Single-Cell Transcriptome Reveals the Regulatory Role of STAT3 in Diquat-Induced Oxidative Stress in Piglet Hepatocytes
Abstract
1. Introduction
2. Results
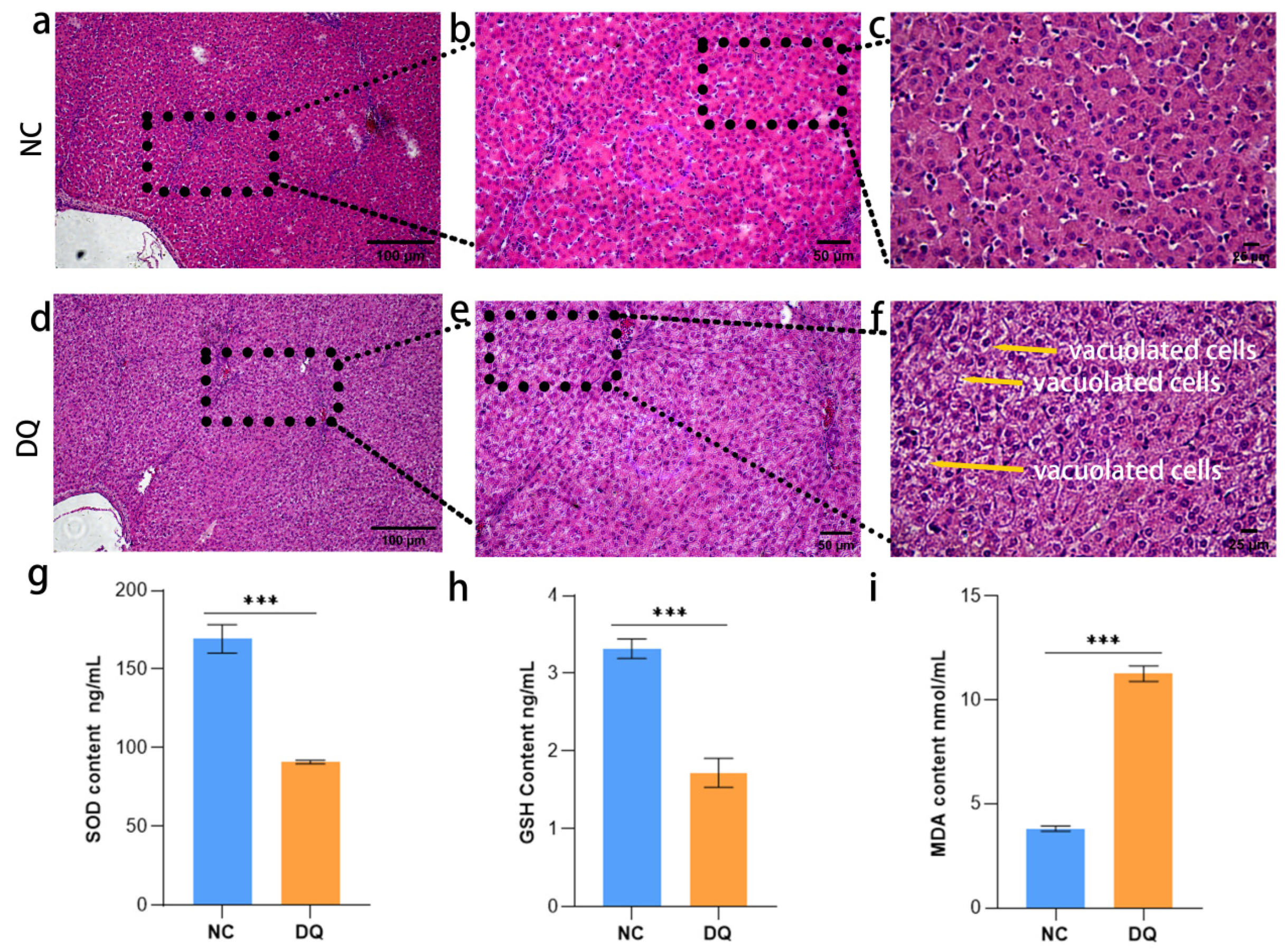
2.1. Detection of DQ-Induced Liver Histopathology and OS-Related Traits
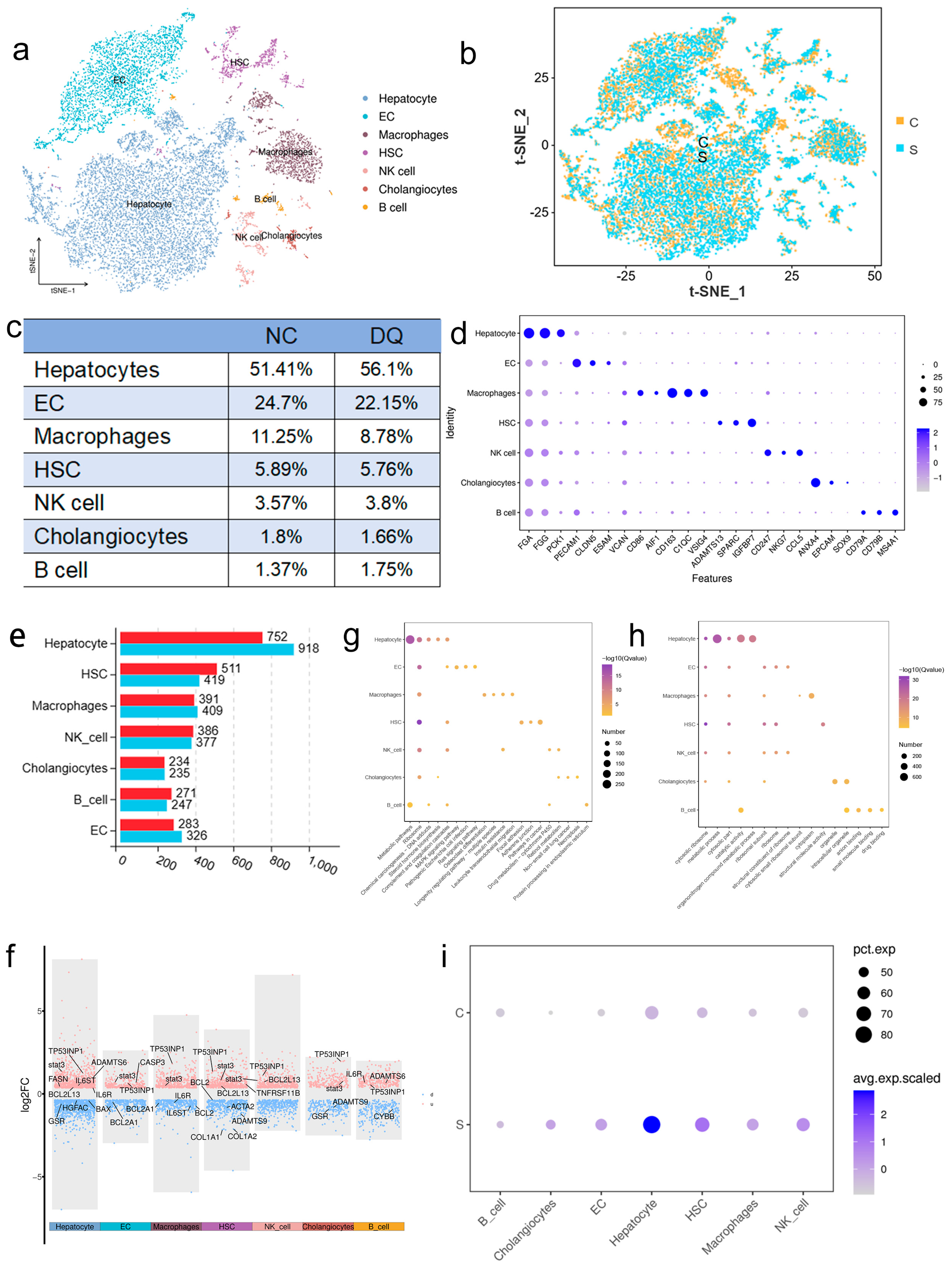
2.2. Single-Cell RNA Sequencing Reveals the Characteristics of Porcine Liver Cell Types
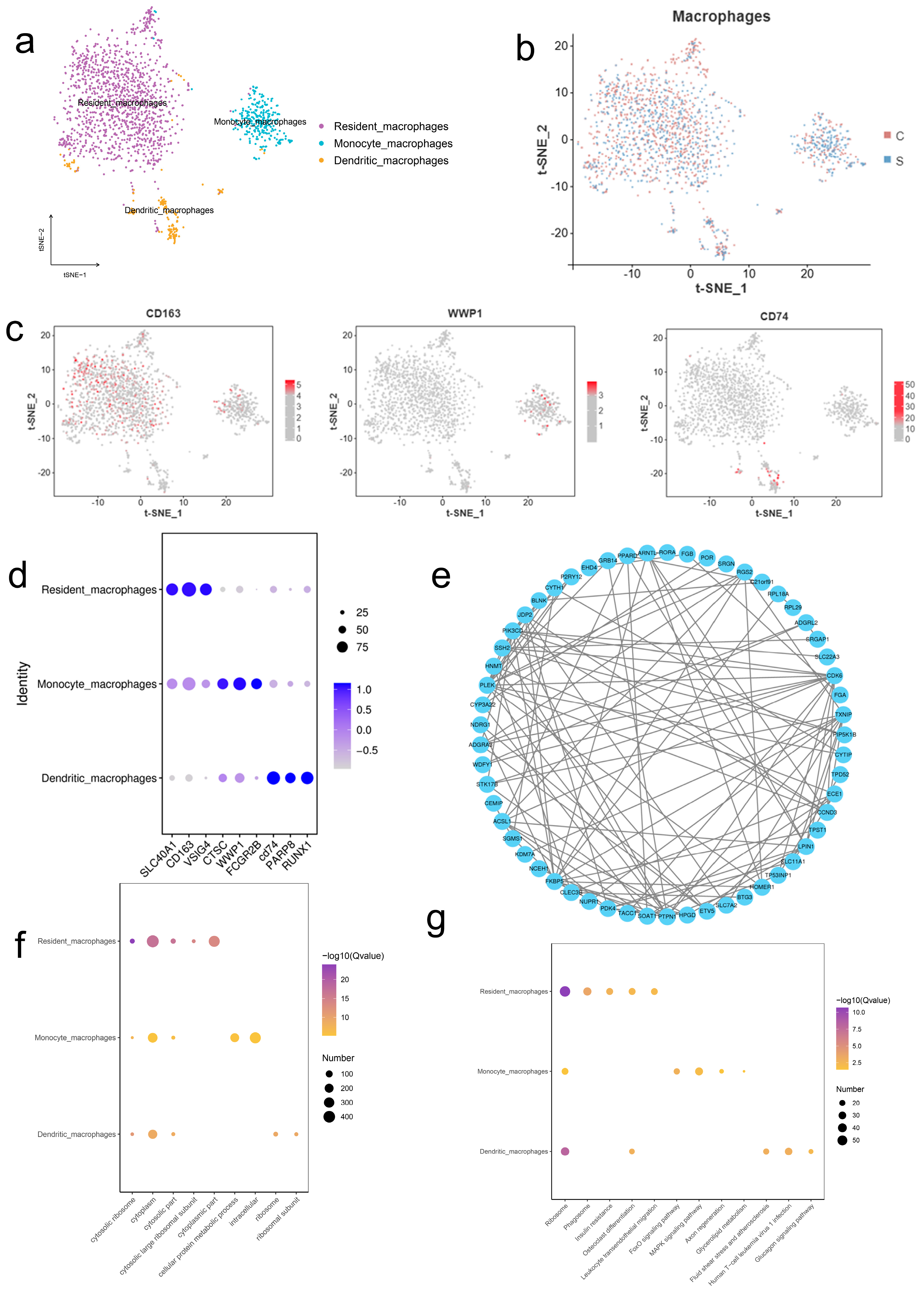
2.3. Single-Cell RNA Sequencing Reveals Macrophage Subsets
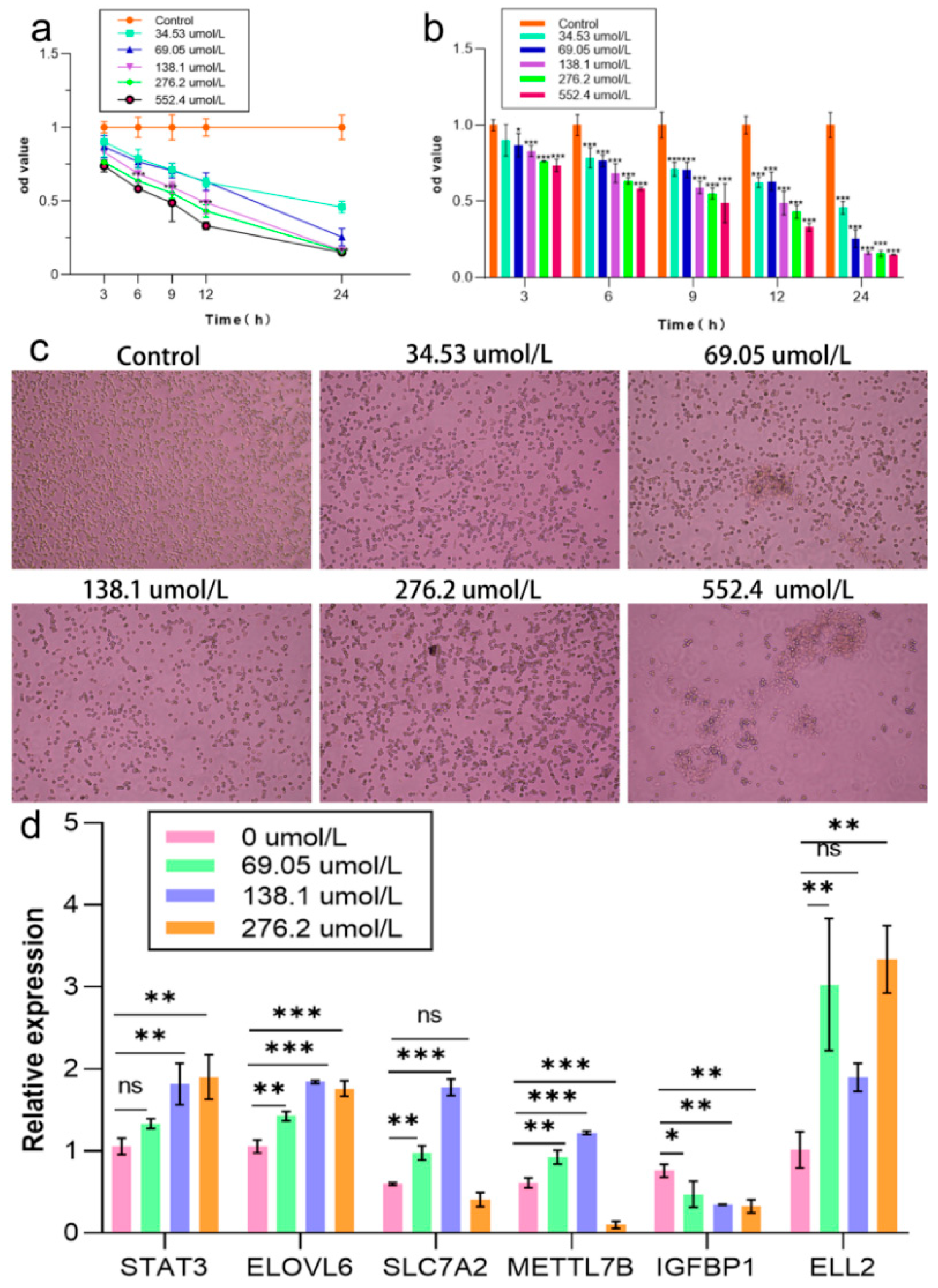
2.4. Establishment of OS Model in NCTC1469 Cells
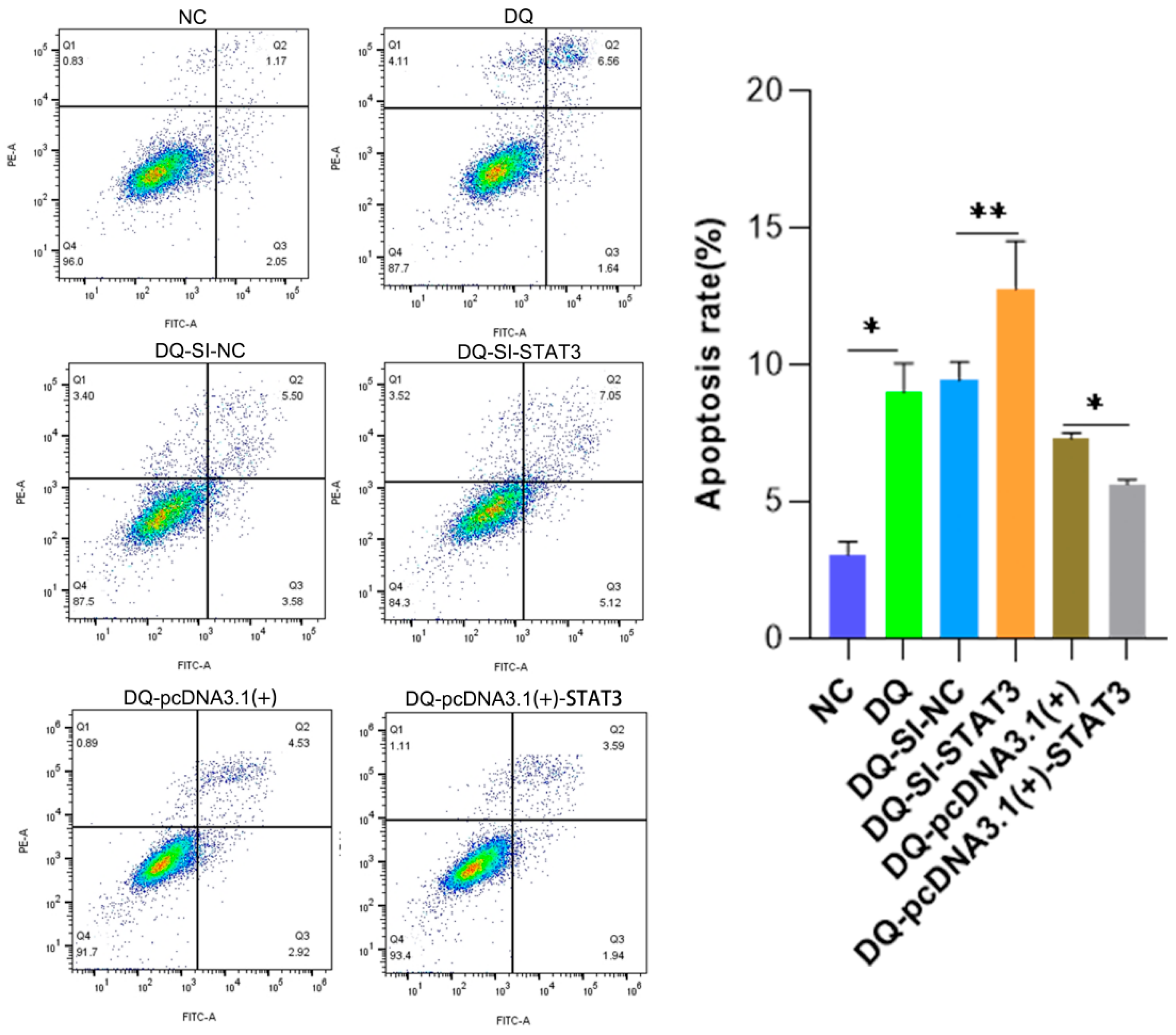
2.5. Annexin V/PI Dual-Staining Flow Cytometry Demonstrates the Role of STAT3
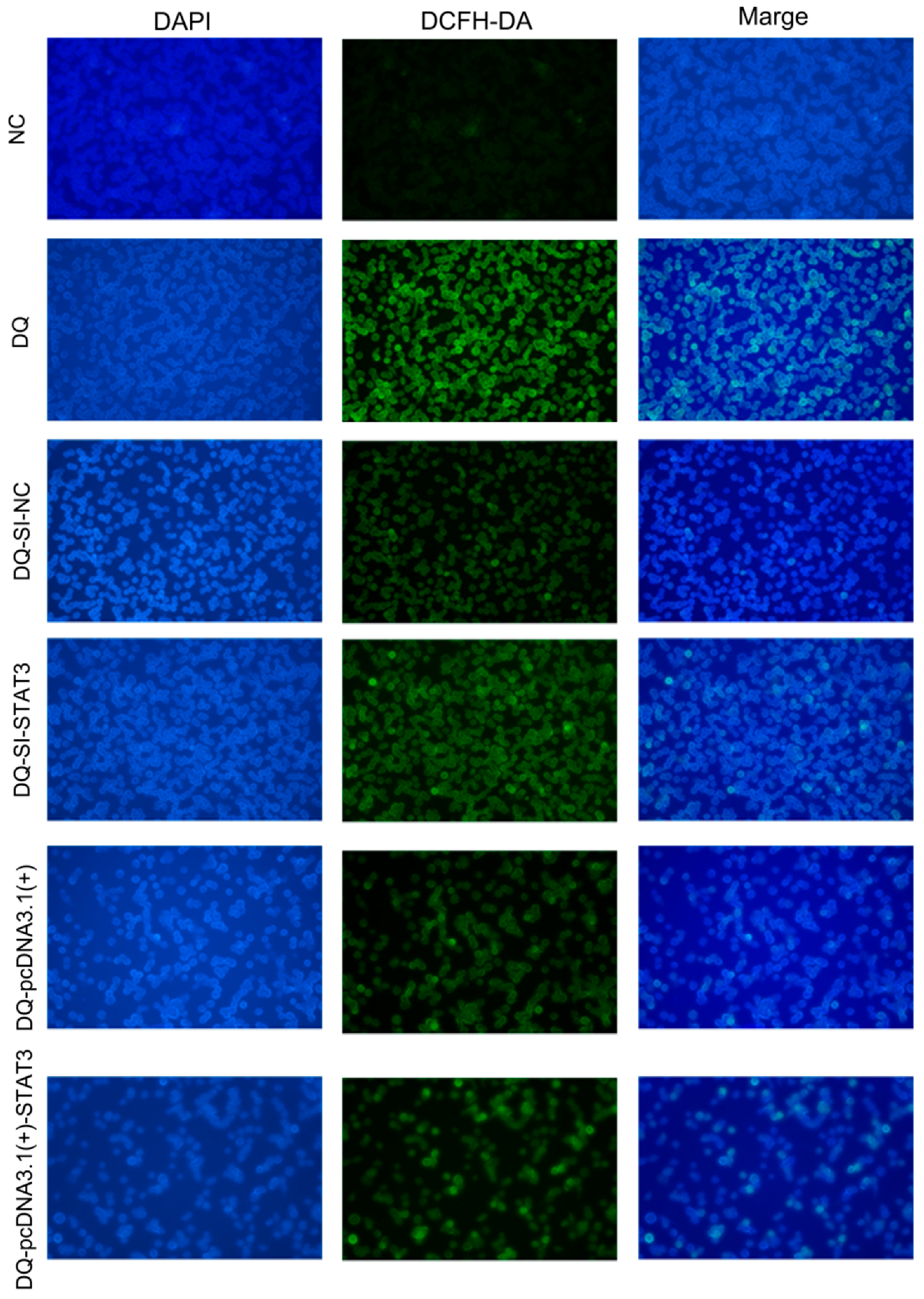
2.6. Fluorescence Microscopy Analysis of ROS in Cells
2.7. Effects of Cellular Antioxidant Genes and Key Genes of Apoptosis
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Cells
4.2. Hematoxylin and Eosin (HE) Staining
4.3. Single-Cell RNA Sequencing
4.4. Cell Culture and Cell Model Establishment
4.5. Cell Viability Assay
4.6. Cell Transfection
4.7. Total RNA Extraction, cDNA Synthesis, and Real-Time RT-PCR
