Cortisol Detection Methods and the Hormone’s Role in Evaluating Circadian Rhythm Disruption
Abstract
1. Introduction
2. Methods
- (TITLE-ABS-KEY (circadian AND (rhythm OR clock OR disruption OR dysregulation)));
- (TITLE-ABS-KEY (cortisol OR hydrocort* OR *corticoid OR corticosteroid));
- #1 AND #2.
- circadian rhythm OR circadian clock OR circadian disruption OR circadian dysregulation (All Fields);
- cortisol or hydrocort* or corticoid or corticosteroid (All Fields);
- #1 AND #2.
- circadian rhythm OR circadian clock OR circadian disruption OR circadian dysregulation);
- cortisol OR hydrocort* OR *corticoid OR corticosteroid;
- #1 AND #2.
3. Methods of Cortisol Level Detection
3.1. Cortisol Level Detection in Biofluids
3.1.1. Saliva
3.1.2. Blood or Serum
3.1.3. Hair
3.1.4. Urine
3.1.5. Interstitial Fluid (ISF)
3.1.6. Sweat
3.2. Emerging Technologies for Cortisol Detection
| Sample Collection | Typical Cortisol Range | Advantages | Disadvantages | Method | Suitability for Assessing Circadian Health |
|---|---|---|---|---|---|
| Saliva | 7 AM–9 AM: 100–750 ng/dL 3 PM–5 PM: <401 ng/dL 11 PM–midnight: <100 ng/dL [33] |
| Suitable | ||
| Blood Serum | 8 AM: 3–23 µg/dL 4 PM: 3–13 µg/dL [33] |
| Suitable | ||
| Hair | 40–128 pg/mL [35] |
|
| Not suitable | |
| Urine | Adult/elderly: <100 µg/24 hr Adolescent: 5–55 µg/24 hr Child: 2–27 µg/24 hr [33] |
| Suitable | ||
| Interstitial Fluid (ISF) | 1–11 ng/mL [59] | Suitable | |||
| Sweat | 8 ng/mL–142 ng/mL [45] |
|
| Suitable |
| Emerging Technology | Sample Size and Population | Methodological Strengths | Limitations/Potential Biases | Stage of Validation |
|---|---|---|---|---|
| Wearable electrochemical sensors (sweat/ISF) [25] | Small pilot/prototype studies; healthy volunteers only | Non-invasive, continuous monitoring potential; wearable integration | No commercial devices; inconsistent benchmarking; sweat matrix effects; calibration and mechanical noise issues | Pre-commercial, prototype stage |
| Nanomaterial-based electrochemical sensors [47] | Review of lab-based nanostructured sensors; no study-level sample | Highlights high sensitivity/selectivity via MIPs, aptamers, etc. | Lack of standardized metrics; sparse real-world benchmarking or LC-MS/MS validation; variable calibration protocols | Lab-based validation (prototype) |
| Paper-based competitive immunosensor [62] | ~3 serum samples (triplicates), healthy adults only | High spike-recovery; simple, cost-effective; rapid detection; good ECL agreement | Very small, homogeneous sample; unblinded; no long-term stability/drift data | Early analytical validation |
4. The Potential of Cortisol in Evaluating Circadian Disruption and Related Health Outcomes
5. Conclusions and Gaps of Knowledge
