p.N370S GBA1 Mutation Influences the Morphology and Lipid Composition of Extracellular Vesicles in Blood Plasma from Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Results
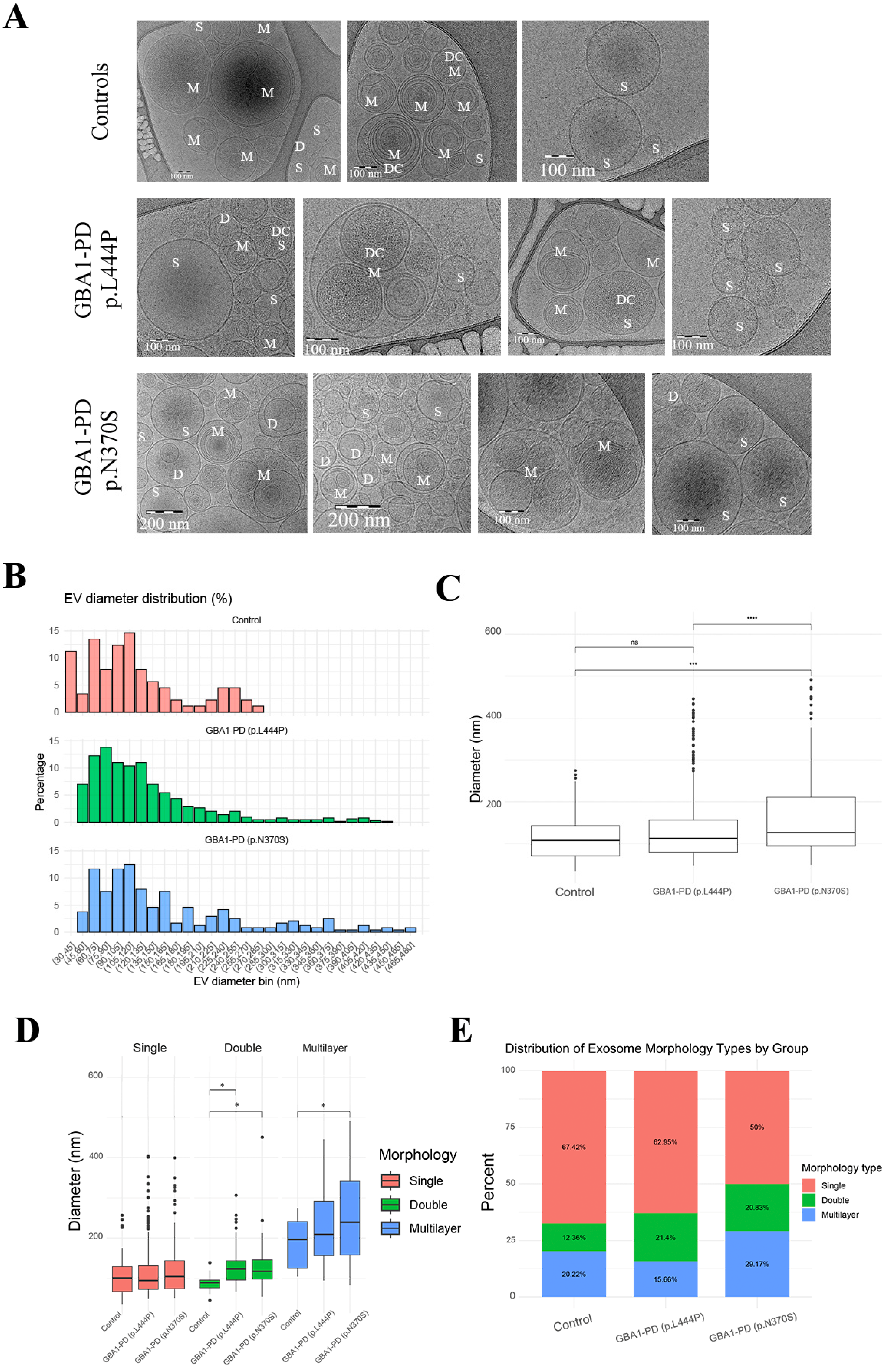
2.1. Morphological Characterization of EVs by Cryo-EM
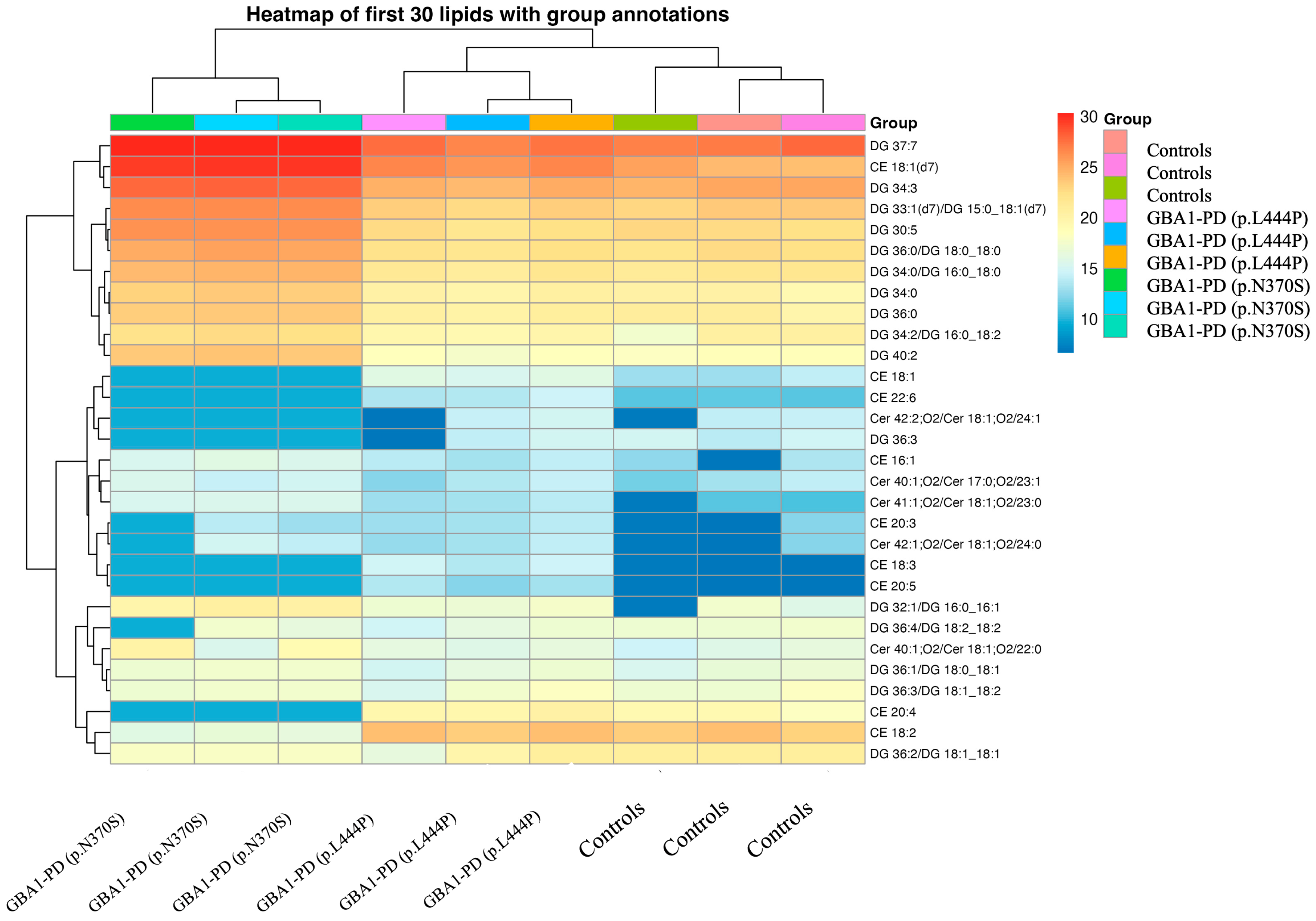
2.2. Lipidomic Profiling of EVs from GBA1-PD Patients Compared to Controls with Stratification by Mutation
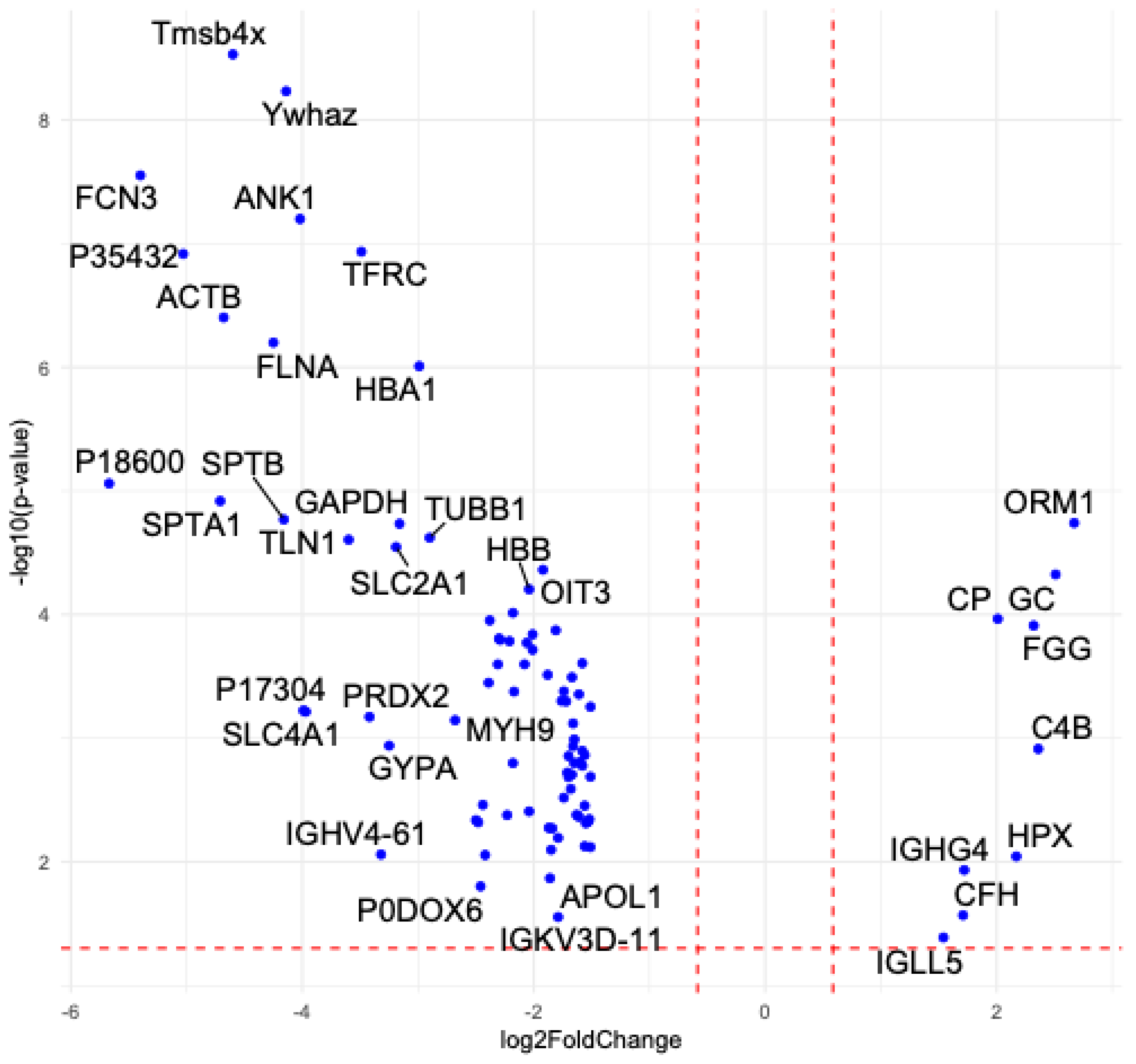
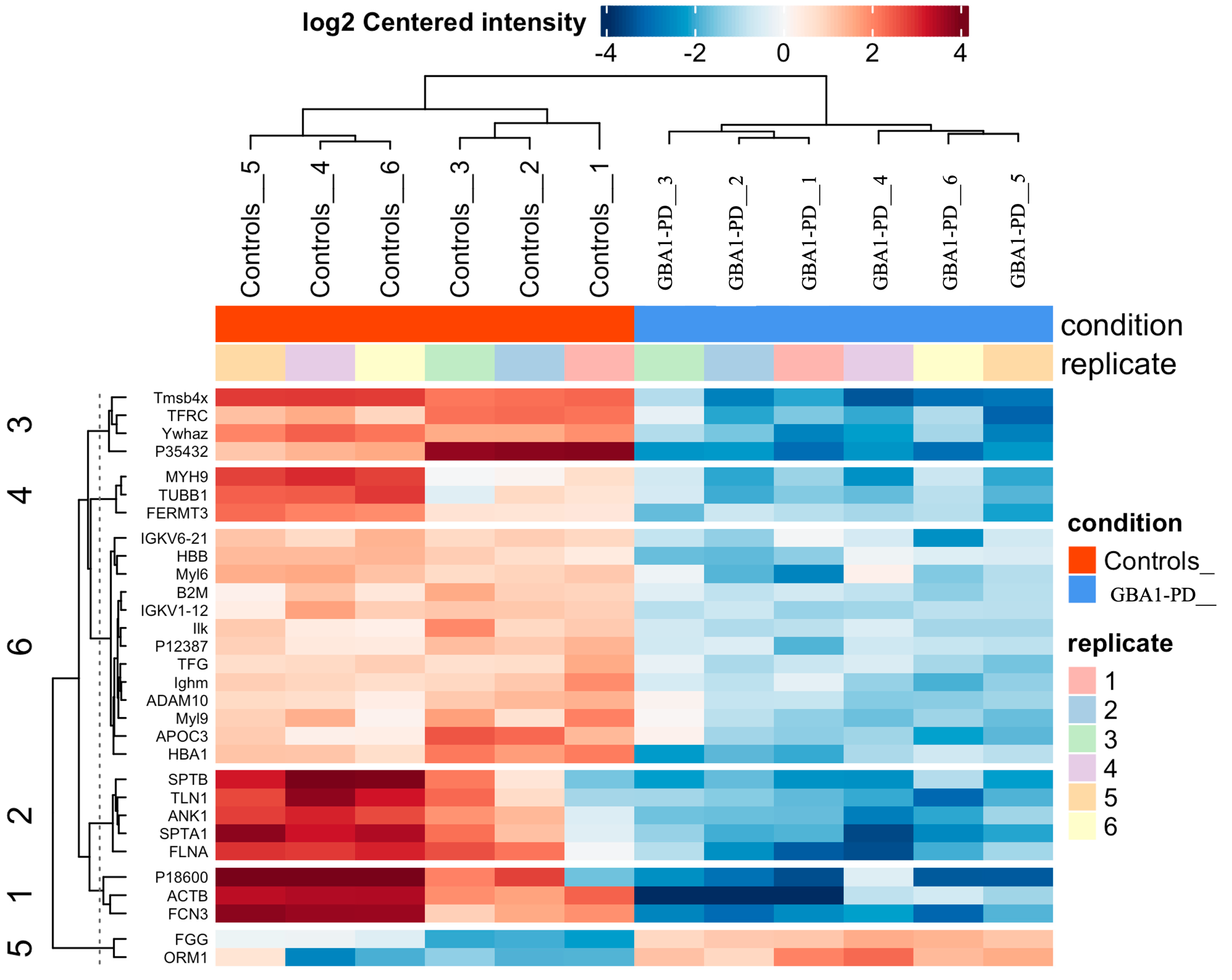
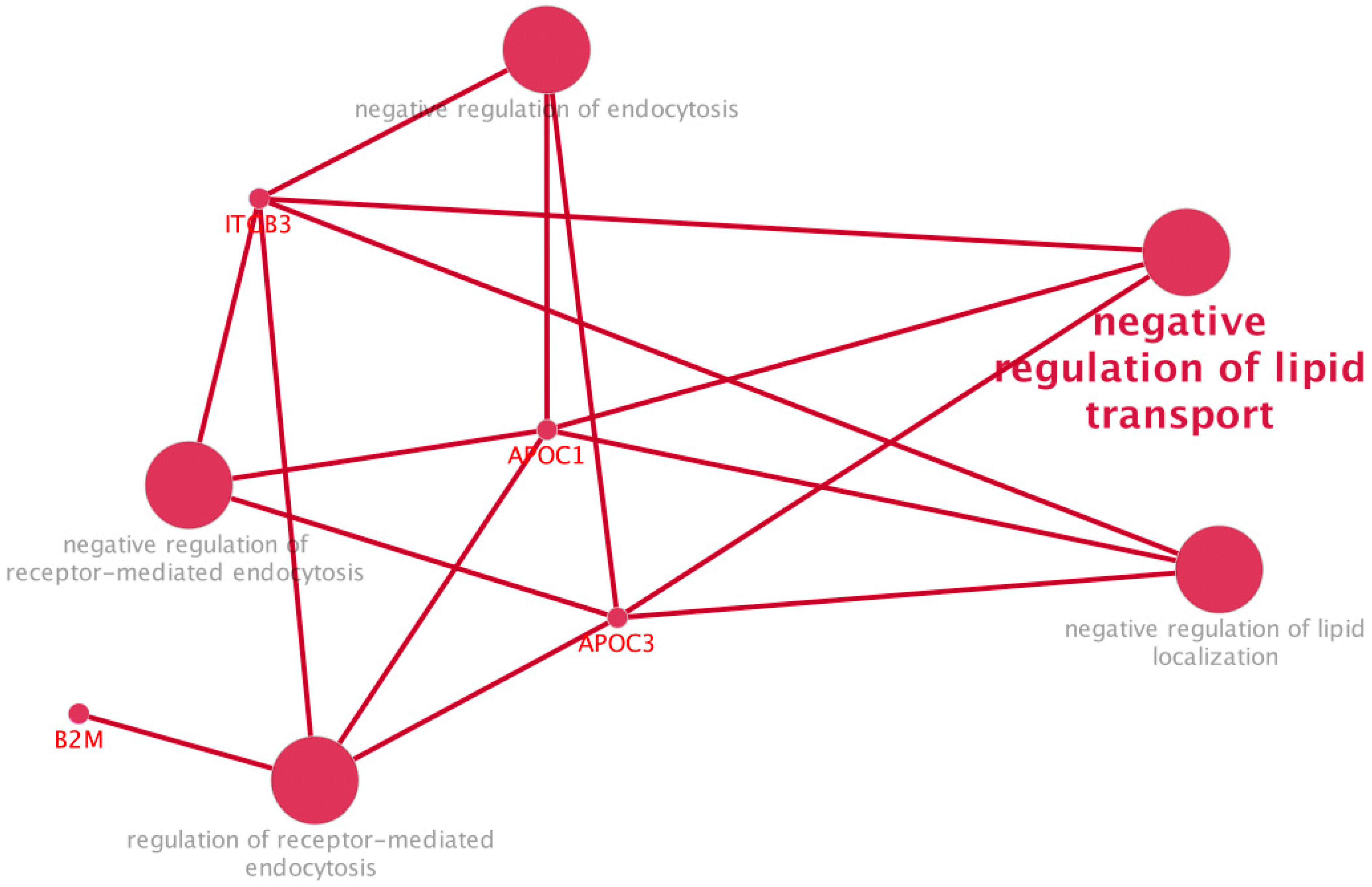
2.3. Proteomic Profiling of EVs from GBA1-PD Patients Compared to Controls
3. Discussion
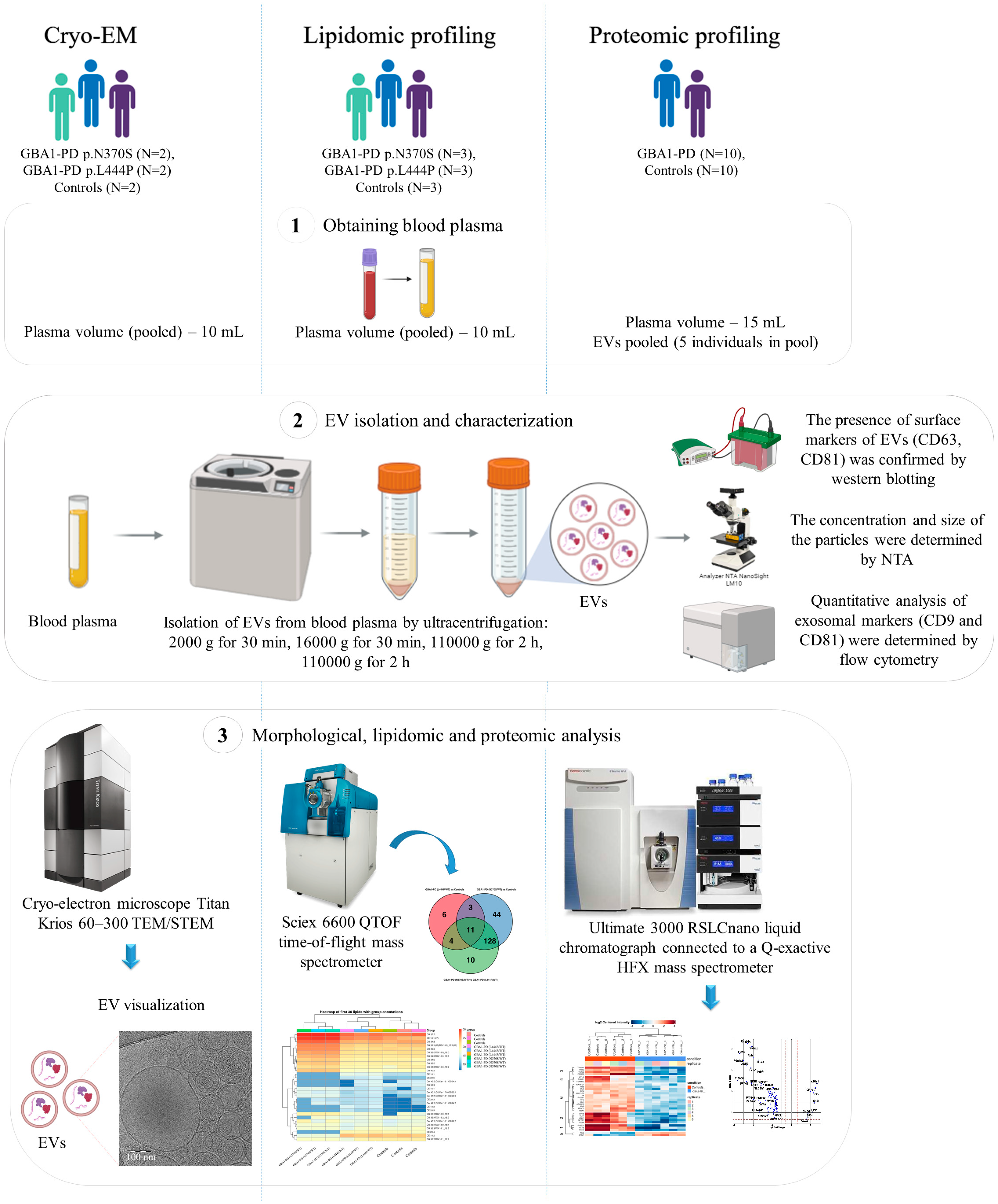
4. Materials and Methods
4.1. Participants
4.2. Isolation of EVs
4.3. Nanoparticle Tracking Analysis (NTA)
4.4. Flow Cytometry
4.5. Cryo-EM
4.6. Western Blotting
4.7. Untargeted Lipidomic Analysis
4.8. Analysis of Lipidome Differential Expression of EVs
4.9. Untargeted Proteomic Analysis
4.10. Analysis of Proteome Differential Expression of EVs
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDS | Calibrant delivery system |
| CEs | Cholesteryl esters |
| Cer | Ceramides |
| Cryo-EM | Cryo-electron microscopy |
| CSF | Cerebrospinal fluid |
| DA neurons | Dopaminergic neurons |
| DED | Direct electron detector |
| DG | Diacylglycerols |
| EV | Extracellular vesicle |
| FC | Fold change |
| FDR | False discovery rate |
| GBA1-PD | GBA1-associated Parkinson’s disease |
| GCase | β-glucocerebrosidase |
| GD | Gaucher disease |
| HPLC-MS/MS | High-performance liquid chromatography–mass spectrometry |
| LPCs | Lysophosphatidylcholines |
| LSD | Lysosomal storage disorder |
| MGs | Monoacylglycerols |
| NAE | N-acylethanolamines |
| NCE | Normalized collision energy |
| NTA | Nanoparticle tracking analysis |
| PCA | Principal component analysis |
| PCs | Phosphatidylcholines |
| PD | Parkinson’s disease |
| PEs | Phosphatidylethanolamines |
| PGs | Phosphatidylglycerols |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| SD | Standard deviation |
| SMs | Sphingomyelins |
| ST | Sterol |
| TEM | Transmission electron microscopy |
| TGs | Triacylglycerols |
References
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma Exosomal α-Synuclein Is Likely CNS-Derived and Increased in Parkinson’s Disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, G.; Han, C.; Ma, K.; Guo, X.; Wan, F.; Kou, L.; Yin, S.; Liu, L.; Huang, J.; et al. Microglia as Modulators of Exosomal Alpha-Synuclein Transmission. Cell Death Dis. 2019, 10, 174. [Google Scholar] [CrossRef]
- Stuendl, A.; Kunadt, M.; Kruse, N.; Bartels, C.; Moebius, W.; Danzer, K.M.; Mollenhauer, B.; Schneider, A. Induction of α-Synuclein Aggregate Formation by CSF Exosomes from Patients with Parkinson’s Disease and Dementia with Lewy Bodies. Brain 2016, 139, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wu, Y.; Liu, G.; Jiang, Y.; Wang, X.; Wang, Z.; Zhang, J.; Feng, T. α-Synuclein in Salivary Extracellular Vesicles as a Potential Biomarker of Parkinson’s Disease. Neurosci. Lett. 2019, 696, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on Extracellular Vesicles: Exosomes and Their Role in Protein Trafficking and Biomarker Potential in Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef]