4.8. Cellular ROS Activity Assay
4.9. Detection of Apoptosis
4.10. Western Blot Analysis
4.11. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murer, S.B.; Aeberli, I.; Braegger, C.P.; Gittermann, M.; Hersberger, M.; Leonard, S.W.; Taylor, A.W.; Traber, M.G.; Zimmermann, M.B. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J. Nutr. 2014, 144, 193–201. [Google Scholar] [CrossRef]
- Beyfuss, K.; Hood, D.A. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018, 23, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an Antioxidant System after Early Weaning in Piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef]
- Chen, J.; Su, Y.; Lin, R.; Lin, F.; Shang, P.; Hussain, R.; Shi, D. Effects of Acute Diquat Poisoning on Liver Mitochondrial Apoptosis and Autophagy in Ducks. Front. Vet. Sci. 2021, 8, 727766. [Google Scholar] [CrossRef]
- Smith, C.V.; Hughes, H.; Lauterburg, B.H.; Mitchell, J.R. Oxidant Stress and Hepatic Necrosis in Rats Treated with Diquat. J. Pharmacol. Exp. Ther. 1985, 235, 172–177. [Google Scholar] [CrossRef]
- Xun, W.; Fu, Q.; Shi, L.; Cao, T.; Jiang, H.; Ma, Z. Resveratrol Protects Intestinal Integrity, Alleviates Intestinal Inflammation and Oxidative Stress by Modulating AhR/Nrf2 Pathways in Weaned Piglets Challenged with Diquat. Int. Immunopharmacol. 2021, 99, 107989. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Zhong, S.; Yu, W.; Wang, J.; Zhu, W.; Yang, T.; Zhao, G.; Jiang, Y.; Li, Y. Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets. Vet. Sci. 2022, 10, 22. [Google Scholar] [CrossRef]
- Sánchez-Valle, V.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–48560. [Google Scholar] [CrossRef] [PubMed]
- Travlos, I.; Rapti, E.; Gazoulis, I.; Kanatas, P.; Tataridas, A.; Kakabouki, I.; Papastylianou, P. The Herbicidal Potential of Different Pelargonic Acid Products and Essential Oils against Several Important Weed Species. Agronomy 2020, 10, 1687. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative Stress as a Crucial Factor in Liver Diseases. World J. Gastroenterol. 2014, 20, 8082–80891. [Google Scholar] [CrossRef]
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef]
- Butturini, E.; Carcereri de Prati, A.; Mariotto, S. Redox Regulation of STAT1 and STAT3 Signaling. Int. J. Mol. Sci. 2020, 21, 7034. [Google Scholar] [CrossRef]
