Nebulized Hybrid Nanoarchaeosomes: Anti-Inflammatory Activity, Anti-Microbial Activity and Cytotoxicity on A549 Cells
Abstract
1. Introduction
2. Results
2.1. Structural Characterization of BSs
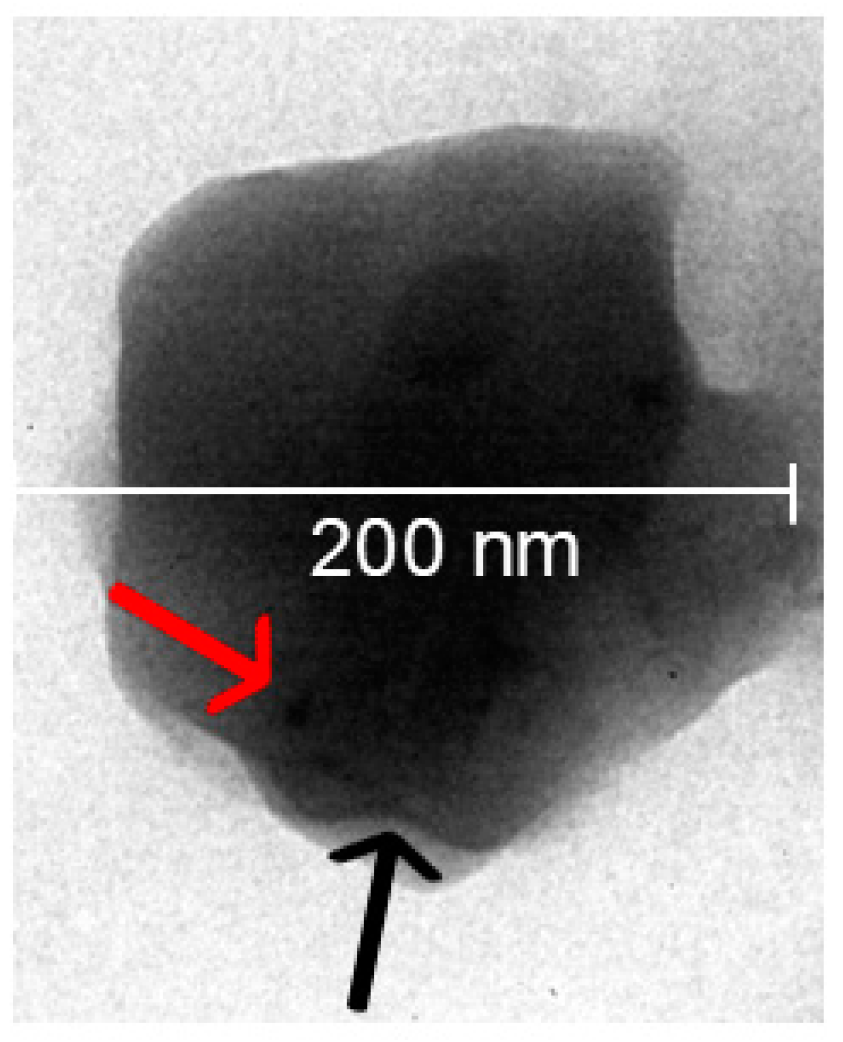
2.2. Structural Characterization of Hybrid nanoARCs
2.3. Cytotoxicity on THP-1 Macrophages and Effect on Lysosomes
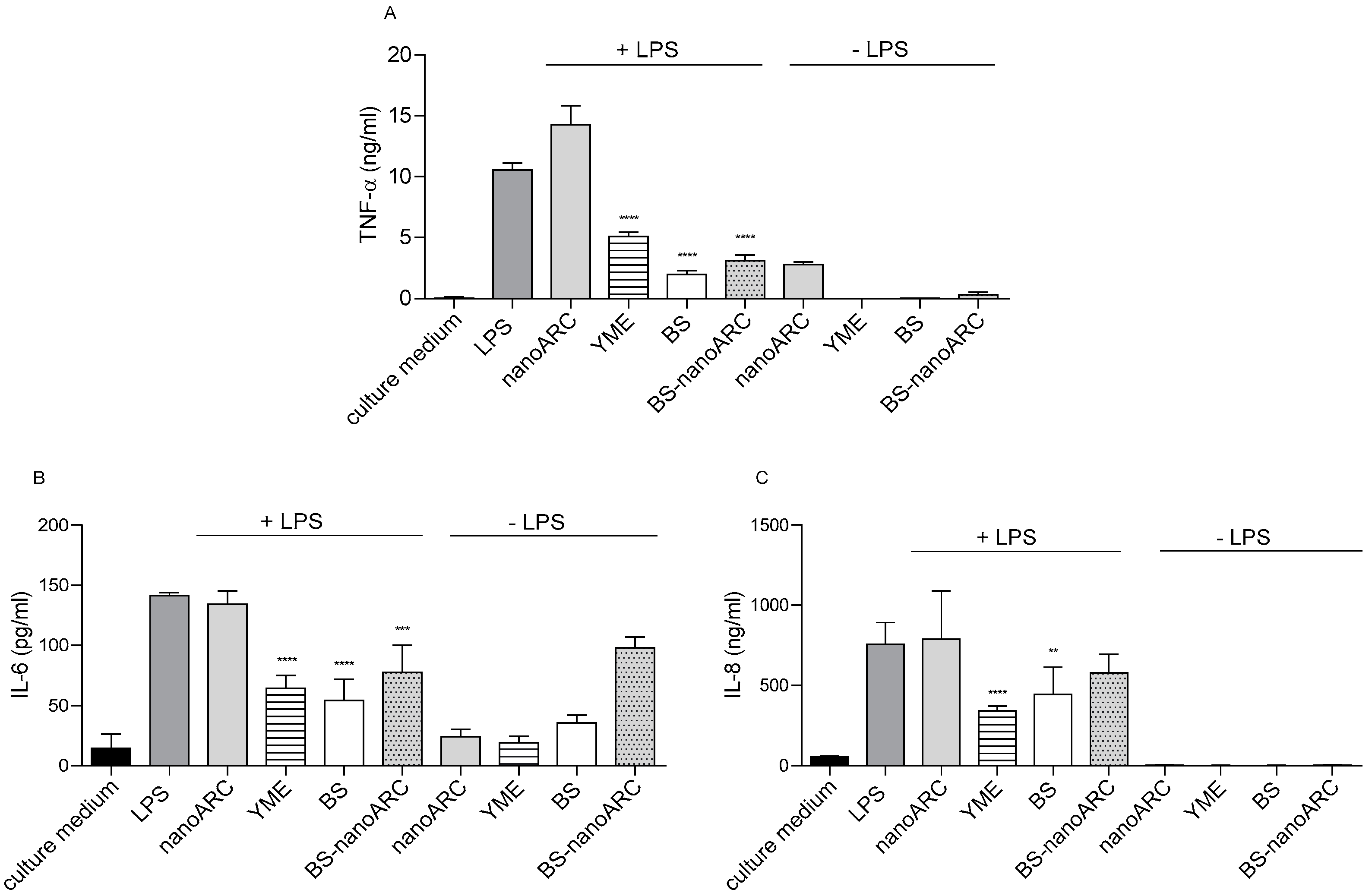
2.4. Anti-Inflammatory and Anti-ROS Activity on THP-1 Macrophages
2.5. Antimicrobial Activity
2.5.1. Anti-Planktonic Activity
2.5.2. Anti-Biofilm Formation Activity
2.5.3. Antifungal Activity
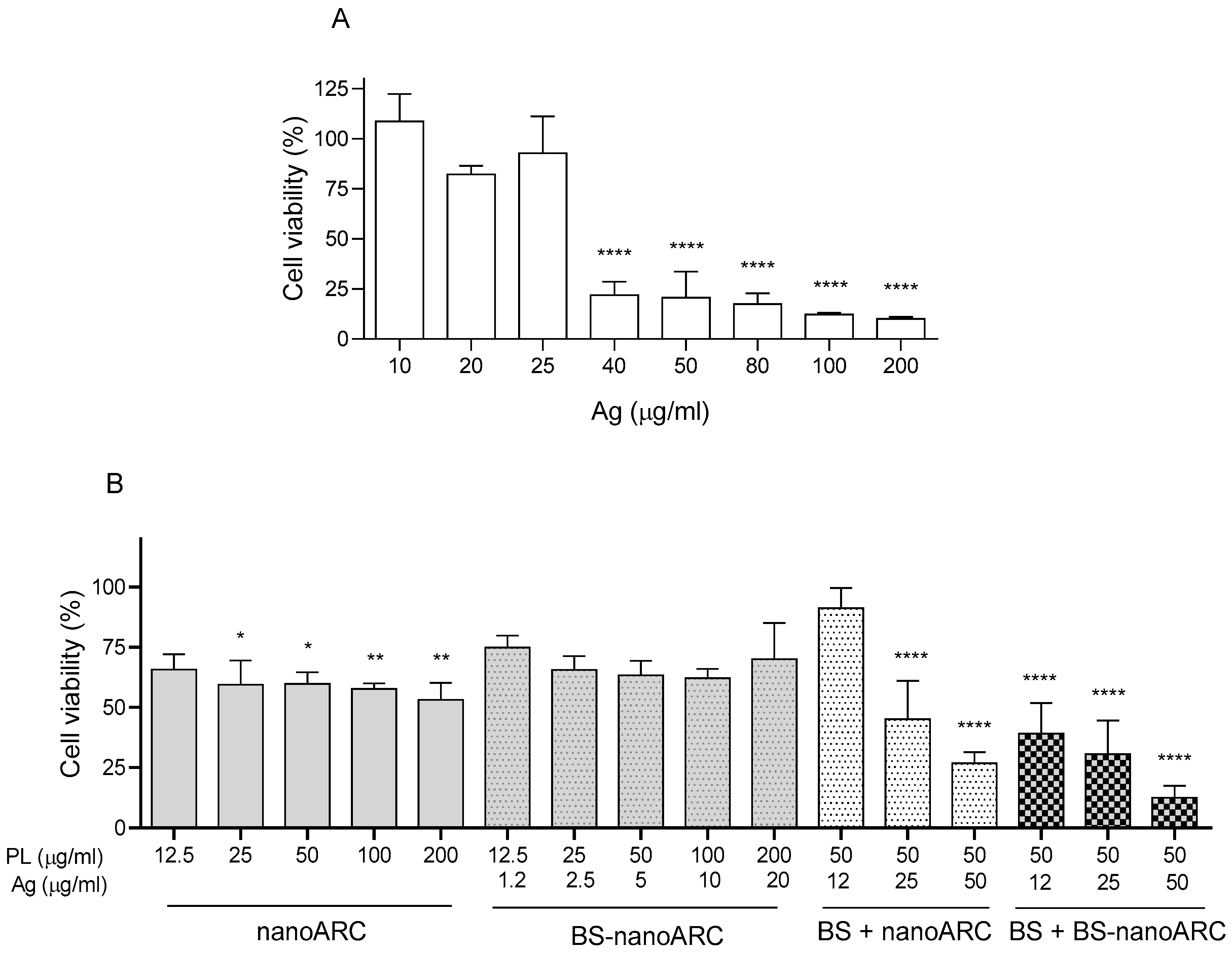
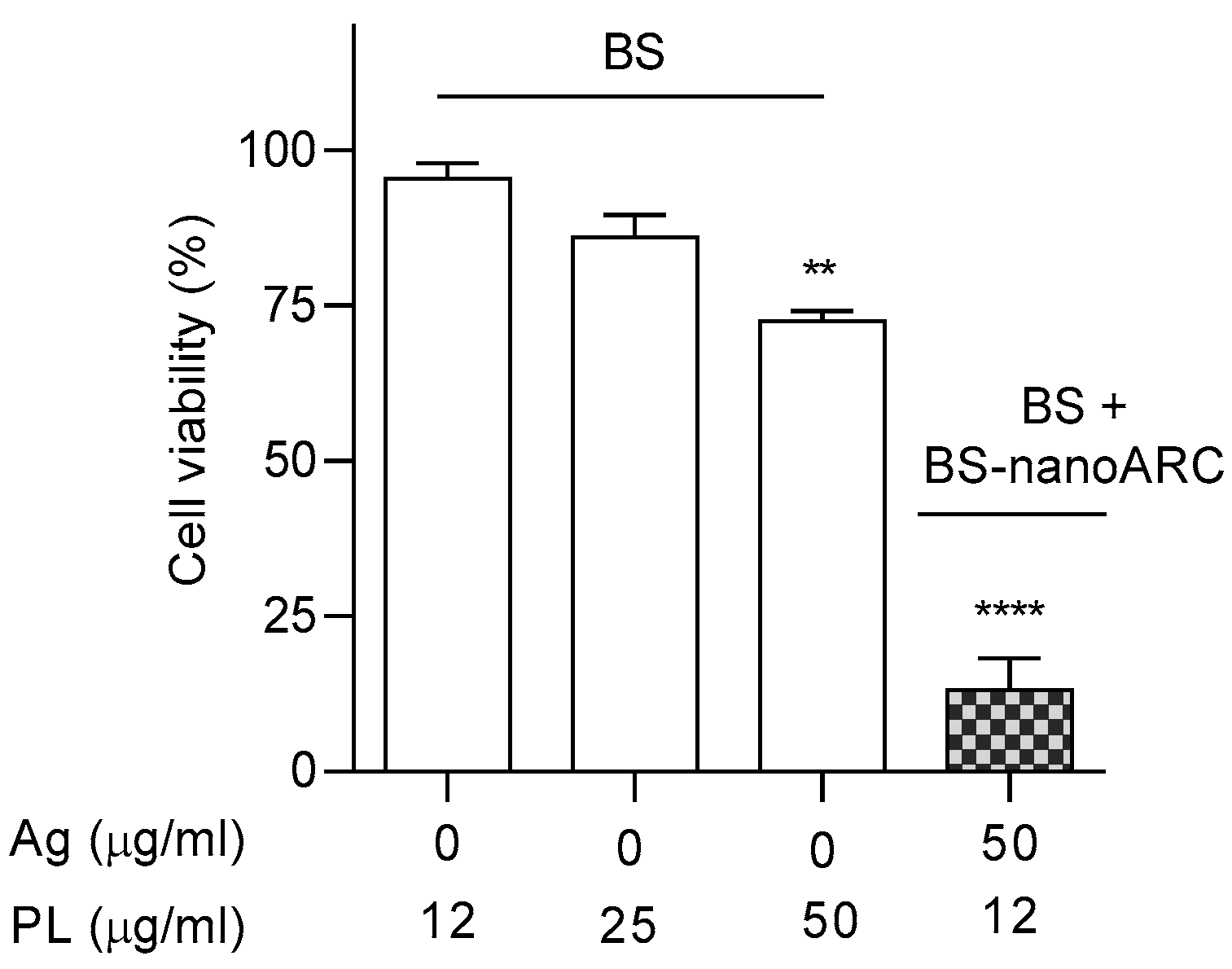
2.6. Cytotoxicity on A549 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Biogenic Silver Nanoparticles (BSs)
4.3. Oxidation State and Quantification of Ag in BSs by X-Ray Absorption
4.4. Raman Spectra of BSs
4.5. Preparation and Structural Characterization of Hybrid nanoARCs
4.6. Antioxidant Activity by ABTS Assay
4.7. Antibacterial Activity
- (i)
- Bacterial strains and growth conditions
- (ii)
- Anti-planktonic activity. Minimum inhibitory concentration (MIC)
- (iii)
- Biofilm-formation inhibition
4.8. Antifungal Activity
- (i)
- Minimum inhibitory concentration (MIC)
- (ii)
- Minimum fungicidal concentration (MFC)
4.9. Cell Lines, Culture Conditions and Cytotoxicity
4.10. Anti-Inflammatory Activity on THP-1 Macrophages
4.11. Intracellular Reactive Oxygen Species (ROS) Production
4.12. Effect on Lysosomes upon Uptake by THP-1 Macrophages and A549 Cells
4.13. Stability to Light, Temperature and Nebulization Stress
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero, E.L.; Morilla, M.J. Ether Lipids from Archaeas in Nano-Drug Delivery and Vaccination. Int. J. Pharm. 2023, 634, 122632. [Google Scholar] [CrossRef]
- Morilla, M.J.; Ghosal, K.; Romero, E.L. More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics 2023, 15, 1828. [Google Scholar] [CrossRef]
- Caimi, A.T.; Yasynska, O.; Rivas Rojas, P.C.; Romero, E.L.; Morilla, M.J. Improved Stability and Biological Activity of Bacterioruberin in Nanovesicles. J. Drug Deliv. Sci. Technol. 2022, 77, 103896. [Google Scholar] [CrossRef]
- Altube, M.J.; Selzer, S.M.; de Farias, M.A.; Portugal, R.V.; Morilla, M.J.; Romero, E.L. Surviving Nebulization-Induced Stress: Dexamethasone in PH-Sensitive Archaeosomes. Nanomedicine 2016, 11, 2103–2117. [Google Scholar] [CrossRef]
- Parra, F.L.; Caimi, A.T.; Altube, M.J.; Cargnelutti, D.E.; Vermeulen, M.E.; de Farias, M.A.; Portugal, R.V.; Morilla, M.J.; Romero, E.L. Make It Simple: (SR-A1+TLR7) Macrophage Targeted NANOarchaeosomes. Front. Bioeng. Biotechnol. 2018, 6, 413650. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Hull, D.R. Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Hu, R.; Yong, K.T.; Roy, I.; Ding, H.; He, S.; Prasad, P.N. Metallic Nanostructures as Localized Plasmon Resonance Enhanced Scattering Probes for Multiplex Dark-Field Targeted Imaging of Cancer Cells. J. Phys. Chem. C 2009, 113, 2676–2684. