Untargeted Plasma Metabolomics Extends the Biomarker Profile of Mitochondrial Neurogastrointestinal Encephalomyopathy
Abstract
1. Introduction
2. Results
2.1. Study Participants
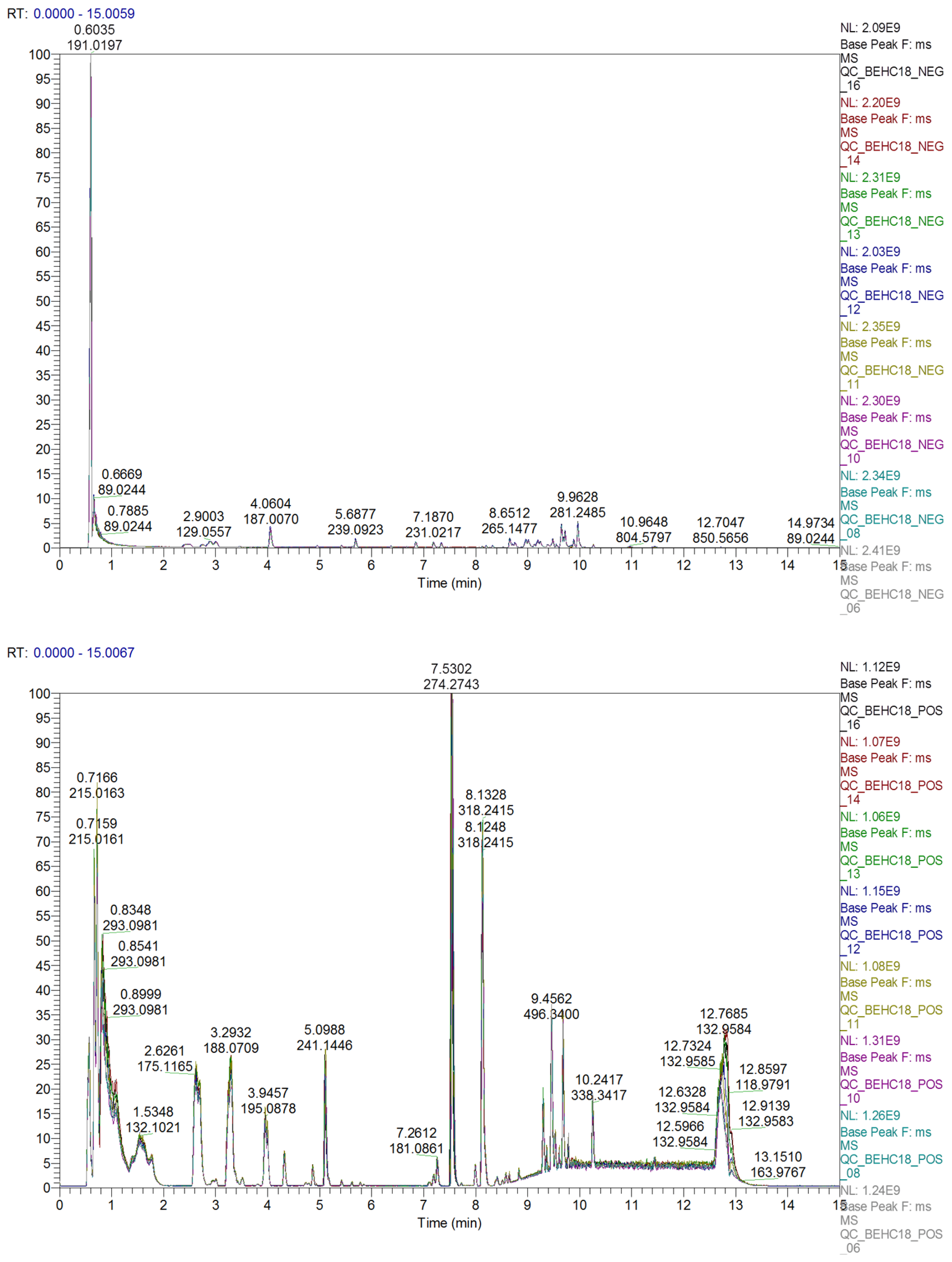
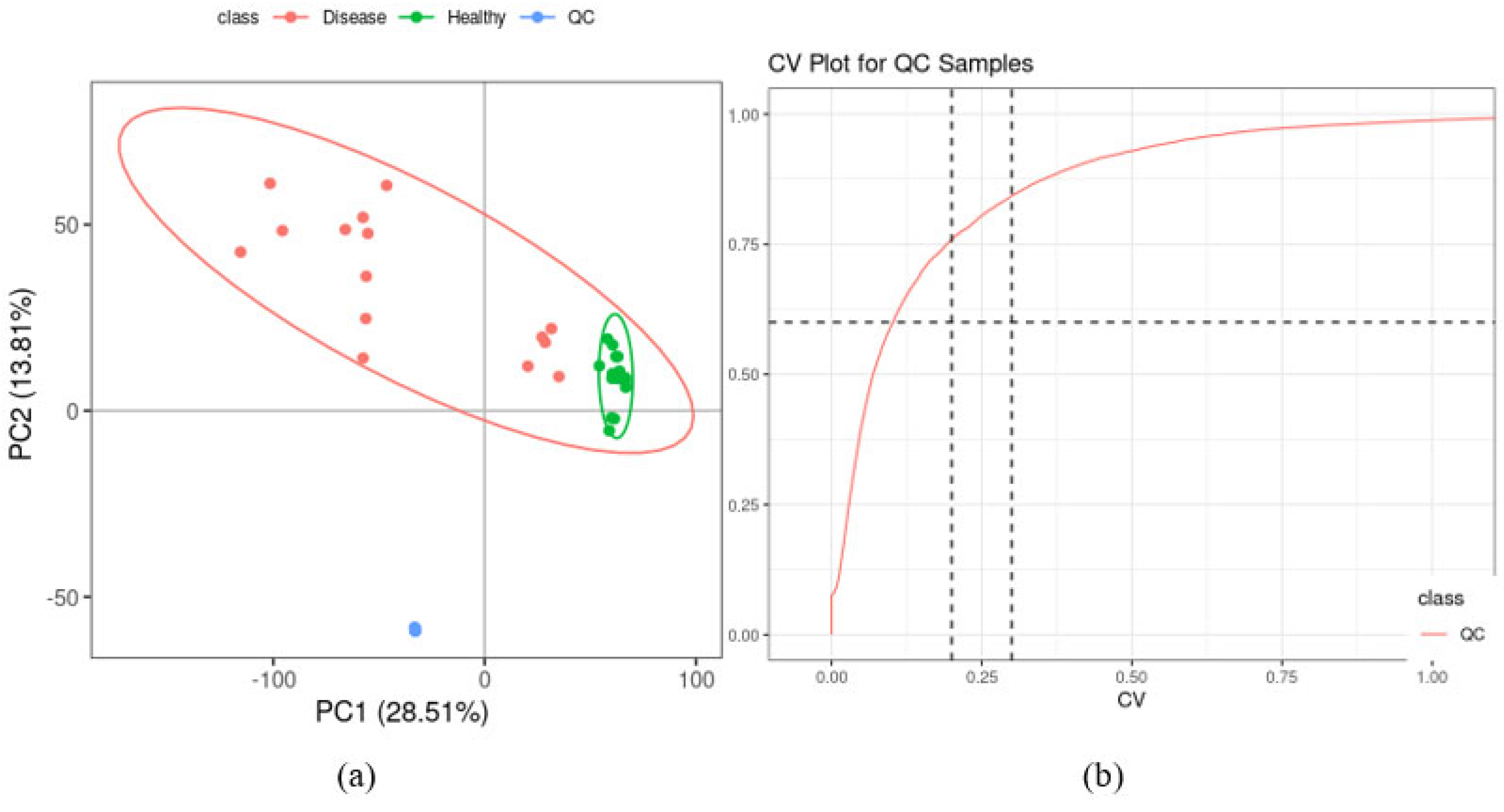
2.2. Quality Control of UHPLC-MS Analysis
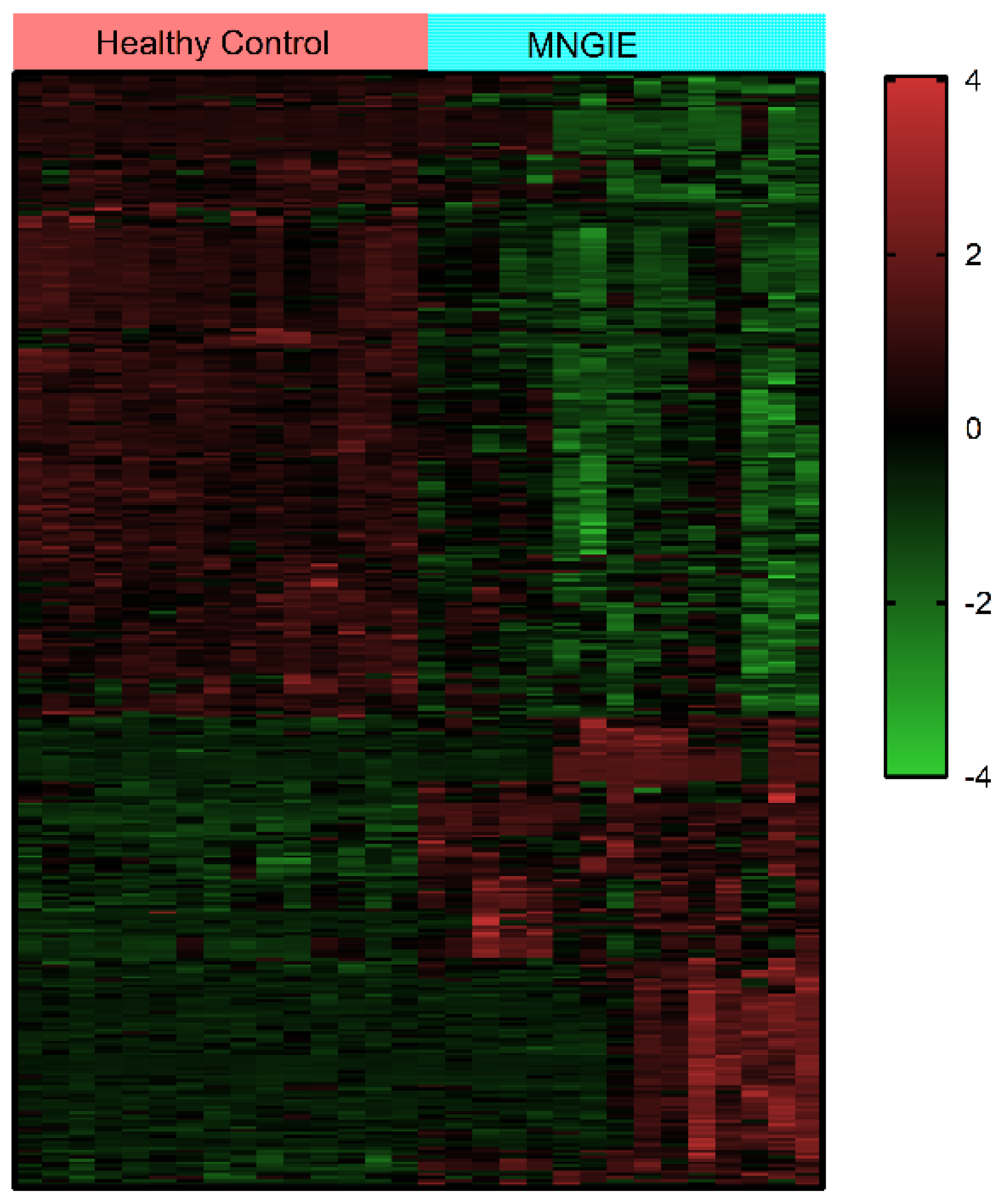
2.3. Metabolite Identification and Classification
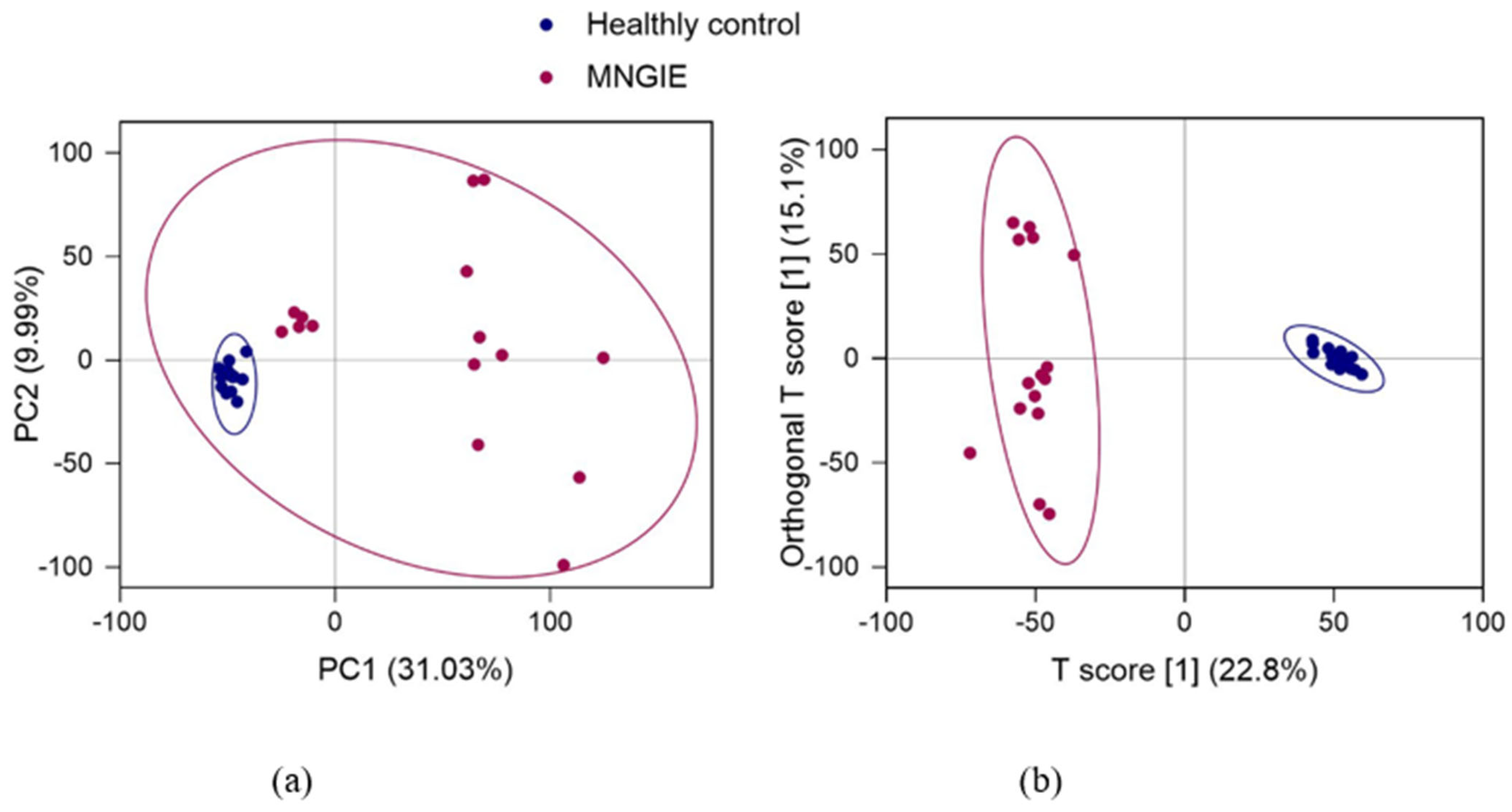
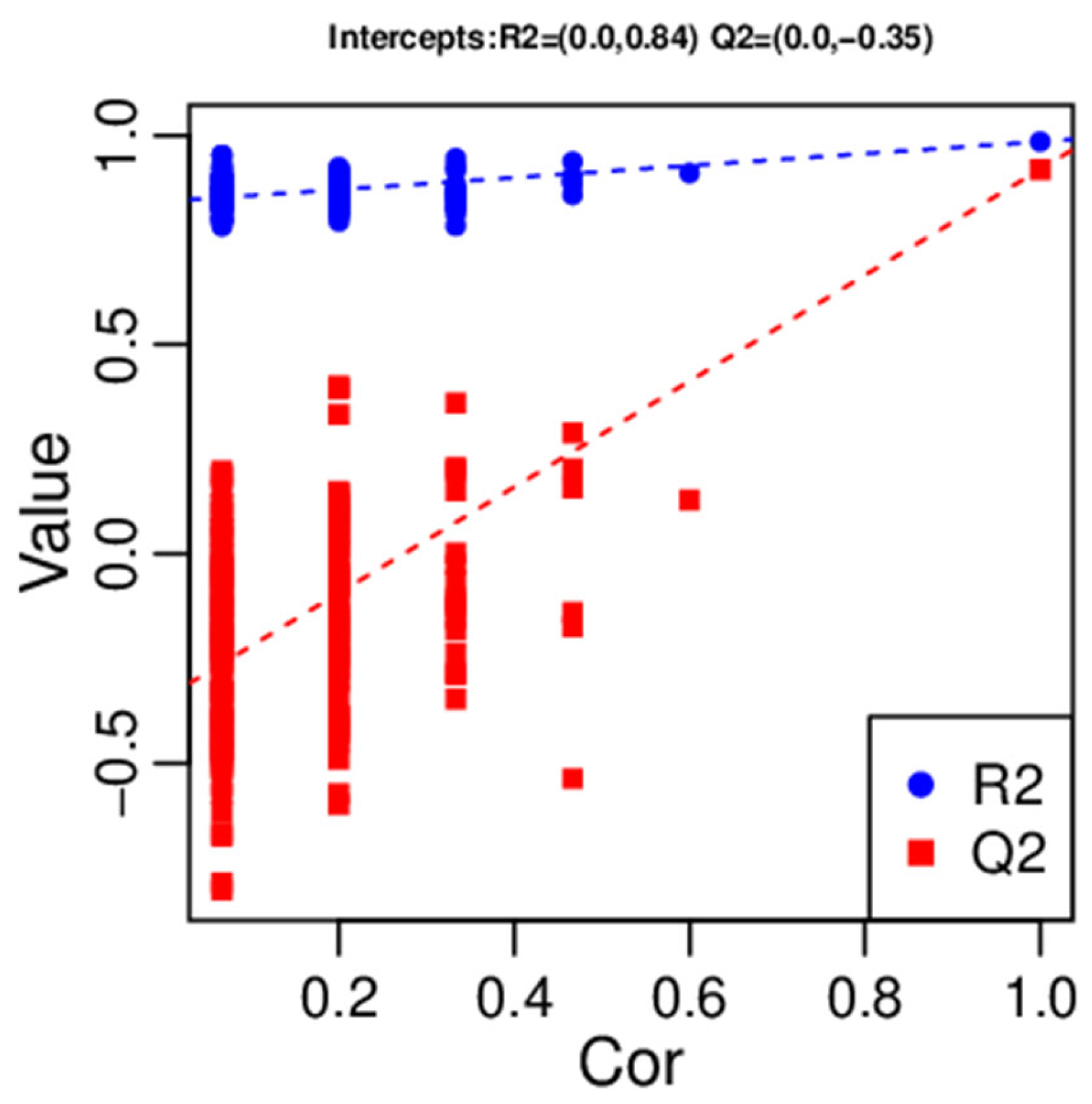
2.4. Multivariate Analysis of Identified Metabolites
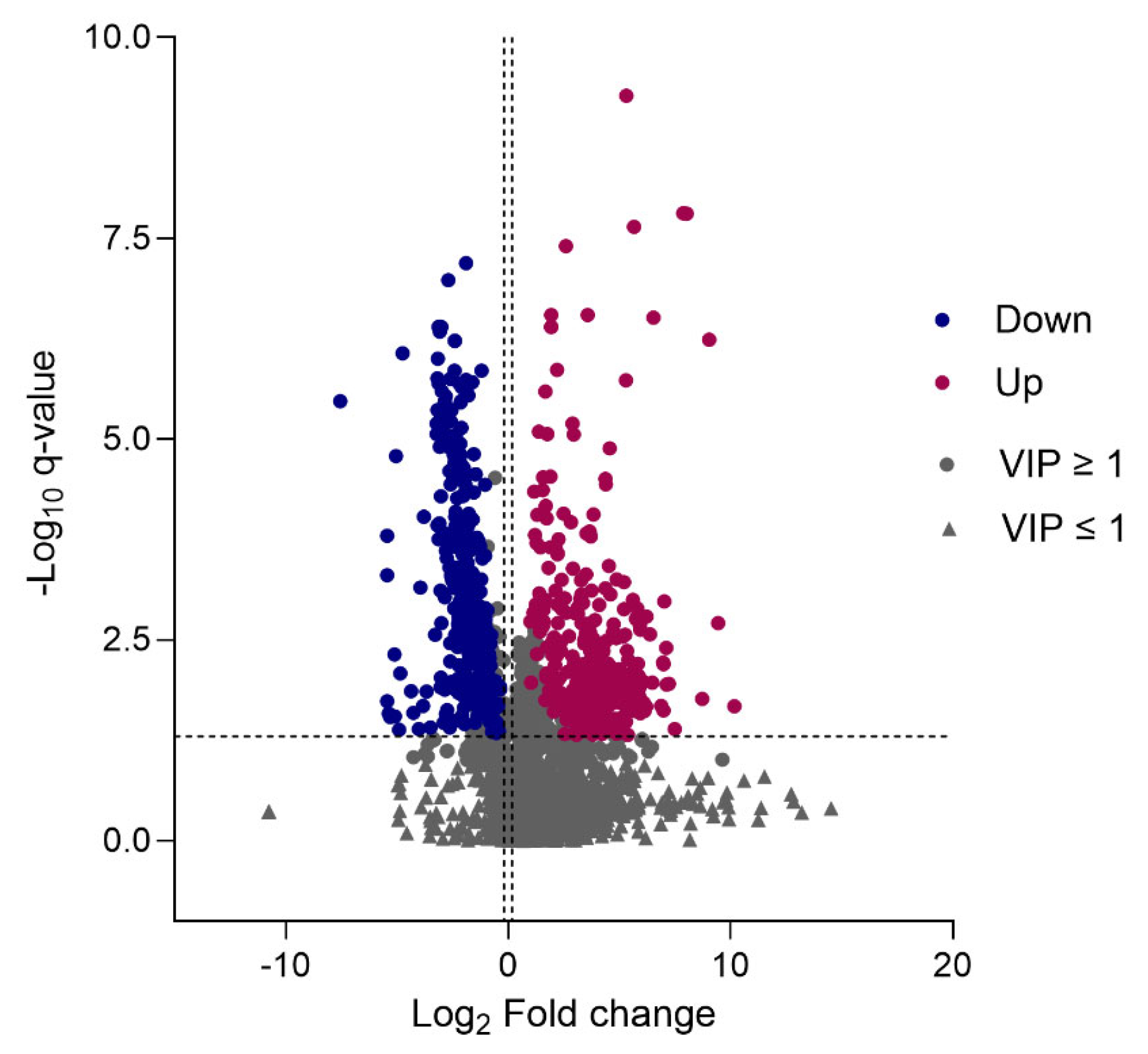
2.5. Differential Metabolite Identification
2.6. Metabolic Pathway Enrichment Analysis of Differential Metabolites
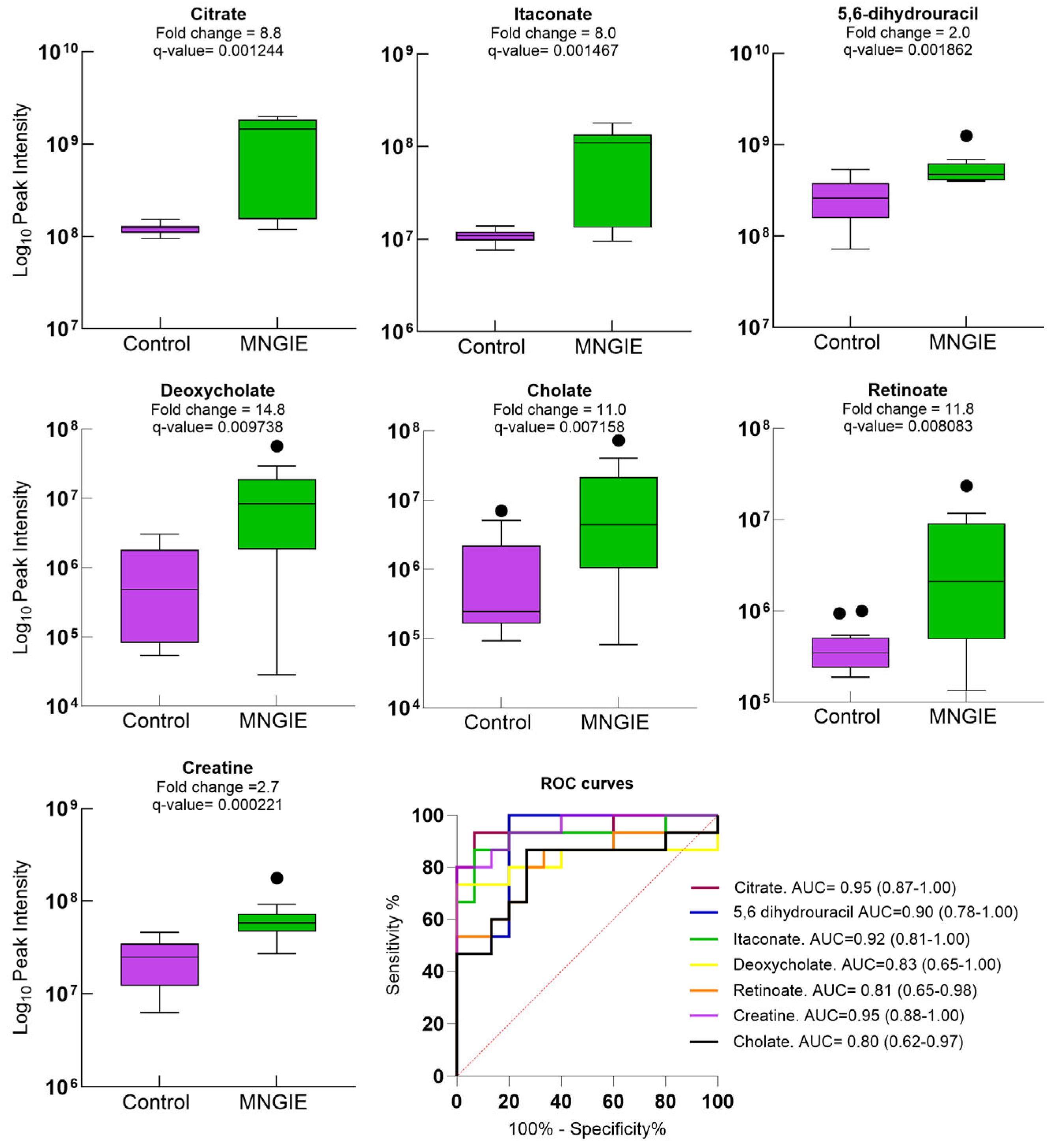