- Lu, F.M.; Yu, Y.R. STAT3 Promotes Schistosome-Induced Liver Injury by Inflammation, Oxidative Stress, Proliferation, and Apoptosis Signal Pathway. Infect. Immun. 2021, 89, e00309-20. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Chen, W.; Qin, M.; Wang, L.X.; Zeng, Y.Q. Characteristics of circRNAs Expression Profiles in the Piglets Intestine Induced by Oxidative Stress. Genes Genomics 2022, 44, 425–433. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.J.; Kostallari, E.; Ibrahim, S.H.; Iwakiri, Y. The Evolving Role of Liver Sinusoidal Endothelial Cells in Liver Health and Disease. Hepatology 2023, 78, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Peiseler, M.; Schwabe, R.; Hampe, J.; Kubes, P.; Heikenwälder, M.; Tacke, F. Immune Mechanisms Linking Metabolic Injury to Inflammation and Fibrosis in Fatty Liver Disease—Novel Insights into Cellular Communication Circuits. J. Hepatol. 2022, 77, 1136–1160. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Fang, X.; Gu, L.; Luo, C.; Chen, L.; Wang, Q. Vitamin D Protects Mice from Diquat-Induced Oxidative Stress through the NF-κB/Nrf2/HO-1 Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6776956. [Google Scholar] [CrossRef]
