Administration of Natural Fish and Algal Oils in Nanoparticle Form to Pregnant Gilts and Newborn Piglets: Biochemical Effects and Spatial–Socio-Economic Implications for Regional Food Systems
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Level of Oxidative Stress
3.2. Activity of Antioxidant Defence Enzymes
3.3. Activity of DNA Repair Enzymes
3.4. Mechanisms Underlying Reduced Activity of Antioxidant and DNA Repair Enzymes
3.5. Socio–Spatial Diffusion of Nutritional Innovation
4. Materials and Methods
4.1. Ethics
4.2. Animals’ Housing and Feeding
4.3. Measurement of the Activity of Antioxidant Defence Enzymes
4.4. Measurement of the Activity of DNA Repair Enzymes: Formamidopirymidyno-DNA Glycosylase (FGP), Thymine-DNA Glycosylase (TDG), and N-Methylpurine DNA Glycosylase (MPG) Was Described in Detail in the Work by Kowalczyk [25]
4.5. Measurement of Malondialdehyde (MDA)
4.6. Statistical Analysis
4.7. Spatial Embedding of the Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase. |
| CAT | Catalase. |
| GPx | Glutathione peroxidase. |
| FPG | Formamidopirymidyno DNA glycosylase. |
| MPG-N | Methylpurine DNA glycosylase. |
| TDG | Thymine-DNA glycosylase. |
| BER | Base Excision Repair. |
| PUFA | Polyunsaturated Fatty Acid. |
| OGG1-8 | Oxoguanine glycosylase. |
| ROS | Reactive Oxygen Species. |
| RNS | Reactive nitrogen Species. |
| EPA | Eicosapentaenoic acid. |
| DHA | Docosahexaenoic acid. |
| (TAC) | Antioxidant capacity |
| MDA | Malondialdehyde. |
| PGE2 | Pro-inflammatory prostaglandins. |
| PGE3 | Anti-inflammatory properties. |
| (εA)-1,N6 | Ethenoadenine. |
| (εC)-3,N4 | Ethenocytosine. |
| (εG)-N2,3 | Ethenoguanine. |
| (εG)-1,N2 | Ethenoguanine. |
| 8oxoG | 8-Oxoguanine. |
| Fpg | Formamidopirymidyno-DNA glycosylase. |
| LA | Linoleic acid. |
| ALA | Alpha lipoic. |
| AA | Arachidonic acid. |
| TABRS | Thiobarbituric Acid-Reactive Substances. |
References
- Surai, P.F. Vitagenes in Avian Biology and Poultry Halth; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020. [Google Scholar]
- Zuo, J.; Zhang, Z.; Luo, M.; Zhou, L.; Nice, E.C.; Zhang, W.; Wang, C.; Huang, C. Redox signaling at the crossroads of human health and disease. Med. Comm. 2022, 3, e127. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug. Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant defenses in eukaryotic cells. In Free Radicals from Basic Science to Medicine; Poli, G., Albano, E., Dianzani, M.U., Eds.; Birkhauser Verlag: Basel, Switzerland, 1993; pp. 365–373. [Google Scholar]
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant enzymes and human health. In Antioxidant Enzyme; El-Missiry, M.A., Ed.; InTechOpen: London, UK, 2012. [Google Scholar]
- Fridovich, I. Superoxide radical and superoxide dismutases. Ann. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Góth, L.; Rass, P.; Páy, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 2004, 8, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Rusterholz, C.; Hahn, S.; Holzgreve, W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 2007, 29, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clin. Lipidol. 2010, 5, 899–911. [Google Scholar] [CrossRef]
- Kim, S.W.; Weaver, A.C.; Shen, Y.B.; Zhao, Y. Improving efficiency of sow productivity: Nutrition and health. J. Anim. Sci. Biotechnol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Ramirez-Tortosa, M.C.; Quiles, J.L.; Palomino, N.; Robles, R.; Mataix, J.; Huertas, J.R. Oxidative stress in erythrocytes from premature and full-term infants during their first 72 h of life. Free Radic. Res. 2003, 37, 317–322. [Google Scholar] [CrossRef]
- Khokhlova, E.V.; Fesenko, Z.S.; Sopova, J.V.; Leonova, E.I. Features of DNA Repair in the Early Stages of Mammalian Embryonic Development. Genes 2020, 11, 1138. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative damage: The biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid Peroxidation—DNA Damage by Malondialdehyde. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 29, 114477. [Google Scholar] [CrossRef]
- Moghadam, F.V.; Pourahmad, R.; Mortazavi, A.; Davoodi, D.; Azizinezhad, R. Use of Fish Oil Nanoencapsulated with Gum Arabic Carrier in Low Fat Probiotic Fermented Milk. Food Sci. Anim. Resour. 2019, 39, 309–323. [Google Scholar] [CrossRef]
- Prorok, P.; Saint-Pierre, C.; Gasparutto, D.; Fedorova, O.S.; Ishchenko, A.A.; Leh, H.; Buckle, M.; Tudek, B.; Saparbaev, M. Highly Mutagenic Exocyclic DNA Adducts Are Substrates for the Human Nucleotide Incision Repair Pathway. PLoS ONE 2012, 7, e51776. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Jaworek, J.; Kot, M.; Sokolowska, B.; Bielen, A.; Janowska, B.; Ciesla, J.M.; Szparecki, G.; Sados, B.; Tudek, B. Inflammation increases oxidative DNA damage repair and stimulates preneoplastic changes in colons of newborn rats. J. Physiol. Pharmacol. 2016, 67, 277–286. [Google Scholar] [PubMed]
