Targeting Mitochondrial Function in Plasmodium falciparum: Insight into Antimalarial Drugs and the Emerging Role of Saccharomyces cerevisiae as a Model System
Abstract
1. Introduction
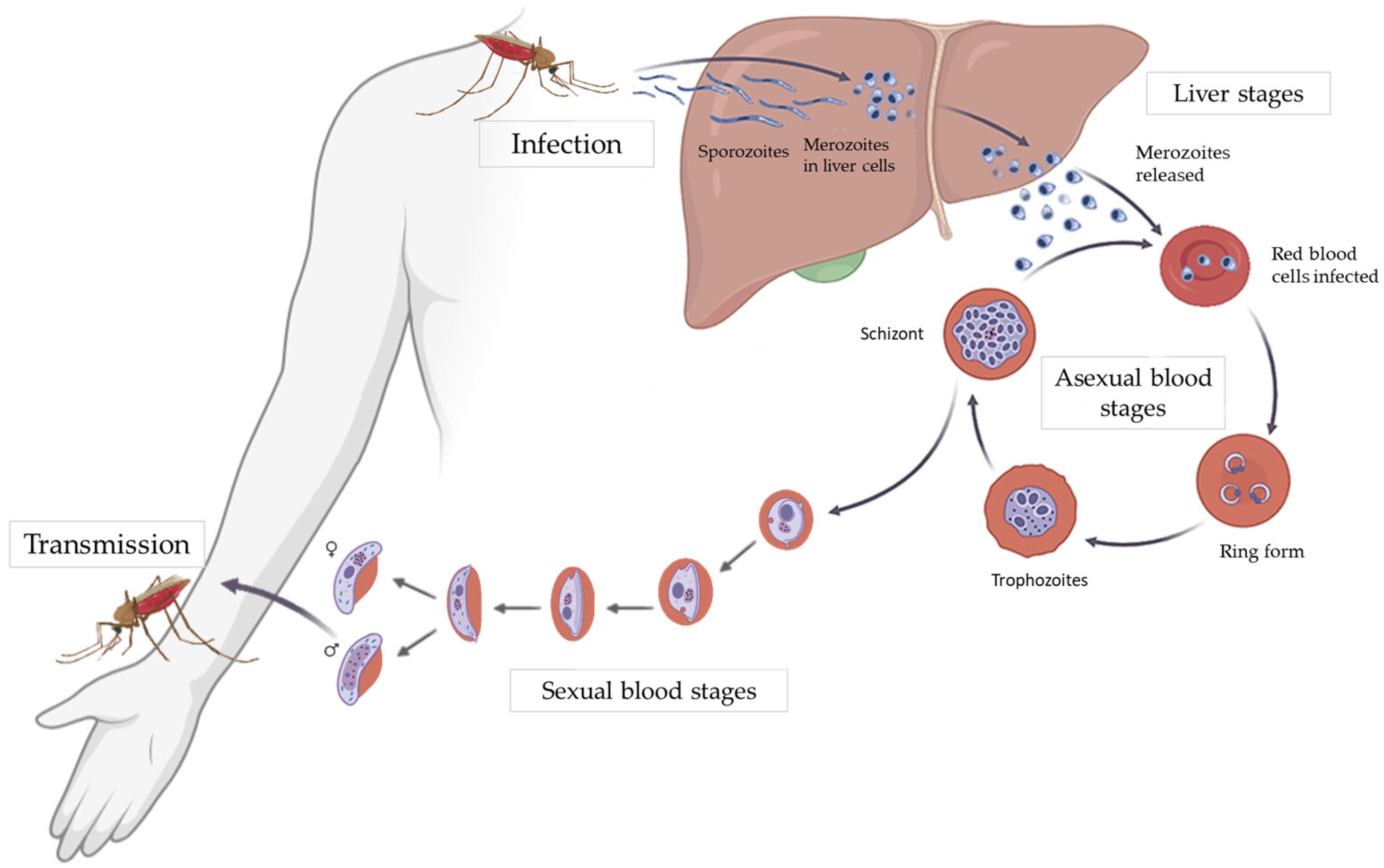
2. Role of Mitochondria in the Parasite Life Cycle
2.1. General Overview of the Parasite Life Cycle
2.2. Mitochondrial Metabolism of the Parasite in the Liver Stage
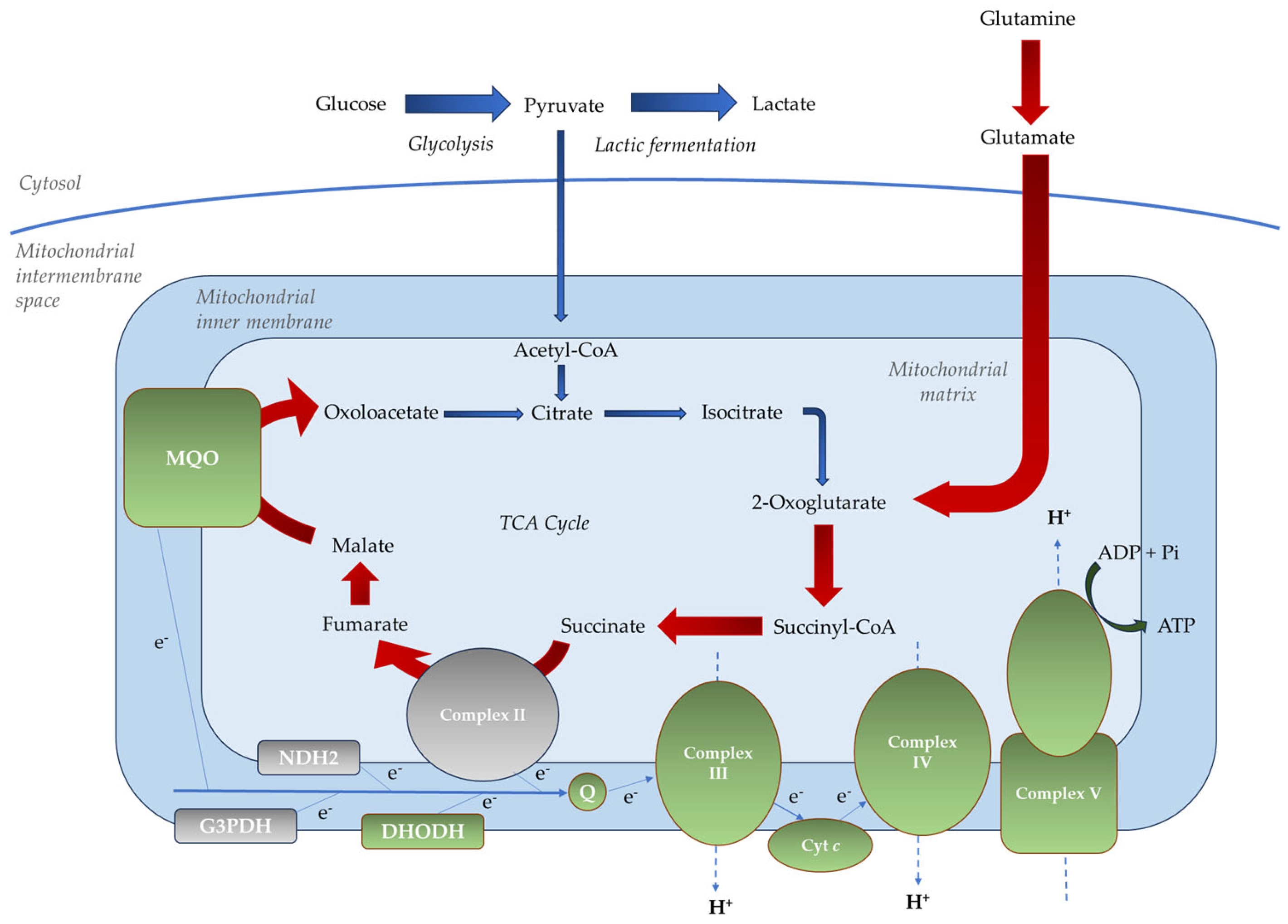
2.3. Mitochondrial Metabolism of the Parasite in the Asexual Blood Stages
- Non-proton motive quinone reductases, such as dihydroorotate dehydrogenase (PfDHODH), malate-quinone oxidoreductase (PfMQO), glycerol 3-phosphate dehydrogenase (PfG3PDH), type II NADH dehydrogenase (PfNDH2, alternative complex I), and succinate dehydrogenase (PfSDH, complex II);
2.4. Mitochondrial Metabolism of the Parasite in the Sexual Blood Stages
3. Antimalarial Agents That Target Mitochondrial Function
3.1. Agents Targeting the mETC
3.1.1. Inhibitors of PfNDH2
3.1.2. Inhibitors of PfSDH
3.1.3. Inhibitors of Complex III
3.1.4. Inhibitors of Complex IV
3.1.5. Inhibitors of Complex V
3.1.6. Inhibitors of PfDHODH
3.1.7. Inhibitors of PfMQO
3.2. Other Mitochondrial Targets
4. Potential Therapeutic Applications of Mitochondria-Targeting Agents Across Different Stages of the Parasite Life Cycle
5. The Yeast S. cerevisiae as a Model for Malaria Research: From General Considerations to Mitochondrial Pharmacological Targeting
5.1. General Advantages of S. cerevisiae as a Model for P. falciparum Research
5.2. Functional and Structural Analogies and Divergences Between the Mitochondrion of P. falciparum and S. cerevisiae
5.3. S. cerevisiae as a Model Organism to Study the Function of P. falciparum Mitochondrial Proteins
5.4. S. cerevisiae as a Model Organism for the Identification and Characterization of Molecular Targets of Promising Antimalarial Compounds
5.5. S. cerevisiae as a Model Organism to Characterize the Mechanism of Action and/or Resistance of Antimalarial Compounds
5.5.1. Plasmodione
5.5.2. Proguanil
5.5.3. ARTs
5.5.4. Primaquine
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | Asexual blood stages |
| ACTs | Artemisinin-based combination therapies |
| ADME | Absorption, distribution, metabolism, and excretion |
| ALA | 5-Aminolevulinic acid |
| ANT | Adenine nucleotide translocator |
| ART | Artemisinin |
| ARTs | Artemisinin and its derivatives |
| CLogP | Calculated logarithm of the partition coefficient |
| CoQ | Coenzyme Q |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CTT1 | Cytosolic catalase |
| CybL | Cytochrome b large |
| CybS | Cytochrome b small |
| Cyt c | Cytochrome c |
| DHA | Dihydroartemisinin |
| EC50 | Half-maximal effective concentration |
| ELQs | Endochin-like quinolones |
| Fp | Flavoprotein |
| G3PDH | Glycerol 3-phosphate dehydrogenase |
| GSK | GlaxoSmithKline |
| HTS | High-throughput screening |
| IC50 | Half-maximal inhibitory concentration |
| Ip | Iron–sulfur cluster protein |
| Ki | Inhibitory constant |
| LSs | Liver stages |
| Mdh1 | Mitochondrial malate dehydrogenase |
| mETC | Mitochondrial electron transport chain |
| MMV | Medicines for Malaria Venture |
| NaFAc | Sodium fluoroacetate |
| ND | Not detected |
| PD | Plasmodione |
| PfACO | Plasmodium falciparum aconitase |
| PfBCKDH | Plasmodium falciparum branched chain ketoacid dehydrogenase complex |
| PfCHA | Plasmodium falciparum Ca2+/H+ antiporter |
| PfCOCP | Plasmodium falciparum citrate/oxoglutarate carrier protein |
| PfDHODH | Plasmodium falciparum dihydroorotate dehydrogenase |
| PfENT1 | Plasmodium falciparum purine transporter |
| PfFH | Plasmodium falciparum fumarate hydratase |
| PfG3PDH | Plasmodium falciparum glycerol 3-phosphate dehydrogenase |
| PfIDH | Plasmodium falciparum isocitrate dehydrogenase |
| PfKDH | Plasmodium falciparum α-ketoglutarate dehydrogenase |
| PfMQO | Plasmodium falciparum malate-quinone oxidoreductase |
| PfNDH2 | Plasmodium falciparum type II NADH dehydrogenase |
| PfPiT | Plasmodium falciparum phosphate transporter |
| PfSDH | Plasmodium falciparum succinate dehydrogenase |
| PHB | Prohibitin |
| Phyre2 | Protein homology/analogY recognition engine |
| Pi | Inorganic phosphate |
| PK | Pharmacokinetic |
| PQ | Primaquine |
| RBCs | Red blood cells |
| ROS | Reactive oxygen species |
| SBS | Sexual blood stages |
| SCID | Severe combined immunodeficiency |
| SFC | Sodium ferrous citrate |
| SOD1 | Cytosolic superoxide dismutase |
| SOD2 | Mitochondrial superoxide dismutase |
| TCA | Tricarboxylic acid |
| TCPs | Target candidate profiles |
| WHO | World Health Organization |
| Δψm | Mitochondrial membrane potential |
References
- World Malaria Report 2024; World Health Organization: Geneva, Switzerland, 2024; ISBN 9789240086173.
- Crabb, B.S.; Cowman, A.; Crabb, B. Plasmodium falciparum Virulence Determinants Unveiled. Genome Biol. 2002, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.B.; Mather, M.W. Mitochondrial Evolution and Functions in Malaria Parasites. Annu. Rev. Microbiol. 2009, 63, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.-P.; Giannangelo, C.; Siddiqui, G.; Creek, D.J. Promising Antimalarial Hits from Phenotypic Screens: A Review of Recently-Described Multi-Stage Actives and Their Modes of Action. Front. Cell Infect. Microbiol. 2023, 13, 1308193. [Google Scholar] [CrossRef]
- Birkholtz, L.M.; Alano, P.; Leroy, D. Transmission-Blocking Drugs for Malaria Elimination. Trends Parasitol. 2022, 38, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Hentzschel, F.; Valkiūnas, G.; Marti, M. Plasmodium Asexual Growth and Sexual Development in the Haematopoietic Niche of the Host. Nat. Rev. Microbiol. 2020, 18, 177–189. [Google Scholar] [CrossRef]
- Appetecchia, F.; Fabbrizi, E.; Fiorentino, F.; Consalvi, S.; Biava, M.; Poce, G.; Rotili, D. Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development. Pharmaceuticals 2024, 17, 962. [Google Scholar] [CrossRef]