2.7. Discriminative Ability of Differential Plasma Metabolites for MNGIE Disease
3. Discussion
- Influence of the exposome: Metabolic profiles are significantly shaped by the exposome, which includes factors such as diet, dietary supplements, medicinal and recreational drugs, personal care products, and occupational exposures. Although known exposome-related metabolites were excluded from our dataset, we cannot entirely rule out the possibility that these exposures influenced the observed metabolic profiles. MNGIE patients typically receive a combination of medications, including analgesics, bowel motility stimulants, anti-emetics, antibiotics, and centrally acting agents tailored to their symptoms. In contrast, healthy controls may be exposed to different confounding factors. Notably, caffeine metabolism emerged as the most significantly downregulated pathway in MNGIE patients. Caffeine is mainly absorbed by the small intestine and is metabolized via demethylation and/or hydroxylation into paraxanthine, theobromine, theophylline, and 1,3,7-trimethyluric acid, all of which were significantly reduced in our patient cohort. Patients with MNGIE exhibit severe gastrointestinal manifestations, including dysmotility, abdominal pain, nausea, dysphagia, pseudo-obstruction, and diarrhoea. These symptoms frequently lead to oral intolerance, progressive weight loss, and malnutrition. The observed differences in caffeine metabolism may reflect comparatively higher caffeine intake among healthy controls.