- Ferenc, K.; Marcinkowski, M.; Olszewski, J.; Kowalczyk, P.; Pilžys, T.; Garbicz, D.; Dib, N.; Świderska, B.; Matyba, P.; Gajewski, Z.; et al. The proteomic profile is altered but not repaired after bariatric surgery in type 2 diabetes pigs. Sci. Rep. 2024, 14, 10235. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Smolińska, K.; Szopa, A.; Sobczyński, J.; Serefko, A.; Dobrowolski, P. Nutritional Quality Implications: Exploring the Impact of a Fatty Acid-Rich Diet on Central Nervous System Development. Nutrients 2024, 16, 1093. [Google Scholar] [CrossRef]
- Mongan, D.; Healy, C.; Jones, H.J.; Zammit, S.; Cannon, M.; Cotter, D.R. Plasma polyunsaturated fatty acids and mental disorders in adolescence and early adulthood: Cross-sectional and longitudinal associations in a general population cohort. Transl. Psychiatry 2021, 11, 321. [Google Scholar]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef]
- Kemse, N.G.; Kale, A.A.; Joshi, S.R. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension Wistar rats improves IL10 and VEGF levels. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 25–32. [Google Scholar] [CrossRef]
- Vericel, E.; Colas, R.; Calzada, C.; Lê, Q.H.; Feugier, N.; Cugnet, C.; Vidal, H.; Laville, M.; Moulin, P.; Lagarde, M. Moderate oral supplementation with docosahexaenoic acid improves platelet function and oxidative stress in type 2 diabetic patients. Thromb. Haemost. 2015, 114, 289–296. [Google Scholar] [CrossRef]
- Mas, E.; Woodman, R.J.; Burke, V.; Puddey, I.B.; Beilin, L.J.; Durand, T.; Mori, T.A. The omega-3 fatty acids EPA and DHA decrease plasma F(2)-isoprostanes: Results from two placebo-controlled interventions. Free Radic. Res. 2010, 44, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Sorg, O.; Granci, V.; Lecumberri, E.; Miralbell, R.; Dupertuis, Y.M.; Pichard, C. Interaction of ω-3 polyunsaturated fatty acids with radiation therapy in two different colorectal cancer cell lines. Clin. Nutr. 2014, 33, 164–170. [Google Scholar] [CrossRef]
- Mathias, P.C.F.; Elmhiri, G.; de Oliveira, J.C.; Delayre-Orthez, C.; Barella, L.F.; Tόfolo, L.P.; Fabricio, G.S.; Chango, A.; Abdennebi-Najar, L. Maternal diet, bioactive molecules, and exercising as reprogramming tools of metabolic programming. Eur. J. Nutr. 2014, 53, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.; Muhlhausler, B.S.; Gibson, R.A.; Xian, Z.C.J. Perinatal maternal dietary supplementation of Ω 3-fatty acids transiently affects bone marrow microenvironment, osteoblast and osteoclast formation, and bone mass in male offspring. Endocrinology 2012, 153, 2455–2465. [Google Scholar] [CrossRef]
- Kosek, V.; Heczkova, M.; Novak, F.; Meisnerova, E.; Novákova, O.; Zelenka, J.; Bechynska, K.; Vrzacova, N.; Suttnar, J. The ω-3 Polyunsaturated Fatty Acids and Oxidative Stress in Long-Term Parenteral Nutrition Dependent Adult Patients: Functional Lipidomics Approach. Nutrients 2020, 12, 2351. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Mori, T.A.; Keelan, J.A.; Waddell, B.J. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 2013, 88, 37. [Google Scholar] [CrossRef]
- Opgenorth, J.; Sordillo, L.M.; van de Haar, M.J. Colostrum supplementation with n-3 fatty acids and α-tocopherol alters plasma polyunsaturated fatty acid profile and decreases an indicator of oxidative stress in newborn calves. J. Dairy Sci. 2020, 103, 3545–3553. [Google Scholar] [CrossRef]
- Luo, W.L.; Luo, Z.; Xu, X.; Zhao, S.; Li, S.H.; Sho, T.; Yao, J.; Zhang, J.; Xu, W.N.; Xu, J.X. The effect of maternal diet with fish oil on oxidative stress and inflammatory reponse in sow and new-born piglets. Oxidative Med. Cell. Longev. 2019, 2019, 6765803. [Google Scholar] [CrossRef]
- Shen, Y.; Wan, H.; Zhu, J.; Fang, Z.; Che, L.; Feng, B.; Lin, Y.; Li, J.; Wu, D.C. Fish oil and olive oil supplementation in late pregnancy and lactation differentially affect oxidative stress and inflammation in sows and piglets. Lipids 2015, 50, 647–658. [Google Scholar] [CrossRef]
- Tanghe, S.; Millet, S.; De Smet, S. Echium oil and linseed oil as alternatives for fish oil in the maternal diet: Blood fatty acid profiles and oxidative status of sows and piglets. J. Anim. Sci. 2013, 91, 3253–3264. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Amador-Licona, N.; Diaz-Murillo, T.A.; Gabriel-Ortiz, G.; Pacheco-Moises, F.P.; Pereyra-Nobara, T.A.; Guizar-Mendoza, J.M.; Barbosa-Sabanero, G.; Orozco-Avina, G.; Moreno-Martinez, S.C.; Luna-Montalban, R.; et al. Omega 3 fatty acids supplementation and oxidative stress in HIV-seropositive patients. A. Clinical Trial. PLoS ONE 2016, 11, e0151637. [Google Scholar] [CrossRef] [PubMed]
- Miskiewicz, E.I.; MacPhee, D.J. Lysis Buffer Choices Are Key Considerations to Ensure Effective Sample Solubilization for Protein Electrophoresis. Methods Mol. Biol. 2019, 1855, 61–72. [Google Scholar] [CrossRef]
- Aebi, H. Methods Enzymol; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA; London, UK, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Venisse, J.S.; Gullner, G.; Brisset, M.N. Evidence for the Involvement of an oxidative stress in the Initation of Infection of Pear by Erwinia Amylovora. Plant Physiolol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef]
