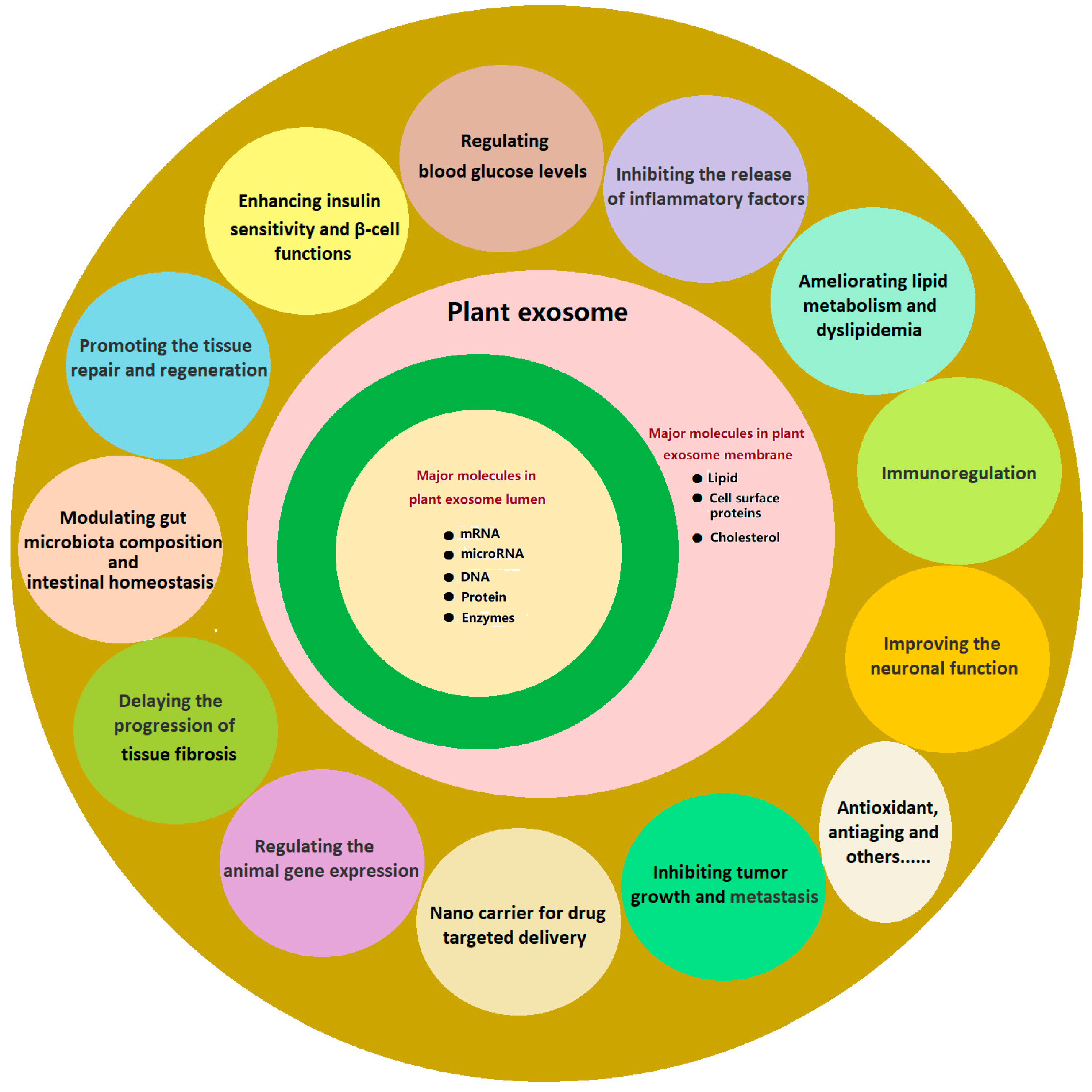
Exosome-like Nanoparticles Extracted from Plant Cells for Diabetes Therapy
Abstract
1. Introduction
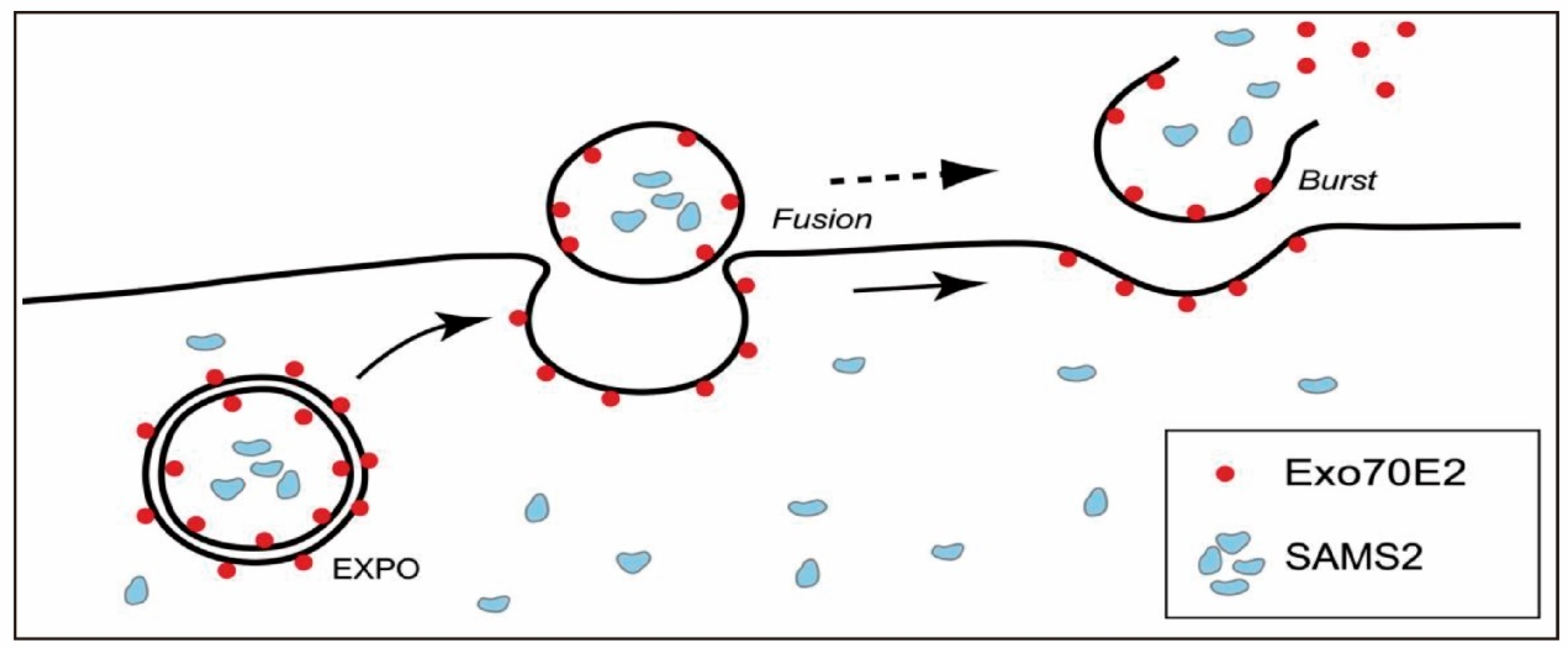
2. Biogenesis Mechanisms of PENPs
3. Structural and Compositional Characteristics of PENPs
3.1. Proteins
3.2. Lipids
3.3. Nucleic Acids
3.4. Functional Small Molecules
4. Extraction and Purification Methods of PENPs
4.1. Pre-Treatment of Plant Tissues
4.1.1. Tissue Disruption Method
4.1.2. Apoplastic Infiltration–Centrifugation Method
4.2. Overview of Extraction and Purification Strategies
4.3. Extraction Strategy Selection and Yield Enhancement Approaches
5. Physicochemical Characterization of PENPs
6. In Vivo Transport and Biodistribution of PENPs
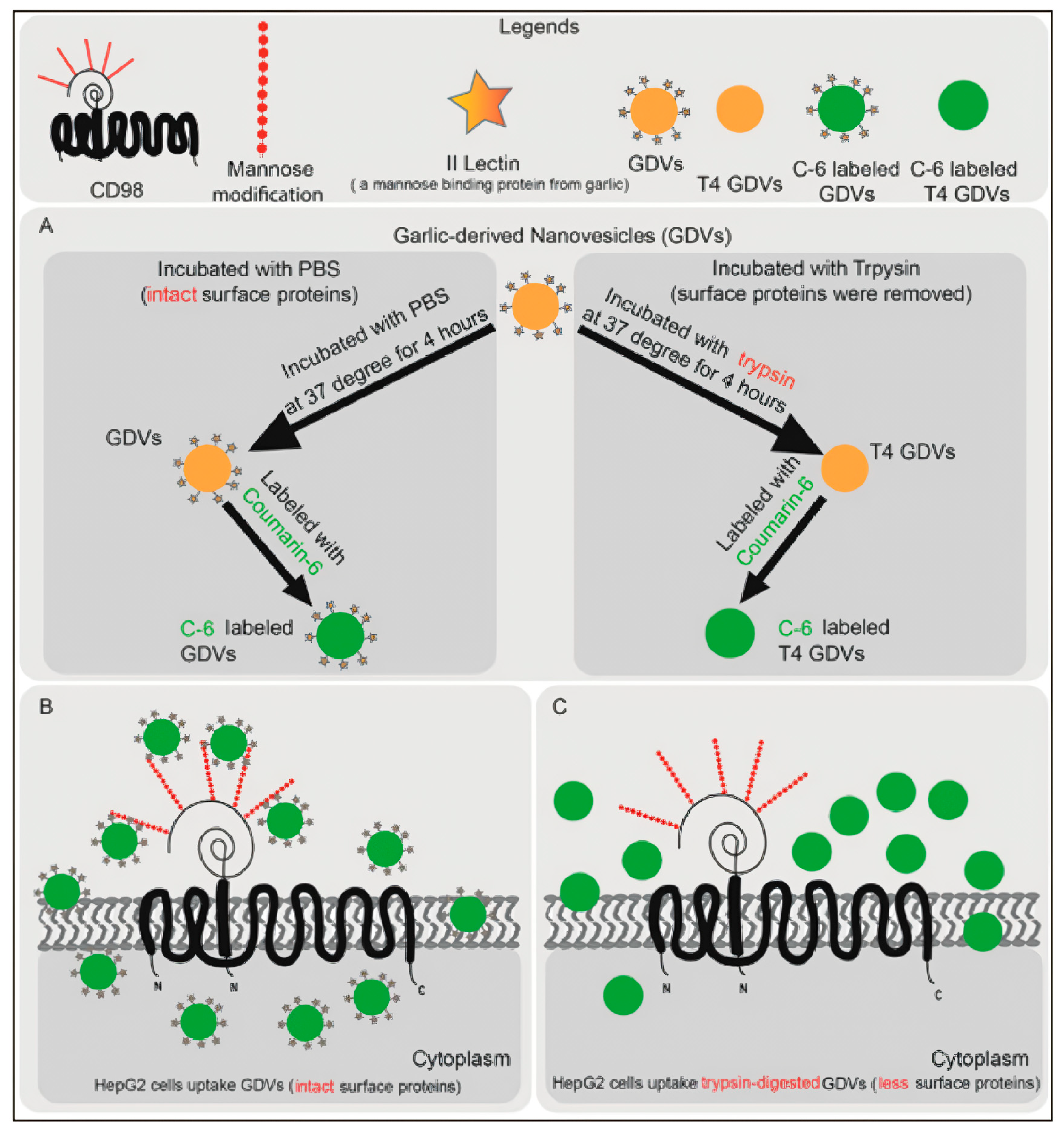
6.1. Cellular Uptake Mechanisms and Intracellular Fate of PENPs
6.2. Biodistribution Patterns and Delivery Strategy-Dependent Effects of PENPs
7. Engineering Strategies and Therapeutic Applications of PENPs as Drug Delivery Platforms
7.1. Surface Engineering and Functionalization of PENPs
7.2. Advantages of PENPs as Drug Carriers
7.3. Drug Loading Techniques
7.4. Drug Delivery Applications