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Park, T.J.; Ajayan, P.; Kubotera, N.; Mousa, S.A.; Linhardt, R.J. Synthesis of Gold and Silver Nanoparticles Stabilized with Glycosaminoglycans Having Distinctive Biological Activities. Biomacromolecules 2009, 10, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles to Chlamydomonas Reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Silver Nanoparticles: Partial Oxidation and Antibacterial Activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Mateo, E.M.; Jiménez, M. Silver Nanoparticle-Based Therapy: Can It Be Useful to Combat Multi-Drug Resistant Bacteria? Antibiotics 2022, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic Synthesis of Silver Nanoparticles and Their Antioxidant and Antibacterial Activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Wypij, M.; Bonde, S.; Yadav, A.; Kratošová, G.; Golińska, P. Biogenic Silver Nanoparticles: What We Know and What Do We Need to Know? Nanomaterials 2021, 11, 2901. [Google Scholar] [CrossRef]
- Adil, M.; Alam, S.; Amin, U.; Ullah, I.; Khan, T.; Muhammad, M.; Ullah, M.; Rehman, A. AMB Express † Efficient Green Silver Nanoparticles-Antibiotic Combinations against Antibiotic-Resistant Bacteria. AMB Expr. 2023, 13, 115. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Georgia Ștefan, M.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.B.; Garcia-Fossa, F.; Radaic, A.; Durán, N.; Fávaro, W.J.; de Jesus, M.B. Biogenic Silver Nanoparticles: In Vitro and in Vivo Antitumor Activity in Bladder Cancer. Eur. J. Pharm. Biopharm. 2020, 151, 162–170. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, J.; Huang, Z.; Jiang, S.; Gu, N. Colloidal Silver Nanoparticles Improve Anti-Leukemic Drug Efficacy via Amplification of Oxidative Stress. Colloids Surf. B. Biointerfaces 2015, 126, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Islam, F.; Asaduzzaman, A.K.M. Biogenic Silver/Silver Chloride Nanoparticles Inhibit Human Cancer Cells Proliferation in Vitro and Ehrlich Ascites Carcinoma Cells Growth in Vivo. Sci. Reports 2022, 12, 8909. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Khuda, F.; Jamil, M.; Ali Khan Khalil, A.; Ullah, R.; Ullah, N.; Naureen, F.; Abbas, M.; Shafiq Khan, M.; Ali, S.; Muhammad Umer Farooqi, H.; et al. Assessment of Antioxidant and Cytotoxic Potential of Silver Nanoparticles Synthesized from Root Extract of Reynoutria Japonica Houtt. Arab. J. Chem. 2022, 15, 104327. [Google Scholar] [CrossRef]
- Lanoix, J.-P.; Pluquet, E.; Lescure, F.X.; Bentayeb, H.; Lecuyer, E.; Boutemy, M.; Dumont, P.; Jounieaux, V.; Schmit, J.L.; Dayen, C.; et al. Bacterial Infection Profiles in Lung Cancer Patients with Febrile Neutropenia. BMC Infect. Dis. 2011, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Dumont-Leblond, N.; Veillette, M.; Racine, C.; Joubert, P.; Duchaine, C. Non-Small Cell Lung Cancer Microbiota Characterization: Prevalence of Enteric and Potentially Pathogenic Bacteria in Cancer Tissues. PLoS ONE 2021, 16, e0249832. [Google Scholar] [CrossRef]