- Zhang, W. Nanoparticle Aggregation: Principles and Modeling. Adv. Exp. Med. Biol. 2014, 811, 19–43. [Google Scholar] [PubMed]
- Hopkins, J.; Tudhope, G.R. Glutathione peroxidase in human red cells in health and disease. Br. J. Haematol. 1973, 25, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar]
- Yilgor, A.; Demir, C. Determination of oxidative stress level and some antioxidant activities in refractory epilepsy Patients. Sci. Rep. 2024, 14, 6688. [Google Scholar] [CrossRef]
- Kajarabille, N.; Hurtado, J.A.; Peña-Quintana, L.; Peña, M.; Ruiz, J.; Diaz-Castro, J.; Rodríguez-Santana, Y.; Martin-Alvarez, E.; López-Frias, M.; Soldado, O.; et al. Omega-3 LCPUFA supplement: A nutritional strategy to prevent maternal and neonatal oxidative stress. Matern. Child Nutr. 2017, 13, e12300. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, C.E.; Olza, J.; Mesa, M.D.; Aguilera, M.; Miles, E.A.; Noakes, P.S.; Vlachava, M.; Kremmyda, L.S.; Diaper, N.D.; Godfrey, K.M.; et al. Fatty acid status and antioxidant defense system in mothers and their newborns after salmon intake during late pregnancy. Nutrition 2017, 33, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Garrel, C.; Alessandri, J.M.; Guesnet, P.; Al-Gubory, K.H. Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. Int. J. Biochem. Cell Biol. 2012, 44, 123–131. [Google Scholar] [CrossRef]
- Lou, Z.; Hurnik, J.F. Peripartum sows in three farrowing crates: Posture patterns and behavioural activities. Appl. Anim. Behav. Sci. 1998, 58, 77–86. [Google Scholar] [CrossRef]
- Ba, X.; Boldogh, I. 8-Oxoguanine DNA glycosylase 1, Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef]
- Puzio, I.; Kapica, M.; Bienko, M.; Valverde Piedra, J.L.; Gajewski, Z.; Wilczak, J.; Kulasek, G.; Zabielski, R. Dietary bioactive substances influenced perinatal bone development in piglets. Livest. Sci. 2007, 108, 72–75. [Google Scholar] [CrossRef]
- Zabielski, R.; Gajewski, Z.; Valverde Piedra, J.L.; Laubitz, D.; Wilczak, J.; Bałasinska, B.; Kulasek, G. The perinatal development of the gastrointestinal tract in piglets can be modified by supplementation of sow diet with bioactive substances. Livest. Sci. 2007, 109, 34–37. [Google Scholar] [CrossRef]
- Tudek, B. Base excision repair modulation as a risk factor for human cancers. Mol. Asp. Med. 2007, 28, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hu, W.; Marnett, L.J.; Tang, M.S. Malondialdehyde, a major endogenous lipid peroxidation product, sensitizes human cells to UV- and BPDE-induced killing and mutagenesis through inhibition of nucleotide excision repair. Mutat. Res. 2006, 601, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Eder, E.; Wacker, M.; Lutz, U.; Nair, J.; Fang, X.; Bartsch, H.; Beland, F.A.; Schlatter, J.; Lutz, W.K. Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem.-Biol. Interact. 2006, 159, 81–89. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Kowalczyk, P.; Tudek, B.; Zabielski, R.; Dziaman, T.; Oliński, R.; van Schooten Frederik, J.; Godschalk, R.W.L. The effect of oxidative stress on nucleotide-excision repair in colon tissue of newborn piglets. Mutat. Res. 2010, 695, 75–80. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Kowalczyk, P.; Tomaszewski, B.; Vasilaki, A.; Maas, L.M.; Moonen, E.J.; Palagani, A.; Godschalk, R.W.L.; Tudek, B.; van Schooten, F.J.; et al. Redox and epigenetic regulation of the APE1 gene in the hippocampus of piglets: The effect of early life exposures. DNA Repair 2014, 18, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A. Effect of fish and fish oil-derived omega-3 fatty acids on lipid oxidation. Redox Rep. 2004, 9, 193–197. [Google Scholar] [CrossRef]
- Wypych, A.; Dunisławska, A.; Grabowska, M.; Michałek, K.; Ożgo, M.; Liput, K.; Herosimczyk, A.; Poławska, E.; Pierzchała, M.; Lepczyński, A. Effects of three-month feeding high-fat diets with different fatty acid composition on kidney histology and expression of genes related to cellular stress and water-electrolyte homeostasis in mice. J. Anim. Feed Sci. 2023, 32, 372–384. [Google Scholar] [CrossRef]
- Wypych, A.; Ożgo, M.; Bernaciak, M.; Herosimczyk, A.; Barszcz, M.; Gawin, K.; Ciechanowicz, A.K.; Kucia, M.; Pierzchała, M.; Poławska, E.; et al. Effect of feeding high fat diets differing in fatty acid composition on oxidative stress markers and protein expression profiles in mouse kidney. J. Anim. Feed Sci. 2024, 33, 170–184. [Google Scholar] [CrossRef]
- Ambrozova, G.; Pekarova, M.; Lojek, A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw 264.7 macrophages. Eur. J. Nutr. 2010, 49, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Fujimoto, K.; Miyazawa, T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J. Nutr. 2000, 130, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- WHO. Healthy Diet—Fact Sheet N°394; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 29 July 2025).