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, K.; Zhao, Y.; Fent, K. Environmental chemicals affect circadian rhythms: An underexplored effect influencing health and fitness in animals and humans. Environ. Int. 2021, 149, 106159. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Mohd Azmi, N.A.S.; Juliana, N.; Azmani, S.; Mohd Effendy, N.; Abu, I.F.; Mohd Fahmi Teng, N.I.; Das, S. Cortisol on Circadian Rhythm and Its Effect on Cardiovascular System. Int. J. Environ. Res. Public Health 2021, 18, 676. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and circadian regulation of cortisol: A short review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2016, 38, 3–45. [Google Scholar] [CrossRef]
- Minnetti, M.; Hasenmajer, V.; Pofi, R.; Venneri, M.A.; Alexandraki, K.I.; Isidori, A.M. Fixing the broken clock in adrenal disorders: Focus on glucocorticoids and chronotherapy. J. Endocrinol. 2020, 246, R13–R31. [Google Scholar] [CrossRef]
- Wang, W.; Duan, X.; Huang, Z.; Pan, Q.; Chen, C.; Guo, L. The GH-IGF-1 Axis in Circadian Rhythm. Front. Mol. Neurosci. 2021, 14, 742294. [Google Scholar] [CrossRef]
- Jones, C.; Gwenin, C. Cortisol level dysregulation and its prevalence-Is it nature’s alarm clock? Physiol. Rep. 2021, 8, e14644. [Google Scholar] [CrossRef]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef]
- Gabinet, N.M. Effects mediated by melatonin and cortisol of artificial light and noise, alone and in combination, on sleep and health. Explor. Neurosci. 2024, 3, 382–417. [Google Scholar] [CrossRef]
- Sertaridou, E.N.; Chouvarda, I.G.; Arvanitidis, K.I.; Filidou, E.K.; Kolios, G.C.; Pnevmatikos, I.N.; Papaioannou, V.E. Melatonin and cortisol exhibit different circadian rhythm profiles during septic shock depending on timing of onset: A prospective observational study. Ann. Intensive Care 2018, 8, 118. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, W.; Chen, Y.; Gong, J.; Wu, Y.; Chen, S.; He, Y.; Yu, H.; Xie, L. The effect of acute sleep deprivation on cortisol level: A systematic review and meta-analysis. Endocr. J. 2024, 71, 753–765. [Google Scholar] [CrossRef]
- Moyers, S.A.; Hagger, M.S. Physical activity and cortisol regulation: A meta-analysis. Biol. Psychol. 2023, 179, 108548. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, T.; Kubokawa, A.; Taketomi, R.; Hatae, K. Effects of day-time exposure to different light intensities on light-induced melatonin suppression at night. J. Physiol. Anthropol. 2015, 34, 27. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; de las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 888292. [Google Scholar] [CrossRef]
- Litvinenko, E.; Merkulova, K.; Postnov, D. Cortisol dynamics and sleep–wake switching: A modeling study. Eur. Phys. J. Spec. Top. 2024, 233, 579–588. [Google Scholar] [CrossRef]
- Liu, P.Y. Rhythms in cortisol mediate sleep and circadian impacts on health. Sleep 2024, 47, zsae151. [Google Scholar] [CrossRef]