- Shi, Q.; Kang, W.; Liu, Z.; Zhu, X. The Role of Exosomes in the Diagnosis of Parkinson’s Disease. Heliyon 2023, 9, e20595. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. Potential Exosome Biomarkers for Parkinson’s Disease Diagnosis: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 5307. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Park. Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef]
- Ramirez, J.; Pancoe, S.X.; Rhoades, E.; Petersson, E.J. The Effects of Lipids on α-Synuclein Aggregation In Vitro. Biomolecules 2023, 13, 1476. [Google Scholar] [CrossRef]
- Schepers, J.; Löser, T.; Behl, C. Lipids and α-Synuclein: Adding Further Variables to the Equation. Front. Mol. Biosci. 2024, 11, 1455817. [Google Scholar] [CrossRef]
- Toffoli, M.; Smith, L.; Schapira, A.H.V. The Biochemical Basis of Interactions between Glucocerebrosidase and Alpha-Synuclein in GBA1 Mutation Carriers. J. Neurochem. 2020, 154, 11–24. [Google Scholar] [CrossRef]
- Do, J.; McKinney, C.; Sharma, P.; Sidransky, E. Glucocerebrosidase and Its Relevance to Parkinson Disease. Mol. Neurodegener. 2019, 14, 36. [Google Scholar] [CrossRef]
- Lesage, S.; Anheim, M.; Condroyer, C.; Pollak, P.; Durif, F.; Dupuits, C.; Viallet, F.; Lohmann, E.; Corvol, J.-C.; Honoré, A.; et al. Large-Scale Screening of the Gaucher’s Disease-Related Glucocerebrosidase Gene in Europeans with Parkinson’s Disease. Hum. Mol. Genet. 2011, 20, 202–210. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multi-Center Analysis of Glucocerebrosidase Mutations in Parkinson Disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.K.; Usenko, T.S.; Tesson, C.; Senkevich, K.A.; Nikolaev, M.A.; Miliukhina, I.V.; Kopytova, A.E.; Timofeeva, A.A.; Yakimovsky, A.F.; Lesage, S.; et al. Mutation Analysis of Parkinson’s Disease Genes in a Russian Data Set. Neurobiol. Aging 2018, 71, 267.e7–267.e10. [Google Scholar] [CrossRef]
- Daykin, E.C.; Ryan, E.; Sidransky, E. Diagnosing Neuronopathic Gaucher Disease: New Considerations and Challenges in Assigning Gaucher Phenotypes. Mol. Genet. Metab. 2021, 132, 49–58. [Google Scholar] [CrossRef]
- Tatiana, S.; Stanislav, N.; Darya, K.; Luiza, G.; Konstantin, S.; Sergey, L.; Elena, V.; Galina, S.; Nikolai, V.; Arthur, K.; et al. Altered Level of Plasma Exosomes in Patients with Gaucher Disease. Eur. J. Med. Genet. 2020, 63, 104038. [Google Scholar] [CrossRef]
- Taguchi, Y.V.; Liu, J.; Ruan, J.; Pacheco, J.; Zhang, X.; Abbasi, J.; Keutzer, J.; Mistry, P.K.; Chandra, S.S. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 2017, 37, 9617–9631. [Google Scholar] [CrossRef] [PubMed]