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- Sinclair, A.J. Update on fatty acids and the brain. Nutrients 2024, 16, 4416. [Google Scholar] [CrossRef]
- Lorenzo, D.D.; Cabodevilla, A.G.; Goldberg, I.J.; Norata, G.D. Cardiac lipid metabolism, mitochondrial function and heart failure. Cardiovasc. Res. 2023, 119, 1905–1914. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin Lipidol. 2021, 16, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Landi, F.; Chew, S.T.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in muscle health and nutrition: A toolkit for healthcare professionals. Clin. Nutr. 2022, 10, 2244–2263. [Google Scholar] [CrossRef]
- Davoudkhani, M. Economic optimization of feeding and shipping strategies in pig-fattening using an individual–based model. Agric. Syst. 2020, 184, 102899. [Google Scholar] [CrossRef]
- Clonan, A.; Roberts, K.E.; Holdsworth, M. Socioeconomic and demographic drivers of red and processed meat consumption: Implications for health and environmental sustainability. Proc. Nutr. Soc. 2016, 75, 367–373. [Google Scholar] [CrossRef]
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. Omega-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef]
- Jawerbaum, A.; Capobianco, E. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta 2011, 32 (Suppl. 2), S212–S217. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.P.; Mark, P.J.; Waddell, B.J. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology 2006, 147, 5568–5574. [Google Scholar] [CrossRef]
- Boog, G. Chronic villitus of unknown etiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 9–15. [Google Scholar]
