad, M.; Bashir, S.; Qurashi, F.; Ghafoor, A.; Ali–Dinar, H. Enhancing Plant Resilience to Biotic and Abiotic Stresses through Exogenously Applied Nanoparticles: A Comprehensive Review of Effects and Mechanism. Phyton 2025, 94, 281. [Google Scholar] [CrossRef]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; Espirito Santo Pereira, A.D.; Sharif, R.; Jogaiah, S.; Paidi, M.K.; Wang, L.; Ali, Q.; et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: Current trends and future directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Narware, J.; Chakma, J.; Singh, S.P.; Prasad, D.R.; Meher, J.; Singh, P.; Bhargava, P.; Sawant, S.B.; Pitambara; Singh, P.J.; et al. Nanomaterial-based biosensors: A new frontier in plant pathogen detection and plant disease management. Front. Bioeng. Biotechnol. 2025, 13, 1570318. [Google Scholar] [CrossRef]
- Smola-Dmochowska, A.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamuła, E.; Dobrzyński, P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 7473. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.M.; Harding, C.A. ‘Overgrowth’ mutants in barley and wheat: New alleles and phenotypes of the ‘Green Revolution’ DELLA gene. J. Exp. Bot. 2013, 64, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Hussain, A.; Cheng, H.; Peng, J. Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 2005, 223, 105–113. [Google Scholar] [CrossRef]
- Tomlinson, L.; Yang, Y.; Emenecker, R.; Smoker, M.; Taylor, J.; Perkins, S.; Smith, J.; MacLean, D.; Olszewski, N.E.; Jones, J.D.G. Using CRISPR/Cas9 genome editing in tomato to create a gibberellin-responsive dominant dwarf DELLA allele. Plant Biotechnol. J. 2019, 17, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wu, S.; Dou, T.; Zhu, H.; Hu, C.; Huo, H.; He, W.; Deng, G.; Sheng, O.; Bi, F.; et al. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 2020, 18, 17–19. [Google Scholar] [CrossRef]
- Kawai, K.; Takehara, S.; Kashio, T.; Morii, M.; Sugihara, A.; Yoshimura, H.; Ito, A.; Hattori, M.; Toda, Y.; Kojima, M.; et al. Evolutionary alterations in gene expression and enzymatic activities of gibberellin 3-oxidase 1 in Oryza. Commun. Biol. 2022, 5, 67. [Google Scholar] [CrossRef]
- Das, A.; Pal, S.; Hasanuzzaman, M.; Adak, M.K.; Sil, S.K. Mitigation of Aluminum Toxicity in Rice Seedlings Using Biofabricated Selenium Nanoparticles and Nitric Oxide: Synergistic Effects on Oxidative Stress Tolerance and Sulfur Metabolism. Chemosphere 2025, 370, 143940. [Google Scholar] [CrossRef]
- Gupta, C.; Ramegowda, V.; Basu, S.; Pereira, A. Using network-based machine learning to predict transcription factors involved in drought resistance. Front. Genet. 2021, 12, 652189. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Zörb, C.; Rengel, Z.; Schubert, S. The expression of the endogenous vacuolar Na+ /H+ antiporters in roots and shoots correlates positively with the salt resistance of wheat (Triticum aestivum L.). Plant Sci. 2005, 169, 959–965. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higer Plants, 2nd ed.; Academic Press: London, UK, 1995; pp. 285–299. [Google Scholar]