8. Mechanisms of PENPs in the Treatment of Diabetes and Its Complications
8.1. Antioxidant and Anti-Inflammatory Activities
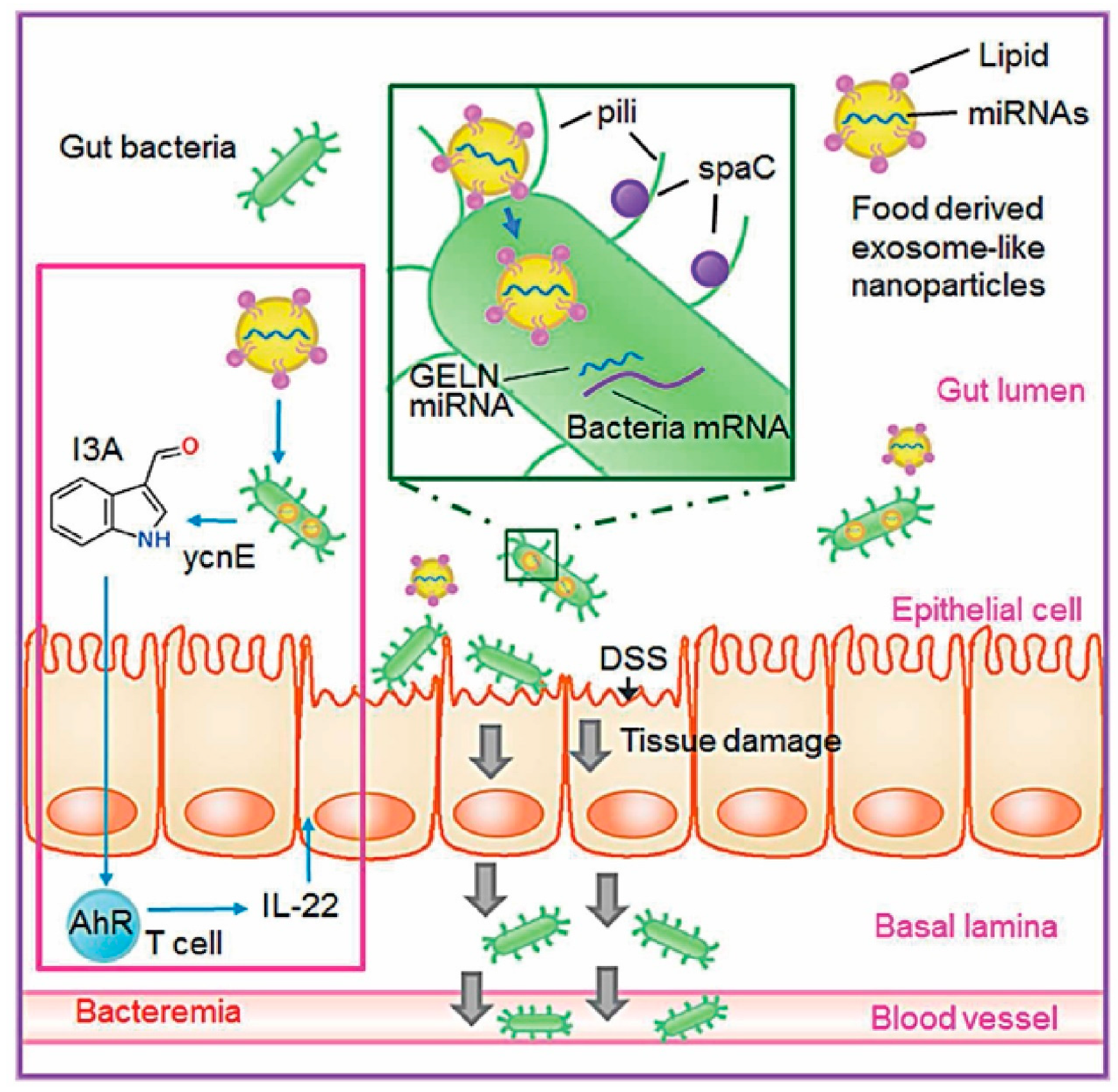
8.2. Modulation of Gut Microbiota and Immune Homeostasis
8.3. Regulation of Glucose Metabolism and Insulin Signaling Pathways
8.4. Promotion of Angiogenesis and Metabolic Reprogramming
9. Application of PENPs in the Treatment of Diabetes and Its Complications
9.1. Antihyperglycemic Effects
9.2. Amelioration of Hepatic Lipid Metabolism Dysregulation
9.3. Therapeutic Applications of PENPs in Diabetic Wound Healing
10. Current Challenges and Future Directions
10.1. Challenges and Optimization Strategies in the Development of PENPs
10.2. Challenges in the Treatment of Diabetes of PENPs
10.3. Barriers in the Treatment of Diabetic Complications
10.4. Translational Barriers and Application Limitations
10.5. Future Research Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. GBD 2021 Diabetes Collaborators: Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234, Erratum in Lancet 2025, 405, 202. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://idf.org/news/idf-diabetes-atlas-11th-edition (accessed on 4 July 2025).