- Arroyo Tardio, P.; Baldini, G.; Seelig, E. Food-induced cortisol secretion is comparable in lean and obese male subjects. Endocr. Connect. 2023, 12, e230126. [Google Scholar] [CrossRef]
- Megha, K.B.; Arathi, A.; Shikha, S.; Alka, R.; Ramya, P.; Mohanan, P.V. Significance of Melatonin in the Regulation of Circadian Rhythms and Disease Management. Mol. Neurobiol. 2024, 61, 5541–5571. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Elahi, A.; Wijns, W.; Shahzad, A. Cortisol detection methods for stress monitoring in connected health. Health Sci. Rev. 2023, 6, 100079. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Sekar, M.; Sriramprabha, R.; Sekhar, P.K.; Bhansali, S.; Ponpandian, N.; Pandiaraj, M.; Viswanathan, C. Review—Towards Wearable Sensor Platforms for the Electrochemical Detection of Cortisol. J. Electrochem. Soc. 2020, 167, 067508. [Google Scholar] [CrossRef]
- Miočević, O.; Cole, C.R.; Laughlin, M.J.; Buck, R.L.; Slowey, P.D.; Shirtcliff, E.A. Quantitative Lateral Flow Assays for Salivary Biomarker Assessment: A Review. Front. Public Health 2017, 5, 133. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H. Clinical and Technical Aspects in Free Cortisol Measurement. Endocrinol. Metab. 2022, 37, 599–607. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.H.; Hong, A.R.; Park, K.S.; Kim, S.W.; Shin, C.S.; Kim, S.Y. Stimulated Salivary Cortisol as a Noninvasive Diagnostic Tool for Adrenal Insufficiency. Endocrinol. Metab. 2020, 35, 628–635. [Google Scholar] [CrossRef]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef]
- Siddiqui, A.; Desai, N.G.; Sharma, S.B.; Aslam, M.; Sinha, U.K.; Madhu, S.V. Association of oxidative stress and inflammatory markers with chronic stress in patients with newly diagnosed type 2 diabetes. Diabetes/Metab. Res. Rev. 2019, 35, e3147. [Google Scholar] [CrossRef]
- Taylor, T.; West, D.J.; Howatson, G.; Jones, C.; Bracken, R.M.; Love, T.D.; Cook, C.J.; Swift, E.; Baker, J.S.; Kilduff, L.P. The impact of neuromuscular electrical stimulation on recovery after intensive, muscle damaging, maximal speed training in professional team sports players. J. Sci. Med. Sport 2015, 18, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sharma, A.K.; Marques, C. On The Application of SiO2/SiC Grating on Ag for High-Performance Fiber Optic Plasmonic Sensing of Cortisol Concentration. Materials 2020, 13, 1623. [Google Scholar] [CrossRef]
- Pagana, K.D.; Pagana, T.; Pagana, T.N. Mosby’s Diagnostic and Laboratory Test Reference; Elsevier: New York, NY, USA, 2015. [Google Scholar]
- Hodes, A.; Lodish, M.B.; Tirosh, A.; Meyer, J.; Belyavskaya, E.; Lyssikatos, C.; Rosenberg, K.; Demidowich, A.; Swan, J.; Jonas, N.; et al. Hair cortisol in the evaluation of Cushing syndrome. Endocrine 2017, 56, 164–174. [Google Scholar] [CrossRef]
- Gonzalez, D.; Jacobsen, D.; Ibar, C.; Pavan, C.; Monti, J.; Fernandez Machulsky, N.; Balbi, A.; Fritzler, A.; Jamardo, J.; Repetto, E.M.; et al. Hair Cortisol Measurement by an Automated Method. Sci. Rep. 2019, 9, 8213. [Google Scholar] [CrossRef]