- Slabu, C.; Zörb, C.; Steffens, D.; Schubert, S. Is salt stress of faba bean (Vicia faba) caused by Na+ or Cl− toxicity? J. Plant Nutr. Soil Sci. 2009, 172, 644–651. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Urbanavičiūtė, I.; Bonfiglioli, L.; Pagnotta, M.A. One Hundred Candidate Genes and Their Roles in Drought and Salt Tolerance in Wheat. Int. J. Mol. Sci. 2021, 22, 6378. [Google Scholar] [CrossRef]
- Stolarska, E.; Paluch-Lubawa, E.; Grabsztunowicz, M.; Kumar Tanwar, U.; Arasimowicz-Jelonek, M.; Phanstiel, O.; Mattoo, A.K.; Sobieszczuk-Nowicka, E. Polyamines as Universal Bioregulators across Kingdoms and Their Role in Cellular Longevity and Death. Crit. Rev. Plant Sci. 2023, 42, 364–384. [Google Scholar] [CrossRef]
- Garg, D.; Singh, H.; Shacham-Diamand, Y. AdapTree: Data-Driven Approach to Assessing Plant Stress Through the AI-Sensor Synergy. Sensors 2025, 25, 3149. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Liang, H.; Bai, Y.; Li, X.; Zhou, J.; Su, C.; Wei, W. Advances in Deep Learning Applications for Plant Disease and Pest Detection: A Review. Remote Sens. 2025, 17, 698. [Google Scholar] [CrossRef]
- Kamarianakis, Z.; Perdikakis, S.; Daliakopoulos, I.N.; Papadimitriou, D.M.; Panagiotakis, S. Design and Implementation of a Low-Cost, Linear Robotic Camera System, Targeting Greenhouse Plant Growth Monitoring. Future Internet 2024, 16, 145. [Google Scholar] [CrossRef]
- Sykes, J.R.; Denby, K.J.; Franks, D.W. Computer vision for plant pathology: A review with examples from cocoa agriculture. Appl. Plant Sci. 2024, 12, e11559. [Google Scholar] [CrossRef]
- Yoosefzadeh Najafabadi, M.; Hesami, M.; Eskandari, M. Machine Learning-Assisted Approaches in Modernized Plant Breeding Programs. Genes 2023, 14, 777. [Google Scholar] [CrossRef]
- Kalbhor, A.; Rohit Borole, R.; Karekar, S.; Deshmukh, N.; Lahane, A. AI and Machine Learning in Precision Agriculture: The Future of Agricultural Precision Agriculture. Int. J. Res. Appl. Sci. Eng. Technol. (IJRASET) 2025, 13, 648–654. [Google Scholar] [CrossRef]
- Mallick, M.T.; Banerjee, S.; Thakur, N.; Saha, H.N.; Chakrabarti, A. Evaluation of State-of-the-Art Deep Learning Techniques for Plant Disease and Pest Detection. Comput. Mater. Contin. 2025, 85, 121–180. [Google Scholar] [CrossRef]
- Uzhinskiy, A. Advanced Technologies and Artificial Intelligence in Agriculture. AppliedMath 2023, 3, 799–813. [Google Scholar] [CrossRef]
- Khatibi, S.M.H.; Ali, J. Harnessing the power of machine learning for crop improvement and sustainable production. Front. Plant Sci. 2024, 15, 1417912. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.M.R.; Noman, I.R.; Aziz, M.M.; Rahaman, M.M.; Islam, M.R.; Manik, M.M.T.G.; Das, K. Transformation of plant breeding using data analytics and information technology: Innovations, applications, and prospective directions. Front. Biosci.-Elite 2025, 17, 27936. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, P.N.H.; Badavath, A.; Srivalli, P. Artificial intelligence and its applications in agriculture: A review. Environ. Conserv. J. 2025, 26, 274–280. [Google Scholar] [CrossRef]