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Yu, M.G.; Gordin, D.; Fu, J.; Park, K.; Li, Q.; King, G.L. Protective Factors and the Pathogenesis of Complications in Diabetes. Endocr. Rev. 2024, 45, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Hu, F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- Gieroba, B.; Kryska, A.; Sroka-Bartnicka, A. Type 2 diabetes mellitus—Conventional therapies and future perspectives in innovative treatment. Biochem. Biophys. Rep. 2025, 42, 102037. [Google Scholar] [CrossRef]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.C., III; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2023, 381, e074068. [Google Scholar] [CrossRef]
- Tsapas, A.; Avgerinos, I.; Karagiannis, T.; Malandris, K.; Manolopoulos, A.; Andreadis, P.; Liakos, A.; Matthews, D.R.; Bekiari, E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann. Intern. Med. 2020, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Uzzan, B.; Cohen, R. Metformin and digestive disorders. Diabetes Metab. 2011, 37, 90–96. [Google Scholar] [CrossRef]
- Fatima, M.; Sadeeqa, S.; Nazir, S.U.R. Metformin and its gastrointestinal problems: A review. Biomed. Res. 2018, 29, 2285–2289. [Google Scholar] [CrossRef]
- Dornhorst, A. Insulinotropic meglitinide analogues. Lancet 2001, 358, 1709–1716. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Patel, M.; Bajwa, N.; Prasad, R.; Vora, L.K. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape. Small 2025, 21, e2502315. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, Y.; Sun, X.; Lin, X.; Koo, S.; Yaremenko, A.V.; Qin, D.; Kong, N.; Farokhzad, O.C.; Tao, W. Cancer nanomedicine toward clinical translation: Obstacles, opportunities, and future prospects. Med 2023, 4, 147–167. [Google Scholar] [CrossRef]
- Afonin, K.A.; Dobrovolskaia, M.A.; Ke, W.; Grodzinski, P.; Bathe, M. Critical review of nucleic acid nanotechnology to identify gaps and inform a strategy for accelerated clinical translation. Adv. Drug Del. Rev. 2022, 181, 114081. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- Sergazy, S.; Adekenov, S.; Khabarov, I.; Adekenova, K.; Maikenova, A.; Aljofan, M. Harnessing Mammalian-and Plant-Derived Exosomes for Drug Delivery: A Comparative Review. Int. J. Mol. Sci. 2025, 26, 4857. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Sasaki, D.; Suzuki, H.; Kusamori, K.; Itakura, S.; Todo, H.; Nishikawa, M. Development of rice bran-derived nanoparticles with excellent anti-cancer activity and their application for peritoneal dissemination. J. Nanobiotechnol. 2024, 22, 114. [Google Scholar] [CrossRef]
- Li, D.; Cao, G.; Yao, X.; Yang, Y.; Yang, D.; Liu, N.; Yuan, Y.; Nishinari, K.; Yang, X. Tartary buckwheat-derived exosome-like nanovesicles against starch digestion and their interaction mechanism. Food Hydrocoll. 2023, 141, 108739. [Google Scholar] [CrossRef]
- Abdel-Mageid, A.D.; Abou-Salem, M.E.S.; Salaam, N.M.H.A.; El-Garhy, H.A.S. The potential effect of garlic extract and curcumin nanoparticles against complication accompanied with experimentally induced diabetes in rats. Phytomedicine 2018, 43, 126–134. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Kim, J.; Zhu, Y.; Chen, S.; Wang, D.; Zhang, S.; Xia, J.; Li, S.; Qiu, Q.; Lee, H.; Wang, J. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnol. 2023, 21, 253. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Chapado, L.A.; Martín-Hernández, R.; Hernández de la Red, S.; Tomé-Carneiro, J.; Gil-Zamorano, J.; Ruiz-Roso, M.B.; Del Saz, A.; Crespo, M.C.; Del Pozo-Acebo, L.; Arantes Ferreira Peres, W.; et al. Connection between miRNA Mediation and the Bioactive Effects of Broccoli (Brassica oleracea var. italica): Exogenous miRNA Resistance to Food Processing and GI Digestion. J. Agric. Food Chem. 2021, 69, 9326–9337. [Google Scholar] [CrossRef]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Bian, Y.; Jiang, X.; Zhu, F.; Yin, F.; Yin, L.; Song, X.; Guo, H.; Liu, J. Garlic-derived exosomes regulate PFKFB3 expression to relieve liver dysfunction in high-fat diet-fed mice via macrophage-hepatocyte crosstalk. Phytomedicine 2023, 112, 154679. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Xie, D.; Canup, B.S.B.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e638. [Google Scholar] [CrossRef]
- Sundaram, K.; Teng, Y.; Mu, J.; Xu, Q.; Xu, F.; Sriwastva, M.K.; Zhang, L.; Park, J.W.; Zhang, X.; Yan, J.; et al. Outer Membrane Vesicles Released from Garlic Exosome-like Nanoparticles (GaELNs) Train Gut Bacteria that Reverses Type 2 Diabetes via the Gut-Brain Axis. Small 2024, 20, e2308680. [Google Scholar] [CrossRef]
