Pharmacokinetic Profiling Using 3H-Labeled Eggshell Membrane and Effects of Eggshell Membrane and Lysozyme Oral Supplementation on DSS-Induced Colitis and Human Gut Microbiota
Abstract
1. Introduction
2. Results
2.1. Proteomic Analysis of Eggshell Membrane Powder
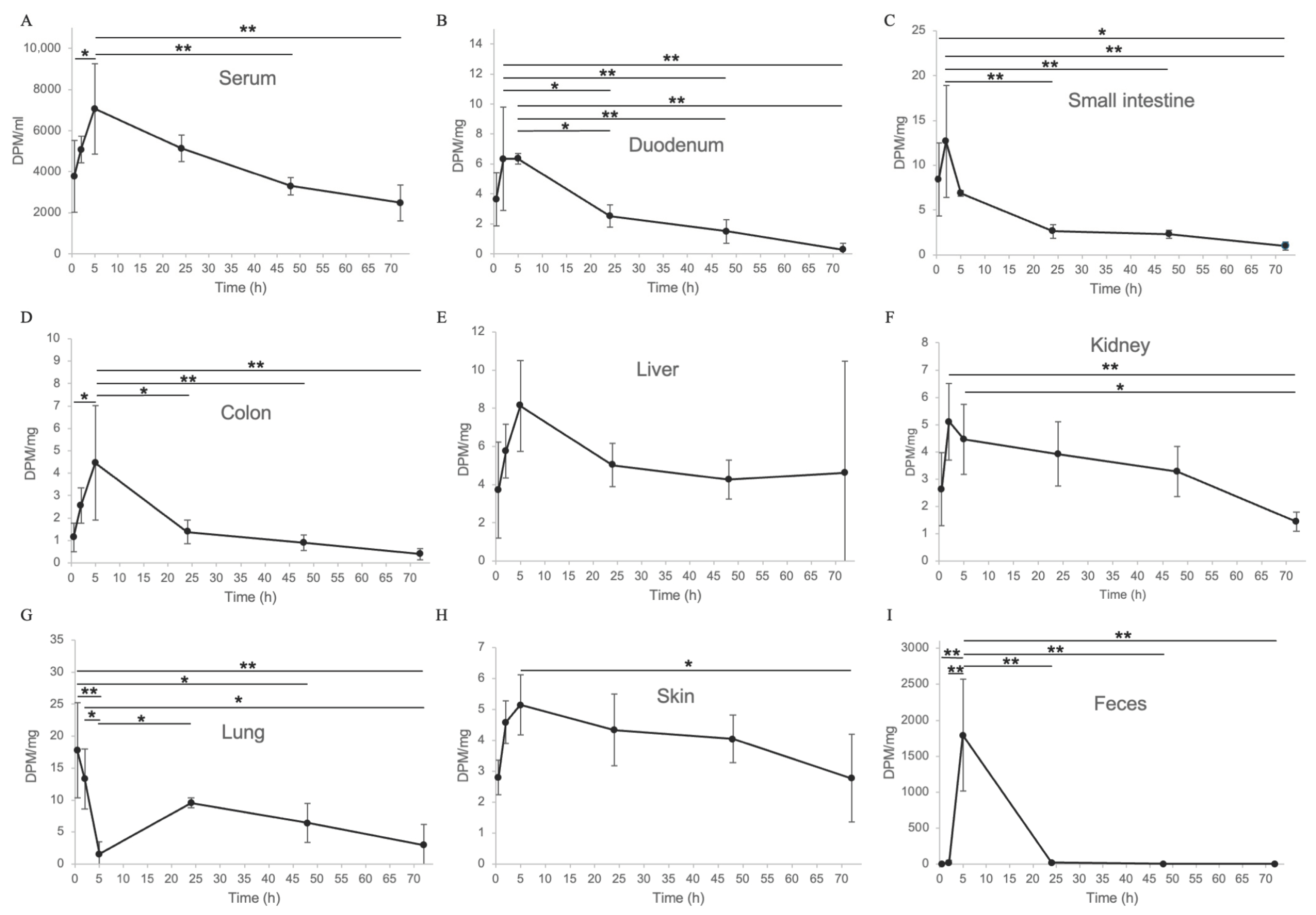
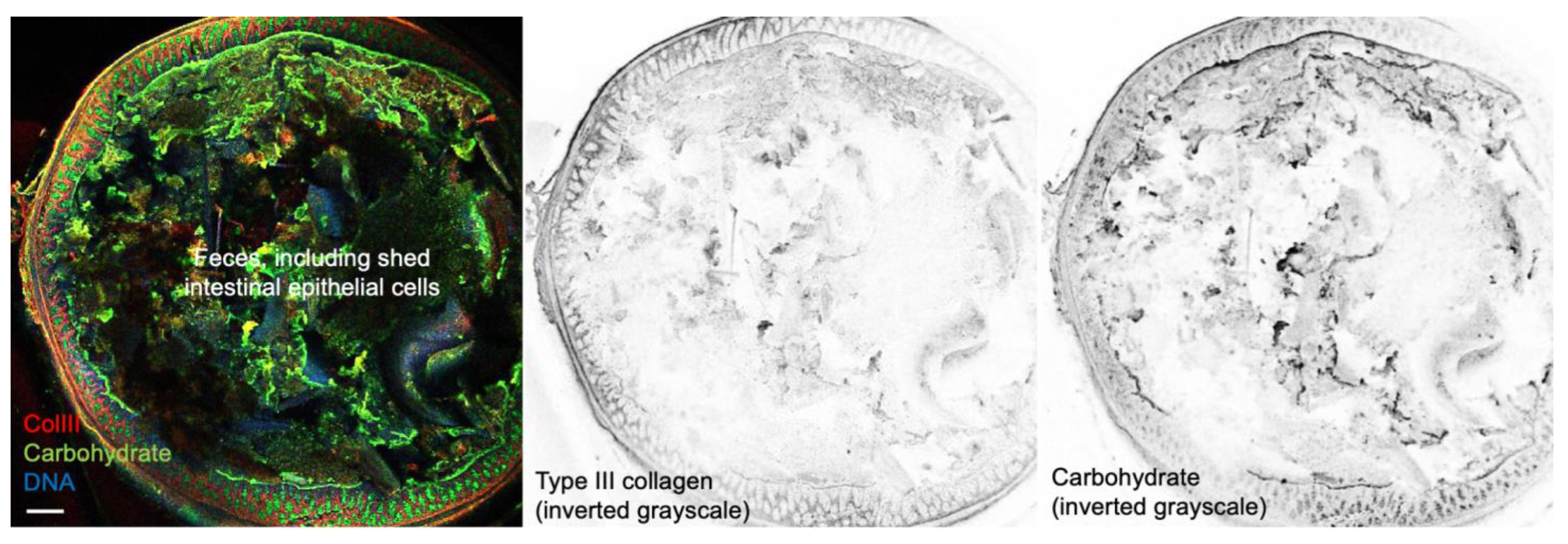
