Murine Cell Line Models for Vascular Mimicry: The Role of YAP/TAZ Signaling
Abstract
1. Introduction
2. Results
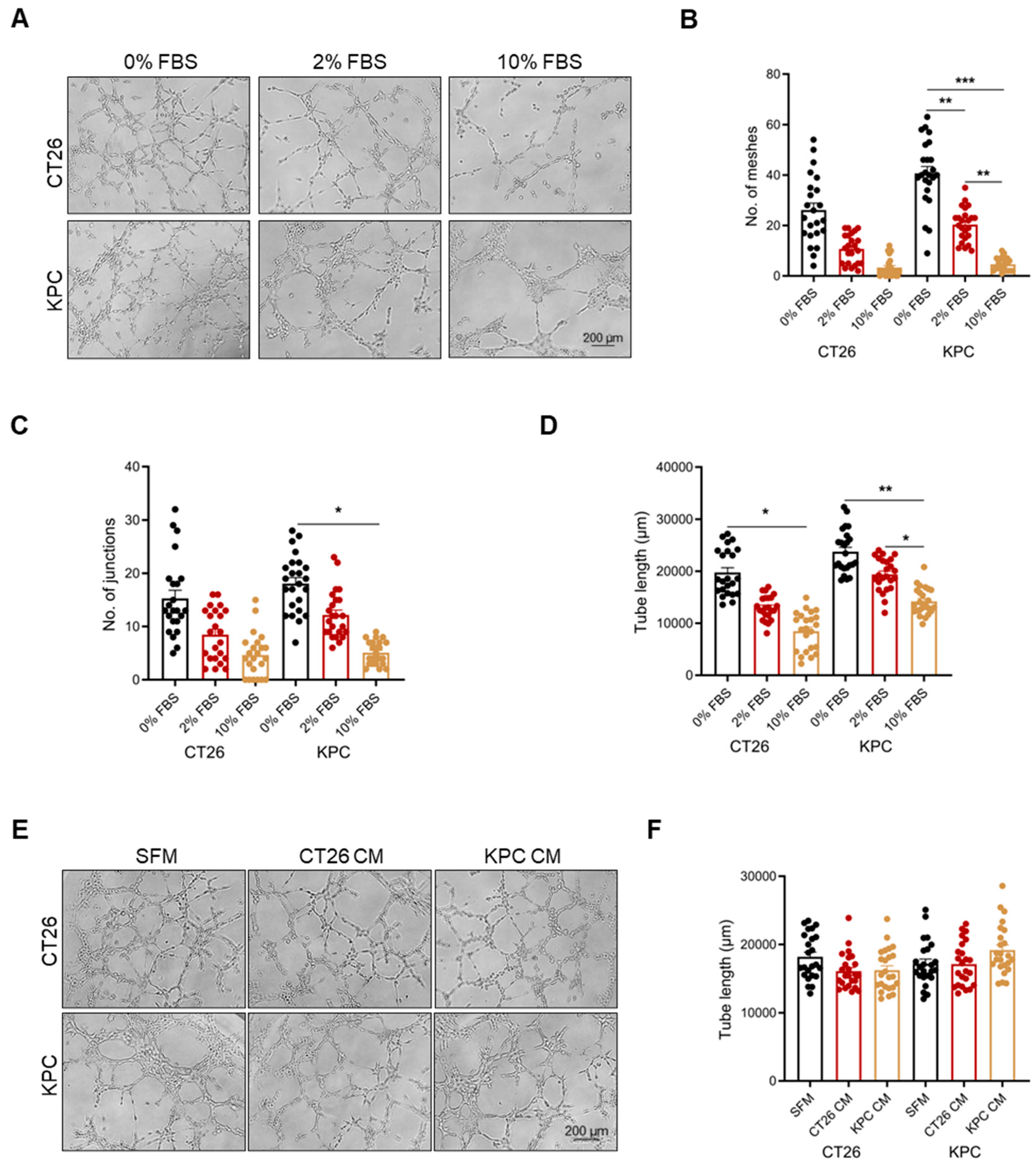
2.1. Vascular Mimicry Is Dependent on Serum Starvation
2.2. Vascular Mimicry Is a Cell-Intrinsic Mechanism Independent of Tumor-Secreted Factors
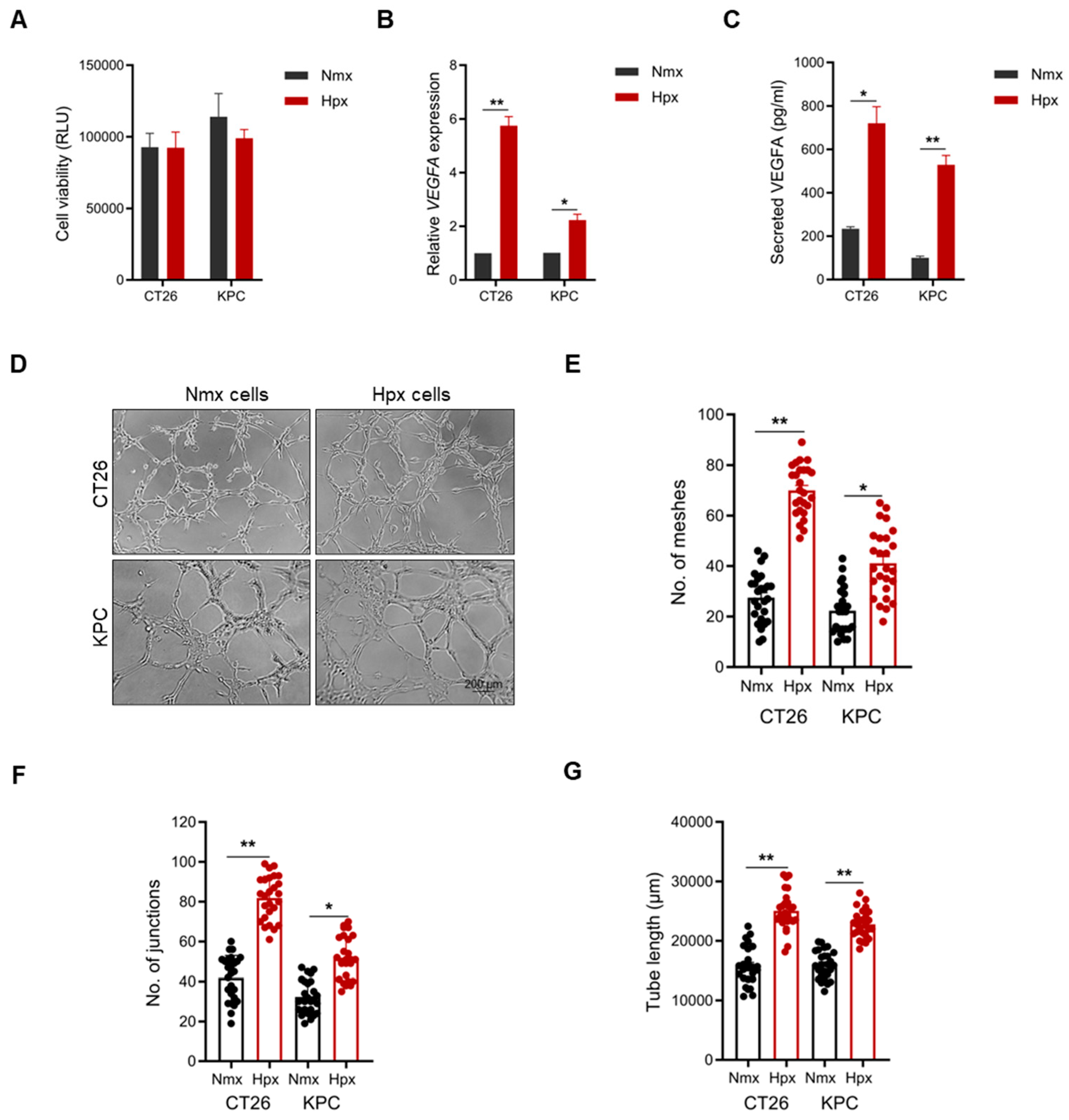
2.3. Hypoxia-Educated Tumor Cells Enhance Vascular Mimicry Formation
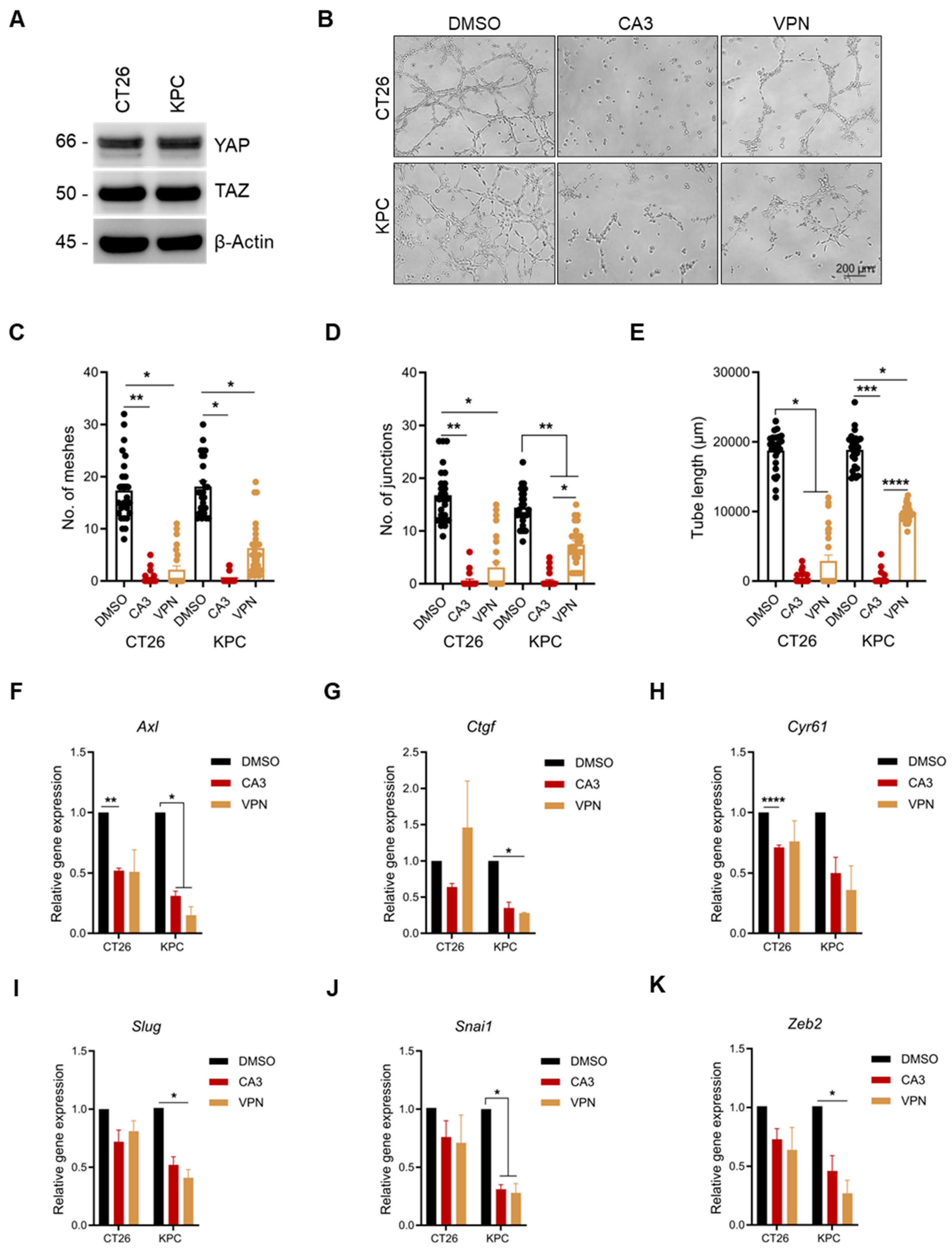
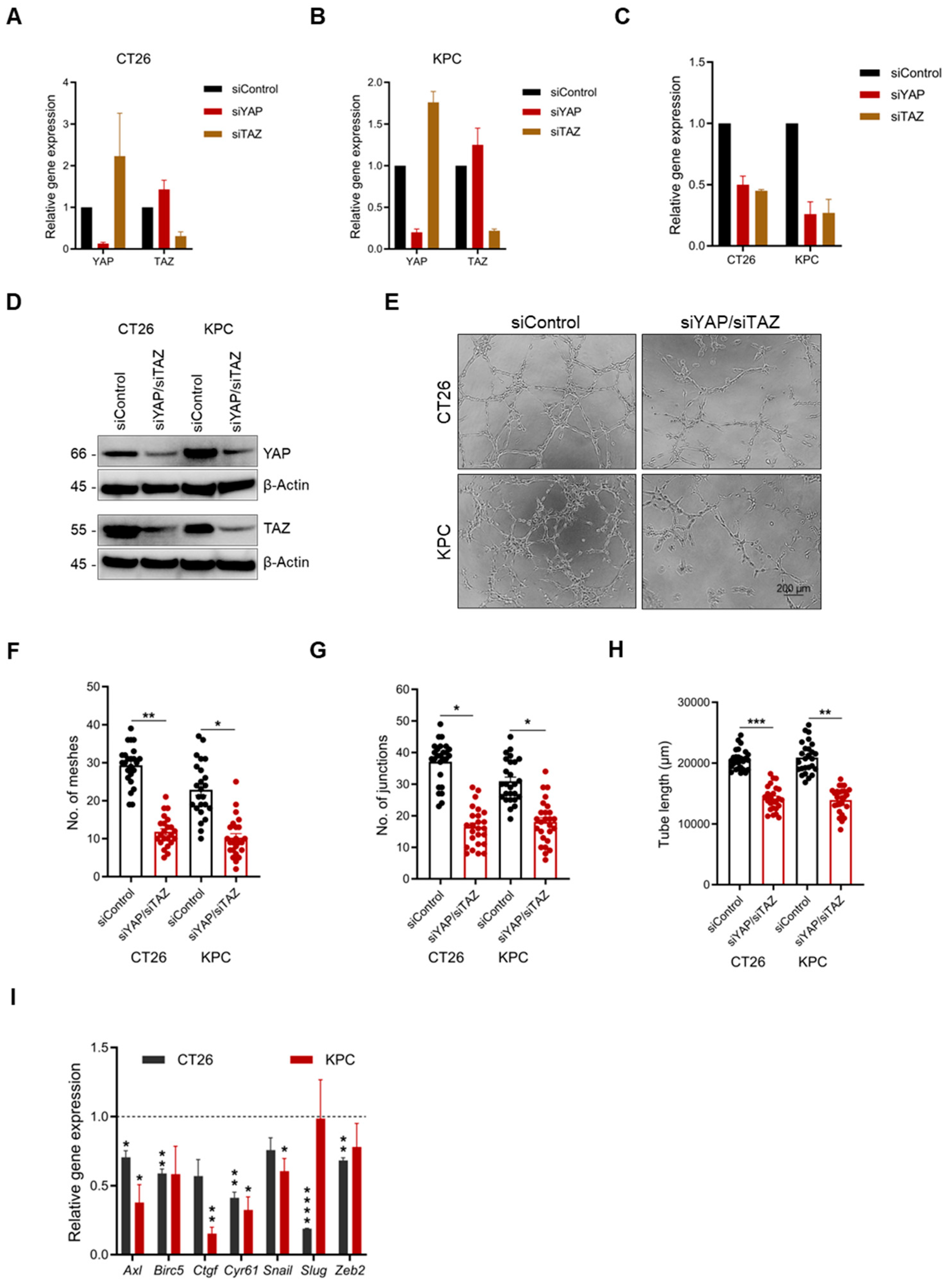
2.4. YAP/TAZ Inhibition Significantly Reduces Vascular Mimicry
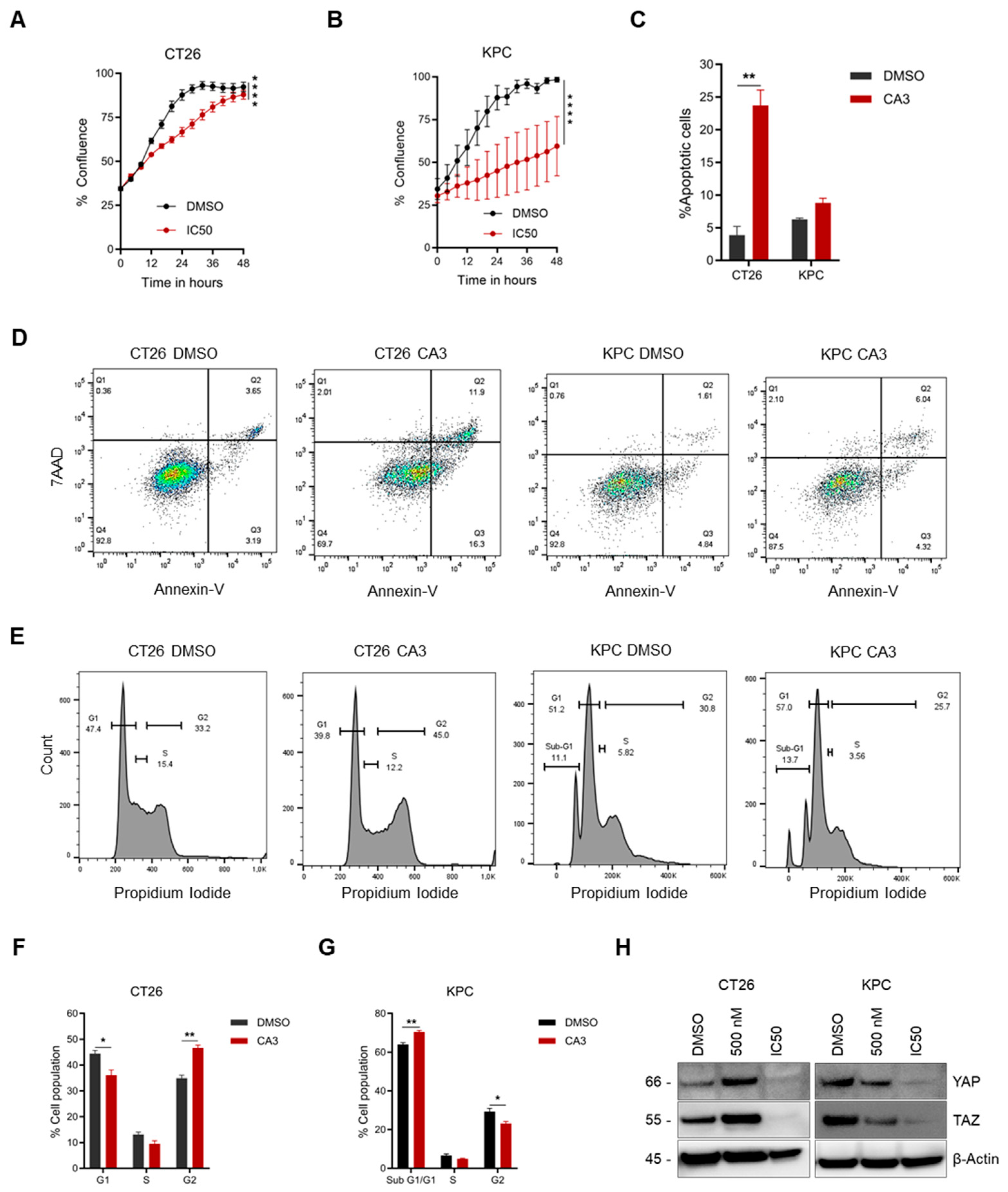
2.5. VM-Inhibiting Dose Attenuates Tumor Cell Chemotaxis
2.6. Determination of IC50 Concentration and Subsequent Functional Effect on Cancer Cells
2.7. Dual YAP/TAZ Gene Knockdown Attenuates VM Formation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Vascular Mimicry Assay
4.3. ELISA Assay
4.4. Quantitative PCR
4.5. Cell Viability Assay
4.6. Chemotaxis Assay
4.7. Colony Formation Assay
4.8. Cell Proliferation Assay
4.9. Apoptosis Assay
4.10. Cell Cycle Analysis
4.11. Western Blot Analysis
4.12. siRNA Silencing
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guelfi, S.; Hodivala-Dilke, K.; Bergers, G. Targeting the tumour vasculature: From vessel destruction to promotion. Nat. Rev. Cancer 2024, 24, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Wechman, S.L.