- Goswami, D.; Minkah, N.K.; Kappe, S.H.I. Malaria Parasite Liver Stages. J. Hepatol. 2022, 76, 735–737. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Kappe, S.H.I. Malaria Parasite Liver Infection and Exoerythrocytic Biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025486. [Google Scholar] [CrossRef]
- Meis, J.F.G.M.; Rijntjes, J.M.; Verhave, J.-P.; Ponnudurai, T.; Hollingdale, M.R.; Smith, J.E.; Sinden, R.E.; Jap, P.H.K.; Meuwissen, J.H.; Yap, S.H. Fine Structure of the Malaria Parasite Plasmodium falciparum in Human Hepatocytes in Vitro. Cell Tissue Res. 1986, 244, 345–350. [Google Scholar] [CrossRef]
- Tarun, A.S.; Peng, X.; Dumpit, R.F.; Ogata, Y.; Silva-Rivera, H.; Camargo, N.; Daly, T.M.; Bergman, L.W.; Kappe, S.H.I. A Combined Transcriptome and Proteome Survey of Malaria Parasite Liver Stages. Proc. Natl. Acad. Sci. USA 2008, 105, 305–310. [Google Scholar] [CrossRef]
- Stanway, R.R.; Bushell, E.; Chiappino-Pepe, A.; Roques, M.; Sanderson, T.; Franke-Fayard, B.; Caldelari, R.; Golomingi, M.; Nyonda, M.; Pandey, V.; et al. Genome-Scale Identification of Essential Metabolic Processes for Targeting the Plasmodium Liver Stage. Cell 2019, 179, 1112–1128.e26. [Google Scholar] [CrossRef]
- Suryavanshi, A.; Chandrashekarmath, A.; Pandey, N.; Balaram, H. Metabolic Flexibility and Essentiality of the Tricarboxylic Acid Cycle in Plasmodium. ACS Infect. Dis. 2025, 11, 335–349. [Google Scholar] [CrossRef]
- Lahree, A.; Mello-Vieira, J.; Mota, M.M. The Nutrient Games—Plasmodium Metabolism during Hepatic Development. Trends Parasitol. 2023, 39, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, S.A.; McConville, M.J. The Plasmodium Tricarboxylic Acid Cycle and Mitochondrial Metabolism. In Encyclopedia of Malaria; Springer: New York, NY, USA, 2014; pp. 1–18. [Google Scholar]
- Evers, F.; Cabrera-Orefice, A.; Elurbe, D.M.; Lindert, M.K.; Boltryk, S.D.; Voss, T.S.; Huynen, M.A.; Brandt, U.; Kooij, T.W.A. Composition and Stage Dynamics of Mitochondrial Complexes in Plasmodium falciparum. Nat. Commun. 2021, 12, 3820. [Google Scholar] [CrossRef] [PubMed]
- MacRae, J.I.; Dixon, M.W.A.; Dearnley, M.K.; Chua, H.H.; Chambers, J.M.; Kenny, S.; Bottova, I.; Tilley, L.; McConville, M.J. Mitochondrial Metabolism of Sexual and Asexual Blood Stages of the Malaria Parasite Plasmodium falciparum. BMC Biol. 2013, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, D.D.; du Toit, F.; Green, K.; Palm, D.; Snoep, J.L. A Detailed Kinetic Model of Glycolysis in Plasmodium falciparum-Infected Red Blood Cells for Antimalarial Drug Target Identification. J. Biol. Chem. 2023, 299, 105111. [Google Scholar] [CrossRef]
- Ginsburg, H. Malaria Parasite Stands Out. Nature 2010, 466, 702–703. [Google Scholar] [CrossRef]
- Oppenheim, R.D.; Creek, D.J.; Macrae, J.I.; Modrzynska, K.K.; Pino, P.; Limenitakis, J.; Polonais, V.; Seeber, F.; Barrett, M.P.; Billker, O.; et al. BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of Toxoplasma Gondii and Plasmodium berghei. PLoS Pathog. 2014, 10, e1004263. [Google Scholar] [CrossRef]
- Srivastava, A.; Philip, N.; Hughes, K.R.; Georgiou, K.; MacRae, J.I.; Barrett, M.P.; Creek, D.J.; McConville, M.J.; Waters, A.P. Stage-Specific Changes in Plasmodium Metabolism Required for Differentiation and Adaptation to Different Host and Vector Environments. PLoS Pathog. 2016, 12, e1006094. [Google Scholar] [CrossRef]
- Rajaram, K.; Tewari, S.G.; Wallqvist, A.; Prigge, S.T. Metabolic Changes Accompanying the Loss of Fumarate Hydratase and Malate–Quinone Oxidoreductase in the Asexual Blood Stage of Plasmodium falciparum. J. Biol. Chem. 2022, 298, 101897. [Google Scholar] [CrossRef]
- Mogi, T.; Kita, K. Diversity in Mitochondrial Metabolic Pathways in Parasitic Protists Plasmodium and Cryptosporidium. Parasitol. Int. 2010, 59, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery. Infect. Drug Resist. 2020, 13, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Ganesan, S.M.; Dass, S.; Morrisey, J.M.; Pou, S.; Nilsen, A.; Riscoe, M.K.; Mather, M.W.; Vaidya, A.B. Mitochondrial Type II NADH Dehydrogenase of Plasmodium falciparum (PfNDH2) Is Dispensable in the Asexual Blood Stages. PLoS ONE 2019, 14, 0214023. [Google Scholar] [CrossRef]
- Boysen, K.E.; Matuschewski, K. Arrested Oocyst Maturation in Plasmodium Parasites Lacking Type II NADH:Ubiquinone Dehydrogenase. J. Biol. Chem. 2011, 286, 32661–32671. [Google Scholar] [CrossRef]
- Hino, A.; Hirai, M.; Tanaka, T.Q.; Watanabe, Y.I.; Matsuoka, H.; Kita, K. Critical Roles of the Mitochondrial Complex II in Oocyst Formation of Rodent Malaria Parasite Plasmodium berghei. J. Biochem. 2012, 152, 259–268. [Google Scholar] [CrossRef]
- Ke, H.; Lewis, I.A.; Morrisey, J.M.; McLean, K.J.; Ganesan, S.M.; Painter, H.J.; Mather, M.W.; Jacobs-Lorena, M.; Llinás, M.; Vaidya, A.B. Genetic Investigation of Tricarboxylic Acid Metabolism during the Plasmodium falciparum Life Cycle. Cell Rep. 2015, 11, 164–174. [Google Scholar] [CrossRef]
- Sheokand, P.K.; Pradhan, S.; Maclean, A.E.; Mühleip, A.; Sheiner, L. Plasmodium falciparum Mitochondrial Complex III, the Target of Atovaquone, Is Essential for Progression to the Transmissible Sexual Stages. Int. J. Mol. Sci. 2024, 25, 9239. [Google Scholar] [CrossRef]
- Painter, H.J.; Morrisey, J.M.; Mather, M.W.; Vaidya, A.B. Specific Role of Mitochondrial Electron Transport in Blood-Stage Plasmodium falciparum. Nature 2007, 446, 88–91. [Google Scholar] [CrossRef]
- Nina, P.B.; Morrisey, J.M.; Ganesan, S.M.; Ke, H.; Pershing, A.M.; Mather, M.W.; Vaidya, A.B. ATP Synthase Complex of Plasmodium falciparum: Dimeric Assembly in Mitochondrial Membranes and Resistance to Genetic Disruption. J. Biol. Chem. 2011, 286, 41312–41322. [Google Scholar] [CrossRef]
- Josling, G.A.; Llinás, M. Sexual Development in Plasmodium Parasites: Knowing When It’s Time to Commit. Nat. Rev. Microbiol. 2015, 13, 573–587. [Google Scholar] [CrossRef]
- Farfour, E.; Charlotte, F.; Settegrana, C.; Miyara, M.; Buffet, P. The Extravascular Compartment of the Bone Marrow: A Niche for Plasmodium falciparum Gametocyte Maturation? Malar. J. 2012, 11, 285. [Google Scholar] [CrossRef]