2.2. In Vivo Distribution of Tritium-Labeled Eggshell Membrane Following Oral Administration in Mice
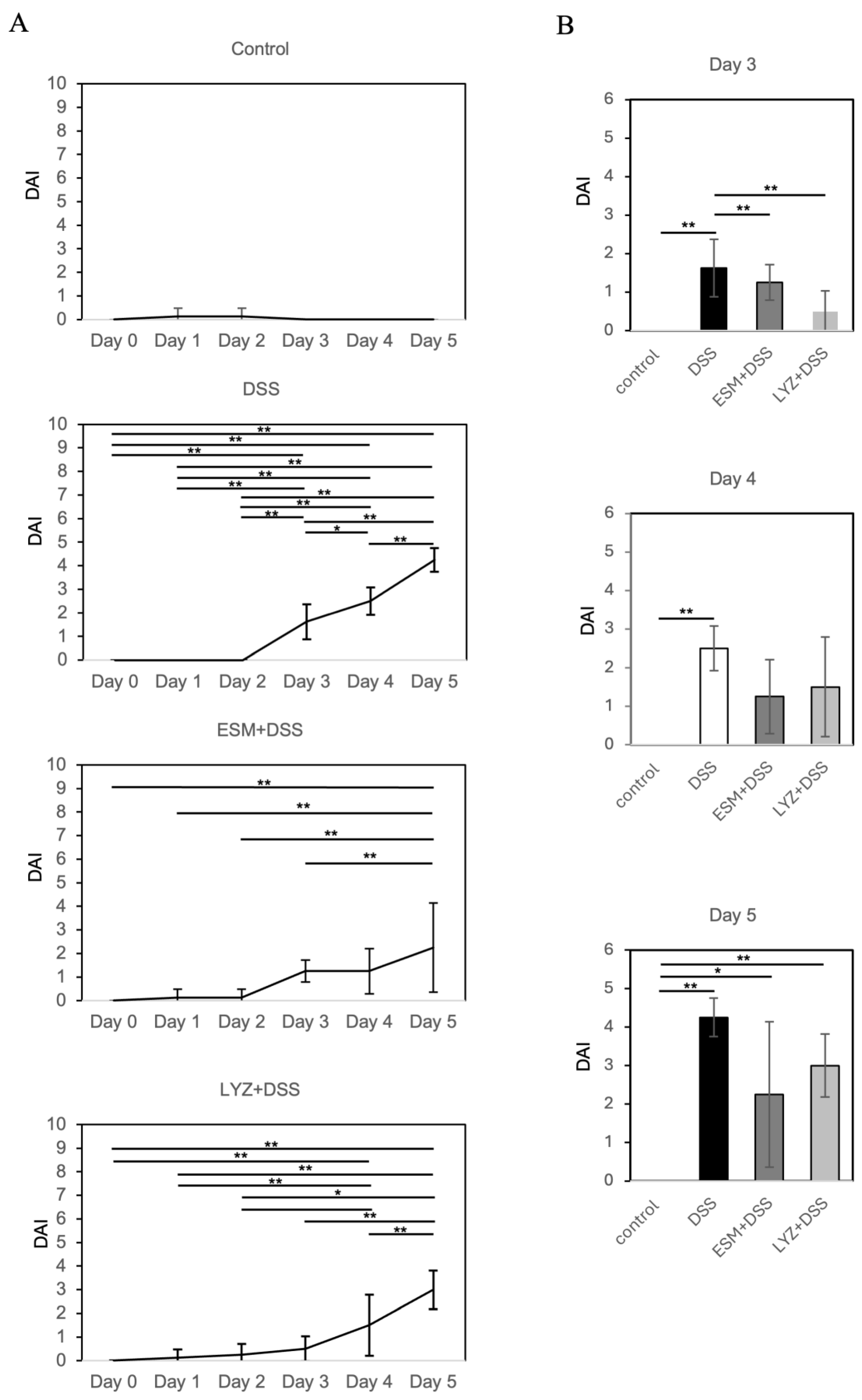
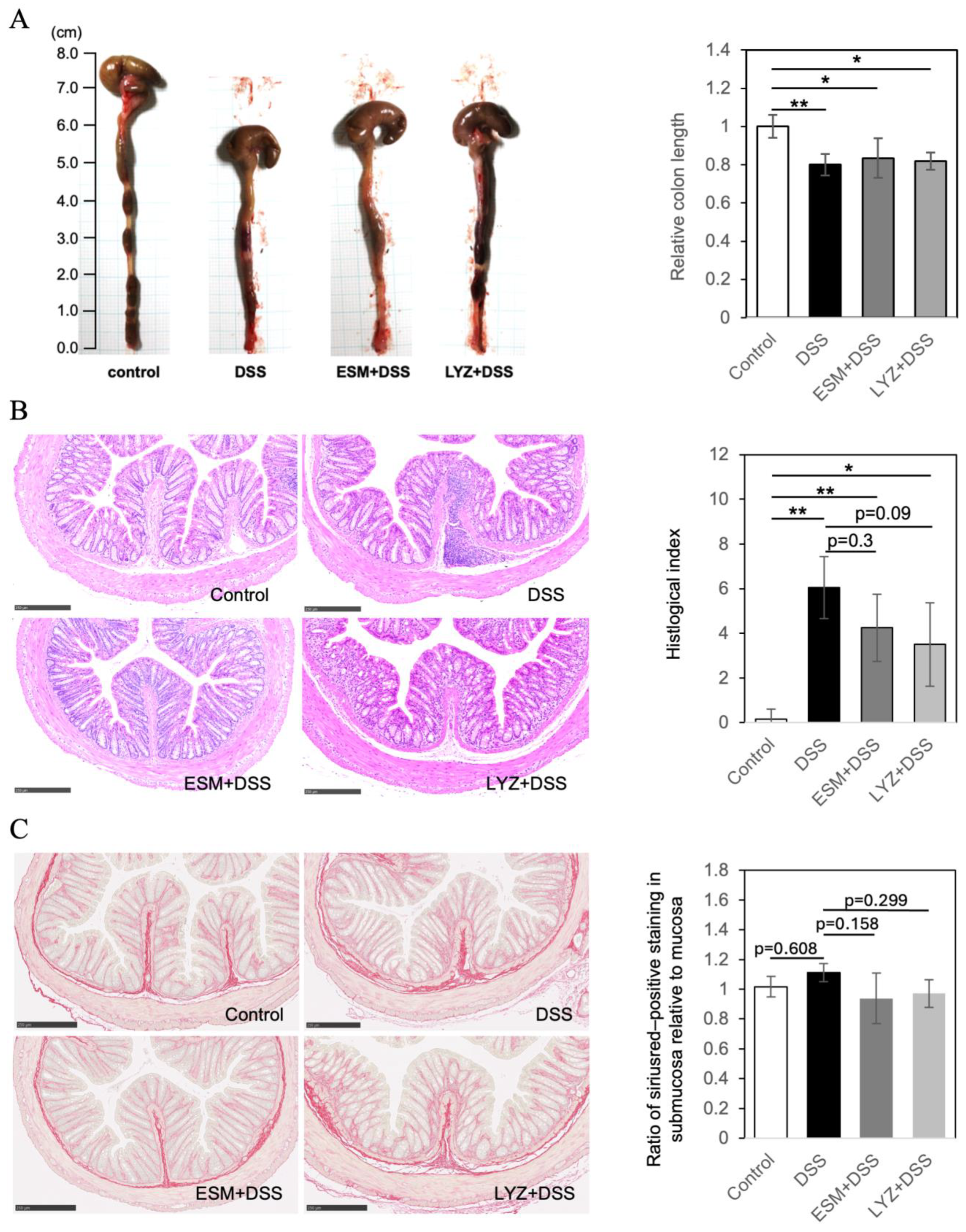