- Parlar, S.C.; Grenn, F.P.; Kim, J.J.; Baluwendraat, C.; Gan-Or, Z. Classification of GBA1 Variants in Parkinson’s Disease: The GBA1-PD Browser. Mov. Disord. 2023, 38, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Krainc, D. Mechanisms of Glucocerebrosidase Dysfunction in Parkinson’s Disease. J. Mol. Biol. 2023, 435, 168023. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.H.; Weinreb, N.J.; Cloyd, J.C.; Tuite, P.J.; Kartha, R.V. GBA1 Mutations: Prospects for Exosomal Biomarkers in α-Synuclein Pathologies. Mol. Genet. Metab. 2020, 129, 35–46. [Google Scholar] [CrossRef]
- Papadopoulos, V.E.; Nikolopoulou, G.; Antoniadou, I.; Karachaliou, A.; Arianoglou, G.; Emmanouilidou, E.; Sardi, S.P.; Stefanis, L.; Vekrellis, K. Modulation of β-Glucocerebrosidase Increases α-Synuclein Secretion and Exosome Release in Mouse Models of Parkinson’s Disease. Hum. Mol. Genet. 2018, 27, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Vincow, E.S.; Merrihew, G.E.; MacCoss, M.J.; Davis, M.Y.; Pallanck, L.J. Glucocerebrosidase Deficiency Promotes Protein Aggregation through Dysregulation of Extracellular Vesicles. PLOS Genet. 2018, 14, e1007694. [Google Scholar] [CrossRef]
- Bae, E.J.; Yang, N.Y.; Song, M.; Lee, C.S.; Lee, J.S.; Jung, B.C.; Lee, H.J.; Kim, S.; Masliah, E.; Sardi, S.P.; et al. Glucocerebrosidase Depletion Enhances Cell-to-Cell Transmission of α-Synuclein. Nat. Commun. 2014, 5, 4755. [Google Scholar] [CrossRef] [PubMed]
- Vassileff, N.; Cheng, L.; Hill, A.F. Extracellular Vesicles—Propagators of Neuropathology and Sources of Potential Biomarkers and Therapeutics for Neurodegenerative Diseases. J. Cell Sci. 2020, 133, jcs243139. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, X.; Chen, L.; Hu, B.; Wang, H.; Huang, W. The Biology, Pathological Roles of Exosomes and Their Clinical Application in Parkinson’s Disease. Neuroscience 2023, 531, 24–38. [Google Scholar] [CrossRef]
- Weiß, A.; Matamoros-Angles, A.; Boros, F.A.; Arnold, P.; Zunke, F. Extracellular Vesicles—Upcoming Biomarkers in Parkinson’s Disease’s Biofluids. Trillium Extracell. Vesicles 2022, 4, 45–51. [Google Scholar] [CrossRef]
- Cerri, S.; Ghezzi, C.; Sampieri, M.; Siani, F.; Avenali, M.; Dornini, G.; Zangaglia, R.; Minafra, B.; Blandini, F. The Exosomal/Total α-Synuclein Ratio in Plasma Is Associated with Glucocerebrosidase Activity and Correlates with Measures of Disease Severity in PD Patients. Front. Cell. Neurosci. 2018, 12, 125. [Google Scholar] [CrossRef]
- Tomlinson, P.R.; Zheng, Y.; Fischer, R.; Heidasch, R.; Gardiner, C.; Evetts, S.; Hu, M.; Wade-Martins, R.; Turner, M.R.; Morris, J.; et al. Identification of Distinct Circulating Exosomes in Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2015, 2, 353–361. [Google Scholar] [CrossRef]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; West, A.B. Ser(P)-1292 LRRK2 in Urinary Exosomes Is Elevated in Idiopathic Parkinson’s Disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Stuendl, A.; Kraus, T.; Chatterjee, M.; Zapke, B.; Sadowski, B.; Moebius, W.; Hobert, M.A.; Deuschle, C.; Brockmann, K.; Maetzler, W.; et al. α-Synuclein in Plasma-Derived Extracellular Vesicles Is a Potential Biomarker of Parkinson’s Disease. Mov. Disord. 2021, 36, 2508–2518. [Google Scholar] [CrossRef]