- Sample size constraints: MNGIE is an ultra-rare disorder with an estimated prevalence of fewer than 1 in 1,000,000 individuals in Europe. Since its initial description by Okamura et al. in 1979, approximately 500 cases have been reported globally [63]. The mean life expectancy is 35 years. Thus, the rarity of MNGIE presents a significant challenge for clinical research, particularly in achieving adequate sample sizes for statistically robust analyses.
- Careful recruitment of age- and sex-matched controls to minimize confounding demographic effects on the metabolome.
- Strict adherence to standardized protocols for sample collection, processing, and storage to reduce pre-analytical variability and ensure that observed metabolic alterations reflect disease-specific changes.
- Application of high-resolution mass spectrometry to enhance analytical sensitivity and selectivity.
- Use of pooled QC samples to monitor instrument performance and correct for technical variability.
- Comprehensive statistical analysis, including PCA and OPLS-DA, to reduce data dimensionality and identify meaningful metabolic patterns.
- Implementation of the Benjamini–Hochberg procedure to control the false discovery rate (FDR) and reduce the likelihood of type I errors.
- 3.
- Lack of targeted metabolite validation: The findings from our untargeted metabolomics analysis were not complemented by targeted metabolite quantification. Such validation will be essential to confirm the diagnostic relevance of the 23 identified biomarkers. However, the computational integration of biomarkers across multiple omics layers, such as the genome, transcriptome, proteome, and metabolome, is more likely to yield insights into underlying molecular and cellular mechanisms and may identify more effective measures of treatment outcomes than relying on a single biomarker. Indeed, integrative analysis approaches can be employed to correlate multi-omics data with disease phenotypes, treatment responses, and patient stratification.
- 4.
- Analytical scope limitations: Since no single chromatography column can separate all metabolites in a sample, we selected a C18 column for this study, as it offers relatively broad coverage for separating non-polar and moderately polar metabolites. Consequently, metabolic disturbances involving highly polar or strongly hydrophilic compounds would not have been captured.
- 5.
- Annotation challenges: Chemical entities assigned to credibility levels 3 to 5 could not be further analyzed due to current limitations in metabolite classification and annotation databases.
4. Materials and Methods
4.1. Study Participants
4.2. Blood Collection
4.3. Untargeted Metabolomic Profiling
4.3.1. Metabolite Extraction
4.3.2. UHPLC-MS Analysis
4.3.3. Metabolite Ion Peak Extraction and Metabolite Identification
4.3.4. Data Preprocessing
4.3.5. Data QC
4.3.6. Classification and Functional Annotation of Detected Metabolites
4.3.7. Statistical Analyses
4.3.8. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirano, M.; Silvestri, G.; Blake, D.M.; Lombes, A.; Minetti, C.; Bonilla, E.; Hays, A.P.; Lovelace, R.E.; Butler, I.; Bertorini, T.E.; et al. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): Clinical, Biochemical, and Genetic Features of an Autosomal Recessive Mitochondrial Disorder. Neurology 1994, 44, 721–727. [Google Scholar] [CrossRef]
- Nishino, I.; Spinazzola, A.; Hirano, M. Thymidine Phosphorylase Gene Mutations in MNGIE, a Human Mitochondrial Disorder. Science 1999, 283, 689–692. [Google Scholar] [CrossRef]
- Nishino, I.; Spinazzola, A.; Hirano, M. MNGIE: From Nuclear DNA to Mitochondrial DNA. Neuromuscul. Disord. 2001, 11, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Nishigaki, Y.; Martí, R. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): A Disease of Two Genomes. Neurologist 2004, 10, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, Y.; Martí, R.; Copeland, W.C.; Hirano, M. Site-Specific Somatic Mitochondrial DNA Point Mutations in Patients with Thymidine Phosphorylase Deficiency. J. Clin. Investig. 2003, 111, 1913–1921. [Google Scholar] [CrossRef]
- Garone, C.; Tadesse, S.; Hirano, M. Clinical and Genetic Spectrum of Mitochondrial Neurogastrointestinal Encephalomyopathy. Brain 2011, 134, 3326–3332. [Google Scholar] [CrossRef]
- Pacitti, D.; Levene, M.; Garone, C.; Nirmalananthan, N.; Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Into the Fourth Decade, What We Have Learned So Far. Front. Genet. 2018, 9, 669. [Google Scholar] [CrossRef]
- Hirano, M. Mitochondrial Neurogastrointestinal Encephalopathy Disease. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Debouverie, M.; Wagner, M.; Ducrocq, X.; Grignon, Y.; Mousson, B.; Weber, M. MNGIE Syndrome in 2 Siblings. Rev. Neurol. 1997, 153, 547–553. [Google Scholar]