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Vascular mimicry: Triggers, molecular interactions and in vivo models. Adv. Cancer Res. 2020, 148, 27–67. [Google Scholar] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, M.J.; Seftor, E.A.; Hess, A.R.; Seftor, R.E. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat. Rev. Cancer 2003, 3, 411–421. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Yang, J.P.; Liao, Y.D.; Mai, D.M.; Xie, P.; Qiang, Y.Y.; Zheng, L.S.; Wang, M.Y.; Mei, Y.; Meng, D.F.; Xu, L.; et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis 2016, 19, 191–200. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Chao, J.T.; Chien, D.S.; Chu, Y.W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef]
- Kuo, C.H.; Wu, Y.F.; Chang, B.I.; Hsu, C.K.; Lai, C.H.; Wu, H.L. Interference in melanoma CD248 function reduces vascular mimicry and metastasis. J. Biomed. Sci. 2022, 29, 98, Erratum in J. Biomed. Sci. 2025, 32, 64. [Google Scholar]
- Provance, O.K.; Oria, V.O.; Tran, T.T.; Caulfield, J.I.; Zito, C.R.; Aguirre-Ducler, A.; Schalper, K.A.; Kluger, H.M.; Jilaveanu, L.B. Vascular mimicry as a facilitator of melanoma brain metastasis. Cell Mol. Life Sci. 2024, 81, 188. [Google Scholar] [CrossRef]