- Zou, J.; Song, Q.; Shaw, P.C.; Wu, Y.; Zuo, Z.; Yu, R. Tangerine Peel-Derived Exosome-like Nanovesicles Alleviate Hepatic Steatosis Induced by Type 2 Diabetes: Evidenced by Regulating Lipid Metabolism and Intestinal Microflora. Int. J. Nanomed. 2024, 19, 10023–10043. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529, Erratum in Cancer Res. 2016, 76, 2845. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, L.Y.; Hong, R.; Song, J.X.; Guo, X.J.; Zhou, W.; Hu, Z.L.; Wang, W.; Wang, Y.L.; Shen, J.G.; et al. Momordica charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front. Pharmacol. 2022, 13, 908830. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wei, Q.; Lv, Y.; Weng, L.; Huang, H.; Wei, Q.; Li, M.; Mao, Y.; Hua, D.; Cai, X.; et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol. Ther. 2022, 30, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, Q.; Bi, X.; Cui, W.; Fang, C.; Gao, J.; Li, J.; Wang, X.; Qu, K.; Qin, X.; et al. Preparation of Pueraria lobata Root-Derived Exosome-Like Nanovesicles and Evaluation of Their Effects on Mitigating Alcoholic Intoxication and Promoting Alcohol Metabolism in Mice. Int. J. Nanomed. 2024, 19, 4907–4921. [Google Scholar] [CrossRef]
- Chi, Y.; Shi, L.; Lu, S.; Cui, H.; Zha, W.; Shan, L.; Shen, Y. Inhibitory effect of Lonicera japonica-derived exosomal miR2911 on human papilloma virus. J. Ethnopharmacol. 2024, 318, 116969. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Xu, H.M.; Liang, Y.J.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Yao, J.; Wang, L.S.; Nie, Y.Q.; et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion. J. Nanobiotechnol. 2023, 21, 309. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef]
- Zhao, B.; Lin, H.; Jiang, X.; Li, W.; Gao, Y.; Li, M.; Yu, Y.; Chen, N.; Gao, J. Exosome-like nanoparticles derived from fruits, vegetables, and herbs: Innovative strategies of therapeutic and drug delivery. Theranostics 2024, 14, 4598–4621. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Wang, J.; Hillmer, S.; Miao, Y.; Lo, S.W.; Wang, X.; Robinson, D.G.; Jiang, L. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco Cells. Plant Cell 2010, 22, 4009–4030. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L.; Pocsfalvi, G. Membrane Transporters in Citrus clementina Fruit Juice-Derived NanoVesicles. Int. J. Mol. Sci. 2019, 20, 6205. [Google Scholar] [CrossRef]
- Ekström, K.; Crescitelli, R.; Pétursson, H.I.; Johansson, J.; Lässer, C.; Olofsson Bagge, R. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast Cancer. BMC Cancer 2022, 22, 50. [Google Scholar] [CrossRef]
- Song, H.; Canup, B.S.B.; Ngo, V.L.; Denning, T.L.; Garg, P.; Laroui, H. Internalization of Garlic-Derived Nanovesicles on Liver Cells is Triggered by Interaction With CD98. ACS Omega 2020, 5, 23118–23128. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, M.; Shao, C.; Ji, N.; Zhang, H.; Li, C. Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis. Molecules 2023, 28, 6182. [Google Scholar] [CrossRef]
- Kırbaş, O.K.; Sağraç, D.; Çiftçi, Ö.C.; Özdemir, G.; Öztürkoğlu, D.; Bozkurt, B.T.; Derman, Ü.C.; Taşkan, E.; Taşlı, P.N.; Özdemir, B.S.; et al. Unveiling the potential: Extracellular vesicles from plant cell suspension cultures as a promising source. Biofactors 2025, 51, e2090. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
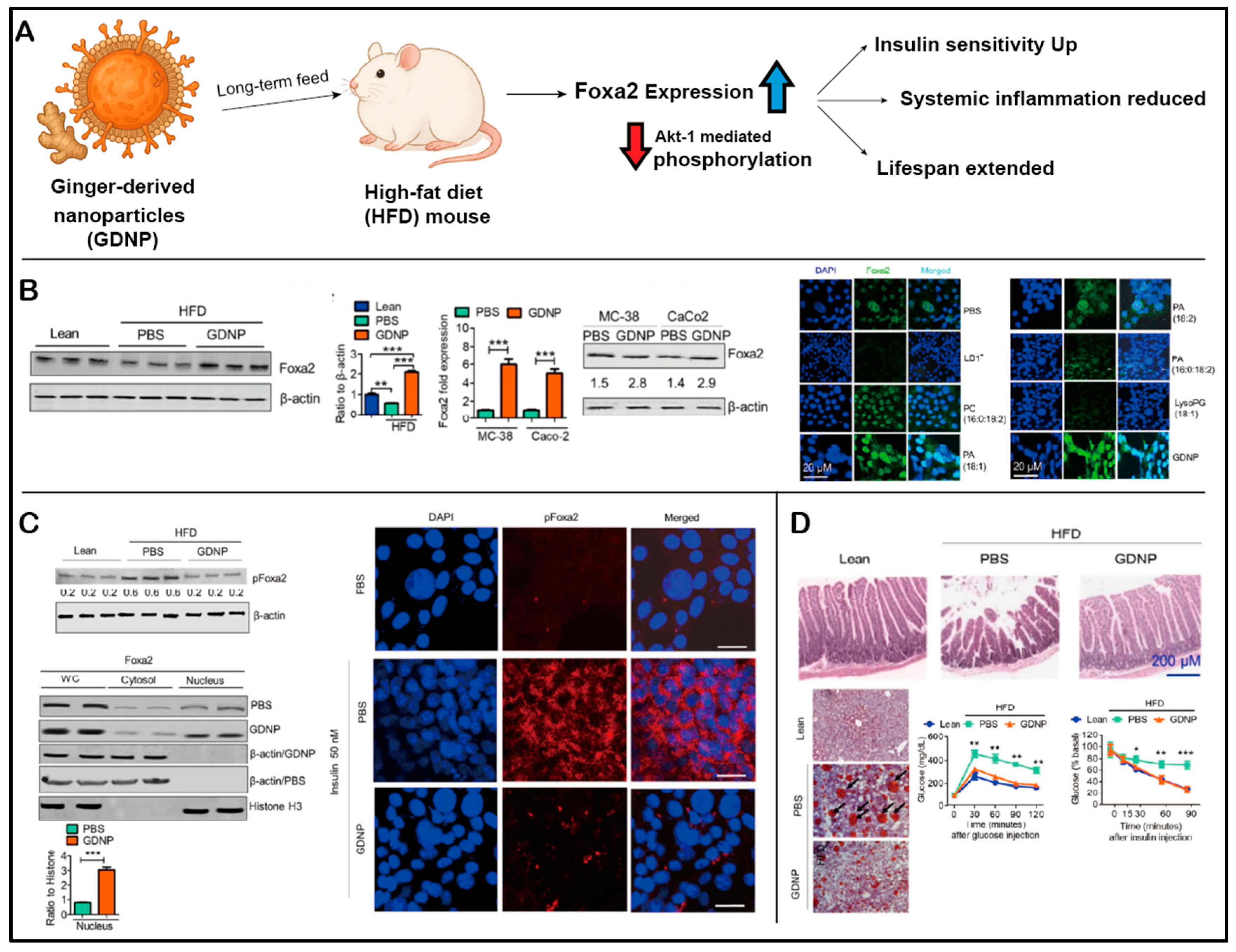
- Kumar, A.; Sundaram, K.; Teng, Y.; Mu, J.; Sriwastva, M.K.; Zhang, L.; Hood, J.L.; Yan, J.; Zhang, X.; Park, J.W.; et al. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics 2022, 12, 1388–1403. [Google Scholar] [CrossRef]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M.K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q.M.; et al. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex into Microglial Exosomes. Small 2021, 18, e2105385. [Google Scholar] [CrossRef]
- Fawzy, M.P.; Hassan, H.A.; Amin, M.U.; Preis, E.; Bakowsky, U.; Fahmy, S.A. Deploying nucleic acids-loaded plant-derived exosomes as green nano gadget in cancer gene therapy. Mater. Adv. 2025, 6, 1230–1261. [Google Scholar] [CrossRef]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience 2019, 21, 308–327, Erratum in iScience 2020, 23, 100869. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Qin, J.; Duan, L. Characterization of miRNA profiling in konjac-derived exosome-like nanoparticles and elucidation of their multifaceted roles in human health. Front. Plant Sci. 2024, 15, 1444683. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef]