- Koren, L.; Mokady, O.; Karaskov, T.; Klein, J.; Koren, G.; Geffen, E. A novel method using hair for determining hormonal levels in wildlife. Anim. Behav. 2002, 63, 403–406. [Google Scholar] [CrossRef]
- Gao, W.; Xie, Q.; Jin, J.; Qiao, T.; Wang, H.; Chen, L.; Deng, H.; Lu, Z. HPLC-FLU detection of cortisol distribution in human hair. Clin. Biochem. 2010, 43, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Hanzu, F.A. Cortisol Measurements in Cushing’s Syndrome: Immunoassay or Mass Spectrometry? Ann. Lab. Med. 2020, 40, 285–296. [Google Scholar] [CrossRef]
- Ghemigian, A.; Cocolos, A.; Neagu, T.P.; Petrova, E.; Nicoleta, D.; Albu, S.E.; Carsote, M. Cushing’s disease—Same condition, different scenarios. Arch. Balk. Med. Union 2018, 53, 135–139. [Google Scholar]
- Shriyan, P.; Sudhir, P.; van Schayck, O.C.P.; Babu, G.R. Corrigendum to “Association of high cortisol levels in pregnancy and altered fetal growth. Results from the MAASTHI, a prospective cohort study, Bengaluru” [The Lancet Regional Health–Southeast Asia 14 (2023) 100196]. Lancet Reg. Health Southeast Asia 2024, 24, 100196. [Google Scholar] [CrossRef]
- Beck, K.R.; Thompson, G.R.; Odermatt, A. Drug-induced endocrine blood pressure elevation. Pharmacol. Res. 2020, 154, 104311. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; LoPilato, A.; Walker, E.F. Psychotropic medication effects on cortisol: Implications for research and mechanisms of drug action. Schizophr. Res. 2019, 213, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, L.; Zhang, M.; Chen, M.; Wu, Y.; Liu, L.; Qi, L.; Zhang, B.; Yang, X.; He, X.; et al. An aptamer-responsive microneedle patch sensor platform combining with hybridization chain reaction amplification for detection of steroid hormone cortisol in skin interstitial fluid. Biosens. Bioelectron. 2025, 269, 116935. [Google Scholar] [CrossRef]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S.H.M. The Detection of Cortisol in Human Sweat: Implications for Measurement of Cortisol in Hair. Ther. Drug Monit. 2014, 36, 30–34. [Google Scholar] [CrossRef]
- Dalirirad, S.; Steckl, A.J. Aptamer-based lateral flow assay for point of care cortisol detection in sweat. Sens. Actuators B Chem. 2019, 283, 79–86. [Google Scholar] [CrossRef]
- Balasamy, S.; Atchudan, R.; Arya, S.; Gunasekaran, B.M.; Nesakumar, N.; Sundramoorthy, A.K. Cortisol: Biosensing and detection strategies. Clin. Chim. Acta 2024, 562, 119888. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015, 63, 124–130. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Matzeu, G.; Florea, L.; Diamond, D. Advances in wearable chemical sensor design for monitoring biological fluids. Sens. Actuators B Chem. 2015, 211, 403–418. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, T.; Li, S.; Huang, J.; Mao, J.; Yang, H.; Gao, S.; Chen, Z.; Lai, Y. A novel strategy for fabricating robust superhydrophobic fabrics by environmentally-friendly enzyme etching. Chem. Eng. J. 2019, 355, 290–298. [Google Scholar] [CrossRef]