- Amusquivar, E.; Herrera, E. Influence of changes in dietary fatty acids during pregnancy on placental and fetal fatty acid profile in the rat. Biol. Neonate 2003, 83, 136–145. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, P.; Kra, G.; Butenko, Y.; Daddam, J.R.; Levin, Y.; Zachut, M. Maternal supplementation with n-3 fatty acids affects placental lipid metabolism, inflammation, oxidative stress, the endocannabinoid system, and the neonate cytokine concentrations in dairy cows. J. Anim. Sci. Biotechnol. 2024, 15, 74. [Google Scholar] [CrossRef]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef]
- Sobrino, A.; Walker, M.E.; Colas, R.A.; Dalli, J. Protective activities of distinct omega-3 enriched oils are linked to their ability to upregulate specialized pro-resolving mediators. PLoS ONE 2020, 15, e0242543. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 freefatty acids suppress macrophage inflammasome activation byinhibiting NF-κB activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef]
- Kulvietis, V.; Zalgeviciene, V.; Dzidziapetrience, J.; Rotomskis, R. Transport of nanoparticles through the placental barier. Tohoku J. Exp. Med. 2011, 225, 225–234. [Google Scholar] [CrossRef]
- Herd, H.; Daum, N.; Jones, A.T.; Huwer, H.; Ghandehari, H.; Leh, C.M. Nanoparticle geometry and surface orientation influences mode of cellular uptake. ACS Nano 2013, 7, 1961–1973. [Google Scholar] [CrossRef]
- Wu, H.; Kong, Y.; Zhao, W.; Wang, F. Measurement of cellular MDA content through MTBE-extraction based TBA assay by eliminating cellular interferences. J. Pharm. Biomed. Anal. 2024, 248, 116332. [Google Scholar] [CrossRef]
