Exploring the Anti-Inflammatory Activity of the Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) in RAW264.7 Cells and Carrageenan-Induced Rat Models
Abstract
1. Introduction
2. Results
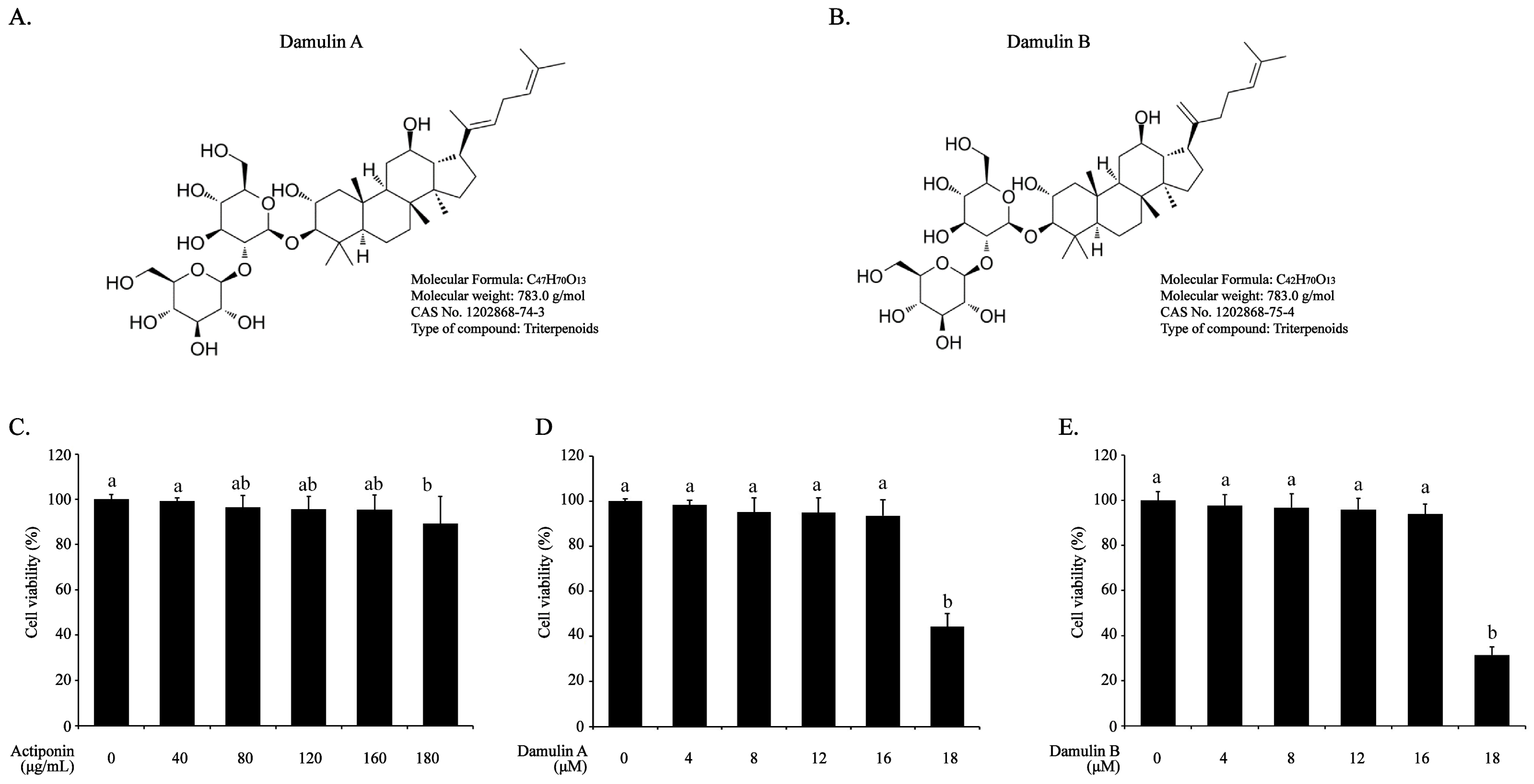
2.1. Effect of AP, DA, and DB on Viability of RAW264.7 Cells
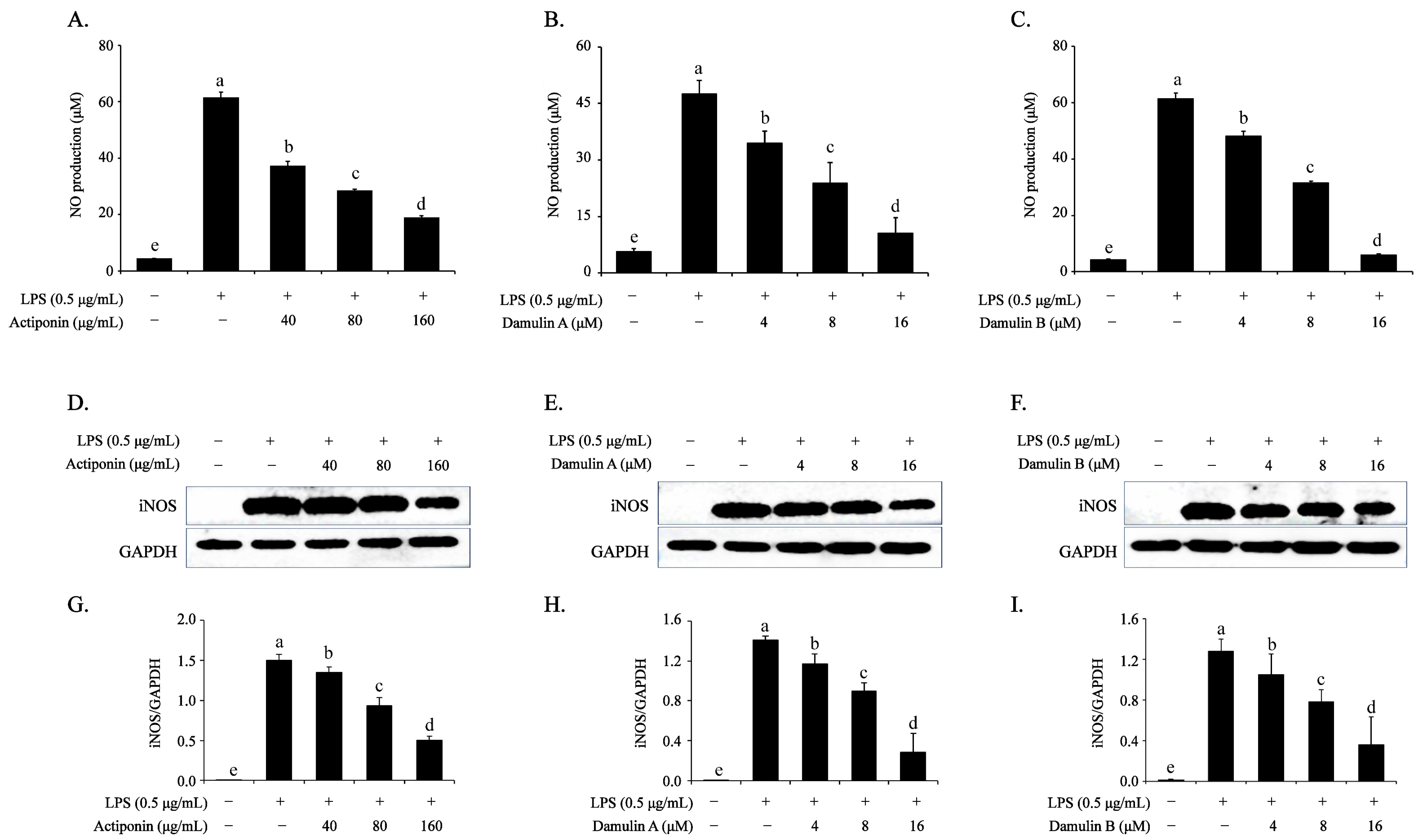