- Barış, Z.; Eminoğlu, T.; Dalgıç, B.; Tümer, L.; Hasanoğlu, A. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): Case Report with a New Mutation. Eur. J. Pediatr. 2010, 169, 1375–1378. [Google Scholar] [CrossRef]
- Cardaioli, E.; Da Pozzo, P.; Malfatti, E.; Battisti, C.; Gallus, G.N.; Gaudiano, C.; MacUcci, M.; Malandrini, A.; Margollicci, M.; Rubegni, A.; et al. A Second MNGIE Patient without Typical Mitochondrial Skeletal Muscle Involvement. Neurol. Sci. 2010, 31, 491–494. [Google Scholar] [CrossRef]
- Bakker, J.A.; Schlesser, P.; Smeets, H.J.M.; Francois, B.; Bierau, J. Biochemical Abnormalities in a Patient with Thymidine Phosphorylase Deficiency with Fatal Outcome. J. Inherit. Metab. Dis. 2010, 33, 139–143. [Google Scholar] [CrossRef]
- Vondráčková, A.; Veselá, K.; Kratochvílová, H.; Kučerová Vidrová, V.; Vinšová, K.; Stránecký, V.; Honzík, T.; Hansíková, H.; Zeman, J.; Tesařová, M. Large Copy Number Variations in Combination with Point Mutations in the TYMP and SCO2 Genes Found in Two Patients with Mitochondrial Disorders. Eur. J. Human. Genet. 2014, 22, 431–434. [Google Scholar] [CrossRef]
- Peedikayil, M.C.; Kagevi, E.I.; Abufarhaneh, E.; Alsayed, M.D.; Alzahrani, H.A. Mitochondrial Neurogastrointestinal Encephalomyopathy Treated with Stem Cell Transplantation: A Case Report and Review of Literature. Hematol./Oncol. Stem Cell Ther. 2015, 8, 85–90. [Google Scholar] [CrossRef]
- Du, J.; Zhang, C.; Liu, F.; Liu, X.; Wang, D.; Zhao, D.; Shui, G.; Zhao, Y.; Yan, C. Distinctive Metabolic Remodeling in TYMP Deficiency beyond Mitochondrial Dysfunction. J. Mol. Med. 2023, 101, 1237–1253. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Reznik, E.; Lee, C.-H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Buzkova, J.; Nikkanen, J.; Ahola, S.; Hakonen, A.H.; Sevastianova, K.; Hovinen, T.; Yki-Järvinen, H.; Pietiläinen, K.H.; Lönnqvist, T.; Velagapudi, V.; et al. Metabolomes of Mitochondrial Diseases and Inclusion Body Myositis Patients: Treatment Targets and Biomarkers. EMBO Mol. Med. 2018, 10, e9091. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Reinstadler, B.; Engelstad, K.; Skinner, O.S.; Stackowitz, E.; Haller, R.G.; Clish, C.B.; Pierce, K.; Walker, M.A.; Fryer, R.; et al. Circulating Markers of NADH-Reductive Stress Correlate with Mitochondrial Disease Severity. J. Clin. Investig. 2021, 131, e136055. [Google Scholar] [CrossRef]
- Shaham, O.; Slate, N.G.; Goldberger, O.; Xu, Q.; Ramanathan, A.; Souza, A.L.; Clish, C.B.; Sims, K.B.; Mootha, V.K. A Plasma Signature of Human Mitochondrial Disease Revealed through Metabolic Profiling of Spent Media from Cultured Muscle Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 1571–1575. [Google Scholar] [CrossRef]
- Maresca, A.; Del Dotto, V.; Romagnoli, M.; La Morgia, C.; Di Vito, L.; Capristo, M.; Valentino, M.L.; Carelli, V.; ER-MITO Study Group. Expanding and Validating the Biomarkers for Mitochondrial Diseases. J. Mol. Med. 2020, 98, 1467–1478. [Google Scholar] [CrossRef]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of Oral Creatine Supplementation in a Patient with MELAS Phenotype and Associated Nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef]
- Borchert, A.; Wilichowski, E.; Hanefeld, F. Supplementation with Creatine Monohydrate in Children with Mitochondrial Encephalomyopathies. Muscle Nerve 1999, 22, 1299–1300. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Roy, B.D.; MacDonald, J.R. A Randomized, Controlled Trial of Creatine Monohydrate in Patients with Mitochondrial Cytopathies. Muscle Nerve 1997, 20, 1502–1509. [Google Scholar] [CrossRef]
- Komura, K.; Hobbiebrunken, E.; Wilichowski, E.K.G.; Hanefeld, F.A. Effectiveness of Creatine Monohydrate in Mitochondrial Encephalomyopathies. Pediatr. Neurol. 2003, 28, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Klopstock, T.; Querner, V.; Schmidt, F.; Gekeler, F.; Walter, M.; Hartard, M.; Henning, M.; Gasser, T.; Pongratz, D.; Straube, A.; et al. A Placebo-Controlled Crossover Trial of Creatine in Mitochondrial Diseases. Neurology 2000, 55, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, C.; Schröder, R.; Müller, K.; Vorgerd, M.; Eggers, J.; Bogdanow, M.; Papassotiropoulos, A.; Fabian, K.; Klockgether, T.; Zange, J. Creatine Has No Beneficial Effect on Skeletal Muscle Energy Metabolism in Patients with Single Mitochondrial DNA Deletions: A Placebo-Controlled, Double-Blind 31P-MRS Crossover Study. Eur. J. Neurol. 2005, 12, 300–309. [Google Scholar] [CrossRef]
- Farr, C.V.; Xiao, Y.; El-Kasaby, A.; Schupp, M.; Hotka, M.; di Mauro, G.; Clarke, A.; Pastor Fernandez, M.; Sandtner, W.; Stockner, T.; et al. Probing the Chemical Space of Guanidino-Carboxylic Acids to Identify the First Blockers of the Creatine-Transporter-1. Mol. Pharmacol. 2024, 106, 319–333. [Google Scholar] [CrossRef]