- Aguilar, R.; Magallon-Tejada, A.; Achtman, A.H.; Moraleda, C.; Joice, R.; Cisteró, P.; Li Wai Suen, C.S.N.; Nhabomba, A.; Macete, E.; Mueller, I.; et al. Molecular Evidence for the Localization of Plasmodium falciparum Immature Gametocytes in Bone Marrow. Blood 2014, 123, 959–966. [Google Scholar] [CrossRef]
- Sturm, A.; Mollard, V.; Cozijnsen, A.; Goodman, C.D.; McFadden, G.I. Mitochondrial ATP Synthase Is Dispensable in Blood-Stage Plasmodium berghei Rodent Malaria but Essential in the Mosquito Phase. Proc. Natl. Acad. Sci. USA 2015, 112, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ke, H.; Morrisey, J.M.; Mather, M.W.; Vaidya, A.B. Genetic Disruption of Isocitrate Dehydrogenase Arrests the Full Development of Sexual Stage Parasites in Plasmodium falciparum. bioRxiv 2022. [Google Scholar] [CrossRef]
- Goodman, C.D.; Buchanan, H.D.; McFadden, G.I. Is the Mitochondrion a Good Malaria Drug Target? Trends Parasitol. 2017, 33, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.L.; Pidathala, C.; Shone, A.E.; Antoine, T.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Targeting the Mitochondrial Electron Transport Chain of Plasmodium falciparum: New Strategies towards the Development of Improved Antimalarials for the Elimination Era. Future Med. Chem. 2013, 5, 1573–1591. [Google Scholar] [CrossRef]
- Phillips, M.A.; Lotharius, J.; Marsh, K.; White, J.; Dayan, A.; White, K.L.; Njoroge, J.W.; El Mazouni, F.; Lao, Y.; Kokkonda, S.; et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl. Med. 2015, 7, 296ra111. [Google Scholar] [CrossRef]
- Bonive-Boscan, A.D.; Acosta, H.; Rojas, A. Metabolic Changes That Allow Plasmodium falciparum Artemisinin-Resistant Parasites to Tolerate Oxidative Stress. Front. Parasitol. 2024, 3, 1461641. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, T.; Li, X.; Michel, T.; Ling, L.; Huang, Z.; Mulaka, M.; Wu, Y.; Gao, H.; Wang, L.; et al. Design, Synthesis, and Biological Evaluation of Multiple Targeting Antimalarials. Acta Pharm. Sin. B 2021, 11, 2900–2913. [Google Scholar] [CrossRef]
- Burnell, E.S. Drugs Targeting Mitochondrial Functions. In Antimalarial Agents: Design and Mechanism of Action; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–402. ISBN 9780081012109. [Google Scholar]
- Cao, Y.; Sun, C.; Wen, H.; Wang, M.; Zhu, P.; Zhong, M.; Li, J.; Chen, X.; Tang, Y.; Wang, J.; et al. A Yeast-Based Drug Discovery Platform To Identify Plasmodium falciparum Type II NADH Dehydrogenase Inhibitors. Antimicrob. Agents Chemother. 2021, 65, e02470-20. [Google Scholar] [CrossRef]
- Ward, S.A.; Fisher, N.; Hill, A.; Mbekeani, A.; Shone, A.; Nixon, G.; Stocks, P.; Gibbons, P.; Amewu, R.; Hong, D.W.; et al. A Novel Drug for Uncomplicated Malaria: Targeted High Throughput Screening (HTS) against the Type II NADH:Ubiquinone Oxidoreductase (PfNdh2) of Plasmodium falciparum. Malar. J. 2010, 9, I14. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Li, X.; Li, J.; Wu, Y.; Yu, J.; Ge, J.; Huang, Z.; Jiang, L.; Rao, Y.; et al. Target Elucidation by Cocrystal Structures of NADH-Ubiquinone Oxidoreductase of Plasmodium falciparum (PfNDH2) with Small Molecule To Eliminate Drug-Resistant Malaria. J. Med. Chem. 2017, 60, 1994–2005. [Google Scholar] [CrossRef]
- Xie, T.; Wu, Z.; Gu, J.; Guo, R.; Yan, X.; Duan, H.; Liu, X.; Liu, W.; Liang, L.; Wan, H.; et al. The Global Motion Affecting Electron Transfer in: Plasmodium falciparum Type II NADH Dehydrogenases: A Novel Non-Competitive Mechanism for Quinoline Ketone Derivative Inhibitors. Phys. Chem. Chem. Phys. 2019, 21, 1805–18118. [Google Scholar] [CrossRef] [PubMed]
- Amporndanai, K.; Pinthong, N.; O’Neill, P.M.; Hong, W.D.; Amewu, R.K.; Pidathala, C.; Berry, N.G.; Leung, S.C.; Ward, S.A.; Biagini, G.A.; et al. Targeting the Ubiquinol-Reduction (Qi) Site of the Mitochondrial Cytochrome Bc1 Complex for the Development of Next Generation Quinolone Antimalarials. Biology 2022, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Rhee, K.H. Antimalarial Activity of Nepodin Isolated from Rumex Crispus. Arch. Pharm. Res. 2013, 36, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, B.C.; He, L.F.; Xiao, C.J.; Jiang, B.; Shen, L. Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species. Molecules 2024, 29, 4759. [Google Scholar] [CrossRef]
- Harada, S.; Inaoka, D.K.; Ohmori, J.; Kita, K. Diversity of Parasite Complex II. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 658–667. [Google Scholar] [CrossRef]
- Komatsuya, K.; Sakura, T.; Shiomi, K.; Ōmura, S.; Hikosaka, K.; Nozaki, T.; Kita, K.; Inaoka, D.K. Siccanin Is a Dual-Target Inhibitor of Plasmodium falciparum Mitochondrial Complex II and Complex III. Pharmaceuticals 2022, 15, 903. [Google Scholar] [CrossRef]
- Sumsakul, W.; Plengsuriyakarn, T.; Chaijaroenkul, W.; Viyanant, V.; Karbwang, J.; Na-Bangchang, K. Antimalarial Activity of Plumbagin in Vitro and in Animal Models. BMC Complement. Altern. Med. 2014, 14, 15. [Google Scholar] [CrossRef]
- Kita, K.; Hirawake, H.; Miyadera, H.; Amino, H.; Takeo, S. Role of Complex II in Anaerobic Respiration of the Parasite Mitochondria from Ascaris suum and Plasmodium falciparum. Biochim. Biophys. Acta Bioenerg. 2002, 1553, 123–139. [Google Scholar] [CrossRef]
- Mi-Ichi, F.; Miyadera, H.; Kobayashi, T.; Takamiya, S.; Waki, S.; Iwata, S.; Shibata, S.; Kita, K. Parasite Mitochondria as a Target of Chemotherapy: Inhibitory Effect of Licochalcone A on the Plasmodium falciparum Respiratory Chain. Ann. N. Y. Acad. Sci. 2005, 1056, 46–54. [Google Scholar] [CrossRef]
- Lunev, S.; Batista, F.A.; Bosch, S.S.; Wrenger, C.; Groves, M.R. Identification and Validation of Novel Drug Targets for the Treatment of Plasmodium falciparum Malaria: New Insights. In Current Topics in Malaria; InTech: Vienna, Austria, 2016. [Google Scholar]