- Peng, W.S.; Gao, M.; Yao, X.F.; Tong, Y.Y.; Zhang, H.H.; He, X. Magnolol Supplementation Alleviates Diquat-Induced Oxidative Stress via PI3K-Akt in Broiler Chickens. Anim. Sci. J. 2023, 94, 13891. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, L.; Zhang, Z.; Xu, D.; Hua, R.; Chen, H.; Li, X.; Duan, J.; Li, Q. Diquat Causes Mouse Testis Injury through Inducing Heme Oxygenase-1-Mediated Ferroptosis in Spermatogonia. Ecotoxicol. Environ. Saf. 2024, 280, 116562. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Zhou, L.; Chen, X.; Sew, W.Q.G.; Herranz, H.; Ye, Z.; Olsen, J.V.; Li, Y.; Nygaard, M.; et al. FOXO-Regulated OSER1 Reduces Oxidative Stress and Extends Lifespan in Multiple Species. Nat. Commun. 2024, 15, 7144. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhao, Y.; He, J.; Zhang, Y.; Zhang, X. PGC-1α Inhibits M2 Macrophage Polarization and Alleviates Liver Fibrosis Following Hepatic Ischemia Reperfusion Injury. Cell Death Discov. 2023, 9, 337. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Xiong, Y.; Teng, L. Protective Effect of Salidroside on Hypoxia-Related Liver Oxidative Stress and Inflammation via Nrf2 and JAK2/STAT3 Signaling Pathways. Food Sci. Nutr. 2021, 9, 5060–5069. [Google Scholar] [CrossRef]
