Reprogrammed Lipid Metabolism-Associated Therapeutic Vulnerabilities in Prostate Cancer
Abstract
1. Introduction
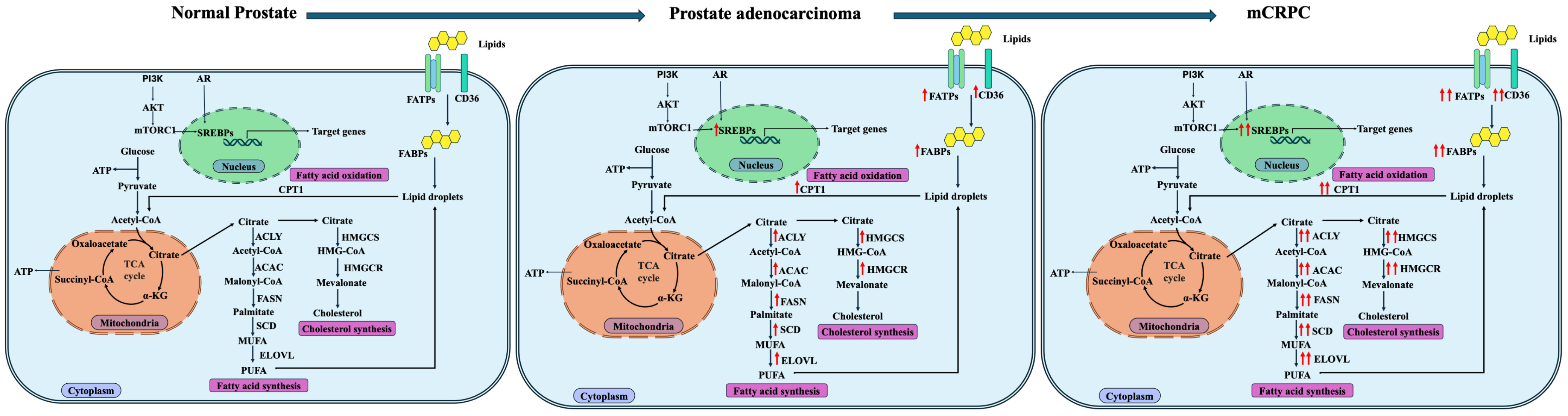
2. Lipid Metabolism in the Normal Prostate
3. Lipid Metabolism in Prostate Cancer
3.1. Increased De Novo Fatty Acid Synthesis in Prostate Cancer
3.2. Increased Fatty Acid Transport and Uptake in Prostate Cancer
3.3. Increased Fatty Acid Beta-Oxidation in Prostate Cancer
3.4. Increased Cholesterol Metabolism in Prostate Cancer
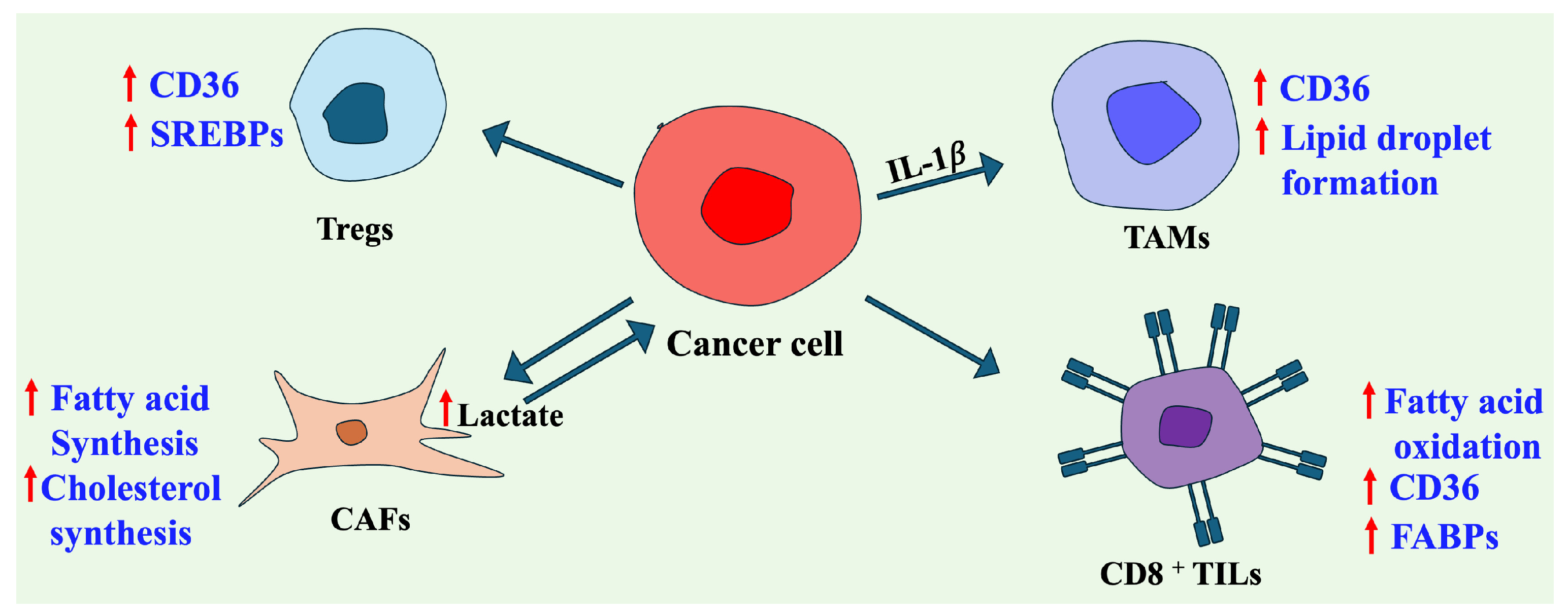
4. Crosstalk Between Lipid Metabolism in the Tumor and Tumor Microenvironment
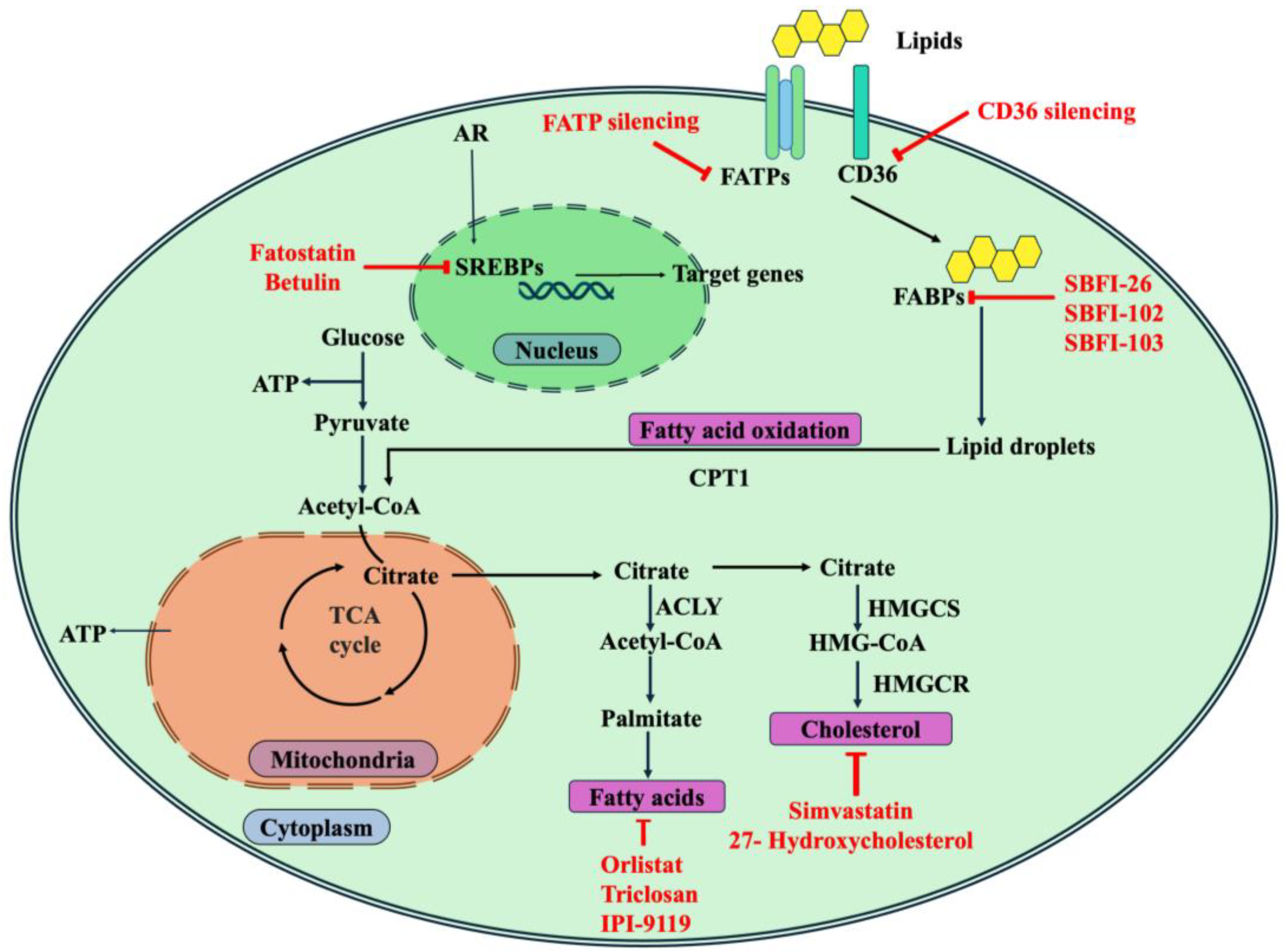
5. Therapeutic Targeting of Lipid Metabolism in Prostate Cancer
5.1. Targeting Transcription Factors
5.2. Targeting Fatty Acid Metabolism
5.3. Targeting Cholesterol Metabolism
6. Future Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAC | Acetyl-CoA carboxylase |
| ACAT | Cholesterol acyltransferase |
| ACLY | Adenosine triphosphate citrate lyase |
| ACSL | Acyl-CoA synthase long-chain proteins |
| AKT | v-Akt murine thymoma viral oncogene (protein kinase B) |
| AMACR | Alpha-Methyl acyl-CoA Racemase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| AR | Androgen receptor |
| AR-FL | Full-length androgen receptor |
| ATP | Adenosine triphosphate |
| BetA | Betulinic acid |
| CaMKK | Calcium/calmodulin-dependent protein kinase kinase |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CRPC | Castration-resistant prostate cancer |
| EGFR | Epidermal Growth Factor Receptor |
| ELOVL | Elongation of very-long-chain fatty acid proteins |
| EMT | Epithelial–mesenchymal transition |
| FABP | Plasma membrane-associated fatty acid-binding protein |
| FAO | Fatty acid oxidation |
| FASN | Fatty acid synthase |
| FATP | Transmembrane fatty acid-transport proteins |
| FOXO | Forkhead box protein |
| HDAC | Histone Deacetylase |
| HDL | High-density lipoprotein |
| HMGCR | 3-Hydroxy-3-methylglutaryl-CoA reductase |
| iCL | Intensive cholesterol-lowering |
| IFNγ | Interferon gamma |
| IHC | Immunohistochemistry |
| INPP5K | Inositol polyphosphate-5-phosphatase K |
| k-RAS | Kirstein rat sarcoma virus |
| LD | Lipid droplets |
| LDL | Low-density lipoprotein |
| LXR | Liver X receptors |
| MAPK | Mitogen-activated protein kinases |
| mCRPC | Metastatic castration resistant prostate cancer |
| MMP | Matrix metalloproteinase |
| mRNA | Messenger ribonucleic acid |
| mTOR | Mammalian target of rapamycin |
| MUFA | Monounsaturated fatty acids |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NEPC | Neuroendocrine prostate cancer |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PCa | Prostate Cancer |
| PI3K | Phosphoinositide 3-kinase |
| PPARγ | Peroxisome proliferator receptor-γ |
| PSA | Prostate-specific antigen |
| PTEN | Phosphatase and tensin homolog |
| PUFA | Polyunsaturated fatty acids |
| RB1 | Retinoblastoma gene 1 |
| RNA | Ribonucleic acid |
| SCAP | Sterol regulatory-element binding protein cleavage-activating protein |
| SCD | Stearoyl-CoA desaturase |
| SFN | Sulforaphane |
| SREBPs | Sterol regulatory-element binding proteins |
| SREBP1 | Sterol regulatory-element binding protein 1 |
| SREBP2 | Sterol regulatory-element binding protein 2 |
| TCGA | The Cancer Genome Atlas |
| TEAD4 | TEA domain containing 4 |
| TME | Tumor microenvironment |
| TP53 | Tumor suppressor gene 53 |
| TRAMP | Transgenic mouse model of PCa |
| VEGF | Vascular endothelial growth factor |
| WNT1 | Wingless-type MMTV integration site family member 1 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Ng, K.L. The Etiology of Prostate Cancer. In Prostate Cancer; Bott, S.R.J., Ng, K.L., Eds.; Exon Publications: Brisbane, Australia, 2021; pp. 17–27. [Google Scholar]
- Halpern, J.A.; Oromendia, C.; Shoag, J.E.; Mittal, S.; Cosiano, M.F.; Ballman, K.V.; Vickers, A.J.; Hu, J.C. Use of Digital Rectal Examination as an Adjunct to Prostate Specific Antigen in the Detection of Clinically Significant Prostate Cancer. J. Urol. 2018, 199, 947–953. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Freter, C. Lipid Metabolism, Apoptosis and Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef]
- Khan, W.; Augustine, D.; Rao, R.S.; Patil, S.; Awan, K.H.; Sowmya, S.V.; Haragannavar, V.C.; Prasad, K. Lipid Metabolism in Cancer: A Systematic Review. J. Carcinog. 2021, 20, 4. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and Cancer: Emerging Roles in Pathogenesis, Diagnosis and Therapeutic Intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef]
- Lima, A.R.; Carvalho, M.; Aveiro, S.S.; Melo, T.; Domingues, M.R.; Macedo-Silva, C.; Coimbra, N.; Jerónimo, C.; Henrique, R.; Bastos, M.d.L.; et al. Comprehensive Metabolomics and Lipidomics Profiling of Prostate Cancer Tissue Reveals Metabolic Dysregulations Associated with Disease Development. J. Proteome Res. 2022, 21, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Takayama, K.; Urano, T.; Obinata, D.; Ikeda, K.; Soga, T.; Takahashi, S.; Inoue, S. ACSL3 Promotes Intratumoral Steroidogenesis in Prostate Cancer Cells. Cancer Sci. 2017, 108, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Balaban, S.; Nassar, Z.D.; Zhang, A.Y.; Hosseini-Beheshti, E.; Centenera, M.M.; Schreuder, M.; Lin, H.-M.; Aishah, A.; Varney, B.; Liu-Fu, F.; et al. Extracellular Fatty Acids Are the Major Contributor to Lipid Synthesis in Prostate Cancer. Mol. Cancer Res. 2019, 17, 949–962. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A Comprehensive Review of the Role of Zinc in Normal Prostate Function and Metabolism; and Its Implications in Prostate Cancer. Arch Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef]