- Duffy, S.; Sleebs, B.E.; Avery, V.M. An Adaptable, Fit-for-Purpose Screening Approach with High-Throughput Capability to Determine Speed of Action and Stage Specificity of Anti-Malarial Compounds. Antimicrob. Agents Chemother. 2024, 68, e0074624. [Google Scholar] [CrossRef]
- Nixon, G.L.; Ward, S.A.; O’Neill, P.M.; Biagini, G.A. Inhibitors of the Plasmodium Mitochondrial Respiratory Chain. In Encyclopedia of Malaria; Springer: New York, NY, USA, 2014; pp. 1–18. [Google Scholar]
- Probst, A.S.; Paton, D.G.; Appetecchia, F.; Bopp, S.; Adams, K.L.; Rinvee, T.A.; Pou, S.; Winter, R.; Du, E.W.; Yahiya, S.; et al. In Vivo Screen of Plasmodium Targets for Mosquito-Based Malaria Control. Nature 2025, 643, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Frueh, L.; Li, Y.; Mather, M.W.; Li, Q.; Pou, S.; Nilsen, A.; Winter, R.W.; Forquer, I.P.; Pershing, A.M.; Xie, L.H.; et al. Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria. ACS Infect. Dis. 2017, 3, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, A.; LaCrue, A.N.; White, K.L.; Forquer, I.P.; Cross, R.M.; Marfurt, J.; Mather, M.W.; Delves, M.J.; Shackleford, D.M.; Saenz, F.E.; et al. Quinolone-3-Diarylethers: A New Class of Antimalarial Drug. Sci. Transl. Med. 2013, 5, 177ra37. [Google Scholar] [CrossRef] [PubMed]
- Pou, S.; Winter, R.W.; Dodean, R.A.; Liebman, K.; Li, Y.; Mather, M.W.; Nepal, B.; Nilsen, A.; Handford, M.J.; Riscoe, T.M.; et al. 3-Position Biaryl Endochin-like Quinolones with Enhanced Antimalarial Performance. ACS Infect. Dis. 2024, 10, 2419–2442. [Google Scholar] [CrossRef]
- Nguyen, W.; Dans, M.G.; Currie, I.; Awalt, J.K.; Bailey, B.L.; Lumb, C.; Ngo, A.; Favuzza, P.; Palandri, J.; Ramesh, S.; et al. 7-N-Substituted-3-Oxadiazole Quinolones with Potent Antimalarial Activity Target the Cytochrome Bc1 Complex. ACS Infect. Dis. 2023, 9, 668–691. [Google Scholar] [CrossRef]
- Beteck, R.M.; Smit, F.J.; Haynes, R.K.; N’Da, D.D. Recent Progress in the Development of Anti-Malarial Quinolones. Malar. J. 2014, 13, 339. [Google Scholar] [CrossRef]
- Song, Z.; Iorga, B.I.; Mounkoro, P.; Fisher, N.; Meunier, B. The Antimalarial Compound ELQ-400 Is an Unusual Inhibitor of the Bc1 Complex, Targeting Both Qo and Qi Sites. FEBS Lett. 2018, 592, 1346–1356. [Google Scholar] [CrossRef]
- Van Schalkwyk, D.A.; Riscoe, M.K.; Pou, S.; Winter, R.W.; Nilsen, A.; Duffey, M.; Moon, R.W.; Sutherland, C.J. Novel Endochin-Like Quinolones Exhibit Potent In Vitro Activity against Plasmodium knowlesi but Do Not Synergize with Proguanil. Antimicrob. Agents Chemother. 2020, 64, e02549-19. [Google Scholar] [CrossRef]
- Kancharla, P.; Dodean, R.A.; Li, Y.; Pou, S.; Pybus, B.; Melendez, V.; Read, L.; Bane, C.E.; Vesely, B.; Kreishman-Deitrick, M.; et al. Lead Optimization of Second-Generation Acridones as Broad-Spectrum Antimalarials. J. Med. Chem. 2020, 63, 6179–6202. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.H.; McConnell, E.V.; Zarella, J.M.B.; Dodean, R.A.; Kancharla, P.; Kelly, J.X.; Doggett, J.S. Acridones Are Highly Potent Inhibitors of Toxoplasma Gondii Tachyzoites. ACS Infect. Dis. 2021, 7, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Yeates, C.L.; Batchelor, J.F.; Capon, E.C.; Cheesman, N.J.; Fry, M.; Hudson, A.T.; Pudney, M.; Trimming, H.; Woolven, J.; Bueno, J.M.; et al. Synthesis and Structure–Activity Relationships of 4-Pyridones as Potential Antimalarials. J. Med. Chem. 2008, 51, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.M.; Manzano, P.; García, M.C.; Chicharro, J.; Puente, M.; Lorenzo, M.; García, A.; Ferrer, S.; Gómez, R.M.; Fraile, M.T.; et al. Potent Antimalarial 4-Pyridones with Improved Physico-Chemical Properties. Bioorg. Med. Chem. Lett. 2011, 21, 5214–5218. [Google Scholar] [CrossRef]
- Calit, J.; Prajapati, S.K.; Benavente, E.D.; Araújo, J.E.; Deng, B.; Miura, K.; Annunciato, Y.; Moura, I.M.R.; Usui, M.; Medeiros, J.F.; et al. Pyrimidine Azepine Targets the Plasmodium Bc1 Complex and Displays Multistage Antimalarial Activity. JACS Au 2024, 4, 3942–3952. [Google Scholar] [CrossRef]
- Awalt, J.K.; Su, W.; Nguyen, W.; Loi, K.; Jarman, K.E.; Penington, J.S.; Ramesh, S.; Fairhurst, K.J.; Yeo, T.; Park, H.; et al. Exploration and Characterization of the Antimalarial Activity of Cyclopropyl Carboxamides That Target the Mitochondrial Protein, Cytochrome b. Eur. J. Med. Chem. 2024, 280, 116921. [Google Scholar] [CrossRef]
- Cruz, F.P.D.; Martin, C.; Buchholz, K.; Lafuente-Monasterio, M.J.; Rodrigues, T.; Sönnichsen, B.; Moreira, R.; Gamo, F.J.; Marti, M.; Mota, M.M.; et al. Drug Screen Targeted at Plasmodium Liver Stages Identifies a Potent Multistage Antimalarial Drug. J. Infect. Dis. 2012, 205, 1278–1286. [Google Scholar] [CrossRef]
- Gomez-Lorenzo, M.G.; Rodríguez-Alejandre, A.; Moliner-Cubel, S.; Martínez-Hoyos, M.; Bahamontes-Rosa, N.; Gonzalez del Rio, R.; Ródenas, C.; Fuente, J.; Lavandera, J.L.; García-Bustos, J.F.; et al. Functional Screening of Selective Mitochondrial Inhibitors of Plasmodium. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 295–303. [Google Scholar] [CrossRef]
- Uyemura, S.A.; Luo, S.; Vieira, M.; Moreno, S.N.J.; Docampo, R. Oxidative Phosphorylation and Rotenone-Insensitive Malate- and NADH-Quinone Oxidoreductases in Plasmodium yoelii yoelii Mitochondria in Situ. J. Biol. Chem. 2004, 279, 385–393. [Google Scholar] [CrossRef]
- BASCO, L.K.; LE BRAS, J. In Vitro Activity of Mitochondrial ATP Synthetase Inhibitors Against Plasmodium falciparum. J. Eukaryot. Microbiol. 1994, 41, 179–183. [Google Scholar] [CrossRef]
- Tougan, T.; Takahashi, K.; Ikegami-Kawai, M.; Horiuchi, M.; Mori, S.; Hosoi, M.; Horii, T.; Ihara, M.; Tsubuki, M. In Vitro and in Vivo Characterization of Anti-Malarial Acylphenoxazine Derivatives Prepared from Basic Blue 3. Malar. J. 2019, 18, 237. [Google Scholar] [CrossRef]
- Phillips, M.A.; White, K.L.; Kokkonda, S.; Deng, X.; White, J.; Mazouni, F.E.; Marsh, K.; Tomchick, D.R.; Manjalanagara, K.; Rudra, K.R.; et al. A Triazolopyrimidine-Based Dihydroorotate Dehydrogenase Inhibitor with Improved Drug-like Properties for Treatment and Prevention of Malaria. ACS Infect. Dis. 2016, 2, 945–957. [Google Scholar] [CrossRef]