- Schoen, A.; Wallace, S.; Holbert, M.F.; Brown-Guidera, G.; Harrison, S.; Murphy, P.; Sanantonio, N.; Van Sanford, D.; Boyles, R.; Mergoum, M.; et al. Reducing the generation time in winter wheat cultivars using speed breeding. Crop Sci. 2023, 63, 2079–2090. [Google Scholar] [CrossRef]
- Assimakopoulos, F.; Vassilakis, C.; Margaris, D.; Kotis, K.; Spiliotopoulos, D. Artificial Intelligence Tools for the Agriculture Value Chain: Status and Prospects. Electronics 2024, 13, 4362. [Google Scholar] [CrossRef]
- Hussein, A.H.A.; Jabbar, K.A.; Mohammed, A.; Jasim, L. Harvesting the future: AI and IoT in agriculture. In E3S Web of Conferences; EDP Sciences: Istanbul, Turkey, 2024; Volume 477, p. 00090. [Google Scholar] [CrossRef]
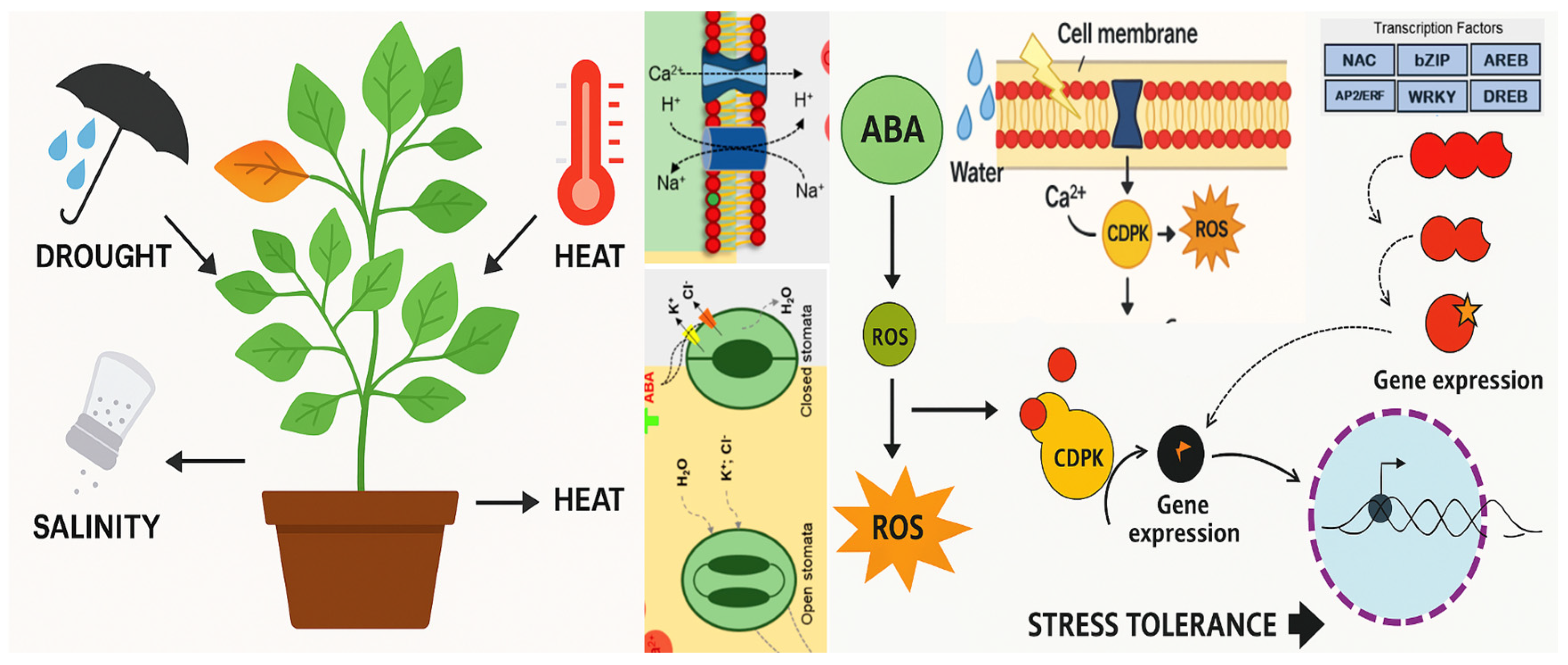

| Pianta | Gene | Stress | Effect of Editing | Reference |
|---|---|---|---|---|
| Rice | OsRR22 | Salinity | Mutants with improved salt tolerance, without compromising performance | [76,77] |
| OsDST | Drought and Salt | Greater tolerance to drought and salinity | [77,78] | |
| OsDERF1 | Drought | enhanced drought resistance | [79] | |
| OsERA1 | Drought (via ABA) | Mutants with longer roots and better response to drought | [80] | |
| OsPYL1/4/6 | Hoy | Knock-out improves heat tolerance and productivity | [4,81] | |
| Wheat | TaERF3 | Drought | Increased water efficiency and photosynthesis under stress | [4,82] |
| TaHKT1;5 | Salt | Improved ionic regulation and salt tolerance | [20,31,36,57,83] | |
| TaDREB2/3, TaHAG1 | Salt/drought | Promotes tolerance, improved multi-source resistance | [4,5,57,84] | |
| Barley | HvITPK1 | Salt | Inositol Trisphosphate 5/6 Kinase | [85] |
| Maize | ZmARGOS8 | Drought | Improve grain yield | [86] |
| ZmTMS5 | Thermosensitive | [87] | ||
| Soybean | GmNAC8, GmNAC12 | Drought | Mutanti più sensibili = ruolo positivo nella tolleranza | [5,31,57,77,88] |
| GmMYB118, GmAITR | Salt/drought | Overexpression → positive role in tolerance | [89] | |
| Tomato | SlARF4, SlHyPRP1 | Salt/drought | Knock-out improves water efficiency and stress tolerance | [90,91] |
| SICBF1 | Cold | C-repeat binding factors (CBFs) | [92] | |
| SlMAPK3 | Hot and Salt | Heat-resistant mutants, with increased HSP; salinity sensitive | [93] | |
| Apple | MdNHX1 | Salt | Salt tolerance high K+/Na+ in leaves | [94] |
| Cotton | GhHB12 | Salt/drought | Increases the tolerance to abiotic stress | [95] |
| Mustard | BnaA6.RGA | Drought | Interacting with the ABA signaling | [96] |
| Chickpea | 4CL | Drought | phenylpropanoid metabolism in the lignin biosynthesis pathway | [97] |
| REV7 | Drought | MYB transcription factor | [97] | |
| Actinidia eriantha | AcePosF21 | Cold | bZIP transcription factor | [98] |
| AceGGP3 | Drought | MYBS1-like and GBF3 transcription factors. AsA | [99] | |
| Peanut | AVP1 | Drought/salt | Biomass, photosynthetic rate, high yield | [100] |