2.2. Inhibitory Effect of AP, DA, and DB on the LPS-Induced NO Production and iNOS Expression in RAW264.7 Cells
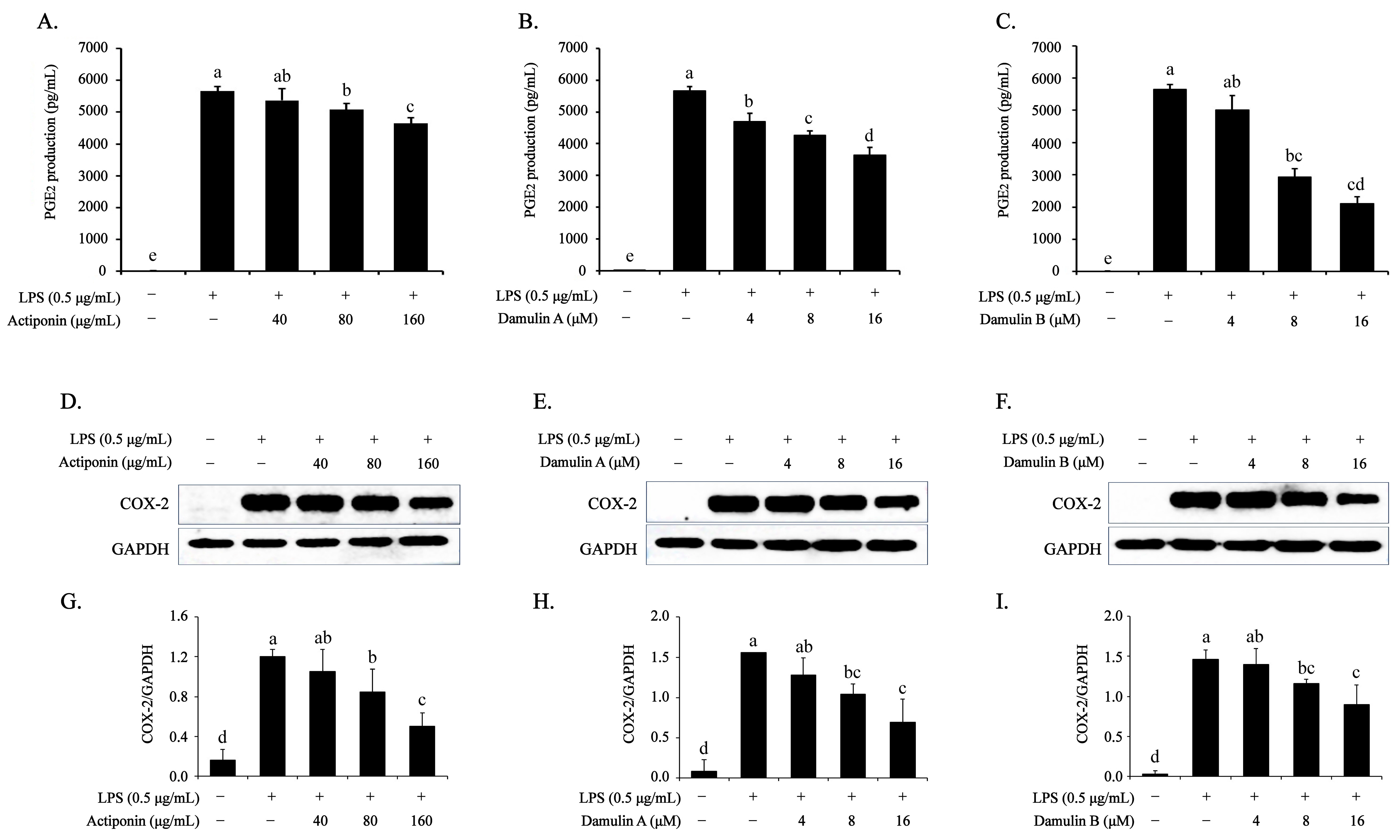
2.3. Inhibitory Effect of AP, DA, and DB on LPS-Induced PGE2 Production and COX-2 Expression in RAW264.7 Cells
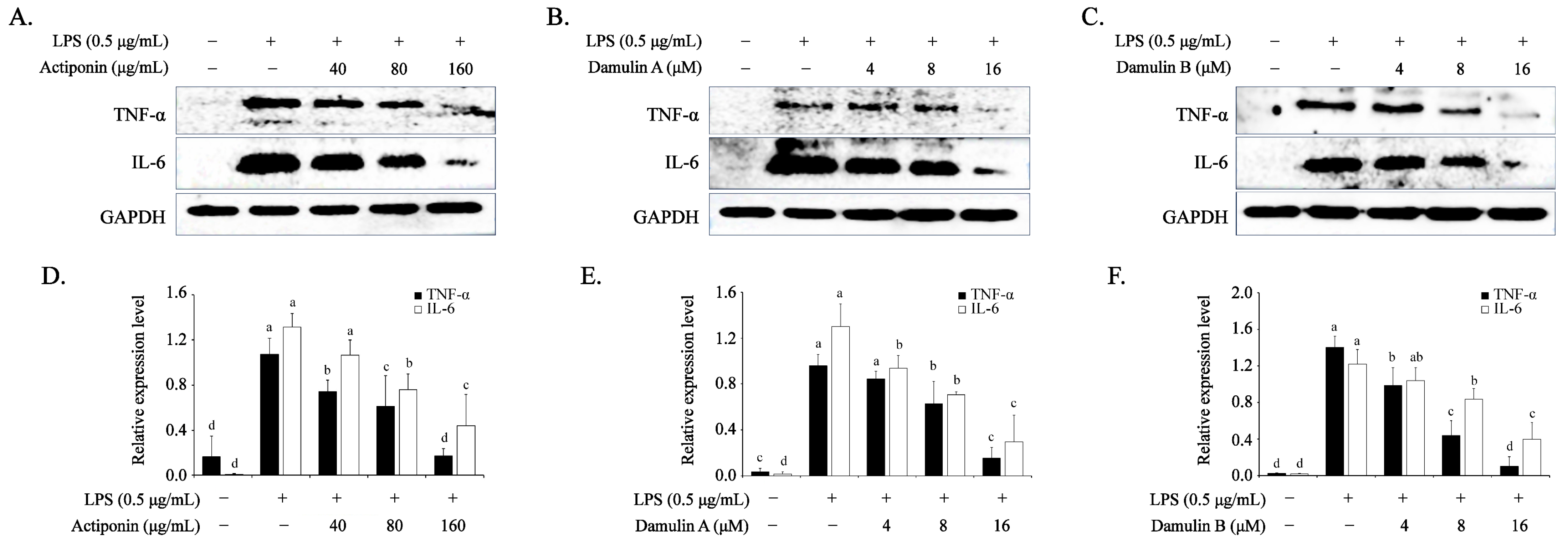