- Leidal, A.M.; Huang, H.H.; Marsh, T.; Solvik, T.; Zhang, D.; Ye, J.; Kai, F.; Goldsmith, J.; Liu, J.Y.; Huang, Y.H.; et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular Vesicles. Nat. Cell Biol. 2020, 22, 187–199. [Google Scholar] [CrossRef]
- Hou, D.; He, F.; Ma, L.; Cao, M.; Zhou, Z.; Wei, Z.; Xue, Y.; Sang, X.; Chong, H.; Tian, C.; et al. The potential atheroprotective role of plant MIR156a as a repressor of monocyte recruitment on inflamed human endothelial Cells. J. Nutr. Biochem. 2018, 57, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Ly, N.P.; Han, H.S.; Kim, M.; Park, J.H.; Choi, K.Y. Plant-derived nanovesicles: Current understanding and applications for cancer therapy. Bioact. Mater. 2023, 22, 365–383. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.Y.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular Vesicles. J. Extracell. Vesicles 2022, 11, e12266. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci. Rep. 2015, 5, 13103. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Melzig, M.F. Extracellular Vesicles from Fresh and Dried Plants-Simultaneous Purification and Visualization Using Gel Electrophoresis. Int. J. Mol. Sci. 2019, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhao, H.; Pan, Q.; Chen, G. Engineering Strategies of Plant-Derived Exosome-like Nanovesicles: Current Knowledge and Future Perspectives. Int. J. Nanomed. 2024, 19, 12793–12815. [Google Scholar] [CrossRef]
- Mun, J.G.; Song, D.H.; Kee, J.Y.; Han, Y. Recent Advances in the Isolation Strategies of Plant-Derived Exosomes and Their Therapeutic Applications. Curr. Issues Mol. Biol. 2025, 47, 144. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Gorgzadeh, A.; Nazari, A.; Ali Ehsan Ismaeel, A.; Safarzadeh, D.; Hassan, J.A.K.; Mohammadzadehsaliani, S.; Kheradjoo, H.; Yasamineh, P.; Yasamineh, S. A state-of-the-art review of the recent advances in exosome isolation and detection methods in viral infection. Virol. J. 2024, 21, 34. [Google Scholar] [CrossRef]
- Viršilė, A.; Samuolienė, G.; Laužikė, K.; Mikalauskienė, E.; Balion, Z.; Jekabsone, A. The Impact of Genotype and Controlled Environment Cultivation Parameters on Tomato-Leaf-Derived Exosome-like Nanoparticle Yield and Properties. Horticulturae 2024, 10, 477. [Google Scholar] [CrossRef]
- Kocholatá, M.; Malý, J.; Kříženecká, S.; Janoušková, O. Diversity of extracellular vesicles derived from calli, cell culture and apoplastic fluid of tobacco. Sci. Rep. 2024, 14, 30111. [Google Scholar] [CrossRef]
- Li, A.; Li, D.; Gu, Y.; Liu, R.; Tang, X.; Zhao, Y.; Qi, F.; Wei, J.; Liu, J. Plant-derived nanovesicles: Further exploration of biomedical function and application potential. Acta Pharm. Sin. B 2023, 13, 3300–3320. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef]
- Zhu, H.; He, W. Ginger: A representative material of herb-derived exosome-like nanoparticles. Front. Nutr. 2023, 10, 1223349. [Google Scholar] [CrossRef]
- Parisse, P.; Rago, I.; Ulloa Severino, L.; Perissinotto, F.; Ambrosetti, E.; Paoletti, P.; Ricci, M.; Beltrami, A.P.; Cesselli, D.; Casalis, L. Atomic force microscopy analysis of extracellular Vesicles. Eur. Biophys. J. 2017, 46, 813–820. [Google Scholar] [CrossRef]
- Chuo, S.T.; Chien, J.C.; Lai, C.P. Imaging extracellular vesicles: Current and emerging methods. J. Biomed. Sci. 2018, 25, 91. [Google Scholar] [CrossRef]
- Koifman, N.; Biran, I.; Aharon, A.; Brenner, B.; Talmon, Y. A direct-imaging cryo-EM study of shedding extracellular vesicles from leukemic monocytes. J. Struct. Biol. 2017, 198, 177–185. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human Cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Kürtösi, B.; Kazsoki, A.; Zelkó, R. A Systematic Review on Plant-Derived Extracellular Vesicles as Drug Delivery Systems. Int. J. Mol. Sci. 2024, 25, 7559. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Sha, A.; Luo, Y.; Xiao, W.; He, J.; Chen, X.; Xiong, Z.; Peng, L.; Zou, L.; Liu, B.; Li, Q. Plant-Derived Exosome-like Nanoparticles: A Comprehensive Overview of Their Composition, Biogenesis, Isolation, and Biological Applications. Int. J. Mol. Sci. 2024, 25, 12092. [Google Scholar] [CrossRef]