- Wenzler, E.; Fraidenburg, D.R.; Scardina, T.; Danziger, L.H. Inhaled Antibiotics for Gram-Negative Respiratory Infections. Clin. Microbiol. Rev. 2016, 29, 581–632. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A. Pre-Clinical Immunotoxicity Studies of Nanotechnology-Formulated Drugs: Challenges, Considerations and Strategy. J. Control. Release 2015, 220, 571–583. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, T.; Artzi, N.; Balyasnikova, I.V.; Barenholz, Y.; La-Beck, N.M.; Brenner, J.S.; Chan, W.C.W.; Decuzzi, P.; Exner, A.A.; Gabizon, A.; et al. Mechanisms and Barriers in Nanomedicine: Progress in the Field and Future Directions. ACS Nano 2024, 18, 13983–13999. [Google Scholar] [CrossRef]
- Giordani, S.; Marassi, V.; Zattoni, A.; Roda, B.; Reschiglian, P. Liposomes Characterization for Market Approval as Pharmaceutical Products: Analytical Methods, Guidelines and Standardized Protocols. J. Pharm. Biomed. Anal. 2023, 236, 115751. [Google Scholar] [CrossRef]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Luiz, H.; Oliveira Pinho, J.; Gaspar, M.M. Advancing Medicine with Lipid-Based Nanosystems—The Successful Case of Liposomes. Biomedicines 2023, 11, 435. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, X.; Shang, M.; Niu, S.; Zhang, W.; Zhang, B.; Huang, W.; Wu, T.; Zhang, T.; Tang, M.; et al. Mitophagy–Lysosomal Pathway Is Involved in Silver Nanoparticle-Induced Apoptosis in A549 Cells. Ecotoxicol. Environ. Saf. 2021, 208, 111463. [Google Scholar] [CrossRef]
- Surface Enhanced Raman Scattering; Chang, R.K., Furtak, T.E., Eds.; Springer: New York, NY, USA, 1982. [Google Scholar]
- Maiti, N.; Tulsi; Kapoor, S.; Tulsi, M. Surface-Enhanced Raman Scattering (SERS) Spectroscopy for Trace Level Detection of Chlorogenic Acid. Adv. Mater. Lett. 2013, 4, 502–506. [Google Scholar] [CrossRef]
- Saravanan, M.; Nanda, A.; Kingsley, S.J. Lactobacillus Delbrueckii Mediated Synthesis of Silver Nanoparticles and Their Evaluation of Antibacterial Efficacy against MDR Clinical Pathogens. In Proceedings of the International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), Chennai, Tamilnadu, India, 28–30 November 2011; pp. 386–390. [Google Scholar] [CrossRef]
- Selvaraj, A. Extracellular Biosynthesis and Biomedical Application of Silver Nanoparticles Synthesized from Baker’s Yeast. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 822–828. [Google Scholar]
- Bothun, G.D. Hydrophobic Silver Nanoparticles Trapped in Lipid Bilayers: Size Distribution, Bilayer Phase Behavior, and Optical Properties. J. Nanobiotechnol. 2008, 6, 13. [Google Scholar] [CrossRef]