2.4. Inhibitory Effect of AP, DA, and DB on LPS-Induced Pro-Inflammatory Cytokines in RAW264.7 Cells
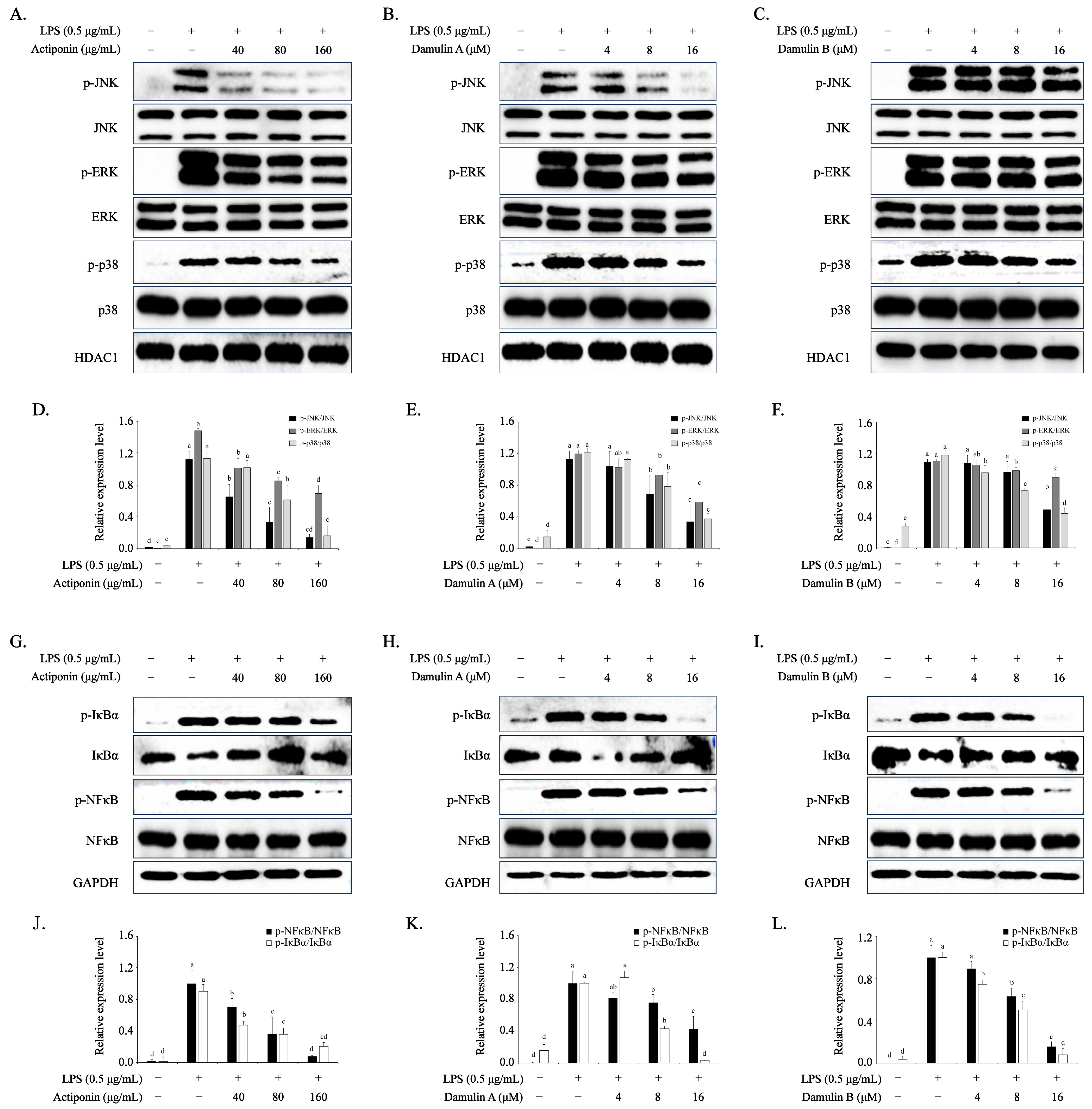
2.5. Inhibition of AP, DA, and DB on NF-κB Signaling Pathway in LPS-Stimulated RAW 264.7 Cells
2.6. Inhibition of AP, DA, and DB on MAPKs in LPS-Stimulated RAW 264.7 Cells
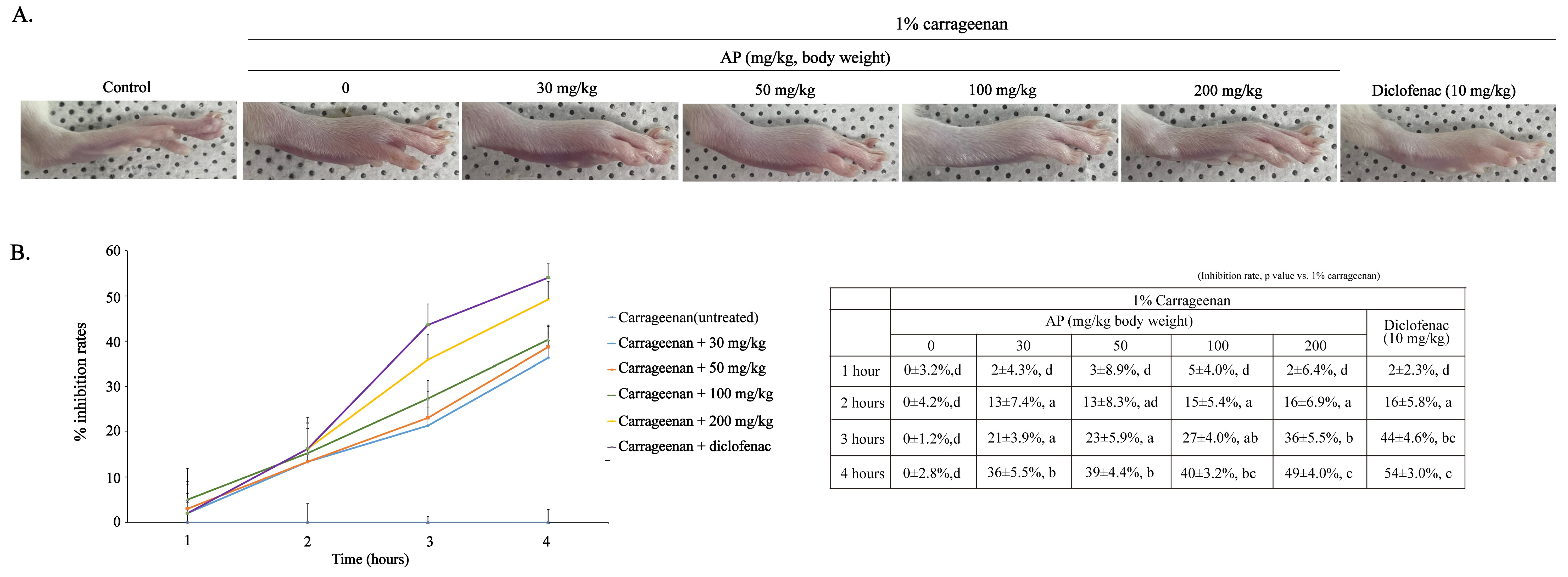
2.7. Effects of AP on Carrageenan-Induced Rat Paw Edema
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of GP Extract (Actiponin ®), DA, and DB
4.3. Cell Culture and Viability Assay
4.4. NO and PGE2 Production Assay
4.5. Protein Extraction and Western Blot Analysis
4.6. Animals Used in the Study
4.7. Inducing Edema in Rat Paws with Carrageenan
- Ct = paw thickness (mm) of the left hind limb at time t.
- C0 = paw thickness (mm) of the left hind limb before carrageenan injection.
- (Ct−C0)control = increase in paw size after carrageenan injection at time t
- (Ct−C0)treated = increase in paw size after carrageenan injection at time t of reference or test drug-treated rats.
- Group 1 (Control) = nothing.