- Kulabukhova, D.G.; Garaeva, L.A.; Emelyanov, A.K.; Senkevich, K.A.; Gracheva, E.V.; Miliukhina, I.V.; Varfolomeeva, E.Y.; Timofeeva, A.A.; Schwartsman, A.L.; Shtam, T.A.; et al. Plasma Exosomes in Inherited Forms of Parkinson’s Disease. Mol. Biol. 2021, 55, 338–345. [Google Scholar] [CrossRef]
- Rani, K.; Mukherjee, R.; Singh, E.; Kumar, S.; Sharma, V.; Vishwakarma, P.; Bharti, P.S.; Nikolajeff, F.; Dinda, A.K.; Goyal, V.; et al. Neuronal Exosomes in Saliva of Parkinson’s Disease Patients: A Pilot Study. Park. Relat. Disord. 2019, 67, 21–23. [Google Scholar] [CrossRef]
- Gleason, A.M.; Woo, E.G.; McKinney, C.; Sidransky, E. The Role of Exosomes in Lysosomal Storage Disorders. Biomolecules 2021, 11, 576. [Google Scholar] [CrossRef]
- Eitan, E.; Suire, C.; Zhang, S.; Mattson, M.P. Impact of Lysosome Status on Extracellular Vesicle Content and Release. Ageing Res. Rev. 2016, 32, 65–74. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Schapira, A.H.; Gardiner, C.; Sargent, I.L.; Wood, M.J.A.; Cooper, J.M. Lysosomal Dysfunction Increases Exosome-Mediated Alpha-Synuclein Release and Transmission. Neurobiol. Dis. 2011, 42, 360–367. [Google Scholar] [CrossRef]
- Wang, X.; Berkowicz, A.; King, K.; Menta, B.; Gabrielli, A.P.; Novikova, L.; Troutwine, B.; Pleen, J.; Wilkins, H.M.; Swerdlow, R.H. Pharmacologic Enrichment of Exosome Yields and Mitochondrial Cargo. Mitochondrion 2022, 64, 136–144. [Google Scholar] [CrossRef]
- Kopytova, A.E.; Usenko, T.S.; Baydakova, G.V.; Nikolaev, M.A.; Senkevich, K.A.; Izyumchenko, A.D.; Tyurin, A.A.; Miliukhina, I.V.; Emelyanov, A.K.; Zakharova, E.Y.; et al. Could Blood Hexosylsphingosine Be a Marker for Parkinson’s Disease Linked with GBA1 Mutations? Mov. Disord. 2022, 37, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.K.; Usenko, T.S.; Kopytova, A.E.; Miliukhina, I.V.; Timofeeva, A.A.; Bezrukova, A.I.; Kulabukhova, D.G.; Baydakova, G.V.; Nikolaev, M.A.; Lavrinova, A.O.; et al. Blood Glucocerebrosidase Activity and α-Synuclein Levels in Patients with GBA1-Associated Parkinson’s Disease and Asymptomatic GBA1 Mutation Carriers. Ann. Clin. Exp. Neurol. 2024, 18, 50–57. [Google Scholar] [CrossRef]
- Pchelina, S.; Baydakova, G.; Nikolaev, M.; Senkevich, K.; Emelyanov, A.; Kopytova, A.; Miliukhina, I.; Yakimovskii, A.; Timofeeva, A.; Berkovich, O.; et al. Blood Lysosphingolipids Accumulation in Patients with Parkinson’s Disease with Glucocerebrosidase 1 Mutations. Mov. Disord. 2018, 33, 1325–1330. [Google Scholar] [CrossRef]