- Apilux, A.; Rengpipat, S.; Suwanjang, W.; Chailapakul, O. Paper-based immunosensor with competitive assay for cortisol detection. J. Pharm. Biomed. Anal. 2020, 178, 112925. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, S.G.; Kim, T.-i. Wearable Devices for Biofluid Monitoring in a Body: From Lab to Commercialization. Korean J. Chem. Eng. 2025, 42, 2011–2036. [Google Scholar] [CrossRef]
- Ferrag, C.; Kerman, K. Grand Challenges in Nanomaterial-Based Electrochemical Sensors. Front. Sens. 2020, 1, 583822. [Google Scholar] [CrossRef]
- Anudevi, S.D.; Ebenezar, K.K.; Hikku, G.S.; Narayan, S. A Decade of Advancement in Nanomaterials-Based Electrochemical Sensors: A Comprehensive Review. Russ. J. Electrochem. 2024, 60, 1175–1197. [Google Scholar] [CrossRef]
- du Plooy, J.; Jahed, N.; Iwuoha, E.; Pokpas, K. Advances in paper-based electrochemical immunosensors: Review of fabrication strategies and biomedical applications. R. Soc. Open Sci. 2023, 10, 230940. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, V.; Castro-Dominguez, B.; James, T.D.; Gamble-Turner, J.M.; Lightman, S.; Reis, N.M. Advancements in Cortisol Detection: From Conventional Methods to Next-Generation Technologies for Enhanced Hormone Monitoring. ACS Sens. 2024, 9, 1666–1681. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- van Smeden, L.; Saris, A.; Sergelen, K.; de Jong, A.M.; Yan, J.; Prins, M.W.J. Reversible Immunosensor for the Continuous Monitoring of Cortisol in Blood Plasma Sampled with Microdialysis. ACS Sens. 2022, 7, 3041–3048. [Google Scholar] [CrossRef]
- Yuan, X.; Ouaskioud, O.; Yin, X.; Li, C.; Ma, P.; Yang, Y.; Yang, P.-F.; Xie, L.; Ren, L. Epidermal Wearable Biosensors for the Continuous Monitoring of Biomarkers of Chronic Disease in Interstitial Fluid. Micromachines 2023, 14, 1452. [Google Scholar] [CrossRef]
- Venugopal, M.; Arya, S.; Chornokur, G.; Bhansali, S. A Realtime and Continuous Assessment of Cortisol in ISF Using Electrochemical Impedance Spectroscopy. Sens. Actuators A Phys. 2011, 172, 154–160. [Google Scholar] [CrossRef]
- Reid, K.J. Assessment of Circadian Rhythms. Neurol. Clin. 2019, 37, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.S.; St. Hilaire, M.A.; McHill, A.W.; Phillips, A.J.K.; Barger, L.K.; Sano, A.; Czeisler, C.A.; Doyle, F.J., III; Klerman, E.B. A classification approach to estimating human circadian phase under circadian alignment from actigraphy and photometry data. J. Pineal Res. 2021, 71, e12745. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef]
- Abbott, S.M.; Malkani, R.G.; Zee, P.C. Circadian disruption and human health: A bidirectional relationship. Eur. J. Neurosci. 2020, 51, 567–583. [Google Scholar] [CrossRef]