- Group 2 (vehicle)= 1% carrageenan + vehicle (0.9% saline).
- Group 3–6 (AP30–200 mg/kg body weight) = 1% carrageenan + 30, 50, 100, and 200 mg/kg body weight of AP dissolved in 0.9% saline, respectively).
- Group 7 (Diclofenac) = 1% carrageenan + 10 mg/kg body weight, diclofenac dissolved in 0.9% saline).
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and antiinflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the medicinal value, bioactive compounds, and pharmacological activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhao, F.F.; Fang, M.; Pu, F.L.; Kong, L.Y.; Liu, J.P. Gynostemma pentaphyllum for dyslipidemia: A systematic review of randomized controlled trials. Front. Pharmacol. 2022, 13, 917521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.Y.; Yue, S.R.; Tan, Y.Y.; Tang, N.; Xu, Y.S.; Zhang, B.J.; Mao, Y.J.; Xue, Z.S.; Lu, A.P.; Liu, B.C.; et al. Gynostemma pentaphyllum extract alleviates NASH in mice: Exploration of inflammation and gut microbiota. Nutrients 2024, 16, 1782. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Luo, Y.; Zhang, J.; Wang, M.; Liao, P.; Cao, L.; Guo, P.; Sun, G.; Sun, X. Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: Insight into the ER-Mediated PI3K/Akt pathway. Int. J. Mol. Sci. 2017, 18, 77. [Google Scholar] [CrossRef]
- Hu, X.; Wang, W.; Zhang, D.; Tian, X.; Zhang, A.; Xu, J.; Feng, F.; Li, W.; Kikuchi, T.; Zhang, J. Phytochemistry, bioactivities and application of Gynostemma pentaphyllum polysaccharide: A review. Int. J. Biol. Macromol. 2025, 322 Pt 1, 144964. [Google Scholar] [CrossRef]
- Shen, Z.; Gao, X.; Huang, D.; Xu, X.; Shen, J. The potential of Gynostemma pentaphyllum in the treatment of hyperlipidemia and its interaction with the LOX1-PI3K-AKT-eNOS pathway. Food Sci. Nut. 2024, 12, 8000–8012. [Google Scholar] [CrossRef]
- Guo, M.; Pei, W.J.; Liu, L.; Chen, K.; Cheng, Y.; Piao, X.L. Neuroprotective effects of gypenosides on LPS-induced anxiety and depression-like behaviors. Int. Immunopharmacol. 2024, 143 Pt 1, 113367. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, H.; Sha, Z.; Zhao, J.; Cai, H.; Xi, R.; Zhao, Z.; Yin, X.; Yang, L.; Liu, C. Gypenoside XLIX inhibiting PI3K/AKT/FOXO1 signaling pathway mediated neuronal mitochondrial autophagy to improve patients with ischemic stroke. Front. Pharmacol. 2025, 16, 1600435. [Google Scholar] [CrossRef]
- Pan, L.; Lan, B.; Li, S.; Jin, Y.; Cui, M.; Xia, Y.; Wei, S.; Huang, H. Gypenoside inhibits gastric cancer proliferation by suppressing glycolysis via the Hippo pathway. Sci. Rep. 2024, 14, 19003. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Wang, Q.; Wang, Z.; Pan, K.; Zhang, J.; Peng, Y.; Wang, G.; Yin, Z.; Sun, J. Simultaneous determination of 13 representative saponins from the total saponins of Gynostemma pentaphyllum in rat plasma using LC-MS/MS for pharmacokinetic study. J. Pharm. Biomed. Anal. 2025, 264, 116970. [Google Scholar] [CrossRef]
- Gauhar, R.; Hwang, S.L.; Jeong, S.S.; Kim, J.E.; Song, H.; Park, D.C.; Song, K.S.; Kim, T.Y.; Oh, W.K.; Huh, T.L. Heat-processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP-activated protein kinase. Biotechnol. Lett. 2012, 34, 1607–1616. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, C.H.; Lee, S.H.; Do, E.; Kim, D.K.; Huh, T.L.; Kim, C.S. Inhibitory effects of heat-processed Gynostemma pentaphyllum extract (Actiponin®) and its components on cartilage breakdown in osteoarthritis. Int. J. Mol. Sci. 2025, 26, 1728. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, M.; Hoang, D.H.; Kovale, L.M.; Lee, J.; Kim, Y.; Lee, C.; Hong, J.; Park, S.; Choe, W.; et al. A hydrodistillate of Gynostemma pentaphyllum and Damulin B prevent cisplatin-induced nephrotoxicity in vitro and in vivo via regulation of AMPKα1 transcription. Nutrients 2022, 14, 4997. [Google Scholar] [CrossRef] [PubMed]