- Zhao, W.J.; Bian, Y.P.; Wang, Q.H.; Yin, F.; Yin, L.; Zhang, Y.L.; Liu, J.H. Blueberry-Derived Exosomes-Like Nanoparticles Ameliorate Nonalcoholic Fatty Liver Disease by Attenuating Mitochondrial Oxidative Stress. Acta Pharmacol. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, F.M.; Caglayan, C.; Satıcı, E. Chemopreventive Effects of Hesperidin against Paclitaxel-Induced Hepatotoxicity and Nephrotoxicity via Amendment of Nrf2/HO-1 and Caspase-3/Bax/Bcl-2 Signaling Pathways. Chem. Biol. Interact. 2022, 365, 110073. [Google Scholar] [CrossRef]
- Abdelrahman, R.S.; Abdel-Rahman, N. Dimethyl Fumarate Ameliorates Acetaminophen-Induced Hepatic Injury in Mice Dependent of Nrf-2/HO-1 Pathway. Life Sci. 2019, 217, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gencer, S.; Gür, C.; İleritürk, M.; Küçükler, S.; Akaras, N.; Şimşek, H.; Kandemir, F.M. The Ameliorative Effect of Carvacrol on Sodium Arsenite-Induced Hepatotoxicity in Rats: Possible Role of Nrf2/HO-1, RAGE/NLRP3, Bax/Bcl-2/Caspase-3, and Beclin-1 Pathways. J. Biochem. Mol. Toxicol. 2024, 38, 23863. [Google Scholar] [CrossRef]