- Nikolaev, M.A.; Kopytova, A.E.; Baidakova, G.V.; Emel’yanov, A.K.; Salogub, G.N.; Senkevich, K.A.; Usenko, T.S.; Gorchakova, M.V.; Koval’chuk, Y.P.; Berkovich, O.A.; et al. Human Peripheral Blood Macrophages As a Model for Studying Glucocerebrosidase Dysfunction. Cell Tissue Biol. 2019, 13, 100–106. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Levy, O.A.; Waters, C.C.; Fahn, S.; Ford, B.; Kuo, S.H.; Mazzoni, P.; Pauciulo, M.W.; Nichols, W.C.; Gan-Or, Z.; et al. Glucocerebrosidase Activity in Parkinson’s Disease with and without GBA Mutations. Brain 2015, 138, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Omer, N.; Giladi, N.; Gurevich, T.; Bar-Shira, A.; Gana-Weisz, M.; Glinka, T.; Goldstein, O.; Kestenbaum, M.; Cedarbaum, J.M.; Mabrouk, O.S.; et al. Glucocerebrosidase Activity Is Not Associated with Parkinson’s Disease Risk or Severity. Mov. Disord. 2022, 37, 190–195. [Google Scholar] [CrossRef]
- Huh, Y.E.; Chiang, M.S.R.; Locascio, J.J.; Liao, Z.; Liu, G.; Choudhury, K.; Kuras, Y.I.; Tuncali, I.; Videnovic, A.; Hunt, A.L.; et al. β-Glucocerebrosidase Activity in GBA-Linked Parkinson Disease: The Type of Mutation Matters. Neurology 2020, 95, E685–E696. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Hammond, D.; Perera, G.; Dobson-Stone, C.; Mueller, N.; Pickford, R.; Kim, W.S.; Kwok, J.B.; Lewis, S.J.G.; Halliday, G.M.; et al. Reduced Glucocerebrosidase Activity in Monocytes from Patients with Parkinson’s Disease. Sci. Rep. 2018, 8, 15446. [Google Scholar] [CrossRef]
- Avenali, M.; Cerri, S.; Ongari, G.; Ghezzi, C.; Pacchetti, C.; Tassorelli, C.; Valente, E.M.; Blandini, F. Profiling the Biochemical Signature of GBA-Related Parkinson’s Disease in Peripheral Blood Mononuclear Cells. Mov. Disord. 2021, 36, 1267–1272. [Google Scholar] [CrossRef]
- Mu, C.; Shao, K.; Su, M.; Guo, Y.; Qiu, Y.; Sun, R.; Sun, S.; Sun, Y.; Liu, C.; Wang, W.; et al. Lysophosphatidylcholine Promoting α-Synuclein Aggregation in Parkinson’s Disease: Disrupting GCase Glycosylation and Lysosomal α-Synuclein Degradation. NPJ Park. Dis. 2025, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Romero, A.; Fernandez-Gonzalez, I.; Riera, J.; Montpeyo, M.; Albert-Bayo, M.; Lopez-Royo, T.; Castillo-Sanchez, P.; Carnicer-Caceres, C.; Arranz-Amo, J.A.; Castillo-Ribelles, L.; et al. Lysosomal Lipid Alterations Caused by Glucocerebrosidase Deficiency Promote Lysosomal Dysfunction, Chaperone-Mediated-Autophagy Deficiency, and Alpha-Synuclein Pathology. NPJ Park. Dis. 2022, 8, 126. [Google Scholar] [CrossRef]
- García-Sanz, P.; Orgaz, L.; Bueno-Gil, G.; Espadas, I.; Rodríguez-Traver, E.; Kulisevsky, J.; Gutierrez, A.; Dávila, J.C.; González-Polo, R.A.; Fuentes, J.M.; et al. N370S-GBA1 Mutation Causes Lysosomal Cholesterol Accumulation in Parkinson’s Disease. Mov. Disord. 2017, 32, 1409–1422. [Google Scholar] [CrossRef]
- Xylaki, M.; Chopra, A.; Weber, S.; Bartl, M.; Outeiro, T.F.; Mollenhauer, B. Extracellular Vesicles for the Diagnosis of Parkinson’s Disease: Systematic Review and Meta-Analysis. Mov. Disord. 2023, 38, 1585–1597. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-Speed Centrifugation Induces Aggregation of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef]