- Minnow, Y.V.T.; Schramm, V.L. Purine and Pyrimidine Pathways as Antimalarial Targets. In Malaria—Recent Advances and New Perspectives; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Calic, P.P.S.; Mansouri, M.; Scammells, P.J.; McGowan, S. Driving Antimalarial Design through Understanding of Target Mechanism. Biochem. Soc. Trans. 2020, 48, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.S.; Rückle, T.; Elliott, S.L.; Ballard, E.; Collins, K.A.; Marquart, L.; Griffin, P.; Chalon, S.; Möhrle, J.J. A Single-Dose Combination Study with the Experimental Antimalarials Artefenomel and DSM265 To Determine Safety and Antimalarial Activity against Blood-Stage Plasmodium falciparum in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 64, e01371-19. [Google Scholar] [CrossRef] [PubMed]
- Booker, M.L.; Bastos, C.M.; Kramer, M.L.; Barker, R.H.; Skerlj, R.; Sidhu, A.B.; Deng, X.; Celatka, C.; Cortese, J.F.; Guerrero Bravo, J.E.; et al. Novel Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase with Anti-Malarial Activity in the Mouse Model. J. Biol. Chem. 2010, 285, 33054–33064. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Lolli, M.L.; Vyas, V.K. A Comprehensive Review of Synthetic Strategies and SAR Studies for the Discovery of PfDHODH Inhibitors as Antimalarial Agents. Part 2: Non-DSM Compounds. Bioorg. Chem. 2024, 153, 107754. [Google Scholar] [CrossRef]
- Palmer, M.J.; Deng, X.; Watts, S.; Krilov, G.; Gerasyuto, A.; Kokkonda, S.; El Mazouni, F.; White, J.; White, K.L.; Striepen, J.; et al. Potent Antimalarials with Development Potential Identified by Structure-Guided Computational Optimization of a Pyrrole-Based Dihydroorotate Dehydrogenase Inhibitor Series. J. Med. Chem. 2021, 64, 6085–6136. [Google Scholar] [CrossRef]
- Sharma, M.; Pandey, V.; Poli, G.; Tuccinardi, T.; Lolli, M.L.; Vyas, V.K. A Comprehensive Review of Synthetic Strategies and SAR Studies for the Discovery of PfDHODH Inhibitors as Antimalarial Agents. Part 1: Triazolopyrimidine, Isoxazolopyrimidine and Pyrrole-Based (DSM) Compounds. Bioorg. Chem. 2024, 146, 107249. [Google Scholar] [CrossRef]
- Nie, Z.; Bonnert, R.; Tsien, J.; Deng, X.; Higgs, C.; Mazouni, F.E.; Zhang, X.; Li, R.; Ho, N.; Feher, V.; et al. Structure-Based Discovery and Development of Highly Potent Dihydroorotate Dehydrogenase Inhibitors for Malaria Chemoprevention. J. Med. Chem. 2025, 68, 590–637. [Google Scholar] [CrossRef]
- Cahyono, A.W.; Fitri, L.E.; Winarsih, S.; Prabandari, E.E.; Waluyo, D.; Pramisandi, A.; Chrisnayanti, E.; Dewi, D.; Siska, E.; Nurlaila, N.; et al. Nornidulin, A New Inhibitor of Plasmodium falciparum Malate: Quinone Oxidoreductase (PfMQO) from Indonesian Aspergillus Sp. BioMCC f.T.8501. Pharmaceuticals 2023, 16, 268. [Google Scholar] [CrossRef]
- Hartuti, E.D.; Inaoka, D.K.; Komatsuya, K.; Miyazaki, Y.; Miller, R.J.; Xinying, W.; Sadikin, M.; Prabandari, E.E.; Waluyo, D.; Kuroda, M.; et al. Biochemical Studies of Membrane Bound Plasmodium falciparum Mitochondrial L-Malate:Quinone Oxidoreductase, a Potential Drug Target. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 191–200. [Google Scholar] [CrossRef]
- Hidayati, A.R.; Melinda; Ilmi, H.; Sakura, T.; Sakaguchi, M.; Ohmori, J.; Hartuti, E.D.; Tumewu, L.; Inaoka, D.K.; Tanjung, M.; et al. Effect of Geranylated Dihydrochalcone from Artocarpus Altilis Leaves Extract on Plasmodium falciparum Ultrastructural Changes and Mitochondrial Malate: Quinone Oxidoreductase. Int. J. Parasitol. Drugs Drug Resist. 2023, 21, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lunev, S.; Dömling, A.; Wrenger, C. Antimalarial Drug Discovery: Structural Insights; University of Groningen: Groningen, The Netherlands, 2018; ISBN 9789403407784. [Google Scholar]
- Jayaraman, V.; Suryavanshi, A.; Kalale, P.; Kunala, J.; Balaram, H. Biochemical Characterization and Essentiality of Plasmodium Fumarate Hydratase. J. Biol. Chem. 2018, 293, 5878–5894. [Google Scholar] [CrossRef] [PubMed]
- Antoine, T.; Fisher, N.; Amewu, R.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Rapid Kill of Malaria Parasites by Artemisinin and Semi-Synthetic Endoperoxides Involves ROS-Dependent Depolarization of the Membrane Potential. J. Antimicrob. Chemother. 2014, 69, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Hikosaka, K.; Balogun, E.O.; Komatsuya, K.; Niikura, M.; Kobayashi, F.; Takahashi, K.; Tanaka, T.; Nakajima, M.; Kita, K. In Vivo Curative and Protective Potential of Orally Administered 5-Aminolevulinic Acid plus Ferrous Ion against Malaria. Antimicrob. Agents Chemother. 2015, 59, 6960–6967. [Google Scholar] [CrossRef]
- Hikosaka, K.; Komatsuya, K.; Suzuki, S.; Kita, K. Mitochondria of Malaria Parasites as a Drug Target. In An Overview of Tropical Diseases; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Burrows, J.N.; Duparc, S.; Gutteridge, W.E.; Hooft van Huijsduijnen, R.; Kaszubska, W.; Macintyre, F.; Mazzuri, S.; Möhrle, J.J.; Wells, T.N.C. New Developments in Anti-Malarial Target Candidate and Product Profiles. Malar. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Biagini, G.A.; Fisher, N.; Shone, A.E.; Mubaraki, M.A.; Srivastava, A.; Hill, A.; Antoine, T.; Warman, A.J.; Davies, J.; Pidathala, C.; et al. Generation of Quinolone Antimalarials Targeting the Plasmodium falciparum Mitochondrial Respiratory Chain for the Treatment and Prophylaxis of Malaria. Proc. Natl. Acad. Sci. USA 2012, 109, 8298–8303. [Google Scholar] [CrossRef]
- Stickles, A.M.; Ting, L.M.; Morrisey, J.M.; Li, Y.; Mather, M.W.; Meermeier, E.; Pershing, A.M.; Forquer, I.P.; Miley, G.P.; Pou, S.; et al. Inhibition of Cytochrome Bc1 as a Strategy for Single-Dose, Multi-Stage Antimalarial Therapy. Am. J. Trop. Med. Hyg. 2015, 92, 1195–1201. [Google Scholar] [CrossRef]
- Smilkstein, M.J.; Pou, S.; Krollenbrock, A.; Bleyle, L.A.; Dodean, R.A.; Frueh, L.; Hinrichs, D.J.; Li, Y.; Martinson, T.; Munar, M.Y.; et al. ELQ-331 as a Prototype for Extremely Durable Chemoprotection against Malaria. Malar. J. 2019, 18, 291. [Google Scholar] [CrossRef]