- Fang, X.; Huang, W.; Sun, Q.; Zhao, Y.; Sun, R.; Liu, F.; Huang, D.; Zhang, Y.; Gao, F.; Wang, B. Melatonin Attenuates Cellular Senescence and Apoptosis in Diabetic Nephropathy by Regulating STAT3 Phosphorylation. Life Sci. 2023, 332, 122108. [Google Scholar] [CrossRef]
- Wang, W.; Liu, M.; Fu, X.; Qi, M.; Zhu, F.; Fan, F.; Wang, Y.; Zhang, K.; Chu, S. Hydroxysafflor yellow A ameliorates alcohol-induced liver injury through PI3K/Akt and STAT3/NF-κB signaling pathways. Phytomedicine 2024, 132, 155814. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kang, K.; Pan, D.; Sun, Y.; Chang, B. FTY720 Attenuates APAP-Induced Liver Injury via the JAK2/STAT3 Signaling Pathway. Int. J. Mol. Med. 2022, 49, 67. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Chao, J.C.; Huang, C.H.; Wu, S.J. Punicalagin from Pomegranate Ameliorates TNF-α/IFN-γ-Induced Inflammatory Responses in HaCaT Cells via Regulation of SIRT1/STAT3 Axis and Nrf2/HO-1 Signaling Pathway. Int. Immunopharmacol. 2024, 130, 111665. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2–ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, J.; Li, H.; Zhang, C.; Zeng, Y.; Wang, J.; Chen, W. Single-Cell Transcriptome Reveals the Regulatory Role of STAT3 in Diquat-Induced Oxidative Stress in Piglet Hepatocytes. Int. J. Mol. Sci. 2025, 26, 9161. https://doi.org/10.3390/ijms26189161
Li Y, Li J, Li H, Zhang C, Zeng Y, Wang J, Chen W. Single-Cell Transcriptome Reveals the Regulatory Role of STAT3 in Diquat-Induced Oxidative Stress in Piglet Hepatocytes. International Journal of Molecular Sciences. 2025; 26(18):9161. https://doi.org/10.3390/ijms26189161
Chicago/Turabian StyleLi, Yunpeng, Jia Li, Hongjin Li, Chu Zhang, Yongqing Zeng, Jin Wang, and Wei Chen. 2025. "Single-Cell Transcriptome Reveals the Regulatory Role of STAT3 in Diquat-Induced Oxidative Stress in Piglet Hepatocytes" International Journal of Molecular Sciences 26, no. 18: 9161. https://doi.org/10.3390/ijms26189161
APA StyleLi, Y., Li, J., Li, H., Zhang, C., Zeng, Y., Wang, J., & Chen, W. (2025). Single-Cell Transcriptome Reveals the Regulatory Role of STAT3 in Diquat-Induced Oxidative Stress in Piglet Hepatocytes. International Journal of Molecular Sciences, 26(18), 9161. https://doi.org/10.3390/ijms26189161