- Chikte, S.; Panchal, N.; Warnes, G. Use of LysoTracker Dyes: A Flow Cytometric Study of Autophagy. Cytom. Part A 2014, 85, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Leist, M.; Gores, G.J. Lysosomes in Cell Death. Oncogene 2004, 23, 2881–2890. [Google Scholar] [CrossRef]
- Oberle, C.; Huai, J.; Reinheckel, T.; Tacke, M.; Rassner, M.; Ekert, P.G.; Buellesbach, J.; Borner, C. Lysosomal Membrane Permeabilization and Cathepsin Release Is a Bax/Bak-Dependent, Amplifying Event of Apoptosis in Fibroblasts and Monocytes. Cell Death Differ. 2010, 17, 1167–1178. [Google Scholar] [CrossRef]
- Wang, R.; Billone, P.S.; Mullett, W.M. Nanomedicine in Action: An Overview of Cancer Nanomedicine on the Market and in Clinical Trials. J. Nanomater. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Jerez, H.E.; Altube, M.J.; Gándola, Y.B.; González, L.; González, M.C.; Morilla, M.J.; Romero, E.L. Macrophage Apoptosis Using Alendronate in Targeted Nanoarchaeosomes. Eur. J. Pharm. Biopharm. 2021, 160, 42–54. [Google Scholar] [CrossRef]
- Sader, H.S.; Huband, M.D.; Castanheira, M.; Flamm, R.K. Pseudomonas aeruginosa antimicrobial susceptibility results from four years (2012 to 2015) of the International Network for Optimal Resistance Monitoring program in the United States. Antimicrob. Agents Chemother. 2017, 61, e02252-16. [Google Scholar] [CrossRef]
- Iglesias, Y.D.; Wilms, T.; Vanbever, R.; Van Bambeke, F. Activity of antibiotics against Staphylococcus aureus in an in vitro model of biofilms in the context of cystic fibrosis: Influence of the culture medium. Antimicrob. Agents Chemother. 2019, 63, e00602-19. [Google Scholar] [CrossRef] [PubMed]
- Altube, M.J.; Caimi, L.I.; Huck-Iriart, C.; Morilla, M.J.; Romero, E.L. Reparation of an Inflamed Air-Liquid Interface Cultured A549 Cells with Nebulized Nanocurcumin. Pharmaceutics 2021, 13, 1331. [Google Scholar] [CrossRef]
- Dailey, L.A.; Schmehl, T.; Gessler, T.; Wittmar, M.; Grimminger, F.; Seeger, W.; Kissel, T. Nebulization of Biodegradable Nanoparticles: Impact of Nebulizer Technology and Nanoparticle Characteristics on Aerosol Features. J. Control. Release 2003, 86, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Altube, M.J.; Perez, N.; Romero, E.L.; Morilla, M.J.; Higa, L.H.; Perez, A.P. Inhaled Lipid Nanocarriers for Pulmonary Delivery of Glucocorticoids: Previous Strategies, Recent Advances and Key Factors Description. Int. J. Pharm. 2023, 642, 123146. [Google Scholar] [CrossRef]
- Corcelli, A.; Lobasso, S. 25 Characterization of Lipids of Halophilic Archaea. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 35, pp. 585–613. [Google Scholar]
- Higa, L.H.; Jerez, H.E.; De Farias, M.A.; Portugal, R.V.; Romero, E.L.; Morilla, M.J. Ultra-Small Solid Archaeolipid Nanoparticles for Active Targeting to Macrophages of the Inflamed Mucosa. Nanomedicine 2017, 12, 1165–1175. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Ibar, A.; Tuttolomondo, M.V.; Desimone, M.F. Dual-Effect Core–Shell Polyphenol Coated Silver Nanoparticles for Tissue Engineering. Nano-Struct. Nano-Objects 2021, 26, 100716. [Google Scholar] [CrossRef]