- Poonam; Gupta, Y.; Gupta, N.; Singh, S.; Wu, L.; Chhikara, B.S.; Rawat, M.; Rathi, B. Multistage Inhibitors of the Malaria Parasite: Emerging Hope for Chemoprotection and Malaria Eradication. Med. Res. Rev. 2018, 38, 1511–1535. [Google Scholar] [CrossRef]
- Dodean, R.A.; Li, Y.; Zhang, X.; Caridha, D.; Madejczyk, M.S.; Jin, X.; Dennis, W.E.; Chetree, R.; Kudyba, K.; McEnearney, S.; et al. Development of Next-Generation Antimalarial Acridones with Radical Cure Potential. J. Med. Chem. 2025, 68, 8817–8840. [Google Scholar] [CrossRef] [PubMed]
- Biamonte, M.A.; Wanner, J.; Roch, K.G. Le Recent Advances in Malaria Drug Discovery. Bioorg. Med. Chem. Lett. 2013, 23, 2829–2843. [Google Scholar] [CrossRef] [PubMed]
- Dorjsuren, D.; Eastman, R.T.; Wicht, K.J.; Jansen, D.; Talley, D.C.; Sigmon, B.A.; Zakharov, A.V.; Roncal, N.; Girvin, A.T.; Antonova-Koch, Y.; et al. Chemoprotective Antimalarials Identified through Quantitative High-Throughput Screening of Plasmodium Blood and Liver Stage Parasites. Sci. Rep. 2021, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.A.; Rückle, T.; Elliott, S.; Marquart, L.; Ballard, E.; Chalon, S.; Griffin, P.; Möhrle, J.J.; McCarthy, J.S. DSM265 at 400 Milligrams Clears Asexual Stage Parasites but Not Mature Gametocytes from the Blood of Healthy Subjects Experimentally Infected with Plasmodium falciparum. Antimicrob. Agents Chemother. 2019, 63, e01837-18. [Google Scholar] [CrossRef]
- Sulyok, M.; Rückle, T.; Roth, A.; Mürbeth, R.E.; Chalon, S.; Kerr, N.; Samec, S.S.; Gobeau, N.; Calle, C.L.; Ibáñez, J.; et al. DSM265 for Plasmodium falciparum Chemoprophylaxis: A Randomised, Double Blinded, Phase 1 Trial with Controlled Human Malaria Infection. Lancet Infect. Dis. 2017, 17, 636–644. [Google Scholar] [CrossRef]
- Murphy, S.C.; Duke, E.R.; Shipman, K.J.; Jensen, R.L.; Fong, Y.; Ferguson, S.; Janes, H.E.; Gillespie, K.; Seilie, A.M.; Hanron, A.E.; et al. A Randomized Trial Evaluating the Prophylactic Activity of DSM265 Against Preerythrocytic Plasmodium falciparum Infection During Controlled Human Malarial Infection by Mosquito Bites and Direct Venous Inoculation. J. Infect. Dis. 2018, 217, 693–702. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Casapia, M.; Chuquiyauri, R.; Hinojosa, J.-C.; Kerr, N.; Rosario, M.; Toovey, S.; Arch, R.H.; Phillips, M.A.; Rozenberg, F.D.; et al. Antimalarial Activity of Single-Dose DSM265, a Novel Plasmodium Dihydroorotate Dehydrogenase Inhibitor, in Patients with Uncomplicated Plasmodium falciparum or Plasmodium vivax Malaria Infection: A Proof-of-Concept, Open-Label, Phase 2a Study. Lancet Infect. Dis. 2018, 18, 874–883. [Google Scholar] [CrossRef]
- WWARN Gametocyte Study Group. Gametocyte Carriage in Uncomplicated Plasmodium falciparum Malaria Following Treatment with Artemisinin Combination Therapy: A Systematic Review and Meta-Analysis of Individual Patient Data. BMC Med. 2016, 14, 79. [Google Scholar] [CrossRef]
- Tilley, L.; Straimer, J.; Gnädig, N.F.; Ralph, S.A.; Fidock, D.A. Artemisinin Action and Resistance in Plasmodium falciparum. Trends Parasitol. 2016, 32, 682–696. [Google Scholar] [CrossRef]
- Jacot, D.; Waller, R.F.; Soldati-Favre, D.; MacPherson, D.A.; MacRae, J.I. Apicomplexan Energy Metabolism: Carbon Source Promiscuity and the Quiescence Hyperbole. Trends Parasitol. 2016, 32, 56–70. [Google Scholar] [CrossRef]
- Bamforth, C.W. Brewing Materials and Processes: A Practical Approach to Beer Excellence; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, ISBN 9780127999548. [Google Scholar]
- Schuster, F.L. Cultivation of Plasmodium spp. Clin. Microbiol. Rev. 2002, 15, 355–364. [Google Scholar] [CrossRef]
- Géry, A.; Basco, L.K.; Heutte, N.; Guillamin, M.; N’Guyen, H.M.T.; Richard, E.; Garon, D.; De Pécoulas, P.E. Long-Term in Vitro Cultivation of Plasmodium falciparum in a Novel Cell Culture Device. Am. J. Trop. Med. Hyg. 2019, 100, 822–827. [Google Scholar] [CrossRef]
- Fischer, G.; Liti, G.; Llorente, B. The Budding Yeast Life Cycle: More Complex than Anticipated? Yeast 2021, 38, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Ludovico, P.; Leão, C. Sugar Metabolism in Yeasts: An Overview of Aerobic and Anaerobic Glucose Catabolism. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin/Heidelberg, Germany, 2006; pp. 101–121. [Google Scholar]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. Ctp1 and Yhm2: Two Mitochondrial Citrate Transporters to Support Metabolic Flexibility of Saccharomyces cerevisiae. Int. J. Mol. Sci. 2024, 25, 1870. [Google Scholar] [CrossRef] [PubMed]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. In Vivo Antioxidant Activity of Common Dietary Flavonoids: Insights from the Yeast Model Saccharomyces cerevisiae. Antioxidants 2024, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe, J.L.; Alvarez-Ruiz, E.; Martin-Plaza, J.J.; Steel, P.G.; Denny, P.W. The Utility of Yeast as a Tool for Cell-Based, Target-Directed High-Throughput Screening. Parasitology 2014, 141, 8–16. [Google Scholar] [CrossRef]
- Frame, I.J.; Deniskin, R.; Rinderspacher, A.; Katz, F.; Deng, S.-X.; Moir, R.D.; Adjalley, S.H.; Coburn-Flynn, O.; Fidock, D.A.; Willis, I.M.; et al. Yeast-Based High-Throughput Screen Identifies Plasmodium falciparum Equilibrative Nucleoside Transporter 1 Inhibitors That Kill Malaria Parasites. ACS Chem. Biol. 2015, 10, 775–783. [Google Scholar] [CrossRef]
- Sweeney, J.M.; Willis, I.M.; Akabas, M.H. Yeast-Based Assay to Identify Inhibitors of the Malaria Parasite Sodium Phosphate Uptake Transporter as Potential Novel Antimalarial Drugs. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 100567. [Google Scholar] [CrossRef]
- Meunier, B.; Fisher, N.; Ransac, S.; Mazat, J.-P.; Brasseur, G. Respiratory Complex III Dysfunction in Humans and the Use of Yeast as a Model Organism to Study Mitochondrial Myopathy and Associated Diseases. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 1346–1361. [Google Scholar] [CrossRef]