- Liu, H.; Deng, Y.; Li, J.; Lin, W.; Liu, C.; Yang, X.; Zhou, Z.; Jiang, Y. Ginger-derived exosome-like nanoparticles: A representative of plant-based natural nanostructured drug delivery system. Front. Bioeng. Biotechnol. 2025, 13, 1569889. [Google Scholar] [CrossRef]
- Bahri, F.; Mansoori, M.; Vafaei, S.; Fooladi, S.; Mir, Y.; Mehrabani, M.; Hozhabri, Y.; Nematollahi, M.H.; Iravani, S. A comprehensive review on ginger-derived exosome-like nanoparticles as feasible therapeutic nano-agents against diseases. Mater. Adv. 2024, 5, 1846–1867. [Google Scholar] [CrossRef]
- Raguraman, R.; Bhavsar, D.; Kim, D.; Ren, X.; Sikavitsas, V.; Munshi, A.; Ramesh, R. Tumor-targeted exosomes for delivery of anticancer drugs. Cancer Lett. 2023, 558, 216093. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Acebo, L.; Hazas, M.-C.L.d.L.; Tomé-Carneiro, J.; Del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef]
- Zhuang, X.; Teng, Y.; Samykutty, A.; Mu, J.; Deng, Z.; Zhang, L.; Cao, P.; Rong, Y.; Yan, J.; Miller, D.; et al. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef]
- Sasaki, D.; Kusamori, K.; Nishikawa, M. Delivery of Corn-Derived Nanoparticles with Anticancer Activity to Tumor Tissues by Modification with Polyethylene Glycol for Cancer Therapy. Pharm. Res. 2023, 40, 917–926. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, W.; Wu, C.; Wang, X.; Chen, J.; Shi, X.; Sha, S.; Li, J.; Liang, X.; Yang, Y.; et al. Lemon-Derived Extracellular Vesicles Nanodrugs Enable to Efficiently Overcome Cancer Multidrug Resistance by Endocytosis-Triggered Energy Dissipation and Energy Production Reduction. Adv. Sci. 2022, 9, 2105274. [Google Scholar] [CrossRef]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.I.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther.-Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, T.; Mansur, S.; Bao, Y. Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications. Molecules 2021, 26, 1135. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, J.; Sohn, Y.; Oh, C.E.; Park, J.H.; Yuk, J.M.; Yeon, J.H. Stability of plant leaf-derived extracellular vesicles according to preservative and storage temperature. Pharmaceutics 2022, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Tinnirello, V.; Rabienezhad Ganji, N.; De Marcos Lousa, C.; Alessandro, R.; Raimondo, S. Exploiting the Opportunity to Use Plant-Derived Nanoparticles as Delivery Vehicles. Plants 2023, 12, 1207. [Google Scholar] [CrossRef]
- Che, K.; Wang, C.; Chen, H. Advancing functional foods: A systematic analysis of plant-derived exosome-like nanoparticles and their health-promoting properties. Front. Nutr. 2025, 12, 1544746. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef] [PubMed]
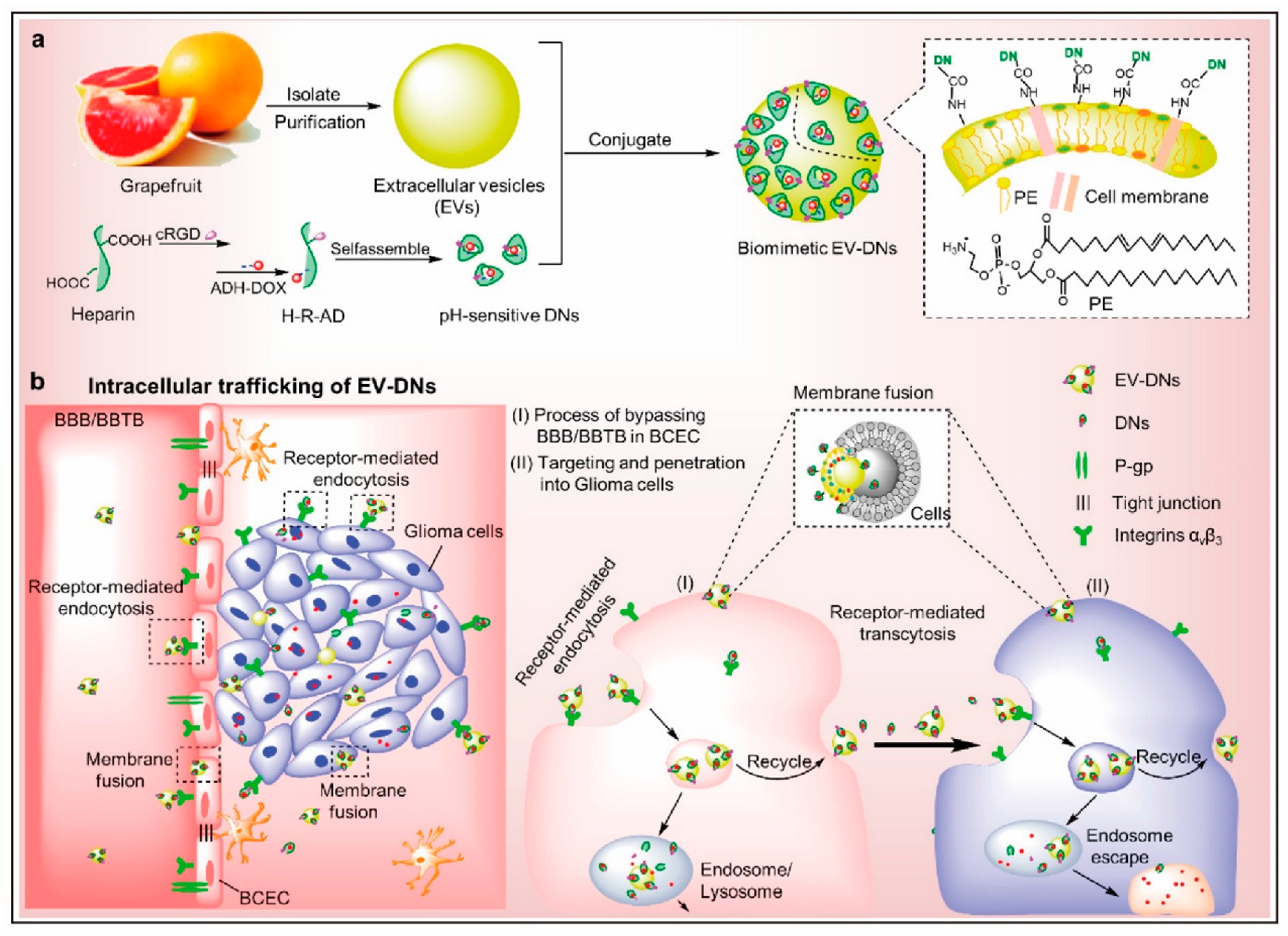
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; Grange, C.; De Rosa, F.G.; Camussi, G. Plant-Derived Extracellular Vesicles as a Delivery Platform for RNA-Based Vaccine: Feasibility Study of an Oral and Intranasal SARS-CoV-2 Vaccine. Pharmaceutics 2023, 15, 974. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Hou, N.; Torii, S.; Saito, N.; Hosaka, M.; Takeuchi, T. Reactive oxygen species-mediated pancreatic β-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology 2008, 149, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Varthaliti, A.; Lygizos, V.; Fanaki, M.; Pergialiotis, V.; Papapanagiotou, A.; Pappa, K.; Theodora, M.; Daskalaki, M.A.; Antsaklis, P.; Daskalakis, G. The Role of IL-6 and TNF-α as Early Biomarkers in the Prediction and Diagnosis of Gestational Diabetes Mellitus. Biomedicines 2025, 13, 1627. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Xia, J.; Qian, D.; Guo, J.; Zhong, L.; Tang, D.; Chen, X.; Peng, W.; Chen, Y.; et al. Natural exosomes-like nanoparticles in mung bean sprouts possesses anti-diabetic effects via activation of PI3K/Akt/GLUT4/GSK-3β signaling pathway. J. Nanobiotechnol. 2023, 21, 349. [Google Scholar] [CrossRef]
- Wang, X.; Tian, R.; Liang, C.; Jia, Y.; Zhao, L.; Xie, Q.; Huang, F.; Yuan, H. Biomimetic nanoplatform with microbiome modulation and antioxidant functions ameliorating insulin resistance and pancreatic β-cell dysfunction for T2DM management. Biomaterials 2025, 313, 122804. [Google Scholar] [CrossRef]
- Miya, M.B.; Ashutosh, M.; Dey, D.; Pathak, V.; Khare, E.; Kalani, K.; Chaturvedi, P.; Singh, V.; Chaturvedi, P.; Kalani, A. Accelerated diabetic wound healing using a chitosan-based nanomembrane incorporating nanovesicles from Aloe barbadensis, Azadirachta indica, and Zingiber officinale. Int. J. Biol. Macromol. 2025, 310, 143169. [Google Scholar] [CrossRef]