- Arreche, R.A.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R.; Vázquez, P.G. Synthesis of Silver Nanoparticles Using Extracts from Yerba Mate (Ilex paraguariensis) Wastes. Waste Biomass Valorization 2020, 11, 245–253. [Google Scholar] [CrossRef]
- Gordon-Falconí, C.; Iannone, M.F.; Zawoznik, M.S.; Cumbal, L.; Debut, A.; Groppa, M.D. Synthesis of Silver Nanoparticles with Remediative Potential Using Discarded Yerba Mate: An Eco-Friendly Approach. J. Environ. Chem. Eng. 2020, 8, 104425. [Google Scholar] [CrossRef]
- Vanin dos Santos Lima, M.; Beloni de Melo, G.; Gracher Teixeira, L.; Grella Miranda, C.; Hermes de Araújo, P.H.; Sayer, C.; Porto Ineu, R.; Leimann, F.V.; Hess Gonçalves, O. Green Synthesis of Silver Nanoparticles Using Ilex paraguariensis Extracts: Antimicrobial Activity and Acetilcolinesterase Modulation in Rat Brain Tissue. Green Chem. Lett. Rev. 2022, 15, 126–136. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and Genotoxicity of Silver Nanoparticles in the Human Lung Cancer Cell Line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, T.; Matsuoka, M. Involvement of Lysosomal Dysfunction in Silver Nanoparticle-Induced Cellular Damage in A549 Human Lung Alveolar Epithelial Cells. J. Occup. Med. Toxicol. 2016, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peiser, L.; Mukhopadhyay, S.; Gordon, S. Scavenger Receptors in Innate Immunity. Curr. Opin. Immunol. 2002, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Brophy, A.; Gorey, B.; Casey, A. Liposomal Encapsulation of Silver Nanoparticles Enhances Cytotoxicity and Causes Induction of Reactive Oxygen Species-Independent Apoptosis. J. Appl. Toxicol. 2018, 38, 616–627. [Google Scholar] [CrossRef]
- De Winther, M.P.J.; Van Dijk, K.W.; Havekes, L.M.; Hofker, M.H. Macrophage Scavenger Receptor Class A: A Multifunctional Receptor in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Altube, M.J.; Cutro, A.; Bakas, L.; Morilla, M.J.; Disalvo, E.A.; Romero, E.L. Nebulizing Novel Multifunctional Nanovesicles: The Impact of Macrophage-Targeted-PH-Sensitive Archaeosomes on a Pulmonary Surfactant. J. Mater. Chem. B 2017, 5, 8083–8095. [Google Scholar] [CrossRef]
- Mbanga, O.; Cukrowska, E.; Gulumian, M. Dissolution Kinetics of Silver Nanoparticles: Behaviour in Simulated Biological Fluids and Synthetic Environmental Media. Toxicol. Reports 2022, 9, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Casey, A. Surface Modification of Silver Nanoparticle (AgNP) by Liposomal Encapsulation Mitigates AgNP-Induced Inflammation. Toxicol. Vitr. 2019, 61, 104641. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Cheung, S.O.F.; Huang, L.; Niu, J.; Tao, C.; Ho, C.M.; Che, C.M.; Tam, P.K.H. Further Evidence of the Anti-Inflammatory Effects of Silver Nanoparticles. ChemMedChem 2009, 4, 1129–1135. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D. Silver Nanoparticle-induced Oxidative Stress, Genotoxicity and Apoptosis in Cultured Cells and Animal Tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the Antioxidant Capacity of Natural Products: A Review on Chemical and Cellular-Based Assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Labrada, A.G.; Isla, D.; Artal, A.; Arias, M.; Rezusta, A.; Pardo, J.; Gálvez, E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends in Cancer 2020, 6, 86–97. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Maynes, M.; Silver, S. Effects of Halides on Plasmid-Mediated Silver Resistance in Escherichia Coli. Appl. Environ. Microbiol. 1998, 64, 5042–5045. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Yuan, X.; Xie, J.; Tai, L.D. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells. Biomaterials 2014, 35, 6707–6715. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.-J.; Choi, J. p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ. Sci. Technol. 2010, 44, 8337–8342. [Google Scholar] [CrossRef]
- Homayouni-Tabrizi, M.; Soltani, M.; Karimi, E.; Namvar, F.; Pouresmaeil, V.; Es-haghi, A. Putative mechanism for anticancer properties of Ag–PP (NPs) extract. IET Nanobiotechnol. 2019, 13, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Bethu, M.S.; Netala, V.R.; Domdi, L.; Tartte, V.; Janapala, V.R. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: An insight into the mechanism. Artif. Cell Nanomed. B 2018, 46, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Bar, L.; Porcar, L.; Gerelli, Y.; Losada-Pérez, P. Resolving the interactions between hydrophilic CdTe quantum dots and positively charged membranes at the nanoscale. J. Colloid Interf. Sci. 2025, 677, 620. [Google Scholar] [CrossRef]
- Anabousi, S.; Kleemann, E.; Bakowsky, U.; Kissel, T.; Schmehl, T.; Gessler, T.; Seeger, W.; Lehr, C.-M.; Ehrhardt, C. Effect of PEGylation on the Stability of Liposomes During Nebulisation and in Lung Surfactant. J. Nanosc. Nanotech. 2006, 6, 3010–3016. [Google Scholar] [CrossRef]
- Zhulina, E.; Borisov, O.V.; Pryamitsyn, V.A. Theory of steric stabilization of colloid dispersions by grafted polymers. J. Colloid Interf. Sci. 1990, 137, 495–511. [Google Scholar] [CrossRef]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized Silver Nanoparticles: Advances and Prospects. Colloids Surfaces B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid Biosynthesis of Silver Nanoparticles from Bacillus Megaterium (NCIM 2326) and Their Antibacterial Activity on Multi Drug Resistant Clinical Pathogens. Colloids Surfaces B Biointerfaces 2011, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- A Rapid and Sensitive Sub-Micro Phosphorus Determination—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=0b19d136-425d-49e6-ae53-139b615bc95a (accessed on 7 July 2024).