- Zara, V.; De Blasi, G.; Ferramosca, A. Assembly of the Multi-Subunit Cytochrome Bc1 Complex in the Yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2022, 23, 10537. [Google Scholar] [CrossRef]
- Maclean, A.E.; Hayward, J.A.; Huet, D.; van Dooren, G.G.; Sheiner, L. The Mystery of Massive Mitochondrial Complexes: The Apicomplexan Respiratory Chain. Trends Parasitol. 2022, 38, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.M.; Lukoyanova, N.; Zhang, Y.; Cabrera-Orefice, A.; Arnold, S.; Meunier, B.; Pinotsis, N.; Maréchal, A. Structure of Yeast Cytochrome c Oxidase in a Supercomplex with Cytochrome Bc1. Nat. Struct. Mol. Biol. 2019, 26, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, A.I.; Smeitink, J.A.M.; Rodenburg, R.J.T. Mitochondrial ATP Synthase: Architecture, Function and Pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Salunke, R.; Mourier, T.; Banerjee, M.; Pain, A.; Shanmugam, D. Highly Diverged Novel Subunit Composition of Apicomplexan F-Type ATP Synthase Identified from Toxoplasma Gondii. PLoS Biol. 2018, 16, e2006128. [Google Scholar] [CrossRef]
- Ganesan, S.M.; Morrisey, J.M.; Ke, H.; Painter, H.J.; Laroiya, K.; Phillips, M.A.; Rathod, P.K.; Mather, M.W.; Vaidya, A.B. Yeast Dihydroorotate Dehydrogenase as a New Selectable Marker for Plasmodium falciparum Transfection. Mol. Biochem. Parasitol. 2011, 177, 29–34. [Google Scholar] [CrossRef]
- Hara, K.; Inada, Y.; Ono, T.; Kuroda, K.; Yasuda-Kamatani, Y.; Ishiguro, M.; Tanaka, T.; Misaka, T.; Abe, K.; Ueda, M. Chimeric yeast G-protein α subunit harboring a 37-residue C-terminal gustducin-specific sequence is functional in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2012, 76, 512–516. [Google Scholar] [CrossRef]
- Sanyal, S.; Kouznetsova, A.; Ström, L.; Björkegren, C. A system for inducible mitochondria-specific protein degradation in vivo. Nat. Commun. 2024, 15, 1454. [Google Scholar] [CrossRef]
- Ito, T.; Kajita, S.; Fujii, M.; Shinohara, Y. Plasmodium Parasite Malate-Quinone Oxidoreductase Functionally Complements a Yeast Deletion Mutant of Mitochondrial Malate Dehydrogenase. Microbiol. Spectr. 2023, 11, e0016823. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Mitochondrial Carriers and Substrates Transport Network: A Lesson from Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 8496. [Google Scholar] [CrossRef]
- De Blasi, G.; Lunetti, P.; Zara, V.; Ferramosca, A. Mitochondrial Citrate Transporters Ctp1-Yhm2 and Respiratory Chain: A Coordinated Functional Connection in Saccharomyces cerevisiae Metabolism. Int. J. Biol. Macromol. 2024, 270, 132364. [Google Scholar] [CrossRef]
- Salcedo-Sora, J.E.; Ward, S.A.; Biagini, G.A. A Yeast Expression System for Functional and Pharmacological Studies of the Malaria Parasite Ca2+/H+ Antiporter. Malar. J. 2012, 11, 254. [Google Scholar] [CrossRef]
- Jenkins, B.J.; Daly, T.M.; Morrisey, J.M.; Mather, M.W.; Vaidya, A.B.; Bergman, L.W. Characterization of a falciparum Orthologue of the Yeast Ubiquinone-Binding Protein, Coq10p. PLoS ONE 2016, 11, e0152197. [Google Scholar] [CrossRef]
- Chellappan, S.; Roy, S.; Nagmoti, J.M.; Tabassum, W.; Hoti, S.L.; Bhattacharyya, M.K.; Nina, P.B. Functional Studies of Plasmodium falciparum’s Prohibitin1 and Prohibitin 2 in Yeast. Indian J. Med. Microbiol. 2020, 38, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Birth, D.; Kao, W.-C.; Hunte, C. Structural Analysis of Atovaquone-Inhibited Cytochrome Bc1 Complex Reveals the Molecular Basis of Antimalarial Drug Action. Nat. Commun. 2014, 5, 4029. [Google Scholar] [CrossRef] [PubMed]
- Mounkoro, P.; Michel, T.; Blandin, S.; Golinelli-Cohen, M.-P.; Davioud-Charvet, E.; Meunier, B. Investigating the Mode of Action of the Redox-Active Antimalarial Drug Plasmodione Using the Yeast Model. Free Radic. Biol. Med. 2019, 141, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Mounkoro, P.; Michel, T.; Golinelli-Cohen, M.-P.; Blandin, S.; Davioud-Charvet, E.; Meunier, B. A Role for the Succinate Dehydrogenase in the Mode of Action of the Redox-Active Antimalarial Drug, Plasmodione. Free Radic. Biol. Med. 2021, 162, 533–541. [Google Scholar] [CrossRef]
- Mounkoro, P.; Michel, T.; Meunier, B. Revisiting the Mode of Action of the Antimalarial Proguanil Using the Yeast Model. Biochem. Biophys. Res. Commun. 2021, 534, 94–98. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, B. The Antimalarial Drug Artemisinin Induces an Additional, Sod1-Supressible Anti-Mitochondrial Action in Yeast. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1285–1294. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, B. The Molecular and Cellular Action Properties of Artemisinins: What Has Yeast Told Us? Microb. Cell 2016, 3, 196–205. [Google Scholar] [CrossRef]
- Laleve, A.; Panozzo, C.; Kühl, I.; Bourand-Plantefol, A.; Ostojic, J.; Sissoko, A.; Tribouillard-Tanvier, D.; Cornu, D.; Burg, A.; Meunier, B.; et al. Artemisinin and Its Derivatives Target Mitochondrial C-Type Cytochromes in Yeast and Human Cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118661. [Google Scholar] [CrossRef]
- Laleve, A.; Vallières, C.; Golinelli-Cohen, M.-P.; Bouton, C.; Song, Z.; Pawlik, G.; Tindall, S.M.; Avery, S.V.; Clain, J.; Meunier, B. The Antimalarial Drug Primaquine Targets Fe–S Cluster Proteins and Yeast Respiratory Growth. Redox Biol. 2016, 7, 21–29. [Google Scholar] [CrossRef]

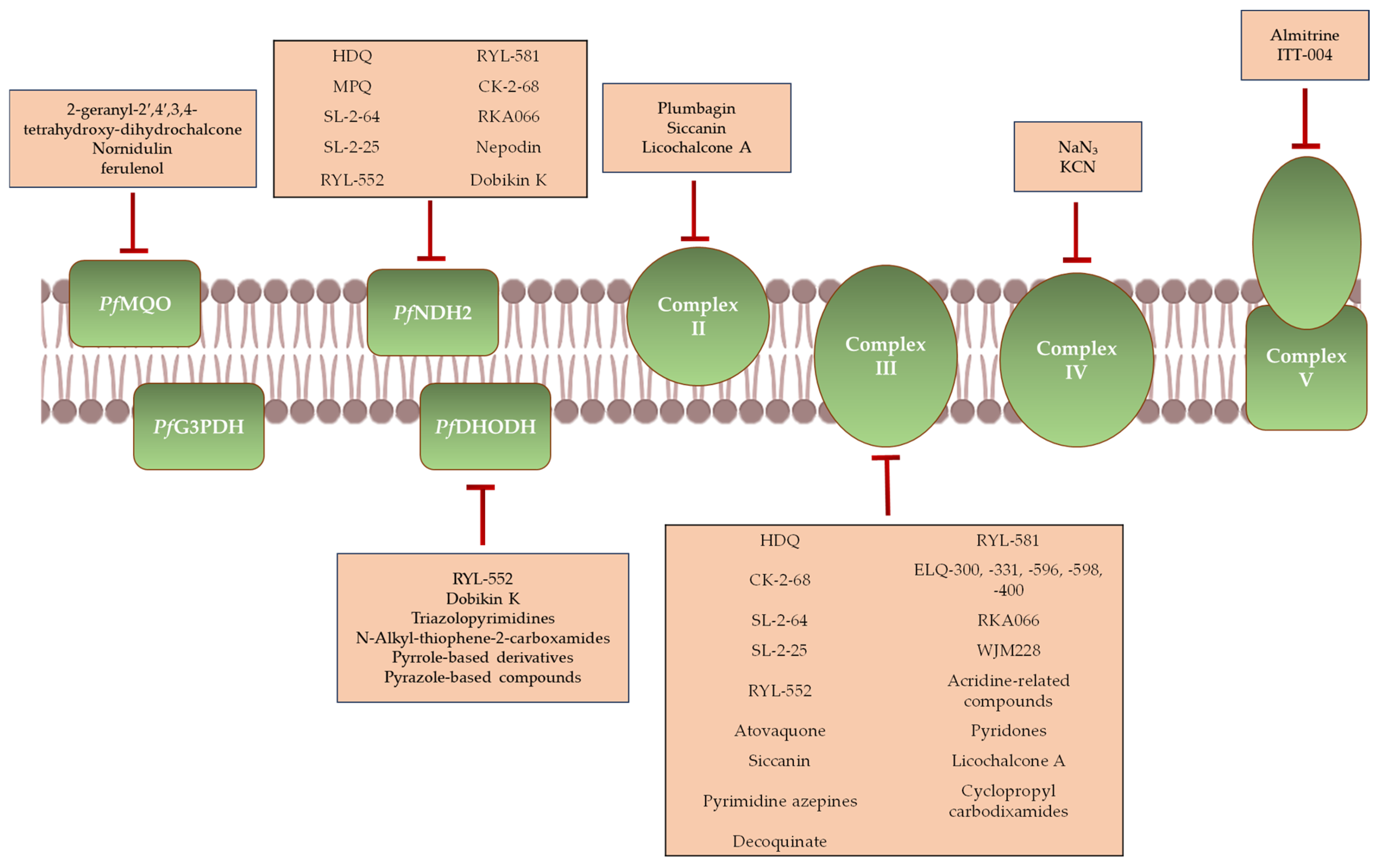
| Compounds | Mitochondrial Target | Plasmodium Life Cycle Stage Target | References | ||
|---|---|---|---|---|---|
| LS | ABS | SBS | |||
| Endochin-like quinolones | |||||
| Hydroxy-2-dodecyl-4-(1H)-quinolone (HDQ) | PfNADH2 complex III (Qi site) | ND | (+) | ND | [25,42,43,44] |
| 7-Chloro-3-methyl-2-{4-[4-(trifluoromethoxy)benzyl]phenyl}quinolin-4(1H)-one (CK-2-68) | PfNADH2 complex III (Qo site) | (+) | (+) | (+) | [25,38,42,43,95] |
| 7-Fluoro-3-methyl-2-{6-[4-(trifluoromethoxy)phenyl]pyridin-3-yl}quinolin-4(1H)-one (SL-2-64) | PfNADH2 complex III | ND | (+) | ND | [38,42] |