- Giordano, A.; Duffy, J.; Epstein, L.J.; Pavlova, M.K. Objective Diagnosis of Circadian Rhythm Disorders. J. Clin. Neurophysiol. 2023, 40, 230–235. [Google Scholar] [CrossRef]
- Reddy, S.; Reddy, V.; Sharma, S. Physiology, Circadian Rhythm. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhang, Y.; Folarin, A.A.; Sun, S.; Cummins, N.; Ranjan, Y.; Rashid, Z.; Stewart, C.; Conde, P.; Sankesara, H.; Laiou, P.; et al. Longitudinal Assessment of Seasonal Impacts and Depression Associations on Circadian Rhythm Using Multimodal Wearable Sensing: Retrospective Analysis. J. Med. Internet. Res. 2024, 26, e55302. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-X.; Jiang, X.-M.; Zheng, Q.-X.; Chen, X.-Q. The association between circadian rhythm of cortisol and shift work regularity among midwives—A multicenter study in Southeast China. Front. Public Health 2022, 10, 965872. [Google Scholar] [CrossRef]
- Negri, M.; Pivonello, C.; Simeoli, C.; Di Gennaro, G.; Venneri, M.A.; Sciarra, F.; Ferrigno, R.; de Angelis, C.; Sbardella, E.; De Martino, M.C.; et al. Cortisol Circadian Rhythm and Insulin Resistance in Muscle: Effect of Dosing and Timing of Hydrocortisone Exposure on Insulin Sensitivity in Synchronized Muscle Cells. Neuroendocrinology 2020, 111, 1005–1028. [Google Scholar] [CrossRef]
- Bavaresco, A.; Mazzeo, P.; Lazzara, M.; Barbot, M. Adipose tissue in cortisol excess: What Cushing’s syndrome can teach us? Biochem. Pharmacol. 2024, 223, 116137. [Google Scholar] [CrossRef] [PubMed]
- Schrader, L.A.; Ronnekleiv-Kelly, S.M.; Hogenesch, J.B.; Bradfield, C.A.; Malecki, K.M.C. Circadian disruption, clock genes, and metabolic health. J. Clin. Investig. 2024, 134, e170998. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, Z.; Wu, M.; Chen, F.; Chen, L. Circadian rhythm regulates the function of immune cells and participates in the development of tumors. Cell Death Discov. 2024, 10, 199. [Google Scholar] [CrossRef]
- Timmons, G.A.; O’Siorain, J.R.; Kennedy, O.D.; Curtis, A.M.; Early, J.O. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front. Immunol. 2020, 11, 1743. [Google Scholar] [CrossRef]
- Robillard, R.; Carpenter, J.S.; Feilds, K.-L.; Hermens, D.F.; White, D.; Naismith, S.L.; Bartlett, D.; Whitwell, B.; Southan, J.; Scott, E.M.; et al. Parallel Changes in Mood and Melatonin Rhythm Following an Adjunctive Multimodal Chronobiological Intervention With Agomelatine in People With Depression: A Proof of Concept Open Label Study. Front. Psychiatry 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, A.-K.; Kajantie, E.; Jones, A.; Pyhälä, R.; Lahti, J.; Heinonen, K.; Eriksson, J.G.; Strandberg, T.E.; Räikkönen, K. Symptoms of attention deficit hyperactivity disorder in children are associated with cortisol responses to psychosocial stress but not with daily cortisol levels. J. Psychiatr. Res. 2011, 45, 1471–1476. [Google Scholar] [CrossRef]