- Šesták, Z.; Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Handbook. Photosynthetica 2004, 42, 186. [Google Scholar] [CrossRef]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of Laurdan Fluorescence Intensity and Polarization to Distinguish between Changes in Membrane Fluidity and Phospholipid Order. Biochim. Biophys. Acta Biomembr. 2002, 1565, 123–128. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, 10–1128. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical Characterization of Lycopene-Loaded Nanostructured Lipid Carrier Formulations for Topical Administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Altube, M.J.; Martínez, M.M.B.; Malheiros, B.; Maffía, P.C.; Barbosa, L.R.S.; Morilla, M.J.; Romero, E.L. Fast Biofilm Penetration and Anti-PAO1 Activity of Nebulized Azithromycin in Nanoarchaeosomes. Mol. Pharm. 2020, 17, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Higa, L.H.; Schilrreff, P.; Briski, A.M.; Jerez, H.E.; de Farias, M.A.; Villares Portugal, R.; Romero, E.L.; Morilla, M.J. Bacterioruberin from Haloarchaea plus Dexamethasone in Ultra-Small Macrophage-Targeted Nanoparticles as Potential Intestinal Repairing Agent. Colloids Surf. B Biointerfaces 2020, 191, 110961. [Google Scholar] [CrossRef] [PubMed]
- Bracesco, N.; Sanchez, A.G.; Contreras, V.; Menini, T.; Gugliucci, A. Recent Advances on Ilex paraguariensis Research: Minireview. J. Ethnopharmacol. 2011, 136, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.I.; de Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A Comprehensive Review on Chemistry, Health Implications, and Technological Considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef]
- El-Sawalhi, S.; Fayad, E.; Porras, G.; Fayad, A.A.; Abdel-Massih, R.M. The Antibacterial Activity of Libanstin from Ilex paraguariensis (Yerba Mate). Fitoterapia 2021, 153, 104962. [Google Scholar] [CrossRef] [PubMed]
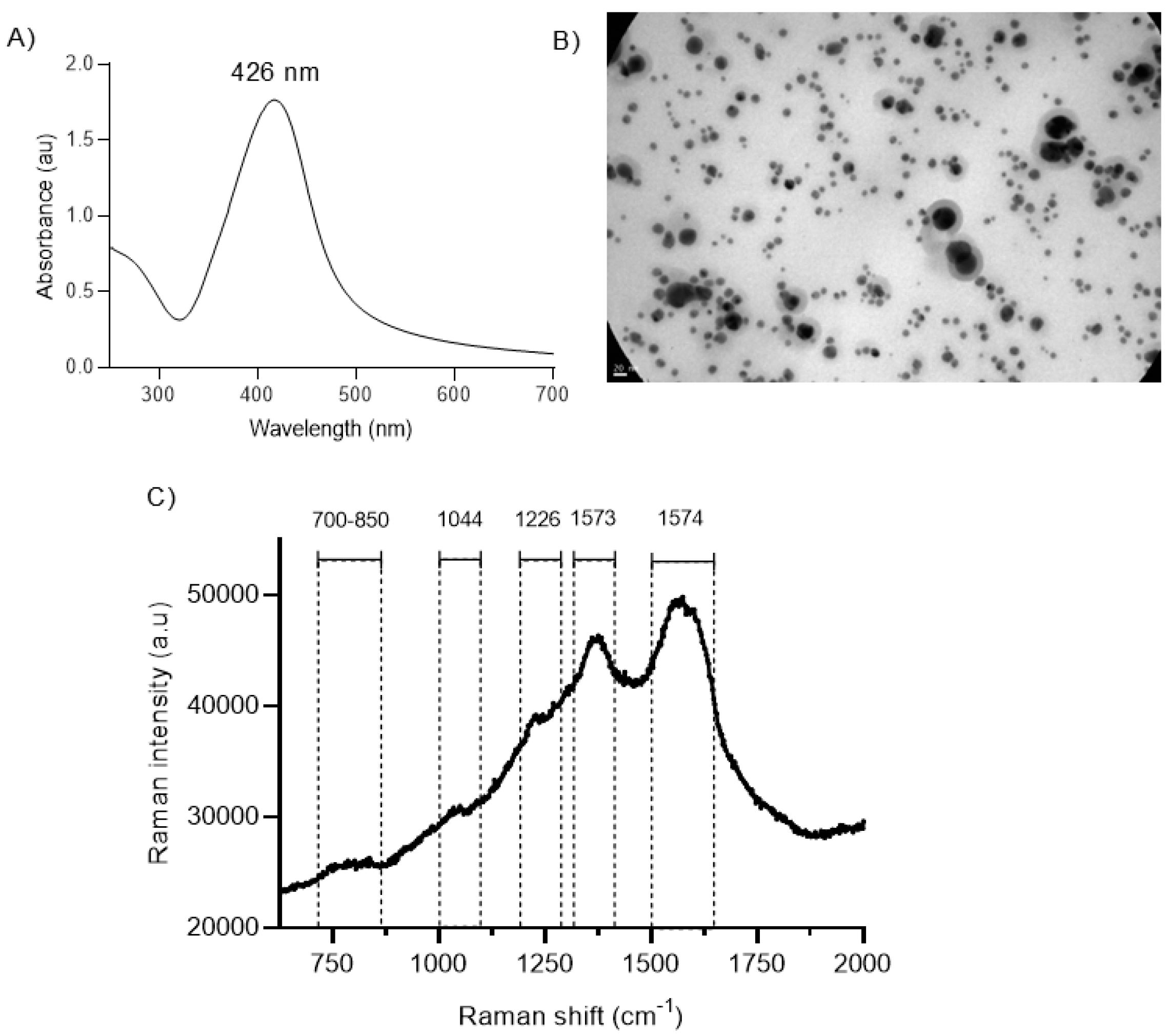
 ; lipid bilayer:
; lipid bilayer:  .
.

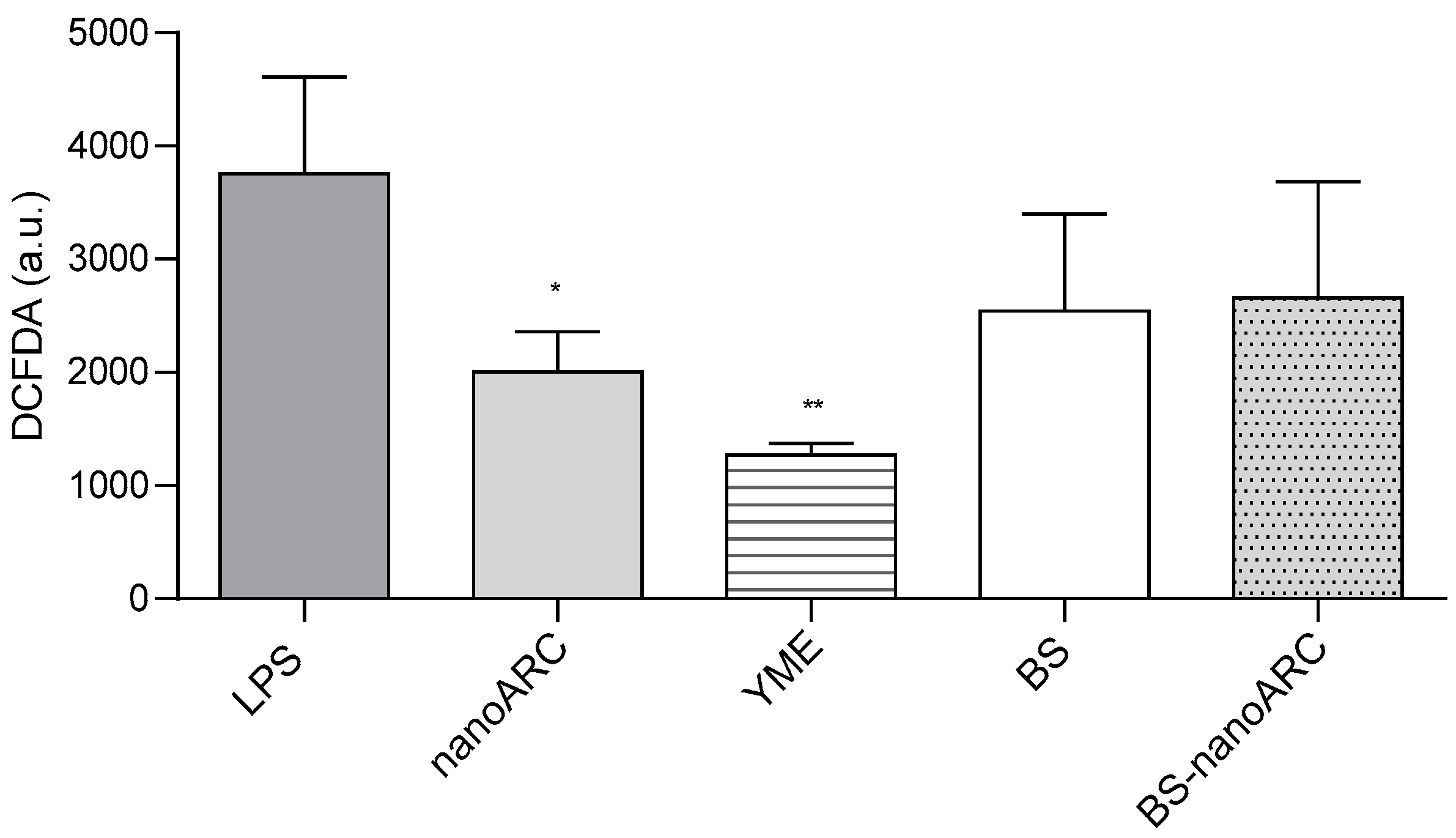

| Formulation | Z-Average (nm ± SD) | PDI ± SD | ζ Potential (mV ± SD) | GP | FA |
|---|---|---|---|---|---|
| BS | 78 ± 43 | 0.44 ± 0.07 | −28 ± 1.0 | ||
| nanoARC | 444 ± 206 | 0.58 ± 0.13 | −55 ± 3.2 | −0.06 | 0.21 |
| BS-nanoARC | 95 ± 37 | 0.61 ± 0.16 | −58 ± 5.5 | −0.26 | 0.21 |
| [BS + BS-nanoARC] | 176 ± 50 | 0.47 | −38 ± 0.6 |
| Formulation | IC50 (μg/mL) | ||
|---|---|---|---|
| Ag | YME | PL | |
| BS | 5 ± 1 | - | - |
| BS-nanoARC | 4 ± 2 | - | 24 ± 17 |
| YME | - | 21 ± 19 | - |
| nanoARC | - | - | >58 ± 37 |
| Formulation | IC50 (μg/mL) | ||
|---|---|---|---|
| Ag | YME | PL | |
| BS | 21.7 | - | - |
| YME | - | >160 | - |
| BS-nanoARC | 6.9 | - | 70 |
| nanoARC | - | - | 133.2 |
| Formulation | MIC (μg/mL) | MBDC (μg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | PAO1 | S. aureus | PAO1 | |||||||||
| Ag | YME | PL | Ag | YME | PL | Ag | YME | PL | Ag | YME | PL | |
| BS | 12.5 | - | - | 6.4 | - | - | 6 | - | - | 7.5 | - | - |
| BS-nanoARC | 13 | - | 130 | 50 | - | 500 | 83 | - | 830 | 83 | - | 830 |