- Zhou, L.; Chang, Y.; Xu, L.; Liu, Z.; Fu, Q.; Yang, Y.; Lin, Z.; Xu, J. The Presence of Vascular Mimicry Predicts High Risk of Clear Cell Renal Cell Carcinoma after Radical Nephrectomy. J. Urol. 2016, 196, 335–342. [Google Scholar] [CrossRef]
- Angara, K.; Borin, T.F.; Arbab, A.S. Vascular Mimicry: A Novel Neovascularization Mechanism Driving Anti-Angiogenic Therapy (AAT) Resistance in Glioblastoma. Transl. Oncol. 2017, 10, 650–660. [Google Scholar] [CrossRef]
- Ayala-Dominguez, L.; Olmedo-Nieva, L.; Munoz-Bello, J.O.; Contreras-Paredes, A.; Manzo-Merino, J.; Martinez-Ramirez, I.; Lizano, M. Mechanisms of Vasculogenic Mimicry in Ovarian Cancer. Front. Oncol. 2019, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Baeten, C.I.; Hillen, F.; Pauwels, P.; de Bruine, A.P.; Baeten, C.G. Prognostic role of vasculogenic mimicry in colorectal cancer. Dis. Colon Rectum 2009, 52, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, F.; Wang, Y.; Li, T.; Xiu, P.; Zhong, J.; Sun, X.; Li, J. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017, 108, 478–487. [Google Scholar] [CrossRef]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.P.; Simms, N.; Polanski, R.; et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef] [PubMed]