- Hirano, M.; Carelli, V.; De Giorgio, R.; Pironi, L.; Accarino, A.; Cenacchi, G.; D’Alessandro, R.; Filosto, M.; Martí, R.; Nonino, F.; et al. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): Position Paper on Diagnosis, Prognosis, and Treatment by the MNGIE International Network. J. Inherit. Metab. Dis. 2021, 44, 376–387. [Google Scholar] [CrossRef]
- Patel, J.; Agasti, A.; Vashishtha, C.; Samarth, A.; Goyal, R.; Oak, P.J.; Sawant, P. An Interesting Case of Intestinal Pseudo-Obstruction: MNGIE. Trop. Gastroenterol. 2011, 32, 138–141. [Google Scholar] [CrossRef]
- Li, Y.; Chvatal-Medina, M.; Trillos-Almanza, M.C.; Bourgonje, A.R.; Connelly, M.A.; Moshage, H.; Bakker, S.J.L.; de Meijer, V.E.; Blokzijl, H.; Dullaart, R.P.F.; et al. Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality. Int. J. Mol. Sci. 2024, 25, 12806. [Google Scholar] [CrossRef]
- Tong, W.; Hannou, S.A.; Wang, Y.; Astapova, I.; Sargsyan, A.; Monn, R.; Thiriveedi, V.; Li, D.; McCann, J.R.; Rawls, J.F.; et al. The Intestine Is a Major Contributor to Circulating Succinate in Mice. FASEB J. 2022, 36, e22546. [Google Scholar] [CrossRef]
- Duong, N.; Bajaj, J.S. The Impact of the Gut Microbiome on Liver Transplantation. Curr. Opin. Organ. Transplant. 2021, 26, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of Fecal Microbial Communities in Patients with Liver Cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Peace, C.G.; O’Neill, L.A. The Role of Itaconate in Host Defense and Inflammation. J. Clin. Investig. 2022, 132, e148548. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Tao, K.; Li, R. Metabolite Itaconate in Host Immunoregulation and Defense. Cell Mol. Biol. Lett. 2023, 28, 100. [Google Scholar] [CrossRef]
- Coelho, C. Itaconate or How I Learned to Stop Avoiding the Study of Immunometabolism. PLoS Pathog. 2022, 18, e1010361. [Google Scholar] [CrossRef]
- Weiss, J.M.; Palmieri, E.M.; Gonzalez-Cotto, M.; Bettencourt, I.A.; Megill, E.L.; Snyder, N.W.; McVicar, D.W. Itaconic Acid Underpins Hepatocyte Lipid Metabolism in Non-Alcoholic Fatty Liver Disease in Male Mice. Nat. Metab. 2023, 5, 981–995. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in Inflammation in the Blood and the Vessel. Front. Pharmacol. 2022, 13, 997403. [Google Scholar] [CrossRef]
- Artru, F.; McPhail, M.J.W.; Triantafyllou, E.; Trovato, F.M. Lipids in Liver Failure Syndromes: A Focus on Eicosanoids, Specialized Pro-Resolving Lipid Mediators and Lysophospholipids. Front. Immunol. 2022, 13, 867261. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Wang, F.; Wu, D.; Qian, J.; Kang, J.; Li, H.; Ma, E. Nutrition Therapy for Mitochondrial Neurogastrointestinal Encephalopathy with Homozygous Mutation of the TYMP Gene. Clin. Nutr. Res. 2015, 4, 132–136. [Google Scholar] [CrossRef]
- Barisic, A.; Ljubas Kelecic, D.; Vranesic Bender, D.; Karas, I.; Brinar, M.; Miletic, V.; Krznaric, Z. Case Report: A Patient with Mitochondrial Neurogastrointestinal Encephalomyopathy and Chronic Intestinal Failure. Front. Nutr. 2022, 9, 983873. [Google Scholar] [CrossRef]
- Kasti, A.; Nikolaki, M.; Pyrousis, I.; Synodinou, K.; Oikonomopoulos, N.; Triantafyllou, K. Intensive Nutrition Support May Benefit Patients with a Rare Mitochondrial Disorder. Nutr. Clin. Pract. 2022, 37, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Kartal, T.; Uzunoğlu, S.; Kaplan, I.; Bulut, F.D.; Derya, K.; Mungan, N. Nutritional Management in MNGIE Disease: A Case Report. Clin. Sci. Nutr. 2025, 7, 63–67. [Google Scholar] [CrossRef]
- Al-Shaer, A.E.; Buddenbaum, N.; Shaikh, S.R. Polyunsaturated Fatty Acids, Specialized pro-Resolving Mediators, and Targeting Inflammation Resolution in the Age of Precision Nutrition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158936. [Google Scholar] [CrossRef] [PubMed]
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Woliński, K.; Płazińska, M.T.; Mikołajczak, P.; Ruchała, M. The Effects of Cannabinoids on the Endocrine System. Endokrynol. Pol. 2018, 69, 705–719. [Google Scholar] [CrossRef]
- Westerbacka, J.; Kotronen, A.; Fielding, B.A.; Wahren, J.; Hodson, L.; Perttilä, J.; Seppänen-Laakso, T.; Suortti, T.; Arola, J.; Hultcrantz, R.; et al. Splanchnic Balance of Free Fatty Acids, Endocannabinoids, and Lipids in Subjects with Nonalcoholic Fatty Liver Disease. Gastroenterology 2010, 139, 1961–1971.e1. [Google Scholar] [CrossRef]
- Kripps, K.; Nakayuenyongsuk, W.; Shayota, B.J.; Berquist, W.; Gomez-Ospina, N.; Esquivel, C.O.; Concepcion, W.; Sampson, J.B.; Cristin, D.J.; Jackson, W.E.; et al. Successful Liver Transplantation in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE). Mol. Genet. Metab. 2020, 130, 58–64. [Google Scholar] [CrossRef]
- Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Approaches to Diagnosis and Treatment. J. Transl. Genet. Genom. 2020, 4, 1–16. [Google Scholar] [CrossRef]
- Tuchman, M.; Ramnaraine, M.L.; O’Dea, R.F. Effects of Uridine and Thymidine on the Degradation of 5-Fluorouracil, Uracil, and Thymine by Rat Liver Dihydropyrimidine Dehydrogenase. Cancer Res. 1985, 45, 5553–5556. [Google Scholar] [CrossRef][Green Version]
- Henricks, L.M.; Jacobs, B.A.W.; Meulendijks, D.; Pluim, D.; van den Broek, D.; de Vries, N.; Rosing, H.; Beijnen, J.H.; Huitema, A.D.R.; Guchelaar, H.-J.; et al. Food-Effect Study on Uracil and Dihydrouracil Plasma Levels as Marker for Dihydropyrimidine Dehydrogenase Activity in Human Volunteers. Br. J. Clin. Pharmacol. 2018, 84, 2761–2769. [Google Scholar] [CrossRef]
- Šarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front. Pharmacol. 2018, 9, 939. [Google Scholar] [CrossRef]
- Dumaswala, R.; Setchell, K.D.; Zimmer-Nechemias, L.; Iida, T.; Goto, J.; Nambara, T. Identification of 3 Alpha,4 Beta,7 Alpha-Trihydroxy-5 Beta-Cholanoic Acid in Human Bile: Reflection of a New Pathway in Bile Acid Metabolism in Humans. J. Lipid Res. 1989, 30, 847–856. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, B.; Zhang, X.; He, Y.; Shao, Y.; Ding, M. Diagnostic and Therapeutic Profiles of Serum Bile Acids in Women with Intrahepatic Cholestasis of Pregnancy-a Pseudo-Targeted Metabolomics Study. Clin. Chim. Acta 2018, 483, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sacquet, E.C.; Gadelle, D.P.; Riottot, M.J.; Raibaud, P.M. Absence of Transformation of Beta-Muricholic Acid by Human Microflora Implanted in the Digestive Tracts of Germfree Male Rats. Appl. Environ. Microbiol. 1984, 47, 1167–1168. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, H.; Tazuma, S.; Kajiyama, G. Protection against Hydrophobic Bile Salt-Induced Cell Membrane Damage by Liposomes and Hydrophilic Bile Salts. Am. J. Physiol. 1993, 264, G835–G839. [Google Scholar] [CrossRef] [PubMed]
- Masclee, A.; Tangerman, A.; van Schaik, A.; van der Hoek, E.W.; van Tongeren, J.H. Unconjugated Serum Bile Acids as a Marker of Small Intestinal Bacterial Overgrowth. Eur. J. Clin. Investig. 1989, 19, 384–389. [Google Scholar] [CrossRef]
- Smirnova, E.; Muthiah, M.D.; Narayan, N.; Siddiqui, M.S.; Puri, P.; Luketic, V.A.; Contos, M.J.; Idowu, M.; Chuang, J.-C.; Billin, A.N.; et al. Metabolic Reprogramming of the Intestinal Microbiome with Functional Bile Acid Changes Underlie the Development of NAFLD. Hepatology 2022, 76, 1811–1824. [Google Scholar] [CrossRef]
- Midzak, A.S.; Chen, H.; Aon, M.A.; Papadopoulos, V.; Zirkin, B.R. ATP Synthesis, Mitochondrial Function, and Steroid Biosynthesis in Rodent Primary and Tumor Leydig Cells. Biol. Reprod. 2011, 84, 976–985. [Google Scholar] [CrossRef]
- Berry, D.C.; Noy, N. All-Trans-Retinoic Acid Represses Obesity and Insulin Resistance by Activating Both Peroxisome Proliferation-Activated Receptor Beta/Delta and Retinoic Acid Receptor. Mol. Cell Biol. 2009, 29, 3286–3296. [Google Scholar] [CrossRef]
- Mercader, J.; Ribot, J.; Murano, I.; Felipe, F.; Cinti, S.; Bonet, M.L.; Palou, A. Remodeling of White Adipose Tissue after Retinoic Acid Administration in Mice. Endocrinology 2006, 147, 5325–5332. [Google Scholar] [CrossRef]
- Takeda, K.; Sriram, S.; Chan, X.H.D.; Ong, W.K.; Yeo, C.R.; Tan, B.; Lee, S.-A.; Kong, K.V.; Hoon, S.; Jiang, H.; et al. Retinoic Acid Mediates Visceral-Specific Adipogenic Defects of Human Adipose-Derived Stem Cells. Diabetes 2016, 65, 1164–1178. [Google Scholar] [CrossRef]
- Gautheron, J.; Lima, L.; Akinci, B.; Zammouri, J.; Auclair, M.; Ucar, S.K.; Ozen, S.; Altay, C.; Bax, B.E.; Nemazanyy, I.; et al. Loss of Thymidine Phosphorylase Activity Disrupts Adipocyte Differentiation and Induces Insulin-Resistant Lipoatrophic Diabetes. BMC Med. 2022, 20, 95. [Google Scholar] [CrossRef]
- Okamura, K.; Santa, T.; Nagae, K.; Omae, T. Congenital Oculoskeletal Myopathy with Abnormal Muscle and Liver Mitochondria. J. Neurol. Sci. 1976, 27, 79–91. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. MetaX: A Flexible and Comprehensive Software for Processing Metabolomics Data. BMC Bioinformatics 2017, 18, 183. [Google Scholar] [CrossRef]
- Di Guida, R.; Engel, J.; Allwood, J.W.; Weber, R.J.M.; Jones, M.R.; Sommer, U.; Viant, M.R.; Dunn, W.B. Non-Targeted UHPLC-MS Metabolomic Data Processing Methods: A Comparative Investigation of Normalisation, Missing Value Imputation, Transformation and Scaling. Metabolomics 2016, 12, 93. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]

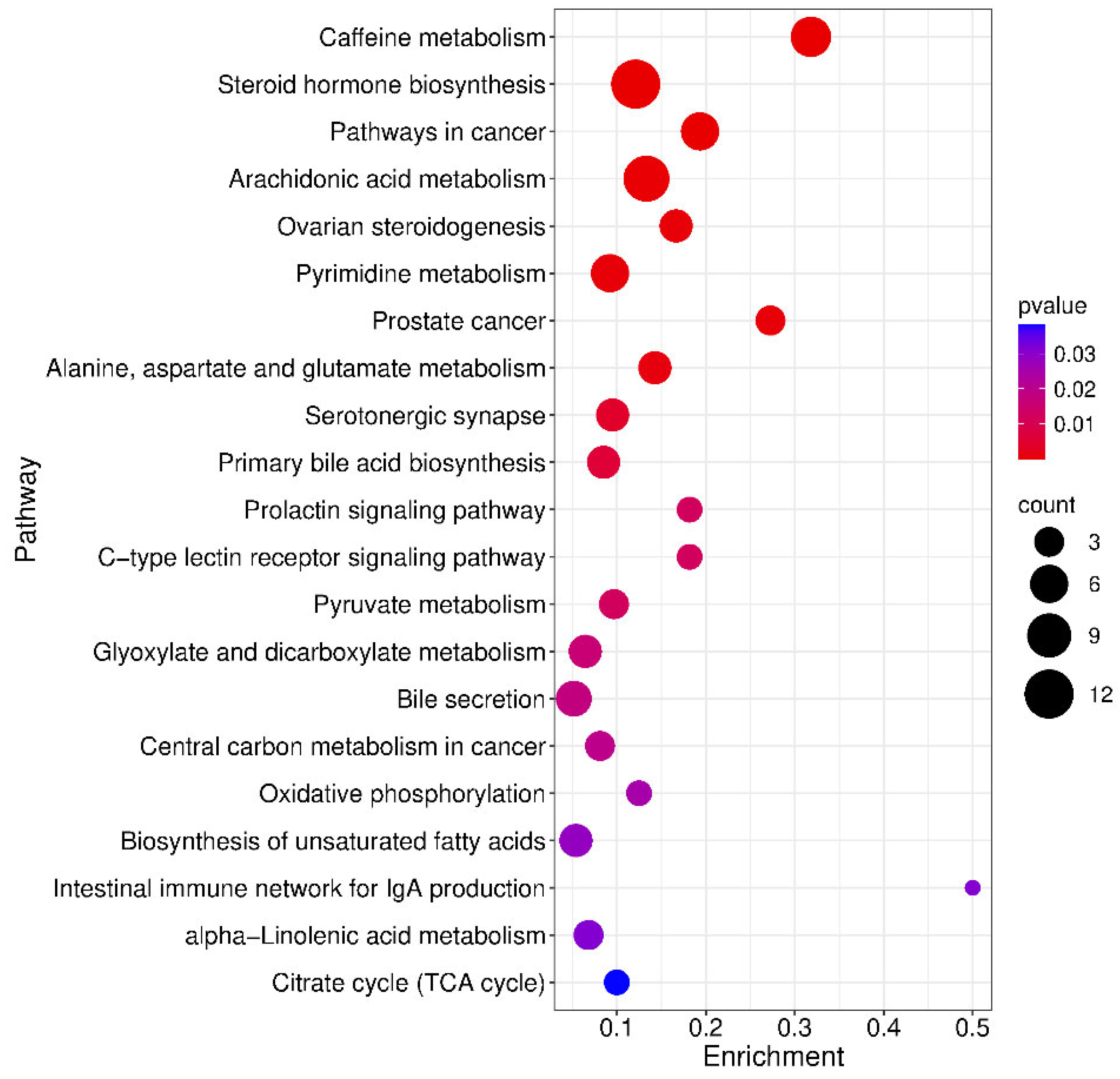
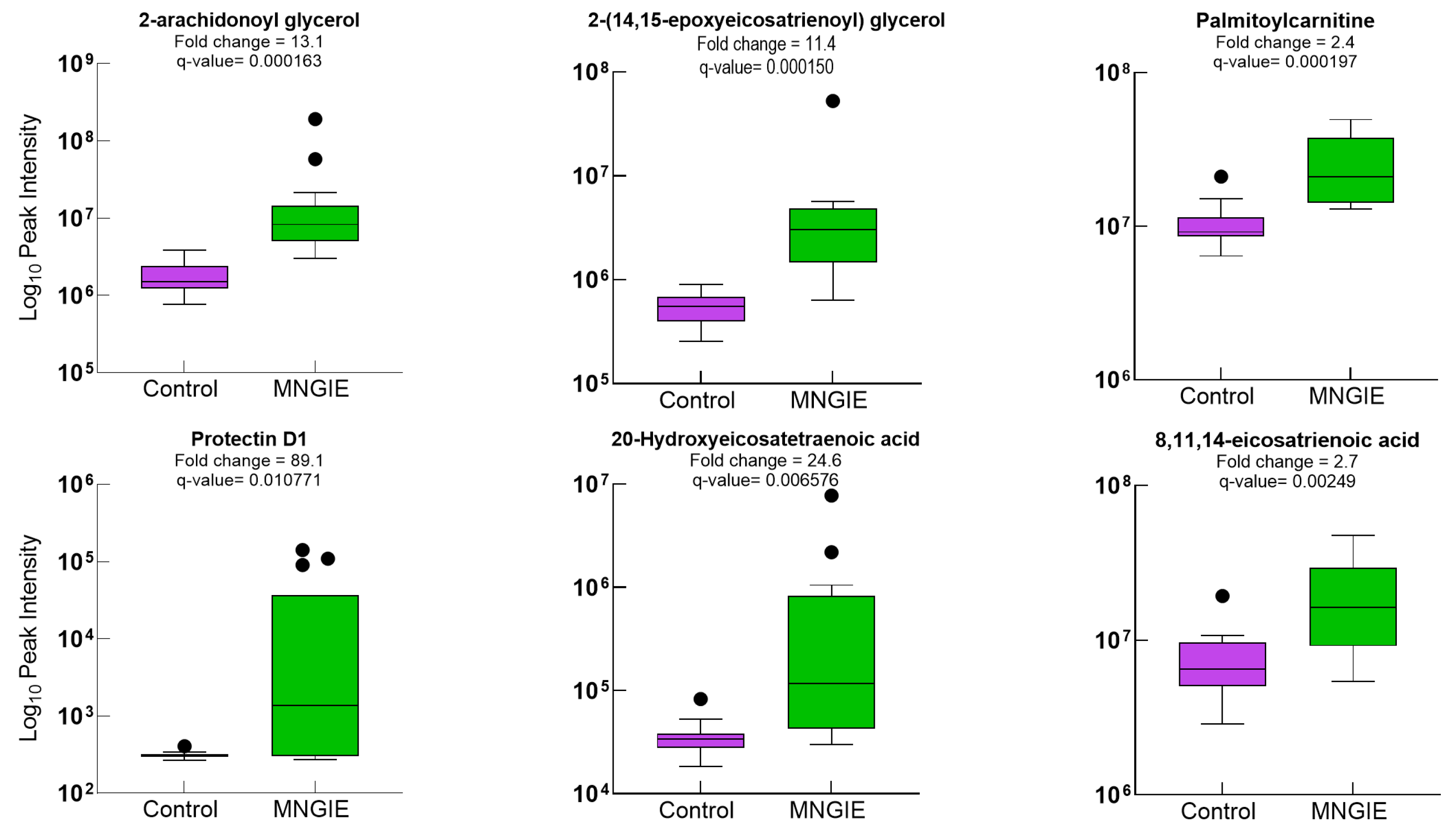
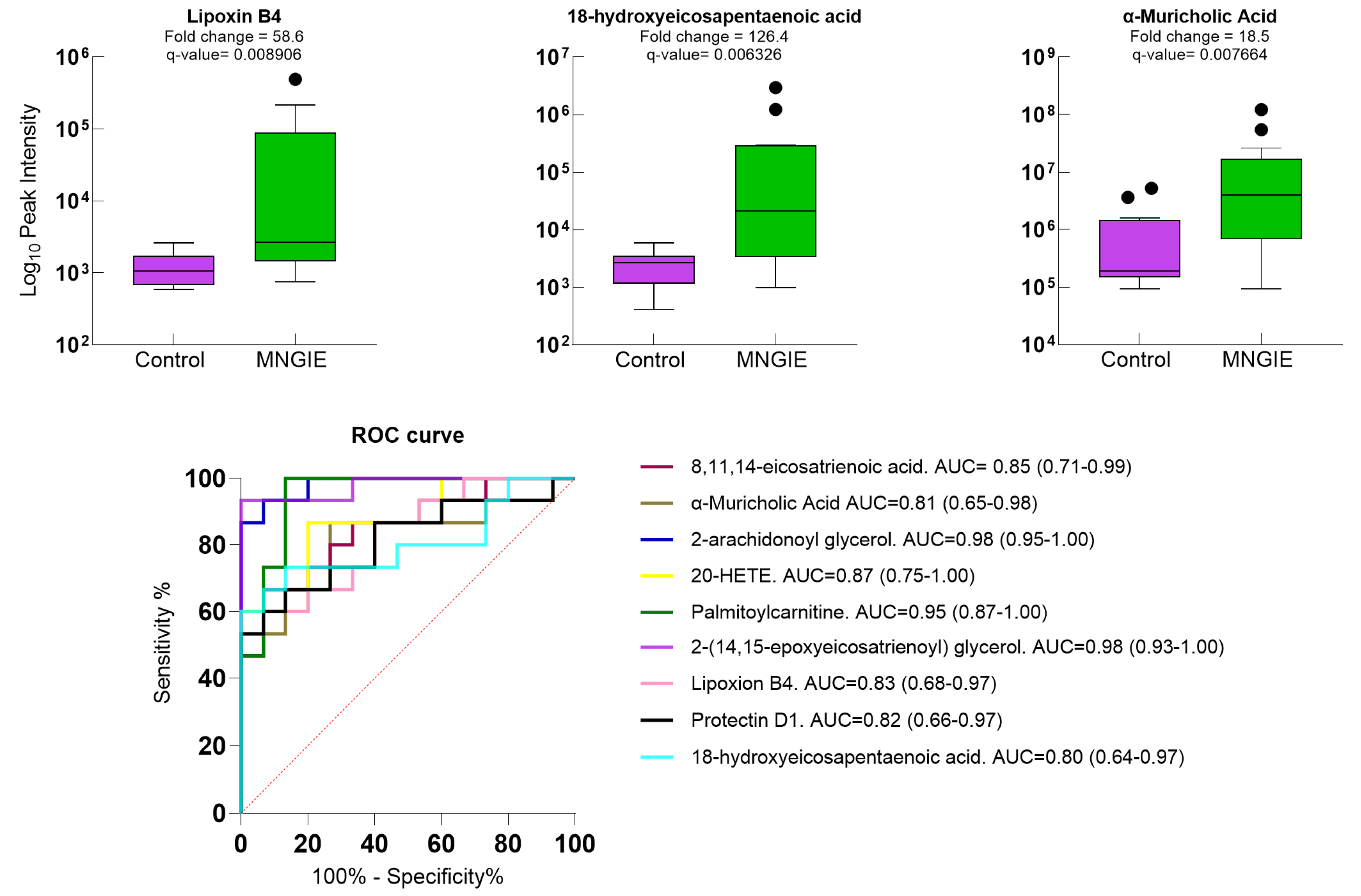
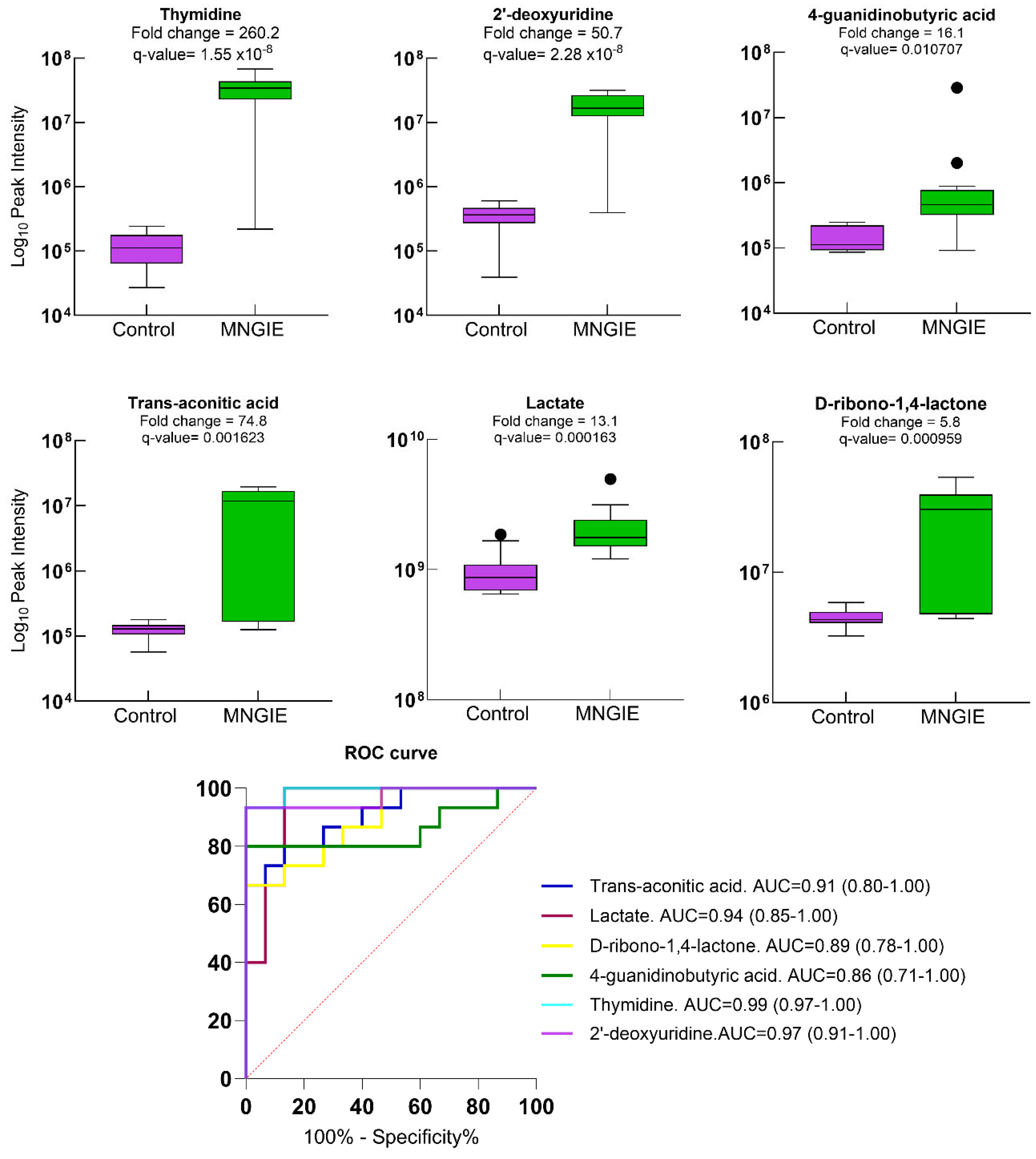
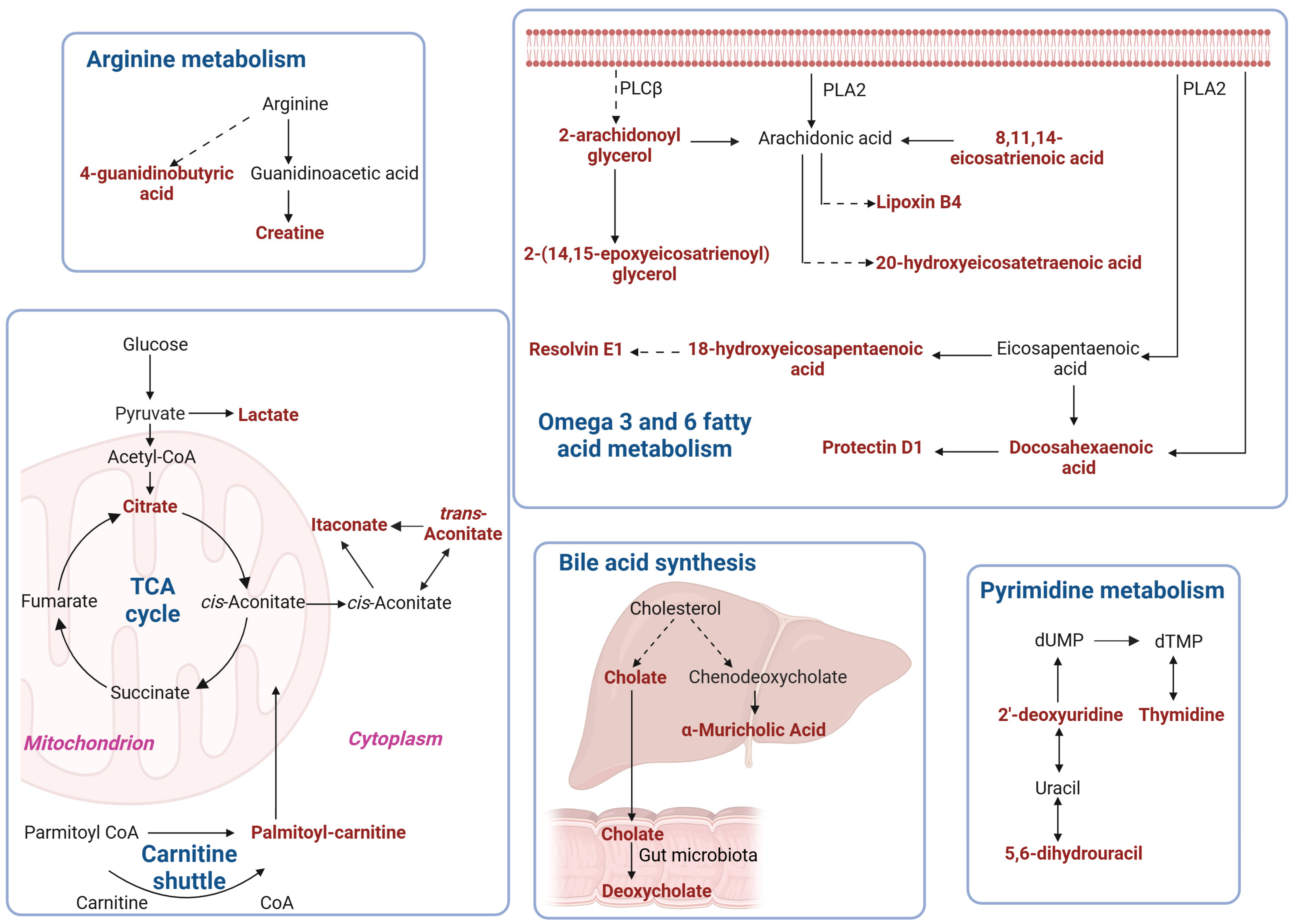