- Kovale, L.; Lee, S.; Song, M.; Lee, J.; Son, H.J.; Sung, Y.K.; Kwack, M.H.; Choe, W.; Kang, I.; Kim, S.S.; et al. Gynostemma pentaphyllum hydrodistillate and its major component damulin B promote hair growth-inducing properties in vivo and in vitro via the Wnt/β-catenin pathway in dermal papilla cells. Nutrients 2024, 16, 985. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Conde-Aranda, J. Inflammation in health and disease: New insights and therapeutic avenues. Int. J. Mol. Sci. 2022, 23, 8392. [Google Scholar] [CrossRef]
- Barbara, J.A.; Van ostade, X.; Lopez, A. Tumour necrosis factor-alpha (TNF-alpha): The good, the bad and potentially very effective. Immunol. Cell Biol. 1996, 74, 434–443. [Google Scholar] [CrossRef]
- Li, W.; Luo, F.; Wu, X.; Fan, B.; Yang, M.; Zhong, W.; Guan, D.; Wang, F.; Wang, Q. Anti-Inflammatory effects and mechanisms of dandelion in RAW264.7 macrophages and zebrafish larvae. Front. Pharmacol. 2022, 13, 906927. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Xie, Q.; Yin, G. Macrophage involvement in idiopathic inflammatory myopathy: Pathogenic mechanisms and therapeutic prospects. J. Inflamm. 2024, 21, 48. [Google Scholar] [CrossRef]
- Müller, J.; von Bernstorff, W.; Heidecke, C.D.; Schulze, T. Differential S1P receptor profiles on M1- and M2-polarized macrophages affect macrophage cytokine production and migration. Biomed. Res. Int. 2017, 2017, 7584621. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, C.; Yao, D.; Li, S.; Lv, J.; Chen, Y.; Huang, X. Anti-inflammatory properties of ophioglonin derived from the fern Ophioglossum vulgatum L. via inactivating NF-κB and MAPK signaling pathways. FEBS Open Bio, 2024; in press. [Google Scholar]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Khajuria, V.; Gupta, S.; Sharma, N.; Kumar, A.; Lone, N.A.; Khullar, M.; Dutt, P.; Sharma, P.R.; Bhagat, A.; Ahmed, Z. Anti-inflammatory potential of hentriacontane in LPS stimulated RAW264.7 cells and mice mode. Biomed. Pharmacothera. 2017, 92, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wen, J.; Pan, Z.; Liu, Y.; Zhu, Y. Celastrus orbiculatus Thunb. extract inhibits inflammatory metabolic adaptation in macrophages and regulates polarization via modulating PKM2. Int. Immunopharmacol. 2024, 144, 113665. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N. Interleukin-6 as a therapeutic target in candidate inflammatory diseases. J. Clin. Pharm. Ther. 2010, 87, 483–487. [Google Scholar] [CrossRef]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Gauhar, R.; Hwang, S.L.; Dao, T.T.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorg. Med. Chem. 2011, 19, 6254–6260. [Google Scholar] [CrossRef]
- Meeran, M.N.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S.N.; Adeghate, E. α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Func. 2020, 11, 965–976. [Google Scholar] [CrossRef]
- Xiang, X.W.; Wang, R.; Yao, L.W.; Zhou, Y.F.; Sun, P.L.; Zheng, B.; Chen, Y.F. Anti-Inflammatory effects of Mytilus coruscus polysaccharide on RAW264.7 cells and DSS-induced colitis in mice. Marine Drugs 2021, 19, 468. [Google Scholar] [CrossRef]
- Kim, D.; Nam, H.J.; Lee, W.; Yim, H.Y.; Ahn, J.Y.; Park, S.W.; Shin, H.R.; Yu, R.; Won, K.J.; Bae, J.S.; et al. PKCα-LSD1-NF-κB-signaling cascade is crucial for epigenetic control of the inflammatory response. Mol. Cell 2018, 398, 411e6. [Google Scholar] [CrossRef]
- Doyle, S.L.; O’Neill, L.A. Toll-like receptors: From the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef]
- Hang, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 alleviates inflammatory response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 pathways in RAW264. 7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef]