- Frégeau-Proulx, L.; Lacouture, A.; Berthiaume, L.; Weidmann, C.; Harvey, M.; Gonthier, K.; Pelletier, J.-F.; Neveu, B.; Jobin, C.; Bastien, D.; et al. Multiple Metabolic Pathways Fuel the Truncated Tricarboxylic Acid Cycle of the Prostate to Sustain Constant Citrate Production and Secretion. Mol. Metab. 2022, 62, 101516. [Google Scholar] [CrossRef]
- Škara, L.; Huđek Turković, A.; Pezelj, I.; Vrtarić, A.; Sinčić, N.; Krušlin, B.; Ulamec, M. Prostate Cancer—Focus on Cholesterol. Cancers 2021, 13, 4696. [Google Scholar] [CrossRef]
- Arunakaran, J.; Balasubramanian, K.; Srinivasan, N.; Aruldhas, M.M.; Govindarajulu, P. Interaction of Androgens and Prolactin on Prostatic Enzymes of the Pyruvate-Malate Cycle Involved in Lipogenesis in Castrated Mature Monkey, Macaca Radiata. Cytobios 1992, 70, 33–40. [Google Scholar]
- Heemers, H.; Vanderhoydonc, F.; Roskams, T.; Shechter, I.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Androgens Stimulate Coordinated Lipogenic Gene Expression in Normal Target Tissues in Vivo. Mol. Cell. Endocrinol. 2003, 205, 21–31. [Google Scholar] [CrossRef]
- Mah, C.Y.; Nassar, Z.D.; Swinnen, J.V.; Butler, L.M. Lipogenic Effects of Androgen Signaling in Normal and Malignant Prostate. Asian J. Urol. 2020, 7, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Daniels, G.; Lee, P.; Monaco, M.E. Lipid Metabolism in Prostate Cancer. Am. J. Clin. Exp. Urol. 2014, 2, 111–120. [Google Scholar]
- Scaglia, N.; Frontini-López, Y.R.; Zadra, G. Prostate Cancer Progression: As a Matter of Fats. Front. Oncol. 2021, 11, 719865. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Gómez de Cedrón, M.; Ramírez de Molina, A. Alterations of Lipid Metabolism in Cancer: Implications in Prognosis and Treatment. Front. Oncol. 2020, 10, 577420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Luo, Y.; Song, L.; Zhang, J.; Wei, C.; Su, S.; Wang, D. Comprehensive Analysis of Cholesterol Metabolism-Related Genes in Prostate Cancer: Integrated Analysis of Single-Cell and Bulk RNA Sequencing. Discov. Oncol. 2025, 16, 1442. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Zhao, X.; Hao, S.; Li, F.; Wang, Y.; Liu, B.; Zhang, D.; Wang, Y.; Zhou, H. Key Events in Cancer: Dysregulation of SREBPs. Front. Pharmacol. 2023, 14, 1130747. [Google Scholar] [CrossRef]
- Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Li, J.; Xu, H.; Zhao, Y.; Yu, X.; Shi, S. Functional Significance of Cholesterol Metabolism in Cancer: From Threat to Treatment. Exp. Mol. Med. 2023, 55, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Stoykova, G.E.; Salzmann-Sullivan, M.; Dzieciatkowska, M.; Liebman, L.N.; Deep, G.; Schlaepfer, I.R. CPT1A Supports Castration-Resistant Prostate Cancer in Androgen-Deprived Conditions. Cells 2019, 8, 1115. [Google Scholar] [CrossRef] [PubMed]
- Pujana-Vaquerizo, M.; Bozal-Basterra, L.; Carracedo, A. Metabolic Adaptations in Prostate Cancer. Br. J. Cancer 2024, 131, 1250–1262. [Google Scholar] [CrossRef]
- Siltari, A.; Syvälä, H.; Lou, Y.-R.; Gao, Y.; Murtola, T.J. Role of Lipids and Lipid Metabolism in Prostate Cancer Progression and the Tumor’s Immune Environment. Cancers 2022, 14, 4293. [Google Scholar] [CrossRef]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis Inhibitors: Therapeutic Opportunities and Challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Kong, P.; Feng, K.; Liu, C.; Sun, T.; Sang, Y.; Duan, X.; Tao, Z.; Liu, W. New Insights into Lipid Metabolism and Prostate Cancer (Review). Int. J. Oncol. 2023, 62, 74. [Google Scholar] [CrossRef]
- Suburu, J.; Chen, Y.Q. Lipids and Prostate Cancer. Prostaglandins Other Lipid Mediat. 2012, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kasomva, K.; Sen, A.; Paulraj, M.G.; Sailo, S.; Raphael, V.; Puro, K.; Assumi, S.R.; Ignacimuthu, S. Roles of MicroRNA in Prostate Cancer Cell Metabolism. Int. J. Biochem. Cell Biol. 2018, 102, 109–116. [Google Scholar] [CrossRef]
- Flavin, R.; Zadra, G.; Loda, M. Metabolic Alterations and Targeted Therapies in Prostate Cancer. J. Pathol. 2011, 223, 284–295. [Google Scholar] [CrossRef]
- Zadra, G.; Photopoulos, C.; Loda, M. The Fat Side of Prostate Cancer. Biochim. Biophys. Acta 2013, 1831, 1518–1532. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pander, A.; Chinnaiyan, A.M. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Graner, E.; Febbo, P.; Weinstein, L.; Bhattacharya, N.; Onody, T.; Bubley, G.; Balk, S.; Loda, M. Fatty Acid Synthase Expression Defines Distinct Molecular Signatures in Prostate Cancer. Mol. Cancer Res. 2003, 1, 707–715. [Google Scholar] [PubMed]