- Tanghe, S.; Missotten, J.; Raes, K.; De Smet, S. The effect of different concentrations of linseed oil or fish oil in the maternal diet on the fatty acid composition and oxidative status of sows and piglets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Bosona, T.; Gebresenbet, G. Cluster building and logistics network integration of local food supply chain. Biosyst. Eng. 2011, 108, 293–302. [Google Scholar] [CrossRef]
- Gollini, I.; Lu, B.; Charlton, M.; Brunsdon, C.; Harris, P. GWmodel: An R Package for Exploring Spatial Heterogeneity Using Geographically Weighted Models. arXiv 2013, arXiv:1306.0413. [Google Scholar] [CrossRef]
- Landaeta-Díaz, L.; Salas, D.; Gutiérrez, V. Mapping Nutritional Inequality: A Primary Socio-Spatial Analysis of Food Deserts in Santiago de Chile. Urban Sci. 2024, 8, 129. [Google Scholar] [CrossRef]
- Rogers, E.M. Diffusion of Innovations, 5th ed.; Free Press: New York, NY, USA, 2003. [Google Scholar]
- Willits-Smith, A.; Brenna, J.T.; Moubarac, J.C.; Herforth, A. Demographic and Socioeconomic Correlates of Disproportionate Beef Consumption. Nutrients 2023, 15, 3795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mierau, J.O.; Riphagen, I.J.; Heiner-Fokkema, M.R.; Dekker, L.H.; Navis, G.J.; Bakker, S.J.L. Types of fish consumption differ across socioeconomic strata and impact plasma omega-3 levels. Eur. J. Nutr. 2024, 63, 435–443. [Google Scholar] [CrossRef]
- de Tonnac, A.; Mourot, J. Effect of dietary sources of n-3 fatty acids on pig performance and technological, nutritional and sensory qualities of pork. Animal 2018, 12, 1527–1535. [Google Scholar] [CrossRef]
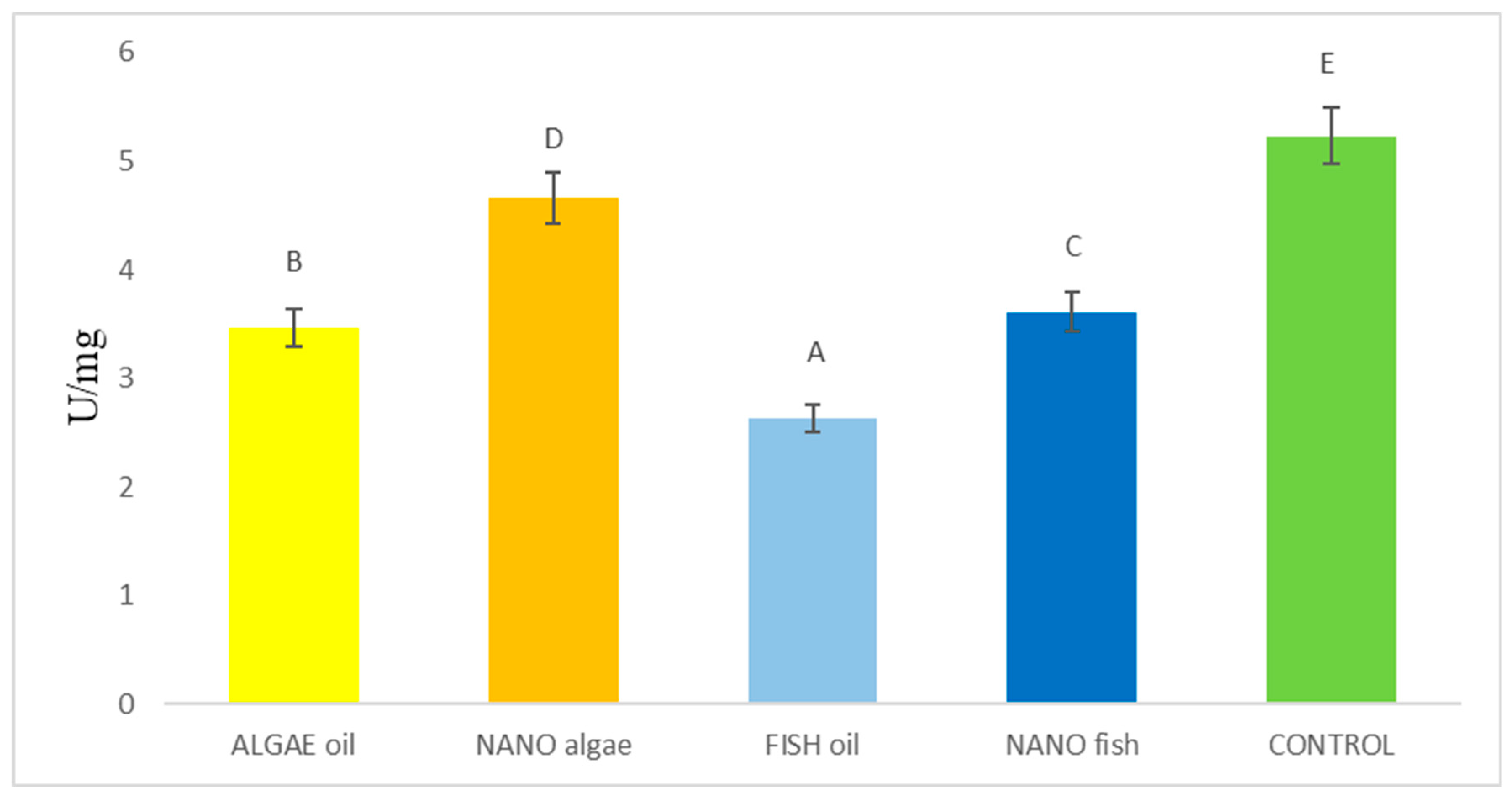
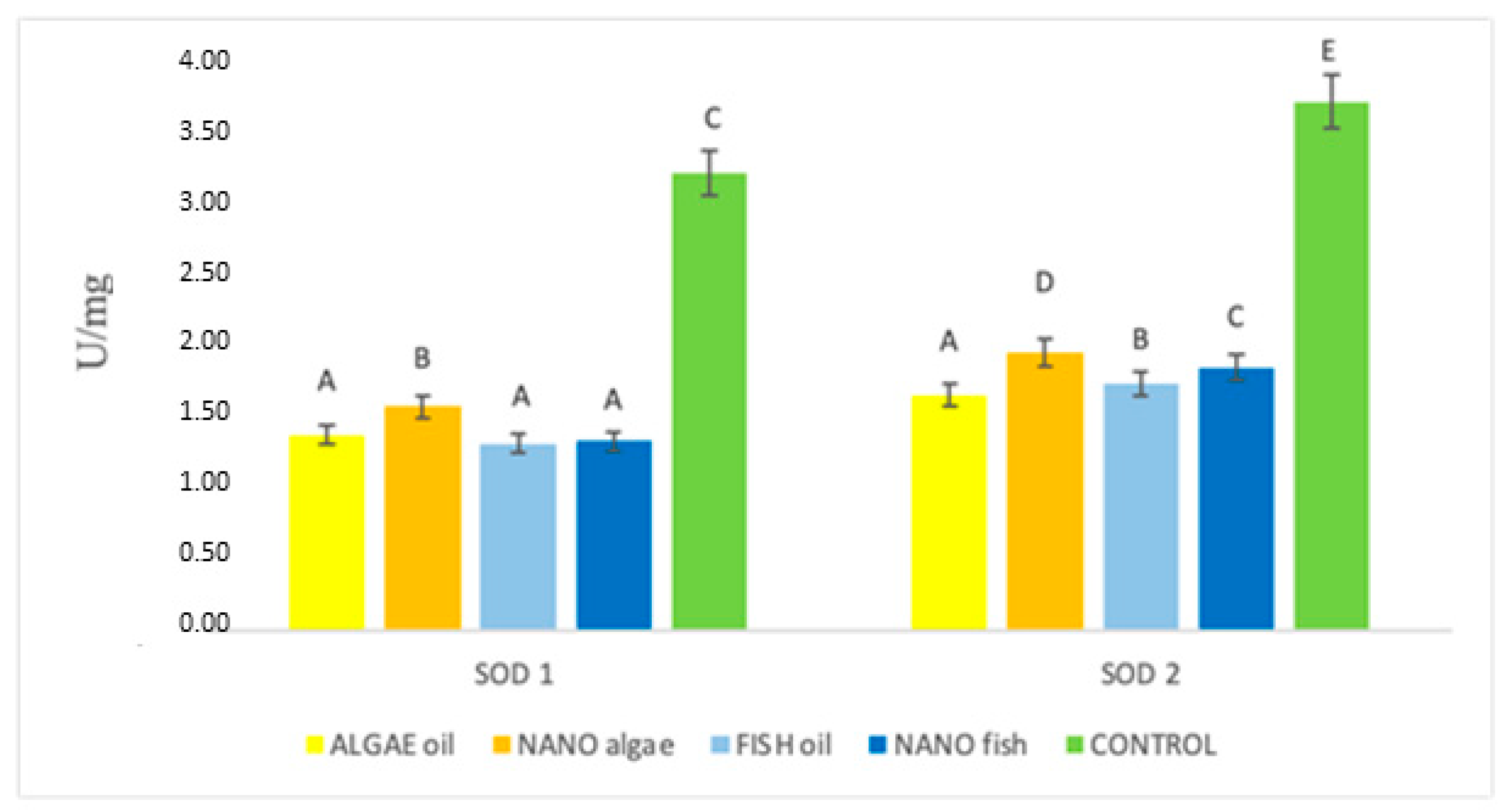
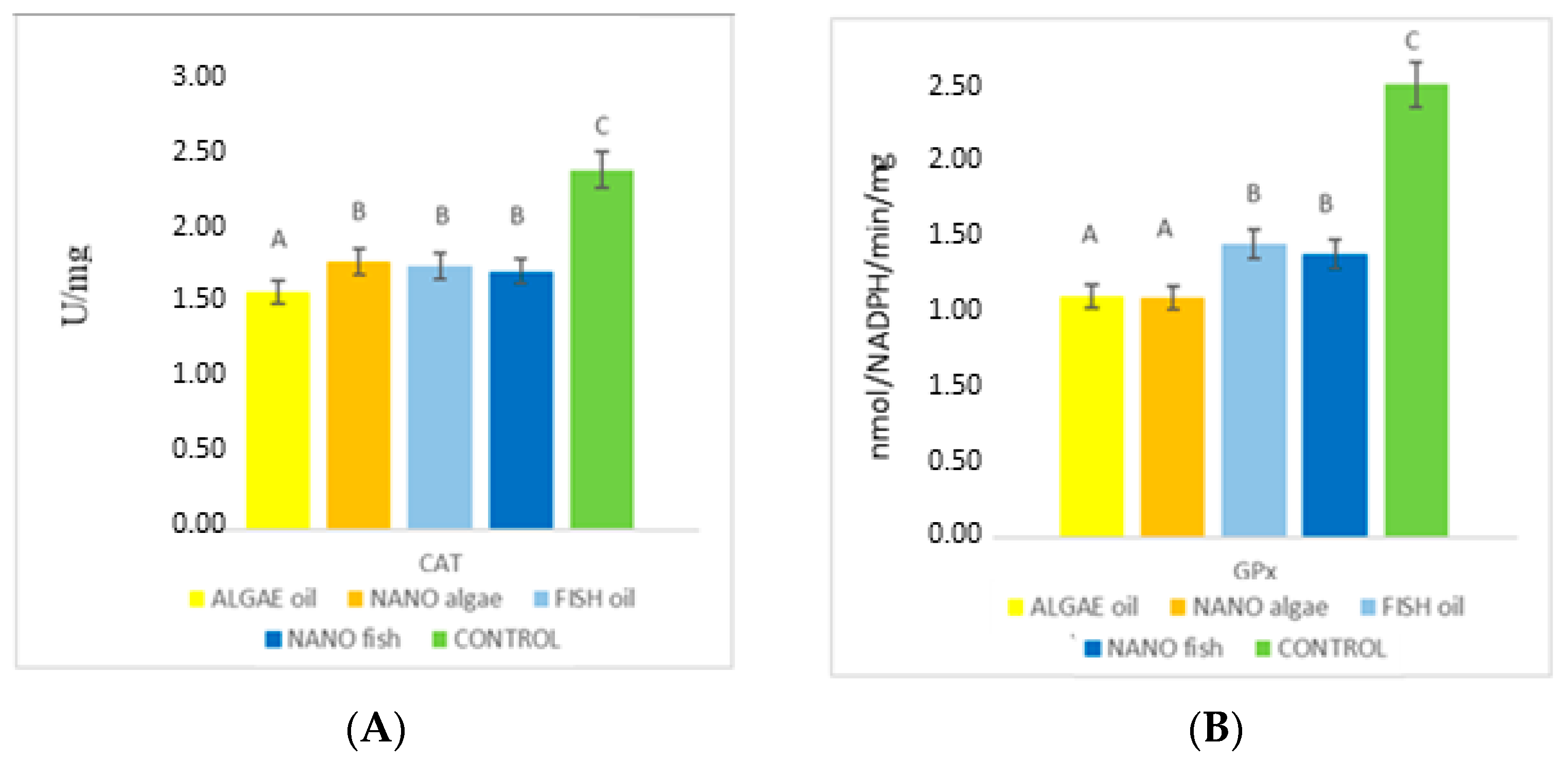
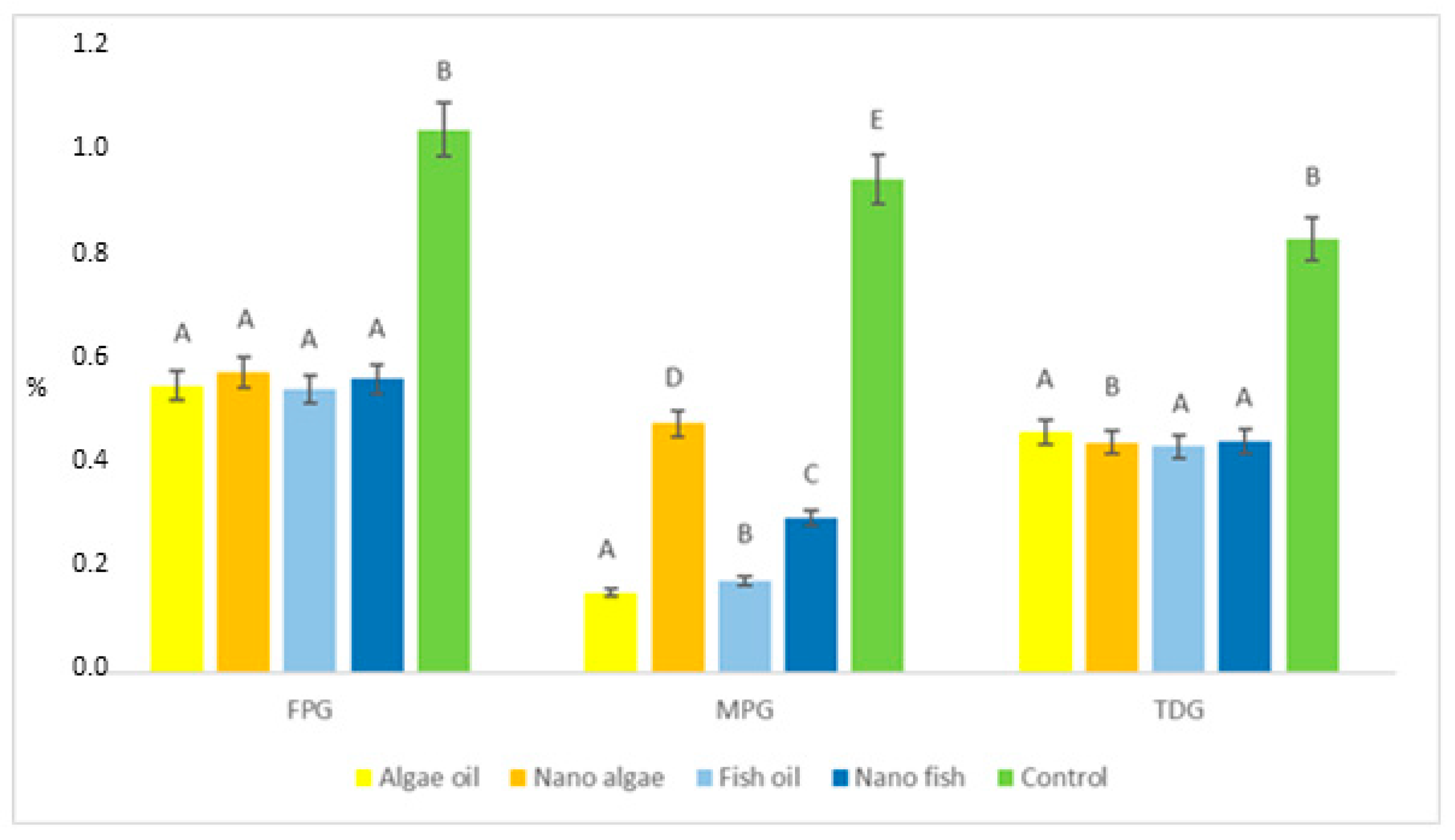
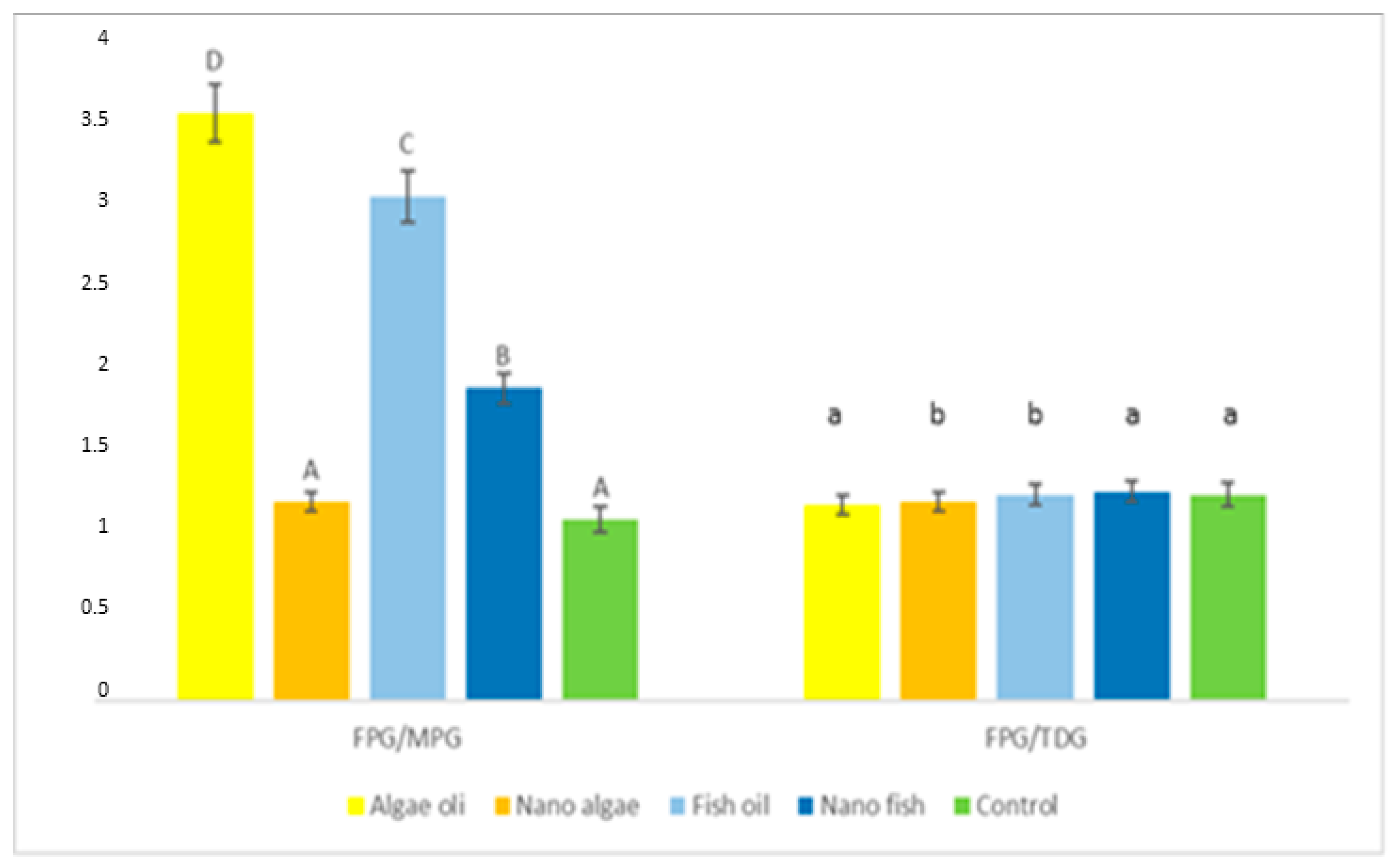