- Chavoshi, H.; Poormolaie, N.; Vahedian, V.; Kazemzadeh, H.; Mir, A.; Nejabati, H.R.; Behroozi, J.; Isazadeh, A.; Hajezimian, S.; Nouri, M.; et al. Vascular mimicry: A potential therapeutic target in breast cancer. Pathol.-Res. Pract. 2022, 234, 153922. [Google Scholar] [CrossRef]
- Guerin, E.; Man, S.; Xu, P.; Kerbel, R.S. A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 2013, 73, 2743–2748. [Google Scholar] [CrossRef]
- Kelland, L.R. Of mice and men: Values and liabilities of the athymic nude mouse model in anticancer drug development. Eur. J. Cancer 2004, 40, 827–836. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Cimpean, A.M.; Raica, M.; Crivellato, E.; Ruggieri, S.; Vacca, A. B16-F10 melanoma cells contribute to the new formation of blood vessels in the chick embryo chorioallantoic membrane through vasculogenic mimicry. Clin. Exp. Med. 2013, 13, 143–147. [Google Scholar] [CrossRef]
- Johnson, D.; Chee, C.E.; Wong, W.; Lam, R.C.T.; Tan, I.B.H.; Ma, B.B.Y. Current advances in targeted therapy for metastatic colorectal cancer—Clinical translation and future directions. Cancer Treat. Rev. 2024, 125, 102700. [Google Scholar] [CrossRef]
- Katsuta, E.; Qi, Q.; Peng, X.; Hochwald, S.N.; Yan, L.; Takabe, K. Pancreatic adenocarcinomas with mature blood vessels have better overall survival. Sci. Rep. 2019, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Benjakul, N.; Prakobphol, N.; Tangshewinsirikul, C.; Dulyaphat, W.; Svasti, J.; Charngkaew, K.; Kangsamaksin, T. Notch