- Wang, Z.; Chao, Z.; Wang, Q.; Zou, F.; Song, T.; Xu, L.; Ning, J.; Cheng, F. EXO1/P53/SREBP1 Axis-Regulated Lipid Metabolism Promotes Prostate Cancer Progression. J. Transl. Med. 2024, 22, 104. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Y.; Gu, M.; Liu, C.; Chen, Q.; Zhan, M.; Wang, Z. Fatty Acid Metabolism Reprogramming in Advanced Prostate Cancer. Metabolites 2021, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; et al. Inhibition of de Novo Lipogenesis Targets Androgen Receptor Signaling in Castration-Resistant Prostate Cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 631–640, Erratum in Proc. Natl. Acad. Sci. USA 2020, 117, 18893. [Google Scholar] [CrossRef]
- Shah, S.; Carriveau, W.J.; Li, J.; Campbell, S.L.; Kopinski, P.K.; Lim, H.-W.; Daurio, N.; Trefely, S.; Won, K.-J.; Wallace, D.C.; et al. Targeting ACLY Sensitizes Castration-Resistant Prostate Cancer Cells to AR Antagonism by Impinging on an ACLY-AMPK-AR Feedback Mechanism. Oncotarget 2016, 7, 43713–43730. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.F.P.; Bonen, A.; Glatz, J.F.C. Fatty Acid Transport across the Cell Membrane: Regulation by Fatty Acid Transporters. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Eeles, R.; Goh, C.; Castro, E.; Bancroft, E.; Guy, M.; Olama, A.A.A.; Easton, D.; Kote-Jarai, Z. The Genetic Epidemiology of Prostate Cancer and Its Clinical Implications. Nat. Rev. Urol. 2014, 11, 18–31. [Google Scholar] [CrossRef] [PubMed]
- M. Swamynathan, M.; Mathew, G.; Aziz, A.; Gordon, C.; Hillowe, A.; Wang, H.; Jhaveri, A.; Kendall, J.; Cox, H.; Giarrizzo, M.; et al. FABP5 Inhibition against PTEN-Mutant Therapy Resistant Prostate Cancer. Cancers 2023, 16, 60. [Google Scholar] [CrossRef]
- Brusselmans, K.; De Schrijver, E.; Verhoeven, G.; Swinnen, J.V. RNA Interference–Mediated Silencing of the Acetyl-CoA-Carboxylase-α Gene Induces Growth Inhibition and Apoptosis of Prostate Cancer Cells. Cancer Res. 2005, 65, 6719–6725. [Google Scholar] [CrossRef]
- Hale, J.S.; Otvos, B.; Sinyuk, M.; Alvarado, A.G.; Hitomi, M.; Stoltz, K.; Wu, Q.; Flavahan, W.; Levison, B.; Johansen, M.L.; et al. Cancer Stem Cell-Specific Scavenger Receptor CD36 Drives Glioblastoma Progression. Stem Cells 2014, 32, 1746–1758. [Google Scholar] [CrossRef]
- Nath, A.; Chan, C. Genetic Alterations in Fatty Acid Transport and Metabolism Genes Are Associated with Metastatic Progression and Poor Prognosis of Human Cancers. Sci. Rep. 2016, 6, 18669. [Google Scholar] [CrossRef]
- Watt, M.J.; Clark, A.K.; Selth, L.A.; Haynes, V.R.; Lister, N.; Rebello, R.; Porter, L.H.; Niranjan, B.; Whitby, S.T.; Lo, J.; et al. Suppressing Fatty Acid Uptake Has Therapeutic Effects in Preclinical Models of Prostate Cancer. Sci. Transl. Med. 2019, 11, eaau5758. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.-O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting Metastasis-Initiating Cells through the Fatty Acid Receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Vona, R.; Iessi, E.; Matarrese, P. Role of Cholesterol and Lipid Rafts in Cancer Signaling: A Promising Therapeutic Opportunity? Front. Cell Dev. Biol. 2021, 9, 622908. [Google Scholar] [CrossRef]
- Kushwaha, P.P.; Verma, S.S.; Shankar, E.; Lin, S.; Gupta, S. Role of Solute Carrier Transporters SLC25A17 and SLC27A6 in Acquired Resistance to Enzalutamide in Castration-resistant Prostate Cancer. Mol. Carcinog. 2022, 61, 397–407. [Google Scholar] [CrossRef]
- Verma, S.; Shankar, E.; Chan, E.R.; Gupta, S. Metabolic Reprogramming and Predominance of Solute Carrier Genes during Acquired Enzalutamide Resistance in Prostate Cancer. Cells 2020, 9, 2535. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, Y.; Gu, Z. FATP5 Modulates Biological Activity and Lipid Metabolism in Prostate Cancer through the TEAD4-Mediated Hippo Signaling. Front. Oncol. 2024, 14, 1442911. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Beesley, C.; Foster, C.S.; Rudland, P.S.; Fujii, H.; Ono, T.; Chen, H.; Smith, P.H.; Ke, Y. Identification of the Messenger RNA for Human Cutaneous Fatty Acid-Binding Protein as a Metastasis Inducer. Cancer Res. 2000, 60, 2390–2398. [Google Scholar]
- Abdulsamad, S.A. Experimental Treatment Efficacy of DmrFABP5 on Prostate Cancer Singly or in Combination with Drugs in Use. Am. J. Cancer Res. 2024, 14, 300–323. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kume, H.; Matsuzaki, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Uemura, M.; Miyagawa, Y.; Tomonaga, T.; Nonomura, N. Proteomic Analysis of Urinary Extracellular Vesicles from High Gleason Score Prostate Cancer. Sci. Rep. 2017, 7, 42961. [Google Scholar] [CrossRef]
- Nitschke, K.; Erben, P.; Waldbillig, F.; Abdelhadi, A.; Weis, C.-A.; Gottschalt, M.; Wahby, S.; Nuhn, P.; Boutros, M.; Michel, M.S.; et al. Clinical Relevance of Gene Expression in Localized and Metastatic Prostate Cancer Exemplified by FABP5. World J. Urol. 2020, 38, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Malki, M.I.; Forootan, S.S.; Adamson, J.; Forootan, F.S.; Chen, D.; Foster, C.S.; Rudland, P.S.; Ke, Y. A Novel Cutaneous Fatty Acid-Binding Protein-Related Signaling Pathway Leading to Malignant Progression in Prostate Cancer Cells. Genes Cancer 2013, 4, 297–314. [Google Scholar] [CrossRef]