- Dronse, J.; Ohndorf, A.; Richter, N.; Bischof, G.N.; Fassbender, R.; Behfar, Q.; Gramespacher, H.; Dillen, K.; Jacobs, H.I.L.; Kukolja, J.; et al. Serum cortisol is negatively related to hippocampal volume, brain structure, and memory performance in healthy aging and Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1154112. [Google Scholar] [CrossRef]
- Puttonen, S.; Viitasalo, K.; Härmä, M. The relationship between current and former shift work and the metabolic syndrome. Scand. J. Work. Environ. Health 2012, 38, 343–348. [Google Scholar] [CrossRef]
- Luther, J.M.; Fogo, A.B. The role of mineralocorticoid receptor activation in kidney inflammation and fibrosis. Kidney Int. Suppl. 2022, 12, 63–68. [Google Scholar] [CrossRef]
- Esdaile, H.; Khan, S.; Mayet, J.; Oliver, N.; Reddy, M.; Shah, A.S.V. The association between the stress hyperglycaemia ratio and mortality in cardiovascular disease: A meta-analysis and systematic review. Cardiovasc. Diabetol. 2024, 23, 412. [Google Scholar] [CrossRef] [PubMed]
- Almand, A.T.; Anderson, A.P.; Hitt, B.D.; Sitko, J.C.; Joy, R.M.; Easter, B.D.; Almand, E.A. The influence of perceived stress on the human microbiome. BMC Res. Notes 2022, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Paul, P.; Khan, A.S.; Sarkar, A.; Laws, S.a.; Chaari, A. Targeting gut microbiota dysbiosis in inflammatory bowel disease: A systematic review of current evidence. Front. Med. 2025, 12, 1435030. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.J.; Colasurdo, E.A.; Li, G.; Shofer, J.B.; Galasko, D.; Quinn, J.F.; Farlow, M.R.; Peskind, E.R. Sex Differences in Basal Cortisol Levels Across Body Fluid Compartments in a Cross-sectional Study of Healthy Adults. J. Endocr. Soc. 2024, 9, bvae220. [Google Scholar] [CrossRef]
- Roelfsema, F.; van Heemst, D.; Iranmanesh, A.; Takahashi, P.; Yang, R.; Veldhuis, J.D. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr. Connect. 2017, 6, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Azam, M.; Tanvir, F.; Saleem, M.; Bilal, A.; Ullah, Q.; Bibi, S.; Ahmad Khan, M. Physiological, Psychological, and Developmental Impacts of Cortisol Production: Sex-and Age-Related Differences in Cortisol Levels and the Diurnal Rhythm of Hormone Secretion. Remit. Rev. 2024, 9, 819–842. [Google Scholar] [CrossRef]
- Kobayashi, H.; Song, C.; Ikei, H.; Park, B.-J.; Kagawa, T.; Miyazaki, Y. Diurnal Changes in Distribution Characteristics of Salivary Cortisol and Immunoglobulin A Concentrations. Int. J. Environ. Res. Public Health 2017, 14, 987. [Google Scholar] [CrossRef]
- Sørensen, S.O.; Pedersen, J.; Rasmussen, M.G.; Kristensen, P.L.; Grøntved, A. Feasibility of home-based sampling of salivary cortisol and cortisone in healthy adults. BMC Res. Notes 2021, 14, 406. [Google Scholar] [CrossRef]
| Factor | Cortisol | Melatonin |
|---|---|---|
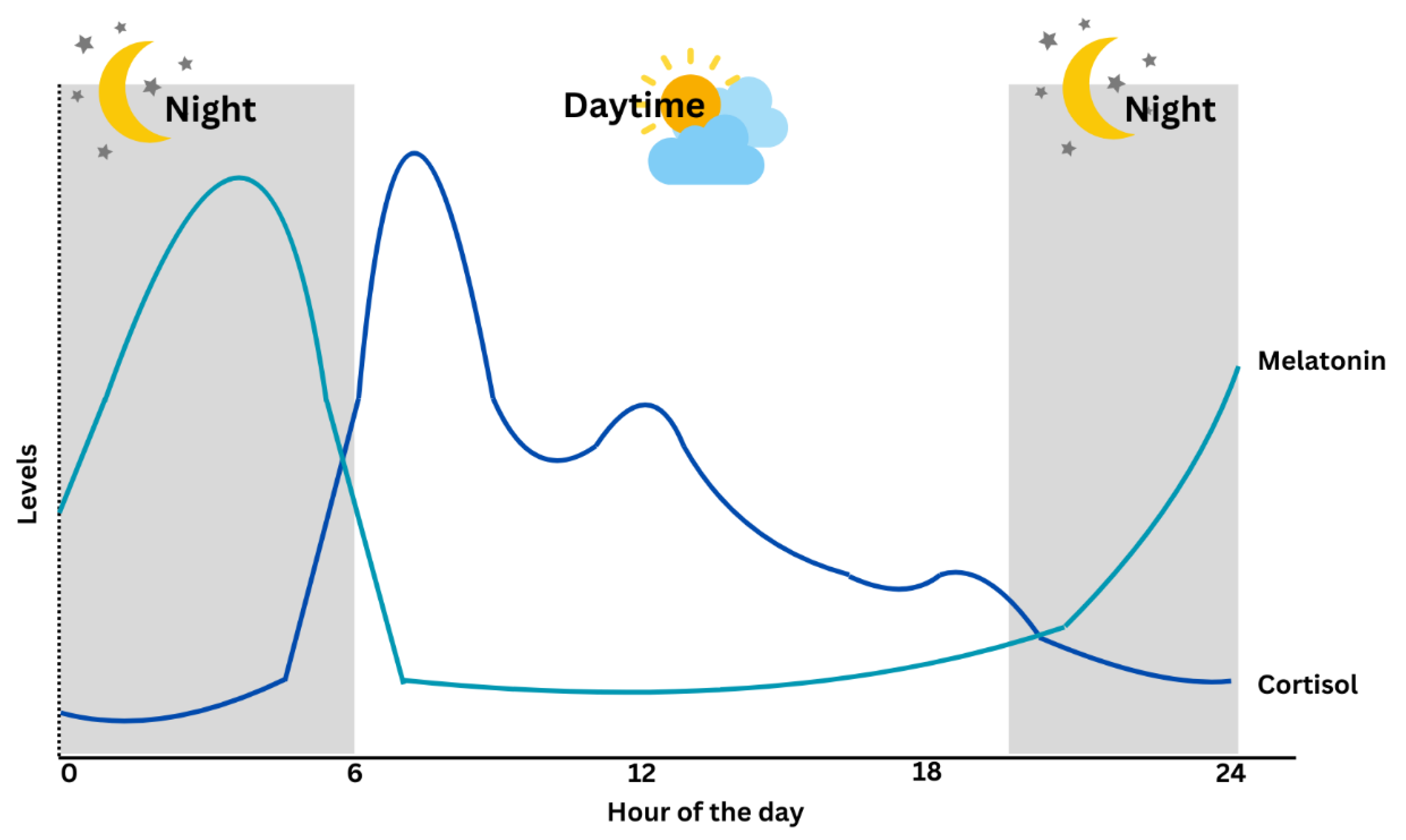