| 3-Methyl-2-{6-[4-(trifluoromethoxy)phenyl]pyridin-3-yl}quinolin-4(1H)-one (SL-2-25) | PfNADH2 complex III | (+) | (+) | ND | [38,42,95] |
| 5-Fluoro-3-methyl-2-[4-[[4-(trifluoromethoxy)phenyl]methyl]phenyl]-1H-quinolin-4-one (RYL-552) | PfNADH2 complex III (Qo site, Qi site) PfDHODH | ND | (+) | ND | [41,45,46] |
| 5-Fluoro-3-methyl-2-[4-[[4-(trifluoromethoxy)phenyl]methyl]phenyl]-1-(N-ethyl-N-propylamino)-4(1H)-quinolone (RYL-581) | PfNADH2 complex III (Qo site, Qi site) | ND | (+) | ND | [41] |
| 1-Methyl-2-pentyl-4(1H)-quinolinone (MPQ) | PfNADH2 | ND | (−) | ND | [43] |
| RKA066 | PfNADH2 complex III (Qisite) | ND | (+) | ND | [47] |
| 6-Chloro-7-methoxy-2-methyl-3-{4-[4-(trifluoromethyl) phenoxy]phenyl}quinolin-4(1H)-one (ELQ-300) | complex III (Qi site) | (+) | (+) | (+) | [57,58,59,96] |
| [6-Chloro-7-methoxy-2-methyl-3-{4-[4-(trifluoromethyl) phenoxy]phenyl}quinolin-4-yl]oxymethyl ethyl carbonate (ELQ-331) | complex III (Qi site) | (+) | (+) | (+) | [58,59,97] |
| 6-Chloro-7-methoxy-2-methyl-3-[4-[4-(trifluoromethoxy)phenyl]phenyl]-1H-quinolin-4-one (ELQ-596) | complex III (Qi site) | ND | (+) | ND | [61] |
| [6-Chloro-7-methoxy-2-methyl-3-[4-[4-(trifluoromethoxy)phenyl]phenyl]quinolin-4-yl]oxymethyl ethyl carbonate (ELQ-598) | complex III (Qi site) | (+) | (+) | ND | [61] |
| WJM228 | complex III (Qo site) | (+) | (+) | (+) | [62] |
| ELQ-400 | complex III (Qo site, Qi site) | (+) | (+) | (+) | [58,64,65,98] |
| Acridine-related compounds | |||||
| WR 249685 | complex III | ND | (+) | ND | [57] |
| T111 | complex III (Qo site, Qi site) | (+) | (+) | (+) | [66,67,99] |
| Naphthoquinones | |||||
| 3-[4-(4-Chlorophenyl)cyclohexyl]-4-hydroxynaphthalene-1,2-dione (Atovaquone) | complex III (Qo site) | (+) | (+) | (+) | [7] |
| 1-(1,8-Dihydroxy-3-methylnaphthalen-2-yl) ethanone (Nepodin) | PfNDH2 | ND | (+) | ND | [48] |
| 5-Hydroxy-2-methyl-1,4-naphthoquinone (Plumbagin) | complex II | ND | (+/−) | ND | [52,53,55] |
| Pyridones | |||||
| GW844520 | complex III (Qi site) | (+) | (+) | ND | [42,47,69,100] |
| GSK932121 | complex III (Qi site) | ND | (+) | ND | [42,47,57] |
| Pyrimidine Azepines | |||||
| PyAz90 | complex III (Qo site) | (+) | (+) | (+) | [70,101] |
| Cyclopropyl carboxamides | |||||
| MMV024397 | complex III (Qo site) | (+) | (+) | (+) | [56,71] |
| WJM280 | complex III (Qo site) | (+) | (+) | (+) | [71] |
| W466 | complex III (Qo site) | (+) | (+) | (+) | [71] |
| W499 | complex III (Qo site) | (+) | (+) | (+) | [71] |
| Terpene compounds | |||||
| Dobikin K | PfNADH2 PfDHODH | ND | (+) | ND | [49] |
| (4aS,6aS,11bR,13aS,13bS)-4,4,6a,9-Tetramethyl-1,2,3,4,4a,5,6,6a,11b,13b-decahydrobenzo[a]furo [2,3,4-mn]xanthen-11-ol (Siccanin) | complex II complex III | ND | (+) | ND | [51] |
| Chalcones | |||||
| (E)-3-[4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl]-1-(4-hydroxyphenyl)prop-2-en-1-one (Licochalcone A) | complex II complex III | ND | (+) | ND | [54,55] |
| 2-geranyl-2′,4′,3,4-tetrahydroxy-dihydrochalcone | PfMQO | ND | (+) | ND | [88] |
| Triazolopyrimidines | |||||
| DSM265 | PfDHODH | (+) | (+) | (−) | [77,78,79,80,102,103,104,105] |
| DSM421 | PfDHODH | (+) | (+) | ND | [77] |
| N-Alkyl-thiophene-2-carboxamides | |||||
| Genz-667348 | PfDHODH | ND | (+) | ND | [42,79,82] |
| Genz-669178 | PfDHODH | ND | (+) | ND | [42] |
| Pyrrole-based derivatives | |||||
| DSM705 | PfDHODH | (+) | (+) | ND | [83,84] |
| DSM873 | PfDHODH | (+) | (+) | ND | [83,84] |
| Pyrazole-based compounds | |||||
| DSM1465 | PfDHODH | (+) | (+) | ND | [85] |
| Other compounds | |||||
| Ethyl-6-decyloxy-7-ethoxy-4-hydroxyquinoline-3-carboxylate (Decoquinate) | complex III (Qo site) | (+) | (+) | (+) | [42,72] |
| Sodium azide (NaN3) | complex IV | ND | (+/−) | ND | [73] |
| Potassium cyanide (KCN) | complex IV | ND | (+/−) | ND | [73,74] |
| Almitrine | complex V | ND | (+) | ND | [55,75] |
| ITT-004 | complex V | ND | (+) | ND | [76] |
| 2,4,7-Trichloro-3,8-dihydroxy-1,9-dimethyl-6-(1-methyl-1-propen-1-yl)-11H-dibenzo[b,e][1,4]dioxepin-11-one (Nornidulin) | PfMQO | ND | (+) | ND | [86] |
| 4-Hydroxy-3-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]chromen-2-one (ferulenol) | PfMQO | ND | (+) | ND | [87] |
| Sodium fluoroacetate (NaFAc) | PfACO | ND | (−) | (+) | [17,89] |
| Mercaptosuccinic acid | PfFH | ND | (+) | ND | [90] |
| ALA/SFC | oxidative stress | ND | (+) | ND | [92,93,94] |
| ART | membrane depolarization other unexplored mechanisms | (−) | (+) | (+) | [106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, S.; Assalve, G.; Lunetti, P.; Kayentao, K.; Dara, A.; Scaramuzzi, D.; Zara, V.; Ferramosca, A. Targeting Mitochondrial Function in Plasmodium falciparum: Insight into Antimalarial Drugs and the Emerging Role of Saccharomyces cerevisiae as a Model System. Int. J. Mol. Sci. 2025, 26, 9150. https://doi.org/10.3390/ijms26189150
Greco S, Assalve G, Lunetti P, Kayentao K, Dara A, Scaramuzzi D, Zara V, Ferramosca A. Targeting Mitochondrial Function in Plasmodium falciparum: Insight into Antimalarial Drugs and the Emerging Role of Saccharomyces cerevisiae as a Model System. International Journal of Molecular Sciences. 2025; 26(18):9150. https://doi.org/10.3390/ijms26189150
Chicago/Turabian StyleGreco, Sara, Graziana Assalve, Paola Lunetti, Kassoum Kayentao, Antoine Dara, Dario Scaramuzzi, Vincenzo Zara, and Alessandra Ferramosca. 2025. "Targeting Mitochondrial Function in Plasmodium falciparum: Insight into Antimalarial Drugs and the Emerging Role of Saccharomyces cerevisiae as a Model System" International Journal of Molecular Sciences 26, no. 18: 9150. https://doi.org/10.3390/ijms26189150
APA StyleGreco, S., Assalve, G., Lunetti, P., Kayentao, K., Dara, A., Scaramuzzi, D., Zara, V., & Ferramosca, A. (2025). Targeting Mitochondrial Function in Plasmodium falciparum: Insight into Antimalarial Drugs and the Emerging Role of Saccharomyces cerevisiae as a Model System. International Journal of Molecular Sciences, 26(18), 9150. https://doi.org/10.3390/ijms26189150