| KEGG Pathway | p Value | KEGG Names | KEGG Identification | Differential Abundance Score and Pathway Regulation Status |
|---|---|---|---|---|
| Caffeine metabolism map 00232 | 2.922 × 10−8 | 7-methylxanthine; 3-methylxanthine; Theobromine; Paraxanthine; Theophylline; 1-Methyluric acid; 1,3,7-trimethyluric acid | C16353 + C16357 + C07480 + C13747 + C07130 + C16359 + C16361 | −1 Down |
| Steroid hormone biosynthesis Map 00140 | 4.579 × 10−8 | 5α-pregnan-3,20-dione; 17α-hydroxyprogesterone; Androsterone; Androsterone glucuronide; Dehydroepiandrosterone; Aldosterone; Etiocholanolone; Estrone; 20-oxopregn-5-en-3-yl hydrogen sulfate; Tetrahydrocortisol; Androstenedione | C03681 + C01176 + C00523 + C11135 + C01227 + C01780 + C04373 + C00468 + C18044 + C05472 + C00280 | −0.8 Down |
| Pathways in cancer map 05200 | 7.263 × 10−6 | Retinoate; Dehydroepiandrosterone (dhea); Prostaglandin e2; Fumaric acid; Androstanolone; Androstenedione | C00777 + C01227 + C00584 + C00122 + C03917 + C00280 | 0 Down |
| Arachidonic acid metabolism Map 00590 | 1.679 × 10−4 | Lipoxin b4; 11-dehydro thromboxane b2; Thromboxane b2; Prostaglandin e2; Prostaglandin a2; 20-hydroxy-(5z,8z,11z,14z)-eicosatetraenoic acid; 15-keto prostaglandin f2α, 11,12-DHET, 15-(S)HPETE, 11,12-EET | C06315 + C05964 + C05963 + C00584 + C05953 + C14748 + C05960 + C14774 + C05966 + C14770 | 0.8 Up |
| Ovarian steroidogenesis Map 04913 | 4.820 × 10−4 | 17α-hydroxyprogesterone; Dehydroepiandrosterone (dhea); Estrone; Androstenedione, 11,12-EET | C01176 + C01227 + C00468 + C00280 + C14770 | −1 Down |
| Pyrimidine metabolism map 00240 | 5.228 × 10−4 | L-glutamine; 5,6-dihydrouracil; Uracil; 2′-deoxyuridine; Thymine; Thymidine | C00064 + C00429 + C00106 + C00526 + C00178 + C00214 | 0.7 Up |
| Prostate cancer map 05215 | 5.652 × 10−4 | Dehydroepiandrosterone (dhea); Androstanolone; Androstenedione | C01227 + C03917 + C00280 | −1 Down |
| Alanine, aspartate and glutamate metabolism map 00250 | 8.844 × 10−4 | Citrate; L-glutamine; Fumaric acid; 2-keto-glutaramic acid | C00158 + C00064 + C00122 + C00940 | 0 Down |
| Serotonergic synapse map 04726 | 4.076 × 10−3 | 11-dehydro thromboxane b2; Thromboxane b2; Prostaglandin e2; Prostaglandin a2, 11,12-EET | C05964 + C05963 + C00584 + C05953 + C14770 | 1 Up |
| Prolactin signaling pathway map04917 | 0.012158 | Estrone; Androstenedione | C00468 + C00280 | −1 Down |
| C-type lectin receptor signaling pathway map 04625 | 0.012158 | Prostaglandin e2; Fucose | C00584 + C01019 | 1 Up |
| Pyruvate metabolism map 00620 | 0.012235 | Fumaric acid; S-lactoylglutathione; 2-butynedioic acid | C00122 + C03451 + C03248 | 0.3 Up |
| Glyoxylate and dicarboxylate metabolism map 00630 | 0.015951 | Citrate; L-glutamine; 4-hydroxy-2-oxoglutaric acid; 3-oxalomalic acid | C00158 + C00064 + C05946 + C01990 | −0.5 Down |
| Bile secretion map 04976 | 0.017976 | Cholate; Deoxycholate; Thromboxane b2; Prostaglandin e2; Bilirubin | C00695 + C04483 + C05963 + C00584 + C00486 | 0.6 Up |
| Central carbon metabolism in cancer map 05230 | 0.019754 | Citrate; L-glutamine; Fumaric acid | C00158 + C00064 + C00122 | 0.3 Up |
| Oxidative phosphorylation Ma p00190 | 0.025202 | Fumaric acid; Ubiquinol 10 | C00122 + C11378 | 0 Down |
| Biosynthesis of unsaturated fatty acids ma p01040 | 0.028497 | Docosahexaenoic acid; Dihomo-gamma-linolenate; Eicosapentaenoate; Nervonic acid | C06429 + C03242 + C06428 + C08323 | 0.8 Up |
| Intestinal immune network for IgA production map 04672 | 0.030961 | Retinoate | C00777 | 1 Up |
| alpha-Linolenic acid metabolism map00592 | 0.031107 | 13(s)-hotre; 12-oxo phytodienoic acid; 9(s)-hotre | C16316 + C01226 + C16326 | 0.3 Up |
| Citrate cycle (TCA cycle) map 00020 | 0.038311 | Citrate; Fumaric acid | C00158 + C00122 | 0.5 Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bax, B.E.; Uçar, S.K. Untargeted Plasma Metabolomics Extends the Biomarker Profile of Mitochondrial Neurogastrointestinal Encephalomyopathy. Int. J. Mol. Sci. 2025, 26, 9107. https://doi.org/10.3390/ijms26189107
Bax BE, Uçar SK. Untargeted Plasma Metabolomics Extends the Biomarker Profile of Mitochondrial Neurogastrointestinal Encephalomyopathy. International Journal of Molecular Sciences. 2025; 26(18):9107. https://doi.org/10.3390/ijms26189107
Chicago/Turabian StyleBax, Bridget E., and Sema Kalkan Uçar. 2025. "Untargeted Plasma Metabolomics Extends the Biomarker Profile of Mitochondrial Neurogastrointestinal Encephalomyopathy" International Journal of Molecular Sciences 26, no. 18: 9107. https://doi.org/10.3390/ijms26189107
APA StyleBax, B. E., & Uçar, S. K. (2025). Untargeted Plasma Metabolomics Extends the Biomarker Profile of Mitochondrial Neurogastrointestinal Encephalomyopathy. International Journal of Molecular Sciences, 26(18), 9107. https://doi.org/10.3390/ijms26189107