- Naeem, A.; Abdulsamad, S.; Zeng, H.; He, G.; Jin, X.; Zhang, J.; Alenezi, B.; Ma, H.; Rudland, P.; Ke, Y. FABP5 Can Substitute for Androgen Receptor in Malignant Progression of Prostate Cancer Cells. Int. J. Oncol. 2023, 64, 18. [Google Scholar] [CrossRef]
- Chkourko Gusky, H.; Diedrich, J.; MacDougald, O.A.; Podgorski, I. Omentum and Bone Marrow: How Adipocyte-rich Organs Create Tumour Microenvironments Conducive for Metastatic Progression. Obes. Rev. 2016, 17, 1015–1029. [Google Scholar] [CrossRef]
- Tan, N.-S.; Shaw, N.S.; Vinckenbosch, N.; Liu, P.; Yasmin, R.; Desvergne, B.; Wahli, W.; Noy, N. Selective Cooperation between Fatty Acid Binding Proteins and Peroxisome Proliferator-Activated Receptors in Regulating Transcription. Mol. Cell. Biol. 2002, 22, 5114–5127. [Google Scholar] [CrossRef]
- Olokpa, E.; Bolden, A.; Stewart, L.V. The Androgen Receptor Regulates PPARγ Expression and Activity in Human Prostate Cancer Cells. J. Cell. Physiol. 2016, 231, 2664–2672. [Google Scholar] [CrossRef]
- Uehara, H.; Takahashi, T.; Oha, M.; Ogawa, H.; Izumi, K. Exogenous Fatty Acid Binding Protein 4 Promotes Human Prostate Cancer Cell Progression. Int. J. Cancer 2014, 135, 2558–2568. [Google Scholar] [CrossRef]
- Uehara, H.; Kobayashi, T.; Matsumoto, M.; Watanabe, S.; Yoneda, A.; Yoshimi, B. Adipose Tissue:Critical Contributor to the Development of Prostate Cancer. J. Med. Investig. 2018, 65, 9–17. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-Mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, K.; Yao, Y.; Reilly, P.T.; Kannan, K.; Kiarash, R.; Mason, J.; Huang, P.; Sawyer, S.K.; Fuerth, B.; Faubert, B.; et al. Carnitine Palmitoyltransferase 1C Promotes Cell Survival and Tumor Growth under Conditions of Metabolic Stress. Genes Dev. 2011, 25, 1041–1051. [Google Scholar] [CrossRef]
- Liu, Y. Fatty Acid Oxidation Is a Dominant Bioenergetic Pathway in Prostate Cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-Z.; Choi, W.-S.; Jain, S.; Dinakaran, D.; Xu, X.; Han, W.H.; Yang, X.-H.; Glubrecht, D.D.; Moore, R.B.; Lemieux, H.; et al. The FABP12/PPARγ Pathway Promotes Metastatic Transformation by Inducing Epithelial-to-Mesenchymal Transition and Lipid-Derived Energy Production in Prostate Cancer Cells. Mol. Oncol. 2020, 14, 3100–3120. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-Z.; Garg, M.; Yang, X.-H.; Godbout, R. Docetaxel-Induced Cell Death Is Regulated by a Fatty Acid-Binding Protein 12-Slug-Survivin Pathway in Prostate Cancer Cells. Int. J. Mol. Sci. 2024, 25, 9669. [Google Scholar] [CrossRef]
- Flaig, T.W.; Salzmann-Sullivan, M.; Su, L.-J.; Zhang, Z.; Joshi, M.; Gijón, M.A.; Kim, J.; Arcaroli, J.J.; Van Bokhoven, A.; Lucia, M.S.; et al. Lipid Catabolism Inhibition Sensitizes Prostate Cancer Cells to Antiandrogen Blockade. Oncotarget 2017, 8, 56051–56065. [Google Scholar] [CrossRef]
- Abudurexiti, M.; Zhu, W.; Wang, Y.; Wang, J.; Xu, W.; Huang, Y.; Zhu, Y.; Shi, G.; Zhang, H.; Zhu, Y.; et al. Targeting CPT1B as a Potential Therapeutic Strategy in Castration-resistant and Enzalutamide-resistant Prostate Cancer. Prostate 2020, 80, 950–961. [Google Scholar] [CrossRef]
- Mah, C.Y.; Nguyen, A.D.T.; Niijima, T.; Helm, M.; Dehairs, J.; Ryan, F.J.; Ryan, N.; Quek, L.-E.; Hoy, A.J.; Don, A.S.; et al. Peroxisomal β-Oxidation Enzyme, DECR2, Regulates Lipid Metabolism and Promotes Treatment Resistance in Advanced Prostate Cancer. Br. J. Cancer 2024, 130, 741–754. [Google Scholar] [CrossRef]
- Zha, S.; Ferdinandusse, S.; Hicks, J.L.; Denis, S.; Dunn, T.A.; Wanders, R.J.; Luo, J.; De Marzo, A.M.; Isaacs, W.B. Peroxisomal Branched Chain Fatty Acid β-Oxidation Pathway Is Upregulated in Prostate Cancer. Prostate 2005, 63, 316–323. [Google Scholar] [CrossRef]
- Zha, S.; Ferdinandusse, S.; Denis, S.; Wanders, R.J.; Ewing, C.M.; Luo, J.; De Marzo, A.M.; Isaacs, W.B. Alpha-Methylacyl-CoA Racemase as an Androgen-Independent Growth Modifier in Prostate Cancer. Cancer Res. 2003, 63, 7365–7376. [Google Scholar]
- Göbel, A.; Pählig, S.; Motz, A.; Breining, D.; Traikov, S.; Hofbauer, L.C.; Rachner, T.D. Overcoming Statin Resistance in Prostate Cancer Cells by Targeting the 3-Hydroxy-3-Methylglutaryl-CoA-Reductase. Biochem. Biophys. Res. Commun. 2024, 710, 149841. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wang, H.; Zhou, X.; Wang, Y.; Xue, L.; Cao, B.; Song, J. The Role of Cholesterol Metabolism in Tumor Therapy, from Bench to Bed. Front. Pharmacol. 2023, 14, 928821. [Google Scholar] [CrossRef] [PubMed]
- di Meo, N.A.; Lasorsa, F.; Rutigliano, M.; Milella, M.; Ferro, M.; Battaglia, M.; Ditonno, P.; Lucarelli, G. The Dark Side of Lipid Metabolism in Prostate and Renal Carcinoma: Novel Insights into Molecular Diagnostic and Biomarker Discovery. Expert. Rev. Mol. Diagn. 2023, 23, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Lasorsa, F.; di Meo, N.A.; Rutigliano, M.; Ferro, M.; Terracciano, D.; Tataru, O.S.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Emerging Hallmarks of Metabolic Reprogramming in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 910. [Google Scholar] [CrossRef]
- Pelton, K.; Freeman, M.R.; Solomon, K.R. Cholesterol and Prostate Cancer. Curr. Opin. Pharmacol. 2012, 12, 751–759. [Google Scholar] [CrossRef]