signaling regulates vasculogenic mimicry and promotes cell morphogenesis and the epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma. PLoS ONE 2022, 17, e0279001. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Zhu, Y.; Wang, Y.; Chen, J.; Zheng, H. SOX2 promotes vasculogenic mimicry by accelerating glycolysis via the lncRNA AC005392.2-GLUT1 axis in colorectal cancer. Cell Death Dis. 2023, 14, 791. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, D.M.; Zhou, X.G.; Yin, N.; Zhang, Y.; Zhang, Z.X.; Li, D.C.; Zhou, J. HIF-2alpha promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget 2017, 8, 47801–47815. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Yuan, C.; Han, T.; Hu, H.; Cui, J.; Jiao, F.; Wang, L. JQ1 effectively inhibits vasculogenic mimicry of pancreatic ductal adenocarcinoma cells via the ERK1/2-MMP-2/9 signaling pathway both in vitro and in vivo. Am. J. Transl. Res. 2019, 11, 1030–1039. [Google Scholar]
- Wei, X.; Chen, Y.; Jiang, X.; Peng, M.; Liu, Y.; Mo, Y.; Ren, D.; Hua, Y.; Yu, B.; Zhou, Y.; et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 2021, 20, 7. [Google Scholar] [CrossRef]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp. Cell Res. 2011, 317, 685–690. [Google Scholar] [CrossRef]