- Tran Huynh, Q.D.; Thi Phan, T.T.; Chu, M.H.; Nguyen, T.V.; Thi Duong, T.L.; Hsu, S.J.; Wang, Y.H.; Pham, N.T.; Nguyen Bui, B.T.; Nguyen, D.K.; et al. Anti-inflammatory glycosides from Gomphandra mollis Merr.: Structural elucidation and mechanistic insights. Phytochemistry 2025, 239, 114583. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rizo, A.B.; Fosado-Rodríguez, R.; Torres-Romero, C.J.; Lara-Riegos, C.J.; Ramírez-Camacho, A.M.; Arroyo, L.A.; Villa de la Torre, E.F.; Ceballos, G.E.; Arana-Argáez, E.V. Models in vivo and in vitro for the study of acute and chronic inflammatory activity: A comprehensive review. Int. Immunopharmacol. 2024, 135, 112292. [Google Scholar] [CrossRef] [PubMed]
- Justil-Guerrero, H.J.; Arroyo-Acevedo, J.L.; Rojas-Armas, J.P.; García-Bustamante, C.O.; Palomino-Pacheco, M.; Almonacid-Román, R.D.; Calva Torres, J.W. Evaluation of bioactive compounds, antioxidant capacity, and anti-Inflammatory effects of lipophilic and hydrophilic extracts of the pericarp of Passiflora tripartita var. mollissima at two stages of ripening. Molecules 2024, 29, 4964. [Google Scholar] [CrossRef] [PubMed]
- Vinegar, R.; Schreiber, W.; Hugo, R. Biphasic development of carrageenan edema in rats. J. Pharmacol. Exp. Ther. 1969, 166, 96–103. [Google Scholar] [CrossRef]
- Crunkhorn, P.; Meacock, S.C. Mediators of the inflammation induced in the rat paw by carrageenan. Br. J. Pharmacol. 1971, 42, 392–402. [Google Scholar] [CrossRef]
- Jang, G.; Lee, S.; Hong, J.; Park, B.; Kim, D.; Kim, C. Anti-Inflammatory effect of 4,5-dicaffeoylquinic acid on RAW264.7 cells and a rat model of inflammation. Nutrients 2021, 13, 3537. [Google Scholar] [CrossRef]
- Park, S.H.; Huh, T.L.; Kim, S.Y.; Oh, M.R.; Tirupathi Pichiah, P.B.; Chae, S.W.; Cha, Y.S. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): A randomized, double-blind, placebo-controlled trial. Obesity 2015, 22, 63–71, Erratum in Obesity 2020, 28, 1770–1773. [Google Scholar] [CrossRef]
- Akinnawo, O.O.; Anyasor, G.N.; Osilesi, O. Aqueous fraction of Alstonia boonei de wild leaves suppressed inflammatory responses in carrageenan and formaldehyde induced arthritic rats. Biomed. Pharmacother. 2017, 86, 95–101. [Google Scholar] [CrossRef]
- Lee, S.A.; Park, B.R.; Moon, S.M.; Han, S.H.; Kim, C.S. Anti-inflammatory potential of Trifolium pratense L. leaf extract in LPS-stimulated RAW264.7 cells and in a rat model of carrageenan-induced inflammation. Arch. Physiol. Biochem. 2018, 126, 74–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.A.; Moon, B.R.; Lee, C.H.; Lee, S.H.; Do, E.; Kim, D.K.; Huh, T.-L.; Kim, C.S. Exploring the Anti-Inflammatory Activity of the Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) in RAW264.7 Cells and Carrageenan-Induced Rat Models. Int. J. Mol. Sci. 2025, 26, 9145. https://doi.org/10.3390/ijms26189145
Lee SA, Moon BR, Lee CH, Lee SH, Do E, Kim DK, Huh T-L, Kim CS. Exploring the Anti-Inflammatory Activity of the Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) in RAW264.7 Cells and Carrageenan-Induced Rat Models. International Journal of Molecular Sciences. 2025; 26(18):9145. https://doi.org/10.3390/ijms26189145
Chicago/Turabian StyleLee, Seul Ah, Bo Ra Moon, Chan Hwi Lee, Sun Hee Lee, Eunju Do, Do Kyung Kim, Tae-Lin Huh, and Chun Sung Kim. 2025. "Exploring the Anti-Inflammatory Activity of the Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) in RAW264.7 Cells and Carrageenan-Induced Rat Models" International Journal of Molecular Sciences 26, no. 18: 9145. https://doi.org/10.3390/ijms26189145
APA StyleLee, S. A., Moon, B. R., Lee, C. H., Lee, S. H., Do, E., Kim, D. K., Huh, T.-L., & Kim, C. S. (2025). Exploring the Anti-Inflammatory Activity of the Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) in RAW264.7 Cells and Carrageenan-Induced Rat Models. International Journal of Molecular Sciences, 26(18), 9145. https://doi.org/10.3390/ijms26189145