- Chimento, A.; Casaburi, I.; Avena, P.; Trotta, F.; De Luca, A.; Rago, V.; Pezzi, V.; Sirianni, R. Cholesterol and Its Metabolites in Tumor Growth: Therapeutic Potential of Statins in Cancer Treatment. Front. Endocrinol. 2019, 9, 807. [Google Scholar] [CrossRef]
- Roy, M.; Kung, H.-J.; Ghosh, P.M. Statins and Prostate Cancer: Role of Cholesterol Inhibition vs. Prevention of Small GTP-Binding Proteins. Am. J. Cancer Res. 2011, 1, 542–561. [Google Scholar]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Lee, H.J.; Li, J.; Vickman, R.E.; Li, J.; Liu, R.; Durkes, A.C.; Elzey, B.D.; Yue, S.; Liu, X.; Ratliff, T.L.; et al. Cholesterol Esterification Inhibition Suppresses Prostate Cancer Metastasis by Impairing the Wnt/β-Catenin Pathway. Mol. Cancer Res. 2018, 16, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhu, N.; Li, H.-F.; Zhao, T.-J.; Zhang, C.-J.; Liao, D.-F.; Qin, L. Cholesterol Homeostasis and Cancer: A New Perspective on the Low-Density Lipoprotein Receptor. Cell. Oncol. 2022, 45, 709–728. [Google Scholar] [CrossRef]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the Tumor Microenvironment: From Cancer Progression to Treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Yu, W.; Lei, Q.; Yang, L.; Qin, G.; Liu, S.; Wang, D.; Ping, Y.; Zhang, Y. Contradictory Roles of Lipid Metabolism in Immune Response within the Tumor Microenvironment. J. Hematol. Oncol. 2021, 14, 187. [Google Scholar] [CrossRef]
- Vasseur, S.; Guillaumond, F. Lipids in Cancer: A Global View of the Contribution of Lipid Pathways to Metastatic Formation and Treatment Resistance. Oncogenesis 2022, 11, 46. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kurupati, R.; Liu, L.; Zhou, X.Y.; Zhang, G.; Hudaihed, A.; Filisio, F.; Giles-Davis, W.; Xu, X.; Karakousis, G.C.; et al. Enhancing CD8+ T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 2017, 32, 377–391.e9. [Google Scholar] [CrossRef]
- Ao, Y.-Q.; Gao, J.; Zhang, L.-X.; Deng, J.; Wang, S.; Lin, M.; Wang, H.-K.; Ding, J.-Y.; Jiang, J.-H. Tumor-Infiltrating CD36+CD8+T Cells Determine Exhausted Tumor Microenvironment and Correlate with Inferior Response to Chemotherapy in Non-Small Cell Lung Cancer. BMC Cancer 2023, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, X.; Song, C.; He, Z.; Wang, R.; Xu, Y.; Jiang, G.; Wan, Y.; Mei, J.; Mao, W. The Role of Lipid Metabolic Reprogramming in Tumor Microenvironment. Theranostics 2023, 13, 1774–1808. [Google Scholar] [CrossRef]
- Kopecka, J.; Godel, M.; Riganti, C. Cholesterol Metabolism: At the Cross Road between Cancer Cells and Immune Environment. Int. J. Biochem. Cell Biol. 2020, 129, 105876. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.d.F.; Andrade, L.N.d.S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-Derived Lipid Mediators: The Crosstalk Between Cancer Cells and Immune Cells. Front. Immunol. 2022, 13, 768606. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid Metabolism in Cancer Progression and Therapeutic Strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Scott, K.F.; Sajinovic, M.; Hein, J.; Nixdorf, S.; Galettis, P.; Liauw, W.; de Souza, P.; Dong, Q.; Graham, G.G.; Russell, P.J. Emerging Roles for Phospholipase A2 Enzymes in Cancer. Biochimie 2010, 92, 601–610. [Google Scholar] [CrossRef]
- Wang, H.; Franco, F.; Tsui, Y.-C.; Xie, X.; Trefny, M.P.; Zappasodi, R.; Mohmood, S.R.; Fernández-García, J.; Tsai, C.-H.; Schulze, I.; et al. CD36-Mediated Metabolic Adaptation Supports Regulatory T Cell Survival and Function in Tumors. Nat. Immunol. 2020, 21, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Wei, J.; Nguyen, T.-L.M.; Shi, H.; Su, W.; Palacios, G.; Dhungana, Y.; Chapman, N.M.; Long, L.; Saravia, J.; et al. Lipid Signalling Enforces Functional Specialization of Treg Cells in Tumours. Nature 2021, 591, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Tang, Y.; Miao, H. Metabolism in Tumor Microenvironment: Implications for Cancer Immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Roy, A.; Dwarakanath, B.S. Metabolic Cooperation and Competition in the Tumor Microenvironment: Implications for Therapy. Front. Oncol. 2017, 7, 68. [Google Scholar] [CrossRef]
- Ippolito, L.; Comito, G.; Parri, M.; Iozzo, M.; Duatti, A.; Virgilio, F.; Lorito, N.; Bacci, M.; Pardella, E.; Sandrini, G.; et al. Lactate Rewires Lipid Metabolism and Sustains a Metabolic–Epigenetic Axis in Prostate Cancer. Cancer Res. 2022, 82, 1267–1282. [Google Scholar] [CrossRef]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1438–1450, Erratum in Cancer Res. 2022, 82, 945. [Google Scholar] [CrossRef]
- Masetti, M.; Carriero, R.; Portale, F.; Marelli, G.; Morina, N.; Pandini, M.; Iovino, M.; Partini, B.; Erreni, M.; Ponzetta, A.; et al. Lipid-Loaded Tumor-Associated Macrophages Sustain Tumor Growth and Invasiveness in Prostate Cancer. J. Exp. Med. 2022, 219, e20210564. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.-X.; Wang, Y.-S.; Zhou, J.-L. Lipid Metabolism, Amino Acid Metabolism, and Prostate Cancer: A Crucial Metabolic Journey. Asian J. Androl. 2024, 26, 123–134. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, G.; Lou, Z. Role of the Sterol Regulatory Element Binding Protein Pathway in Tumorigenesis. Front. Oncol. 2020, 10, 1788. [Google Scholar] [CrossRef]