- Song, S.; Xie, M.; Scott, A.W.; Jin, J.; Ma, L.; Dong, X.; Skinner, H.D.; Johnson, R.L.; Ding, S.; Ajani, J.A. A Novel YAP1 Inhibitor Targets CSC-Enriched Radiation-Resistant Cells and Exerts Strong Antitumor Activity in Esophageal Adenocarcinoma. Mol. Cancer Ther. 2018, 17, 443–454. [Google Scholar] [CrossRef]
- Takemoto, M.; Tanaka, T.; Tsuji, R.; Togashi, Y.; Higashi, M.; Fumino, S.; Tajiri, T. The synergistic antitumor effect of combination therapy with a MEK inhibitor and YAP inhibitor on pERK-positive neuroblastoma. Biochem. Biophys. Res. Commun. 2021, 570, 41–46. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Oria, V.O.; Zhang, H.; Zito, C.R.; Rane, C.K.; Ma, X.Y.; Provance, O.K.; Tran, T.T.; Adeniran, A.; Kluger, Y.; Sznol, M.; et al. Coupled fibromodulin and SOX2 signaling as a critical regulator of metastatic outgrowth in melanoma. Cell Mol. Life Sci. 2022, 79, 377. [Google Scholar] [CrossRef]
- Mabeta, P. Paradigms of vascularization in melanoma: Clinical significance and potential for therapeutic targeting. Biomed. Pharmacother. 2020, 127, 110135. [Google Scholar] [CrossRef]
- Azad, T.; Ghahremani, M.; Yang, X. The Role of YAP and TAZ in Angiogenesis and Vascular Mimicry. Cells 2019, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.E.; Elimam, R.; Zgheib, A.; Annabi, B. A Role for the Hippo/YAP1 Pathway in the Regulation of In Vitro Vasculogenic Mimicry in Glioblastoma Cells. J. Cell Mol. Med. 2024, 28, e70304. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Alimbetov, D.; Askarova, S.; Umbayev, B.; Davis, T.; Kipling, D. Pharmacological Targeting of Cell Cycle, Apoptotic and Cell Adhesion Signaling Pathways Implicated in Chemoresistance of Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1690. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Hill, D.S.; Daignault, S.M.; Lui, G.Y.L.; Sharp, D.M.; Gabrielli, B.; Weninger, W.; Haass, N.K. Cell Cycle Phase-Specific Drug Resistance as an Escape Mechanism of Melanoma Cells. J. Investig. Dermatol. 2016, 136, 1479–1489. [Google Scholar] [CrossRef]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L., 3rd; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 2018, 293, 11230–11240. [Google Scholar] [CrossRef]
- Roy, M.E.; Veilleux, C.; Paquin, A.; Gagnon, A.; Annabi, B. Transcriptional regulation of CYR61 and CTGF by LM98: A synthetic YAP-TEAD inhibitor that targets in-vitro vasculogenic mimicry in glioblastoma cells. Anti-Cancer Drugs 2024, 35, 709–719. [Google Scholar] [CrossRef]
- Lim, D.; Cho, J.G.; Yun, E.; Lee, A.; Ryu, H.Y.; Lee, Y.J.; Yoon, S.; Chang, W.; Lee, M.S.; Kwon, B.S.; et al. MicroRNA 34a-AXL Axis Regulates Vasculogenic Mimicry Formation in Breast Cancer Cells. Genes 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Staton, C.A.; Stribbling, S.M.; Tazzyman, S.; Hughes, R.; Brown, N.J.; Lewis, C.E. Current methods for assaying angiogenesis in vitro and in vivo. Int. J. Exp. Pathol. 2004, 85, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of Angiogenesis Assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righetti, M.; Primorac, A.-M.; Erler, J.T.; Oria, V.O. Murine Cell Line Models for Vascular Mimicry: The Role of YAP/TAZ Signaling. Int. J. Mol. Sci. 2025, 26, 9129. https://doi.org/10.3390/ijms26189129
Righetti M, Primorac A-M, Erler JT, Oria VO. Murine Cell Line Models for Vascular Mimicry: The Role of YAP/TAZ Signaling. International Journal of Molecular Sciences. 2025; 26(18):9129. https://doi.org/10.3390/ijms26189129
Chicago/Turabian StyleRighetti, Matilde, Ana-Maria Primorac, Janine Terra Erler, and Victor Oginga Oria. 2025. "Murine Cell Line Models for Vascular Mimicry: The Role of YAP/TAZ Signaling" International Journal of Molecular Sciences 26, no. 18: 9129. https://doi.org/10.3390/ijms26189129
APA StyleRighetti, M., Primorac, A.-M., Erler, J. T., & Oria, V. O. (2025). Murine Cell Line Models for Vascular Mimicry: The Role of YAP/TAZ Signaling. International Journal of Molecular Sciences, 26(18), 9129. https://doi.org/10.3390/ijms26189129