| YME | - | 4500 | - | - | 4500 | - | - | 2300 | - | - | 2300 | - |
| nanoARC | - | - | >1000 | - | - | >1000 | - | - | >1000 | - | - | >250 |
| Formulation | MIC (μg/mL) | MFC (μg/mL) | ||||
|---|---|---|---|---|---|---|
| Ag | YME | PL | Ag | YME | PL | |
| BS | 12.5 | - | - | 25 | - | - |
| BS-nanoARC | 50 | - | 500 | >100 | - | >1000 |
| YME | - | >1000 | - | - | >1000 | - |
| nanoARC | - | - | >1000 | - | - | >1000 |
| Formulation | IC50 (μg/mL) | ||
|---|---|---|---|
| Ag | YME | PL | |
| BS | 35.9 | - | - |
| YME | - | >160 | - |
| BS-nanoARC | 20 | - | 200 |
| [BS + BS-nanoARC] | 12 | - | 50 |
| nanoARC | - | - | >200 |
| Formulation | Days of Storage | Nebulization | Z-Average (nm) | PDI | ζ Potential (mV) | ABTS IC50 (μg Ag/mL) | % PL Recuperation After Nebulization |
|---|---|---|---|---|---|---|---|
| BS | 0 | − | 59 | 0.452 | −34.9 | 7 | − |
| 30 | − | 74 | 0.493 | −35.1 | 7 | − | |
| 0 | + | 56 | 0.428 | −25.6 | − | − | |
| 30 | + | 59.95 | 0.929 | −25.3 | − | − | |
| BS-nanoARC | 0 | − | 68 | 0.436 | −44 | 6.1 | − |
| 30 | − | 144.6 | 0.722 | −65.4 | 6.4 | − | |
| 0 | + | 79 | 0.389 | −42.1 | − | 40 | |
| 30 | + | >1000 | 1 | −38 | − | 40 | |
| [BS + BS-nanoARC] | 0 | − | 190 | 0.451 | −39 | 7 | − |
| 30 | − | 125.4 | 0.506 | −66.2 | 6.5 | − | |
| 0 | + | 200 | 0.422 | −32.5 | − | 30 | |
| 30 | + | >1000 | 1 | −66.2 | − | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabile, S.G.G.; Perez, N.; Jerez, H.E.; Simioni, Y.R.; Butassi, E.; Mizrahi, M.D.; Nobile, M.L.; Perez, A.P.; Morilla, M.J.; Higa, L.H.; et al. Nebulized Hybrid Nanoarchaeosomes: Anti-Inflammatory Activity, Anti-Microbial Activity and Cytotoxicity on A549 Cells. Int. J. Mol. Sci. 2025, 26, 392. https://doi.org/10.3390/ijms26010392
Stabile SGG, Perez N, Jerez HE, Simioni YR, Butassi E, Mizrahi MD, Nobile ML, Perez AP, Morilla MJ, Higa LH, et al. Nebulized Hybrid Nanoarchaeosomes: Anti-Inflammatory Activity, Anti-Microbial Activity and Cytotoxicity on A549 Cells. International Journal of Molecular Sciences. 2025; 26(1):392. https://doi.org/10.3390/ijms26010392
Chicago/Turabian StyleStabile, Sofia Giuliana Guerin, Noelia Perez, Horacio Emanuel Jerez, Yamila Roxana Simioni, Estefanía Butassi, Martin Daniel Mizrahi, Matias Leonardo Nobile, Ana Paula Perez, Maria Jose Morilla, Leticia Herminia Higa, and et al. 2025. "Nebulized Hybrid Nanoarchaeosomes: Anti-Inflammatory Activity, Anti-Microbial Activity and Cytotoxicity on A549 Cells" International Journal of Molecular Sciences 26, no. 1: 392. https://doi.org/10.3390/ijms26010392
APA StyleStabile, S. G. G., Perez, N., Jerez, H. E., Simioni, Y. R., Butassi, E., Mizrahi, M. D., Nobile, M. L., Perez, A. P., Morilla, M. J., Higa, L. H., & Romero, E. L. (2025). Nebulized Hybrid Nanoarchaeosomes: Anti-Inflammatory Activity, Anti-Microbial Activity and Cytotoxicity on A549 Cells. International Journal of Molecular Sciences, 26(1), 392. https://doi.org/10.3390/ijms26010392