- Shao, W.; Machamer, C.E.; Espenshade, P.J. Fatostatin Blocks ER Exit of SCAP but Inhibits Cell Growth in a SCAP-Independent Manner. J. Lipid Res. 2016, 57, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.-S.; Lee, Y.-R.; Fung, J.; Katon, J.M.; et al. An Aberrant SREBP-Dependent Lipogenic Program Promotes Metastatic Prostate Cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.-T.; Hu, P.; Huang, W.-C. Fatostatin Displays High Antitumor Activity in Prostate Cancer by Blocking SREBP-Regulated Metabolic Pathways and Androgen Receptor Signaling. Mol. Cancer Ther. 2014, 13, 855–866. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.B.; Chung, L.W.K.; Huang, W.-C. Anti-Cancer Efficacy of SREBP Inhibitor, Alone or in Combination with Docetaxel, in Prostate Cancer Harboring P53 Mutations. Oncotarget 2015, 6, 41018–41032. [Google Scholar] [CrossRef]
- Kim, S.-H.; Singh, K.B.; Hahm, E.-R.; Lokeshwar, B.L.; Singh, S.V. Withania Somnifera Root Extract Inhibits Fatty Acid Synthesis in Prostate Cancer Cells. J. Tradit. Complement. Med. 2020, 10, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-F.; Jiang, W.-P.; Basavaraj, P.; Huang, S.-Y.; Ruangsai, P.; Wu, J.-B.; Huang, G.-J.; Huang, W.-C. Cell Suspension Culture Extract of Eriobotrya Japonica Attenuates Growth and Induces Apoptosis in Prostate Cancer Cells via Targeting SREBP-1/FASN-Driven Metabolism and AR. Phytomedicine 2021, 93, 153806. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Huang, G.-J.; Wu, H.-C.; Kao, M.-C.; Huang, W.-C. Ganoderma Tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells. Molecules 2018, 23, 2539. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Deep, G.; Singh, R.P.; Agarwal, C.; Agarwal, R. Silibinin Inhibits Aberrant Lipid Metabolism, Proliferation and Emergence of Androgen-Independence in Prostate Cancer Cells via Primarily Targeting the Sterol Response Element Binding Protein 1. Oncotarget 2014, 5, 10017–10033. [Google Scholar] [CrossRef]
- Deep, G.; Kumar, R.; Nambiar, D.K.; Jain, A.K.; Ramteke, A.M.; Serkova, N.J.; Agarwal, C.; Agarwal, R. Silibinin Inhibits Hypoxia-induced HIF-1α-mediated Signaling, Angiogenesis and Lipogenesis in Prostate Cancer Cells: In Vitro Evidence and in Vivo Functional Imaging and Metabolomics. Mol. Carcinog. 2017, 56, 833–848. [Google Scholar] [CrossRef]
- Flaig, T.W.; Gustafson, D.L.; Su, L.-J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A Phase I and Pharmacokinetic Study of Silybin-Phytosome in Prostate Cancer Patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Huang, Y.; Li, W.; Xie, Y.; Zhang, D.; Niu, Y.; Zhao, Y. SREBF1-Based Metabolic Reprogramming in Prostate Cancer Promotes Tumor Ferroptosis Resistance. Cell Death Discov. 2025, 11, 75. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.-T.; Josson, S.; Mukhopadhyay, N.K.; Kim, J.; Freeman, M.R.; Huang, W.-C. MicroRNA-185 and 342 Inhibit Tumorigenicity and Induce Apoptosis through Blockade of the SREBP Metabolic Pathway in Prostate Cancer Cells. PLoS ONE 2013, 8, e70987. [Google Scholar] [CrossRef]
- Guan, M.; Su, L.; Yuan, Y.-C.; Li, H.; Chow, W.A. Nelfinavir and Nelfinavir Analogs Block Site-2 Protease Cleavage to Inhibit Castration-Resistant Prostate Cancer. Sci. Rep. 2015, 5, 9698. [Google Scholar] [CrossRef]
- Sukjoi, W.; Ngamkham, J.; Attwood, P.V.; Jitrapakdee, S. Targeting Cancer Metabolism and Current Anti-Cancer Drugs. In Reviews on New Drug Targets in Age-Related Disorders: Part II; Springer: Cham, Switzerland, 2021; pp. 15–48. [Google Scholar]
- Schcolnik-Cabrera, A.; Chávez-Blanco, A.; Domínguez-Gómez, G.; Taja-Chayeb, L.; Morales-Barcenas, R.; Trejo-Becerril, C.; Perez-Cardenas, E.; Gonzalez-Fierro, A.; Dueñas-González, A. Orlistat as a FASN Inhibitor and Multitargeted Agent for Cancer Therapy. Expert Opin. Investig. Drugs 2018, 27, 475–489. [Google Scholar] [CrossRef]
- Kridel, S.J.; Axelrod, F.; Rozenkrantz, N.; Smith, J.W. Orlistat Is a Novel Inhibitor of Fatty Acid Synthase with Antitumor Activity. Cancer Res. 2004, 64, 2070–2075. [Google Scholar] [CrossRef]
- Rae, C.; Fragkoulis, G.I.; Chalmers, A.J. Cytotoxicity and Radiosensitizing Activity of the Fatty Acid Synthase Inhibitor C75 Is Enhanced by Blocking Fatty Acid Uptake in Prostate Cancer Cells. Adv. Radiat. Oncol. 2020, 5, 994–1005. [Google Scholar] [CrossRef]
- Sadowski, M.C.; Pouwer, R.H.; Gunter, J.H.; Lubik, A.A.; Quinn, R.J.; Nelson, C.C. The Fatty Acid Synthase Inhibitor Triclosan: Repurposing an Anti-Microbial Agent for Targeting Prostate Cancer. Oncotarget 2014, 5, 9362–9381. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Kim, S.-H.; Hahm, E.-R.; Pore, S.K.; Jacobs, B.L.; Singh, S. V Prostate Cancer Chemoprevention by Sulforaphane in a Preclinical Mouse Model Is Associated with Inhibition of Fatty Acid Metabolism. Carcinogenesis 2018, 39, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Heuer, T.S.; Ventura, R.; Mordec, K.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G. FASN Inhibition and Taxane Treatment Combine to Enhance Anti-Tumor Efficacy in Diverse Xenograft Tumor Models through Disruption of Tubulin Palmitoylation and Microtubule Organization and FASN Inhibition-Mediated Effects on Oncogenic Signaling and Gene Expression. EBioMedicine 2017, 16, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.; Mordec, K.; Waszczuk, J.; Wang, Z.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G.; Heuer, T.S. Inhibition of de Novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine 2015, 2, 808–824. [Google Scholar] [CrossRef]