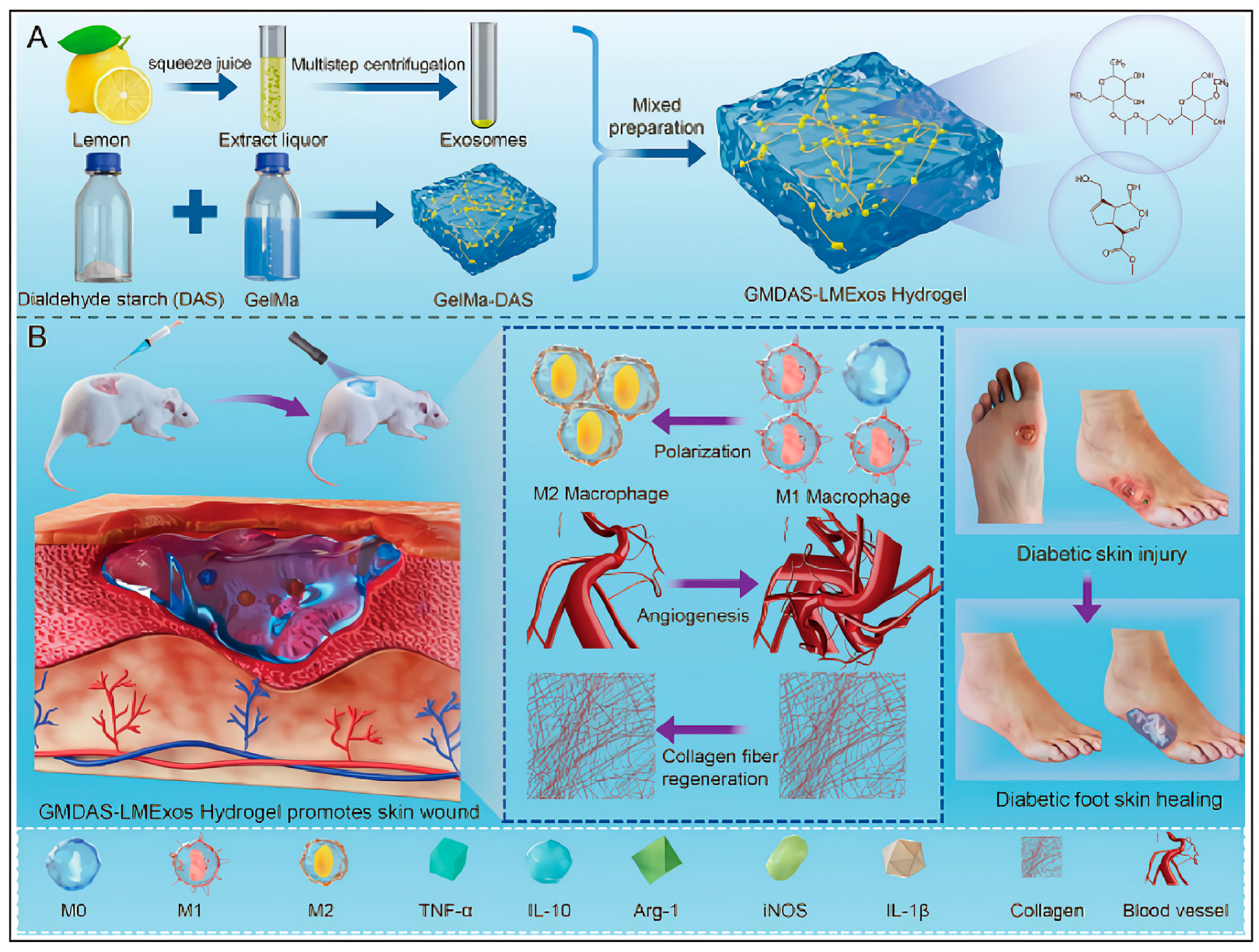
- Jin, E.; Yang, Y.; Cong, S.; Chen, D.; Chen, R.; Zhang, J.; Hu, Y.; Chen, W. Lemon-derived nanoparticle-functionalized hydrogels regulate macrophage reprogramming to promote diabetic wound healing. J. Nanobiotechnol. 2025, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ding, Y.; Wang, S.; Jiang, L. Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies. Metabolites 2025, 15, 397. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Yang, L.; Luo, S.; Deng, Y. Gut microbiota and its metabolites regulate insulin resistance: Traditional Chinese medicine insights for T2DM. Front. Microbiol. 2025, 16, 1554189. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Chong, S.; Lin, M.; Chong, D.; Jensen, S.; Lau, N.S. A systematic review on gut microbiota in type 2 diabetes mellitus. Front. Endocrinol. 2025, 15, 1486793. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yi, G.; Cao, G.; Midgley, A.C.; Yang, Y.; Yang, D.; Liu, W.; He, Y.; Yao, X.; Li, G. Dual-Carriers of Tartary Buckwheat-Derived Exosome-like Nanovesicles Synergistically Regulate Glucose Metabolism in the Intestine-Liver Axis. Small 2025, 21, e2410124. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.; Jurado-Aguilar, J.; Wahli, W.; Palomer, X.; Vázquez-Carrera, M. Increased hepatic gluconeogenesis and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2024, 35, 1062–1077. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
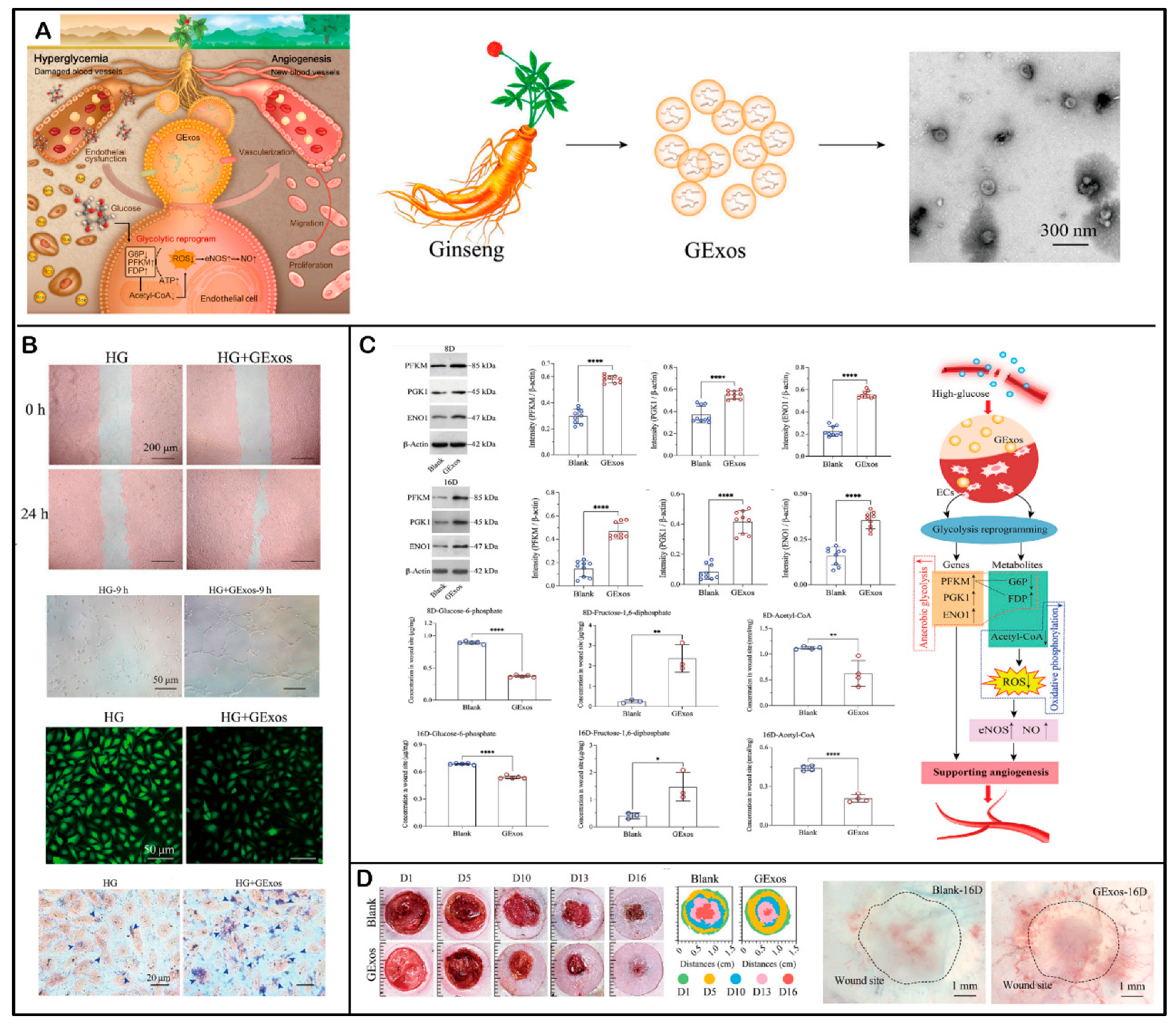
- Tan, M.; Liu, Y.; Xu, Y.; Yan, G.; Zhou, N.; Chen, H.; Jiang, Z.; Peng, L. Plant-Derived Exosomes as Novel Nanotherapeutics Contrive Glycolysis Reprogramming-Mediated Angiogenesis for Diabetic Ulcer Healing. Biomater. Res. 2024, 28, 0035. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive loaded novel nano-formulations for targeted drug delivery and their therapeutic potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Sun, D.S.; Wang, K.L.; Shang, D.Y. Nanomedicine of Plant Origin for the Treatment of Metabolic Disorders. Front. Bioeng. Biotechnol. 2022, 9, 811917. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, A.; Ma, Q.; Yan, X.; Bian, L.; Zhang, P.; Wu, J. Nanoparticles prepared from pterostilbene reduce blood glucose and improve diabetes complications. J. Nanobiotechnol. 2021, 19, 191. [Google Scholar] [CrossRef]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; He, X.; Huang, K. Plant derived Exosome-Like nanoparticles and their therapeutic applications in glucolipid metabolism diseases. J. Agric. Food Chem. 2025, 73, 6385–6399. [Google Scholar] [CrossRef]
- Yan, L.; Cao, Y.; Hou, L.; Luo, T.; Li, M.; Gao, S.; Wang, L.; Sheng, K.; Zheng, L. Ginger exosome-like nanoparticle-derived miRNA therapeutics: A strategic inhibitor of intestinal inflammation. J. Adv. Res. 2025, 69, 1–15. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.F.; Liu, Y.; Lv, Q.Z.; Liu, G.L.; Zhang, J.G.; Li, X.Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front. Pharmacol. 2019, 9, 1566. [Google Scholar] [CrossRef]
- Lonardo, A.; Lombardini, S.; Ricchi, M.; Scaglioni, F.; Loria, P. Review article: Hepatic steatosis and insulin resistance. Aliment. Pharmacol. Ther. 2005, 22 (Suppl. 2), 64–70. [Google Scholar] [CrossRef]
- Guan, Y.; Niu, H.; Liu, Z.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Yin, R.Y.; Tian, J.X. Extracellular vesicles: Mechanisms and prospects in type 2 diabetes and its complications. Front. Endocrinol. 2025, 15, 1521281. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Jin, J.; Fu, Z.H.; Wang, G.M.; Lei, X.H.; Xu, J.; Wang, J.Z. Extracellular vesicle-based drug overview: Research landscape, quality control and nonclinical evaluation strategies. Signal Transduct. Target. Ther. 2025, 10, 255. [Google Scholar] [CrossRef] [PubMed]



| Plant Sources | Dominant Lipid Species and Composition | Potential In Vivo Targeting | Ref. |
|---|---|---|---|
| Grapefruit | PE (~46%), PC (~29%) | Intestine (enhanced uptake), liver (preferential accumulation) | [64] |
| Ginger | PA (~38%), DGDG (~33%), MGDG (~21%) | Small intestine (uptake by epithelial cells, oral) | [65] |
| Oat | PC (~30%), DGDG (~29.8%) | Brain (crosses BBB; microglial uptake) | [66] |
| Method | Isolation Principle | Purity | Scalability | Vesicle Integrity | Advantages | Limitations |
|---|---|---|---|---|---|---|