| Antioxidant Enzymes | Algal Oil | Nano Algal | Fish Oil | Nano Fish | Control | SE Pooled |
|---|---|---|---|---|---|---|
| SOD1 (U/mg) | 1.37 A | 1.57 B | 1.31 A | 1.33 A | 3.19 C | 0.038 |
| SOD2 (U/mg) | 1.65 A | 1.94 D | 1.73 B | 1.83 C | 3.74 E | 0.0171 |
| CAT (U/mg) | 1.47 A | 1.66 B | 1.63 B | 1.60 B | 2.23 C | 0.017 |
| GPx (nmol/NADPH/min/mg) | 1.49 A | 1.48 A | 1.81 B | 1.75 B | 2.80 C | 0.031 |
| DNA glycosylases | ||||||
| FPG (fmol/µg of protein/h) | 0.551 A | 0.577 A | 0.544 A | 0.564 A | 1.042 B | 0.0089 |
| MPG (fmol/µg of protein/h) | 0.154 A | 0.478 D | 0.177 B | 0.297 C | 0.947 E | 0.0038 |
| TDG (fmol/µg of protein/h) | 0.462 A | 0.442 A | 0.434 A | 0.444 A | 0.832 B | 0.0114 |
| FPG/MPG | 3.58 | 1.21 A | 3.07 C | 1.90 B | 1.10 A | 0.0326 |
| FPG/TDG | 1.19 | 1.31 | 1.25 | 1.27 | 1.25 | 0.0138 |
| MDA (µg/L) (lipid peroxidation) | 3.46 B | 4.66 D | 2.62 A | 3.61 C | 5.23 E | 0.0179 |
| Item | Day of Pregnancy | |
|---|---|---|
| Till 90 | 90–114 | |
| Nutritional value (per kg diet) | ||
| Crude protein, g | 145 | 175 |
| Lysine, g | 8.0 | 10.0 |
| Methionine | 3.0 | 3.0 |
| Methionine + Cystine | 6.0 | 7.0 |
| Threonine, g | 6.0 | 6.5 |
| Tryptophan, g | 2.0 | 2.0 |
| Crude fibre | 50.0 | 36.0 |
| Calcium, g | 8.0 | 9.0 |
| Total phosphorus | 6.0 | 6.5 |
| Digestible phosphorus, g | 2.0 | 3.0 |
| Vitamin A, I.U | 13,000 | 12,500 |
| Vitamin E, mg | 100 | 100 |
| Vitamin D, I.U. | 2000 | 2000 |
| Metabolizable energy, MJ | 12.0 | 13.0 |
| Ingredient | Norsan Omega-3 Arktis (Per 10 mL) | Norsan Omega-3 Vegan (Per 10 mL) |
|---|---|---|
| Components | ||
| Sunflower oil | - | - |
| Fish oil | 8.8 g | - |
| Algal oil | - | 7.0 g |
| Olive oil | - | 2.2 g |
| Fatty acids’ composition | ||
| SFA | 1.8 g | - |