- Khwairakpam, A.; Shyamananda, M.; Sailo, B.; Rathnakaram, S.; Padmavathi, G.; Kotoky, J.; Kunnumakkara, A. ATP Citrate Lyase (ACLY): A Promising Target for Cancer Prevention and Treatment. Curr. Drug Targets 2015, 16, 156–163. [Google Scholar] [CrossRef]
- Gao, Y.; Islam, M.S.; Tian, J.; Lui, V.W.Y.; Xiao, D. Inactivation of ATP Citrate Lyase by Cucurbitacin B: A Bioactive Compound from Cucumber, Inhibits Prostate Cancer Growth. Cancer Lett. 2014, 349, 15–25. [Google Scholar] [CrossRef]
- Liu, S.; Lai, J.; Feng, Y.; Zhuo, Y.; Zhang, H.; Chen, Y.; Li, J.; Mei, X.; Zeng, Y.; Su, J.; et al. Acetyl-CoA Carboxylase 1 Depletion Suppresses de Novo Fatty Acid Synthesis and Mitochondrial β-Oxidation in Castration-Resistant Prostate Cancer Cells. J. Biol. Chem. 2023, 299, 102720. [Google Scholar] [CrossRef]
- Beckers, A.; Organe, S.; Timmermans, L.; Scheys, K.; Peeters, A.; Brusselmans, K.; Verhoeven, G.; Swinnen, J.V. Chemical Inhibition of Acetyl-CoA Carboxylase Induces Growth Arrest and Cytotoxicity Selectively in Cancer Cells. Cancer Res. 2007, 67, 8180–8187. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; d’Oelsnitz, S.; Kaczocha, M. FABP5 Coordinates Lipid Signaling That Promotes Prostate Cancer Metastasis. Sci. Rep. 2019, 9, 18944. [Google Scholar] [CrossRef]
- Al-Jameel, W.; Gou, X.; Forootan, S.S.; Fayi, M.S.A.; Rudland, P.S.; Forootan, F.S.; Zhang, J.; Cornford, P.A.; Hussain, S.A.; Ke, Y. Inhibitor SBFI26 Suppresses the Malignant Progression of Castration-Resistant PC3-M Cells by Competitively Binding to Oncogenic FABP5. Oncotarget 2017, 8, 31041–31056, Erratum in Oncotarget 2020, 11, 3025. [Google Scholar] [CrossRef]
- Carbonetti, G.; Converso, C.; Clement, T.; Wang, C.; Trotman, L.C.; Ojima, I.; Kaczocha, M. Docetaxel/Cabazitaxel and Fatty Acid Binding Protein 5 Inhibitors Produce Synergistic Inhibition of Prostate Cancer Growth. Prostate 2020, 80, 88–98. [Google Scholar] [CrossRef]
- Al-Jameel, W.; Gou, X.; Jin, X.; Zhang, J.; Wei, Q.; Ai, J.; Li, H.; Al-Bayati, A.; Platt-Higgins, A.; Pettitt, A.; et al. Inactivated FABP5 Suppresses Malignant Progression of Prostate Cancer Cells by Inhibiting the Activation of Nuclear Fatty Acid Receptor PPARγ. Genes Cancer 2019, 10, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ochoa, P.; Rodríguez-Zapater, S.; Anel, A.; Esteban, L.M.; Camón-Fernández, A.; Espilez-Ortiz, R.; Gil-Sanz, M.J.; Borque-Fernando, Á. Prostate Cancer and the Mevalonate Pathway. Int. J. Mol. Sci. 2024, 25, 2152. [Google Scholar] [CrossRef] [PubMed]
- Kochuparambil, S.T.; Al-Husein, B.; Goc, A.; Soliman, S.; Somanath, P.R. Anticancer Efficacy of Simvastatin on Prostate Cancer Cells and Tumor Xenografts Is Associated with Inhibition of Akt and Reduced Prostate-Specific Antigen Expression. J. Pharmacol. Exp. Ther. 2011, 336, 496–505. [Google Scholar] [CrossRef]
- Longo, J.; Mullen, P.J.; Yu, R.; van Leeuwen, J.E.; Masoomian, M.; Woon, D.T.S.; Wang, Y.; Chen, E.X.; Hamilton, R.J.; Sweet, J.M.; et al. An Actionable Sterol-Regulated Feedback Loop Modulates Statin Sensitivity in Prostate Cancer. Mol. Metab. 2019, 25, 119–130. [Google Scholar] [CrossRef]
- Kong, Y.; Cheng, L.; Mao, F.; Zhang, Z.; Zhang, Y.; Farah, E.; Bosler, J.; Bai, Y.; Ahmad, N.; Kuang, S.; et al. Inhibition of Cholesterol Biosynthesis Overcomes Enzalutamide Resistance in Castration-Resistant Prostate Cancer (CRPC). J. Biol. Chem. 2018, 293, 14328–14341. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, M.T.; Petillo, S.; Santoni, A.; Cippitelli, M. Liver X Receptors: Regulators of Cholesterol Metabolism, Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2020, 11, 584303. [Google Scholar] [CrossRef] [PubMed]
- Bouchareb, E.; Dallel, S.; De Haze, A.; Damon-Soubeyrand, C.; Renaud, Y.; Baabdaty, E.; Vialat, M.; Fabre, J.; Pouchin, P.; De Joussineau, C.; et al. Liver X Receptors Enhance Epithelial to Mesenchymal Transition in Metastatic Prostate Cancer Cells. Cancers 2024, 16, 2776. [Google Scholar] [CrossRef]
- Hoang, J.-J.; Baron, S.; Volle, D.H.; Lobaccaro, J.-M.A.; Trousson, A. Lipids, LXRs and Prostate Cancer: Are HDACs a New Link? Biochem. Pharmacol. 2013, 86, 168–174. [Google Scholar] [CrossRef]
- Chen, T.; Xu, J.; Fu, W. EGFR/FOXO3A/LXR-α Axis Promotes Prostate Cancer Proliferation and Metastasis and Dual-Targeting LXR-α/EGFR Shows Synthetic Lethality. Front. Oncol. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Raza, S.; Meyer, M.; Goodyear, C.; Hammer, K.D.P.; Guo, B.; Ghribi, O. The Cholesterol Metabolite 27-Hydroxycholesterol Stimulates Cell Proliferation via ERβ in Prostate Cancer Cells. Cancer Cell Int. 2017, 17, 52. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Sekine, Y.; Oka, D.; Nakazawa, S.; Tsuji, Y.; Nakayama, H.; Suzuki, K. Simvastatin Induces Autophagy and Inhibits Proliferation in Prostate Cancer Cells. Anticancer Res. 2023, 43, 5377–5386. [Google Scholar] [CrossRef]