| Circadian Pattern | Peaks in the early morning (around 7–8 AM), declines throughout the day [10]. | Rises in the evening, peaks during the night, decreases in the early morning [11]. |
| Stability [12,13] | Highly stable and reproducible over time. | More sensitive to environmental factors like light exposure. |
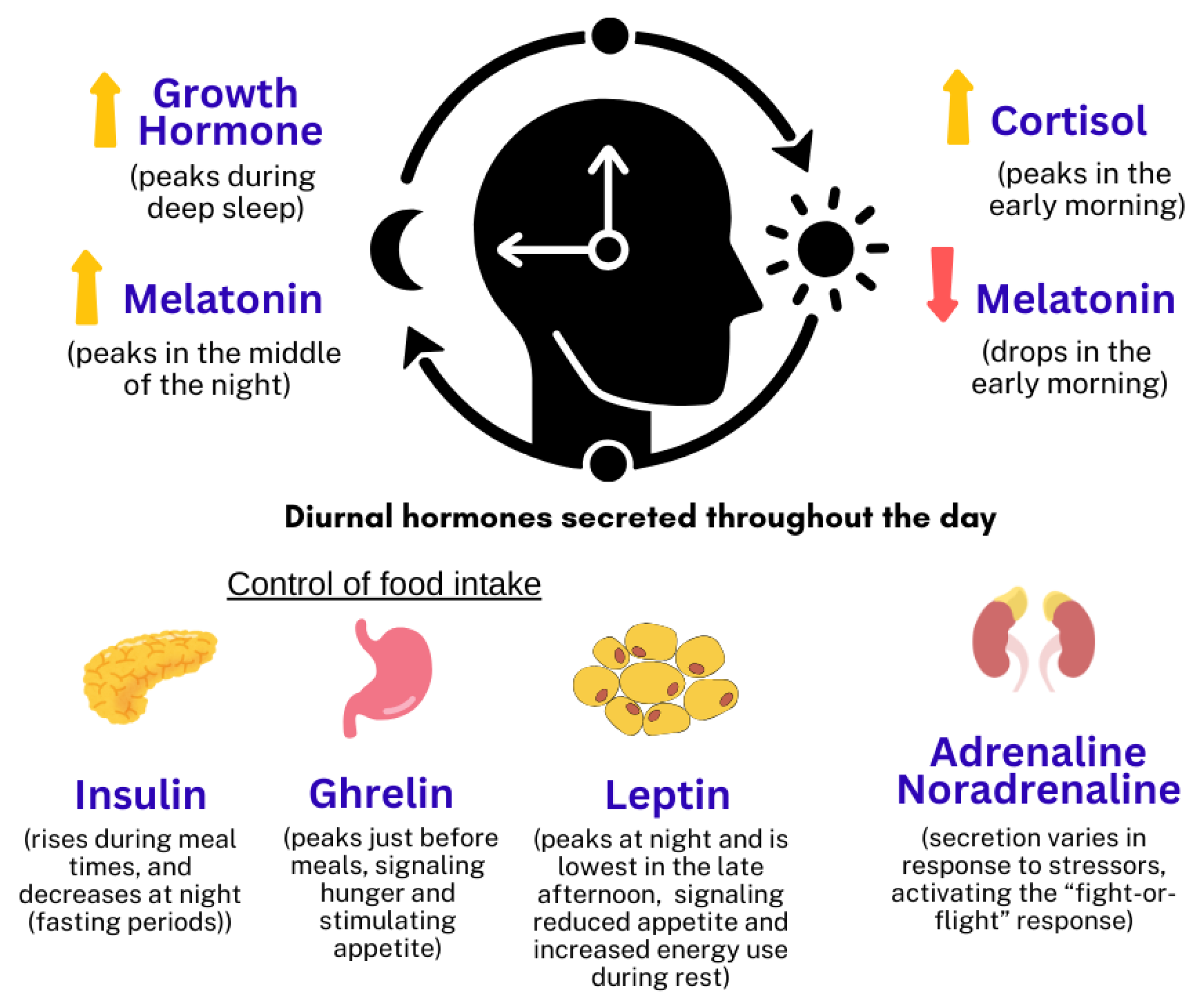
| Influencing Factors | Stress, sleep quality, physical activity [14,15,16]. | Light exposure, age [17,18]. |
| System | Specific Effect | Changes to Cortisol | Type of Study | References |
|---|---|---|---|---|
| Endocrine | Insulin resistance, impaired glucose tolerance, central fat deposition → obesity and metabolic syndrome. | Elevated or flattened cortisol rhythm | Animal models, Human studies (Shift workers) | [72,73,74] |
| Nervous | i. Impaired cognitive functions: Hippocampal atrophy, reduced neurogenesis → accelerating dementia and cognitive decline. | Chronic cortisol elevation | Human study | [79] |
| ii. Increased risk of mood disorders Linked to MDD, anxiety, bipolar disorder, PTSD, ADHD, schizophrenia, and Alzheimer’s. | Elevated cortisol, except for PTSD which is due to reduced cortisol level | Animal models, Human studies, Meta-analysis | [77,78] | |
| Cardiovascular | Hypertension, hyperlipidemia, and endothelial dysfunction leading to atherosclerosis → higher risk for CVD. | Elevated cortisol | Human studies (General population, Shift workers) | [80,81,82] |
| Digestive | Altered gut microbiota, increased gastrointestinal inflammation. | Fluctuations in cortisol | Human study | [83] |
| Immune | Immune suppression, reduced cytokine production, and chronic inflammation. | Fluctuations in cortisol levels with circadian rhythm | Animal models, Human studies | [75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juliana, N.; Maluin, S.M.; Effendy, N.M.; Abu, I.F.; Azmani, S. Cortisol Detection Methods and the Hormone’s Role in Evaluating Circadian Rhythm Disruption. Int. J. Mol. Sci. 2025, 26, 9141. https://doi.org/10.3390/ijms26189141
Juliana N, Maluin SM, Effendy NM, Abu IF, Azmani S. Cortisol Detection Methods and the Hormone’s Role in Evaluating Circadian Rhythm Disruption. International Journal of Molecular Sciences. 2025; 26(18):9141. https://doi.org/10.3390/ijms26189141
Chicago/Turabian StyleJuliana, Norsham, Sofwatul Mokhtarah Maluin, Nadia Mohd Effendy, Izuddin Fahmy Abu, and Sahar Azmani. 2025. "Cortisol Detection Methods and the Hormone’s Role in Evaluating Circadian Rhythm Disruption" International Journal of Molecular Sciences 26, no. 18: 9141. https://doi.org/10.3390/ijms26189141
APA StyleJuliana, N., Maluin, S. M., Effendy, N. M., Abu, I. F., & Azmani, S. (2025). Cortisol Detection Methods and the Hormone’s Role in Evaluating Circadian Rhythm Disruption. International Journal of Molecular Sciences, 26(18), 9141. https://doi.org/10.3390/ijms26189141