- Morandi, M.I.; Busko, P.; Ozer-Partuk, E.; Khan, S.; Zarfati, G.; Elbaz-Alon, Y.; Abou Karam, P.; Napso Shogan, T.; Ginini, L.; Gil, Z.; et al. Extracellular Vesicle Fusion Visualized by Cryo-Electron Microscopy. PNAS Nexus 2022, 1, pgac156. [Google Scholar] [CrossRef] [PubMed]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular Vesicles from Activated Platelets: A Semiquantitative Cryo-Electron Microscopy and Immuno-Gold Labeling Study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.N.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-Electron Microscopy of Extracellular Vesicles in Fresh Plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of Extracellular Vesicles in Human Ejaculates Revealed by Cryo-Electron Microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-Electron Microscopy of Extracellular Vesicles from Cerebrospinal Fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef]
- López de Frutos, L.; Almeida, F.; Murillo-Saich, J.; Conceição, V.A.; Guma, M.; Queheberger, O.; Giraldo, P.; Miltenberger-Miltenyi, G. Serum Phospholipid Profile Changes in Gaucher Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10387. [Google Scholar] [CrossRef] [PubMed]
- Guedes, L.C.; Chan, R.B.; Gomes, M.A.; Conceição, V.A.; Machado, R.B.; Soares, T.; Xu, Y.; Gaspar, P.; Carriço, J.A.; Alcalay, R.N.; et al. Serum Lipid Alterations in GBA-Associated Parkinson’s Disease. Park. Relat. Disord. 2017, 44, 58–65. [Google Scholar] [CrossRef]
- Kurzawa-Akanbi, M.; Tammireddy, S.; Fabrik, I.; Gliaudelytė, L.; Doherty, M.K.; Heap, R.; Matečko-Burmann, I.; Burmann, B.M.; Trost, M.; Lucocq, J.M.; et al. Altered Ceramide Metabolism Is a Feature in the Extracellular Vesicle-Mediated Spread of Alpha-Synuclein in Lewy Body Disorders. Acta Neuropathol. 2021, 142, 961–984. [Google Scholar] [CrossRef]
- Grigor’eva, E.V.; Kopytova, A.E.; Yarkova, E.S.; Pavlova, S.V.; Sorogina, D.A.; Malakhova, A.A.; Malankhanova, T.B.; Baydakova, G.V.; Zakharova, E.Y.; Medvedev, S.P.; et al. Biochemical Characteristics of IPSC-Derived Dopaminergic Neurons from N370S GBA Variant Carriers with and without Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 4437. [Google Scholar] [CrossRef] [PubMed]
- Schöndorf, D.C.; Aureli, M.; McAllister, F.E.; Hindley, C.J.; Mayer, F.; Schmid, B.; Sardi, S.P.; Valsecchi, M.; Hoffmann, S.; Schwarz, L.K.; et al. IPSC-Derived Neurons from GBA1-Associated Parkinson’s Disease Patients Show Autophagic Defects and Impaired Calcium Homeostasis. Nat. Commun. 2014, 5, 4028. [Google Scholar] [CrossRef]
- Fernandes, H.J.R.; Hartfield, E.M.; Christian, H.C.; Emmanoulidou, E.; Zheng, Y.; Booth, H.; Bogetofte, H.; Lang, C.; Ryan, B.J.; Sardi, S.P.; et al. ER Stress and Autophagic Perturbations Lead to Elevated Extracellular α-Synuclein in GBA-N370S Parkinson’s IPSC-Derived Dopamine Neurons. Stem Cell Rep. 2016, 6, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Bezrukova, A.I.; Basharova, K.S.; Emelyanov, A.K.; Rybakov, A.V.; Miliukhina, I.V.; Pchelina, S.N.; Usenko, T.S. Autophagy Process in Parkinson’s Disease Depends on Mutations in the GBA1 and LRRK2 Genes. Biochem. Genet. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Fredriksen, K.; Aivazidis, S.; Sharma, K.; Burbidge, K.J.; Pitcairn, C.; Zunke, F.; Gelyana, E.; Mazzulli, J.R. Pathological α-Syn Aggregation Is Mediated by Glycosphingolipid Chain Length and the Physiological State of α-Syn In Vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2108489118. [Google Scholar] [CrossRef]
- Galvagnion, C.; Marlet, F.R.; Cerri, S.; Schapira, A.H.V.; Blandini, F.; Di Monte, D.A. Sphingolipid Changes in Parkinson L444P GBA Mutation Fibroblasts Promote α-Synuclein Aggregation. Brain 2022, 145, 1038–1051. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Cerri, S.; Ghezzi, C.; Ongari, G.; Croce, S.; Avenali, M.; Zangaglia, R.; Di Monte, D.A.; Valente, E.M.; Blandini, F. GBA Mutations Influence the Release and Pathological Effects of Small Extracellular Vesicles from Fibroblasts of Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 2215. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kojima, M.; Kurosawa, T.; Sasaki, R.; Ichihara, S.; Hiraku, Y.; Tomimoto, H.; Murata, M.; Oikawa, S. Proteomic Profiling of Exosomal Proteins for Blood-Based Biomarkers in Parkinson’s Disease. Neuroscience 2018, 392, 121–128. [Google Scholar] [CrossRef]
- Jiang, R.; Rong, C.; Ke, R.; Meng, S.; Yan, X.; Ke, H.; Wu, S.; Azim, A. Differential Proteomic Analysis of Serum Exosomes Reveals Alterations in Progression of Parkinson Disease. Medicine 2019, 98, e17478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Liu, X.; Zhang, J.; Gao, Y.; Li, S.; Chang, C.; Liu, X.; Yang, G. Comparative Proteomic Analysis of Plasma Exosomes Reveals the Functional Contribution of N-Acetyl-alpha-glucosaminidase to Parkinson’s Disease. Neural Regen. Res. 2025, 20, 2998–3012. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of Exosomes in Central Nervous System Diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef]
- Dubiela, P.; Szymańska-Rożek, P.; Eljaszewicz, A.; Lipiński, P.; Hasiński, P.; Giersz, D.; Walewska, A.; Tynecka, M.; Moniuszko, M.; Tylki-Szymańska, A. Alpha-Synuclein MRNA Level Found Dependent on L444P Variant in Carriers and Gaucher Disease Patients on Enzyme Replacement Therapy. Biomolecules 2023, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Flores-León, M.; Outeiro, T.F. Expanding Our Understanding of Synucleinopathies: Proteinopathy, Proteinopenia, and Lipidopathy. FEBS J. 2025, 292, 4774–4788. [Google Scholar] [CrossRef] [PubMed]
- Sarchione, A.; Marchand, A.; Taymans, J.M.; Chartier-Harlin, M.C. Alpha-Synuclein and Lipids: The Elephant in the Room? Cells 2021, 10, 2452. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Pucha, K.A.; Mustapic, M.; Exarchos, T.P.; Vlamos, P.; Kapogiannis, D. Lipidomic Analysis of Plasma Extracellular Vesicles Derived from Alzheimer’s Disease Patients. Cells 2024, 13, 702. [Google Scholar] [CrossRef]