| Cassava, cucumber, Tomato | eIF4E, CsLOB1, SlDmr6-1 | Virus or bacteria | Broad resistance through gene knockouts | [101,102,103] |
| Poplar | CarNac3 | Drought | Transcription factor from chickpea | [104,105] |
| PagHCF106 | Drought | Modulating stomatal aperture | [106] | |
| PagHB7/PagABF4–PagEPFL9 | Drought | Regulates Stomatal Density | [107] |
| Biostimulant | Application | Main Effects | Crops | Ref. |
|---|---|---|---|---|
| Seaweed extracts (Ascophyllum nodosum, Ecklonia maxima) | Foliar spray, soil drench | Promote root and shoot growth, enhance photosynthesis, improve tolerance to drought, salinity, and temperature stress | Wheat, tomato, maize | [135] |
| Humic and fulvic acids | Soil amendment, fertigation | Improve nutrient uptake (N, P, Fe, Zn), stimulate root development, enhance soil microbial activity | Maize, soybean, cucumber | [136] |
| Protein hydrolysates and amino acids | Foliar spray, seed treatment, fertigation | Stimulate N metabolism, increase photosynthetic efficiency, improve yield and fruit quality under stress | Tomato, lettuce, grapevine | [137] |
| Microbial biostimulants (PGPR: Azospirillum, Bacillus, Pseudomonas; Mycorrhiza: Glomus spp.) | Seed coating, soil inoculation, root dipping | Enhance nutrient uptake (P, N), improve root architecture, boost stress tolerance and pathogen resistance | Maize, wheat, legumes | [138] |
| Chitosan and biopolymers | Foliar spray, seed coating | Act as defense elicitors, strengthen antioxidant response, improve resistance to pathogens | Strawberry, tomato, pepper | [139] |
| Silicon-based compounds | Foliar spray, soil application | Reinforce cell walls, reduce lodging, increase tolerance to salinity, drought, and heavy metals | Rice, cucumber, barley | [140] |
| Typology | Method |
|---|---|
| Integrating genomics and advanced phenotyping |
|
| |
| Using Artificial Intelligence (AI) |
|
| |
| Pan-genomics and super pan-genomics |
|
| |
| De novo domestication and use of wild relatives |
|
| |
| Accelerated breeding pipeline |
|
| |
| “Horses for courses” approach |
|
| Multi-location and station tests |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnotta, M.A. Molecular Breeding for Abiotic Stress Tolerance in Crops: Recent Developments and Future Prospectives. Int. J. Mol. Sci. 2025, 26, 9164. https://doi.org/10.3390/ijms26189164
Pagnotta MA. Molecular Breeding for Abiotic Stress Tolerance in Crops: Recent Developments and Future Prospectives. International Journal of Molecular Sciences. 2025; 26(18):9164. https://doi.org/10.3390/ijms26189164
Chicago/Turabian StylePagnotta, Mario A. 2025. "Molecular Breeding for Abiotic Stress Tolerance in Crops: Recent Developments and Future Prospectives" International Journal of Molecular Sciences 26, no. 18: 9164. https://doi.org/10.3390/ijms26189164
APA StylePagnotta, M. A. (2025). Molecular Breeding for Abiotic Stress Tolerance in Crops: Recent Developments and Future Prospectives. International Journal of Molecular Sciences, 26(18), 9164. https://doi.org/10.3390/ijms26189164