| MUFA | 3.9 g | - |
| PUFA | 2.3 g | 4.4 g |
| Omega-3 | 2.0 g | 4.0 g |
| EPA | 750 mg | 1218 mg |
| DPA | 95 mg | 314 mg |
| DHA | 975 mg | 2316 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, P.; Sobol, M.; Makulska, J.; Węglarz, A.; Kurylczyk, A.; Skiba, G. Administration of Natural Fish and Algal Oils in Nanoparticle Form to Pregnant Gilts and Newborn Piglets: Biochemical Effects and Spatial–Socio-Economic Implications for Regional Food Systems. Int. J. Mol. Sci. 2025, 26, 9158. https://doi.org/10.3390/ijms26189158
Kowalczyk P, Sobol M, Makulska J, Węglarz A, Kurylczyk A, Skiba G. Administration of Natural Fish and Algal Oils in Nanoparticle Form to Pregnant Gilts and Newborn Piglets: Biochemical Effects and Spatial–Socio-Economic Implications for Regional Food Systems. International Journal of Molecular Sciences. 2025; 26(18):9158. https://doi.org/10.3390/ijms26189158
Chicago/Turabian StyleKowalczyk, Paweł, Monika Sobol, Joanna Makulska, Andrzej Węglarz, Apoloniusz Kurylczyk, and Grzegorz Skiba. 2025. "Administration of Natural Fish and Algal Oils in Nanoparticle Form to Pregnant Gilts and Newborn Piglets: Biochemical Effects and Spatial–Socio-Economic Implications for Regional Food Systems" International Journal of Molecular Sciences 26, no. 18: 9158. https://doi.org/10.3390/ijms26189158
APA StyleKowalczyk, P., Sobol, M., Makulska, J., Węglarz, A., Kurylczyk, A., & Skiba, G. (2025). Administration of Natural Fish and Algal Oils in Nanoparticle Form to Pregnant Gilts and Newborn Piglets: Biochemical Effects and Spatial–Socio-Economic Implications for Regional Food Systems. International Journal of Molecular Sciences, 26(18), 9158. https://doi.org/10.3390/ijms26189158