- Mohamed, A.; Molendijk, J.; Hill, M.M. Lipidr: A Software Tool for Data Mining and Analysis of Lipidomics Datasets. J. Proteome Res. 2020, 19, 2890–2897. [Google Scholar] [CrossRef]
- Vaudel, M.; Barsnes, H.; Berven, F.S.; Sickmann, A.; Martens, L. SearchGUI: An Open-Source Graphical User Interface for Simultaneous OMSSA and X!Tandem Searches. Proteomics 2011, 11, 996–999. [Google Scholar] [CrossRef]
- Zorina, E.; Ronzhina, N.; Legina, O.; Klopov, N.; Zgoda, V.; Naryzhny, S. Proteoform Patterns in Hepatocellular Carcinoma Tissues: Aspects of Oncomarkers. Proteomes 2025, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant Computational Platform for Mass Spectrometry-Based Shotgun Proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Zhang, X.; Smits, A.H.; Van Tilburg, G.B.A.; Ovaa, H.; Huber, W.; Vermeulen, M. Proteome-Wide Identification of Ubiquitin Interactions Using UbIA-MS. Nat. Protoc. 2018, 13, 530–550. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape Plugin: Pathway Insights Using Integrated Experimental and In Silico Data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usenko, T.S.; Kopytova, A.E.; Izyumchenko, A.D.; Kulabukhova, D.G.; Silantyev, A.S.; Kazakova, V.D.; Basharova, K.S.; Bezrukova, A.I.; Garaeva, L.A.; Pichkur, E.B.; et al. p.N370S GBA1 Mutation Influences the Morphology and Lipid Composition of Extracellular Vesicles in Blood Plasma from Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 9152. https://doi.org/10.3390/ijms26189152
Usenko TS, Kopytova AE, Izyumchenko AD, Kulabukhova DG, Silantyev AS, Kazakova VD, Basharova KS, Bezrukova AI, Garaeva LA, Pichkur EB, et al. p.N370S GBA1 Mutation Influences the Morphology and Lipid Composition of Extracellular Vesicles in Blood Plasma from Patients with Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(18):9152. https://doi.org/10.3390/ijms26189152
Chicago/Turabian StyleUsenko, Tatiana S., Alena E. Kopytova, Artem D. Izyumchenko, Darya G. Kulabukhova, Artemiy S. Silantyev, Victoria D. Kazakova, Katerina S. Basharova, Anastasia I. Bezrukova, Luiza A. Garaeva, Evgeny B. Pichkur, and et al. 2025. "p.N370S GBA1 Mutation Influences the Morphology and Lipid Composition of Extracellular Vesicles in Blood Plasma from Patients with Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 18: 9152. https://doi.org/10.3390/ijms26189152
APA StyleUsenko, T. S., Kopytova, A. E., Izyumchenko, A. D., Kulabukhova, D. G., Silantyev, A. S., Kazakova, V. D., Basharova, K. S., Bezrukova, A. I., Garaeva, L. A., Pichkur, E. B., Artynyuk, A. V., Miliukhina, I. V., Timofeeva, A. A., Miroshnikova, V. V., Naryzhny, S. N., Emelyanov, A. K., Zakharzhevskaya, N. B., Konevega, A. L., Shtam, T. A., & Pchelina, S. N. (2025). p.N370S GBA1 Mutation Influences the Morphology and Lipid Composition of Extracellular Vesicles in Blood Plasma from Patients with Parkinson’s Disease. International Journal of Molecular Sciences, 26(18), 9152. https://doi.org/10.3390/ijms26189152