- Rushworth, L.K.; Loveridge, C.; Salji, M.; MacLeod, M.; Mui, E.; Sumpton, D.; Neilson, M.; Hedley, A.; Alexander, L.; McCartney, E.; et al. Phase II Proof-of-concept Study of Atorvastatin in Castration-resistant Prostate Cancer. BJU Int. 2023, 131, 236–243. [Google Scholar] [CrossRef] [PubMed]
| Agents | Mechanism | Reference |
|---|---|---|
| Fatostatin | Inhibits SREBPs. Suppresses migration, invasion, and proliferation, and induces apoptosis in LNCaP and C4-2B cells. | [106] |
| Ganoderma tsugae extract | Inhibits SREBPs. Inhibits migration and invasion and induces apoptosis in LNCaP and C4-2 cells. | [110] |
| miR-185 And M342 | Inhibits SREBPs. Inhibits proliferation, clonogenic survival, and migration and promotes apoptosis in LNCaP and C4-2B cells. | [115] |
| Nelfinavir | Inhibits SREBPs. Inhibits proliferation and induces apoptosis in PC3 and DU145 cells. | [116] |
| Eriobotrya japonica extract | Inhibits SREBP1. Promotes cytotoxicity and apoptosis, and inhibits migration and invasion in LNCaP and C4-2 cells. | [109] |
| Silibinin | Inhibits SREBPs and activates AMPK. Decreases angiogenesis in LNCaP and 22Rv1 cells. | [111,112] |
| Betulin | Inhibits SREBP1. Induces ferroptosis in LNCaP and PC3 cells. | [110] |
| Agents | Mechanism | Reference |
|---|---|---|
| Orlistat | Inhibits FASN. Inhibits migration and angiogenesis and induces cytotoxicity and apoptosis in PC3, DU145, and LNCaP cells. | [117,118,119] |
| Triclosan | Inhibits FASN. Induces cytotoxicity, cell cycle arrest, and apoptosis in LNCaP, C4-2B, PC3, and 22Rv1 cells. | [121] |
| IPI-9119 | Inhibits FASN, AR-FL/AR-V7. Inhibits cell growth, induces ER Stress, and apoptosis in LNCaP, C4-2, C4-2B, LNCaP-95, and 22RV1 cells. | [39] |
| C75 | Inhibits FASN. Induces cytotoxicity, enhances sensitivity to ionizing radiation in PC3 and LNCaP cells. | [120] |
| TVB-3166 | Inhibits FASN. Inhibits cell viability, clonogenic survival, and induces apoptosis in 22RV1 cells. | [123,124] |
| Sulforaphane | Inhibits ACAC and FASN. Induces apoptosis in LNCaP and 22Rv1 cells. | [122] |
| Withania somnifera extract | Inhibits FASN, ACAC, and SREBP1. Inhibits clonogenic survival and proliferation and induces apoptosis in LNCaP and 22Rv1 cells. | [108] |
| Bms-303141 | Inhibits ACLY. Sensitizes 22Rv1 CRPC cells to enzalutamide treatment. | [40] |
| SBFI26 | Inhibits FABP5. Supresses proliferation, migration, invasion, and clonogenic survival in PC3M cells. | [132] |
| SBFI-102 and SBFI-103 | Inhibits FABP5. Supresses proliferation, migration, invasion, and clonogenic survival in PC3, DU145, and 22Rv1 cells. | [131] |
| Dmrfabp5 | Inhibits FABP5. Supresses proliferation, migration, invasion, and clonogenic survival in LNCaP, DU145, and 22Rv1 cells. | [54] |
| Agents | Mechanism | Reference |
|---|---|---|
| Simvastatin | Inhibits HMGCR. Inhibits proliferation, migration, invasion, and clonogenic survival in LNCaP cells, and induces autophagy in PC3 cells | [134,142] |
| Simvastatin and enzalutamide | Inhibits HMGCR. Sensitize MR49F, C4-2R, and 22Rv1 cells to enzalutamide. | [136] |
| GW3965 (LXR-α agonist) + Afatinib (EGFR inhibitor) | Activates LXR-α and inhibits EGFR. Inhibits proliferation in PC3 cells. | [140] |
| 27-hydroxycholesterol | Involves ERβ activation, disruption of lipids, and potential effects on DNA damage repair and other signaling pathways | [141] |
| Drug | Target | Phase | Population | Trial ID |
|---|---|---|---|---|
| Denifanstat (TVB-2640) + Enzalutamide | FASN inhibitor | Phase I | mCRPC | NCT05743621 |
| Ezetimibe ± Simvastatin | Cholesterol absorption/synthesis | Phase II | Localized PCa on active surveillance | NCT06437574 |
| Aspirin ± Atorvastatin added to first-line CRPC therapy | HMG-CoA reductase and Cyclooxygenase (COX) | Phase III | Men starting first-line CRPC therapy | NCT03819101 |
| Atorvastatin with ADT (SPECTRE trial) | HMG-CoA reductase inhibitor | Phase II | CRPC | ISRCTN16951765 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parupathi, P.; Devarakonda, L.S.; Francois, E.; Amjed, M.; Kumar, A. Reprogrammed Lipid Metabolism-Associated Therapeutic Vulnerabilities in Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 9132. https://doi.org/10.3390/ijms26189132
Parupathi P, Devarakonda LS, Francois E, Amjed M, Kumar A. Reprogrammed Lipid Metabolism-Associated Therapeutic Vulnerabilities in Prostate Cancer. International Journal of Molecular Sciences. 2025; 26(18):9132. https://doi.org/10.3390/ijms26189132
Chicago/Turabian StyleParupathi, Prashanth, Lakshmi Sirisha Devarakonda, Ekniel Francois, Mehak Amjed, and Avinash Kumar. 2025. "Reprogrammed Lipid Metabolism-Associated Therapeutic Vulnerabilities in Prostate Cancer" International Journal of Molecular Sciences 26, no. 18: 9132. https://doi.org/10.3390/ijms26189132
APA StyleParupathi, P., Devarakonda, L. S., Francois, E., Amjed, M., & Kumar, A. (2025). Reprogrammed Lipid Metabolism-Associated Therapeutic Vulnerabilities in Prostate Cancer. International Journal of Molecular Sciences, 26(18), 9132. https://doi.org/10.3390/ijms26189132