| Differential centrifugation (DC) | Sequential centrifugation with gradually increasing g force to pellet particles | Low to moderate | High | Moderate to low | Simple, low cost, widely accessible | High risk of co-pelleting proteins and organelle fragments, variability between batches |
| Density-gradient ultracentrifugation (DGUC) | Separation by buoyant density in sucrose or iodixanol medium | High | Low | Moderate | High resolution and purity | Time-consuming, low-throughput, possible osmotic stress |
| Ultrafiltration (UF, including TFF) | Filtration based on membrane pore size and continuous flow | Moderate | High | Moderate to high | Suitable for large volumes, relatively fast | Membrane fouling, loss of small vesicles |
| Size-exclusion chromatography (SEC) | Gel filtration to separate vesicles from proteins and small molecules | High | Moderate | High | Gentle on vesicles, preserves bioactivity | Limited-throughput, dilution and reduced yield |
| Polyethylene glycol precipitation (PEG) | Polymer induced precipitation of vesicles | Low to moderate | High | Moderate to low | Rapid, inexpensive, scalable | Co-precipitation of contaminants, polymer residues |
| Immunoaffinity capture | Isolation based on specific vesicle surface markers such as TET8 or PEN1 | Very high | Low | High | High specificity for target subpopulations | High cost, dependence on antibody availability, limited yield |
| Asymmetric flow field-flow fractionation (AF4) | Fractionation of vesicles by size under a flow field | High | Low to moderate | High | High resolution, maintains structural integrity | Specialized instrumentation, method complexity |
| Aqueous two-phase system (ATPS) | Partitioning of vesicles between two immiscible polymer phases | Moderate to high | High | High | Gentle on vesicles, potentially scalable | Need for removal of residual polymers, optimization required |
| Electrophoresis–dialysis hybrid | Use of electric field with dialysis membrane to remove charged impurities | High | Low | High | Effective for charged impurities, precise separation | Low-throughput, labor-intensive |
| Microfluidic platforms | On-chip vesicle sorting by hydrodynamic or affinity based principles | High | Low to moderate | High | Rapid, automated, requires minimal sample | Scale up not established, device-specific variability |
| Route of Administration | Plant Sources | Primary Target Organs/Tissues | Key Advantages | Applicable Disease Models | Ref. |
|---|---|---|---|---|---|
| Oral Administration | Ginger, Grapefruit, Ginseng | Distal small intestine, cecum, colon, liver | High stability, strong dependence on the enterohepatic axis | Inflammatory bowel disease, intestinal cancer, non-alcoholic fatty liver disease | [98,109,110] |
| Intravenous Administration | Ginseng, Corn | Liver, spleen, systemic circulation | Bypasses first-pass metabolism, high systemic delivery efficiency | Cancer, liver diseases, cerebral ischemia, and other systemic diseases | [111,112] |
| Intraperitoneal Administration | Grapefruit, Ginseng | Liver, spleen, kidneys, lungs | Stable pharmacokinetics, suitable for animal studies | Inflammation, systemic immune-related diseases | [47,70,111] |
| Intranasal Delivery | Grapefruit | Lungs, brain | Non-invasive, bypasses the BBB | Brain tumors, neuroinflammation, pulmonary diseases | [70,114] |
| Transdermal Delivery | Safflower, Ginseng | Dermis, subcutaneous tissue | Targeted to wound sites, suitable for localized therapy | Wound healing, chronic dermatitis | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Guo, Y.; Msomi, N.Z.; Islam, M.S.; Chu, M. Exosome-like Nanoparticles Extracted from Plant Cells for Diabetes Therapy. Int. J. Mol. Sci. 2025, 26, 9155. https://doi.org/10.3390/ijms26189155
Xiao X, Guo Y, Msomi NZ, Islam MS, Chu M. Exosome-like Nanoparticles Extracted from Plant Cells for Diabetes Therapy. International Journal of Molecular Sciences. 2025; 26(18):9155. https://doi.org/10.3390/ijms26189155
Chicago/Turabian StyleXiao, Xin, Yuliang Guo, Nontokozo Zimbili Msomi, Md. Shahidul Islam, and Maoquan Chu. 2025. "Exosome-like Nanoparticles Extracted from Plant Cells for Diabetes Therapy" International Journal of Molecular Sciences 26, no. 18: 9155. https://doi.org/10.3390/ijms26189155
APA StyleXiao, X., Guo, Y., Msomi, N. Z., Islam, M. S., & Chu, M. (2025). Exosome-like Nanoparticles Extracted from Plant Cells for Diabetes Therapy. International Journal of Molecular Sciences, 26(18), 9155. https://doi.org/10.3390/ijms26189155