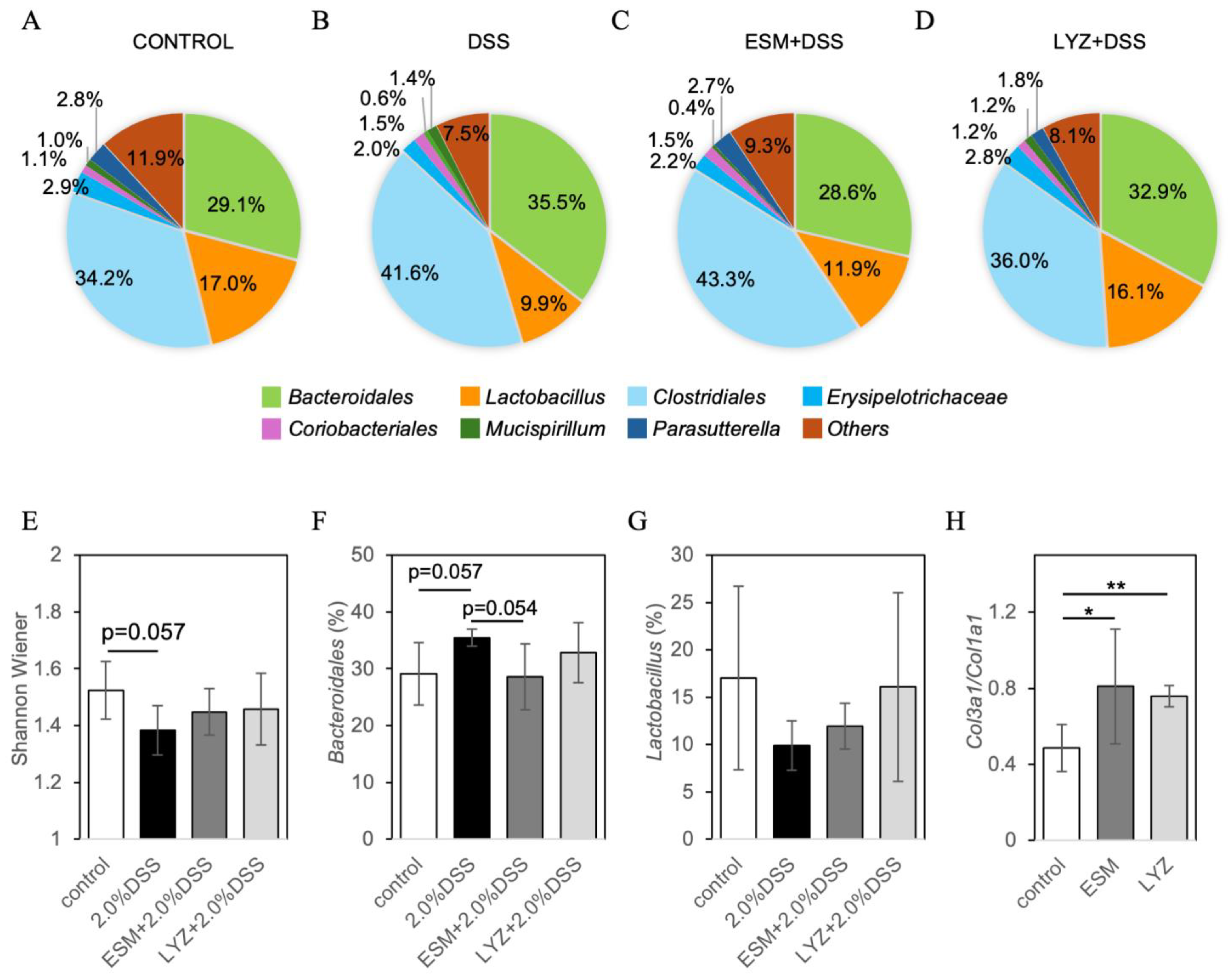
2.3. DSS-Induced Colitis Mouse Model and Eggshell Membrane Supplementation: Effects on Intestinal Pathology and Gut Microbiota
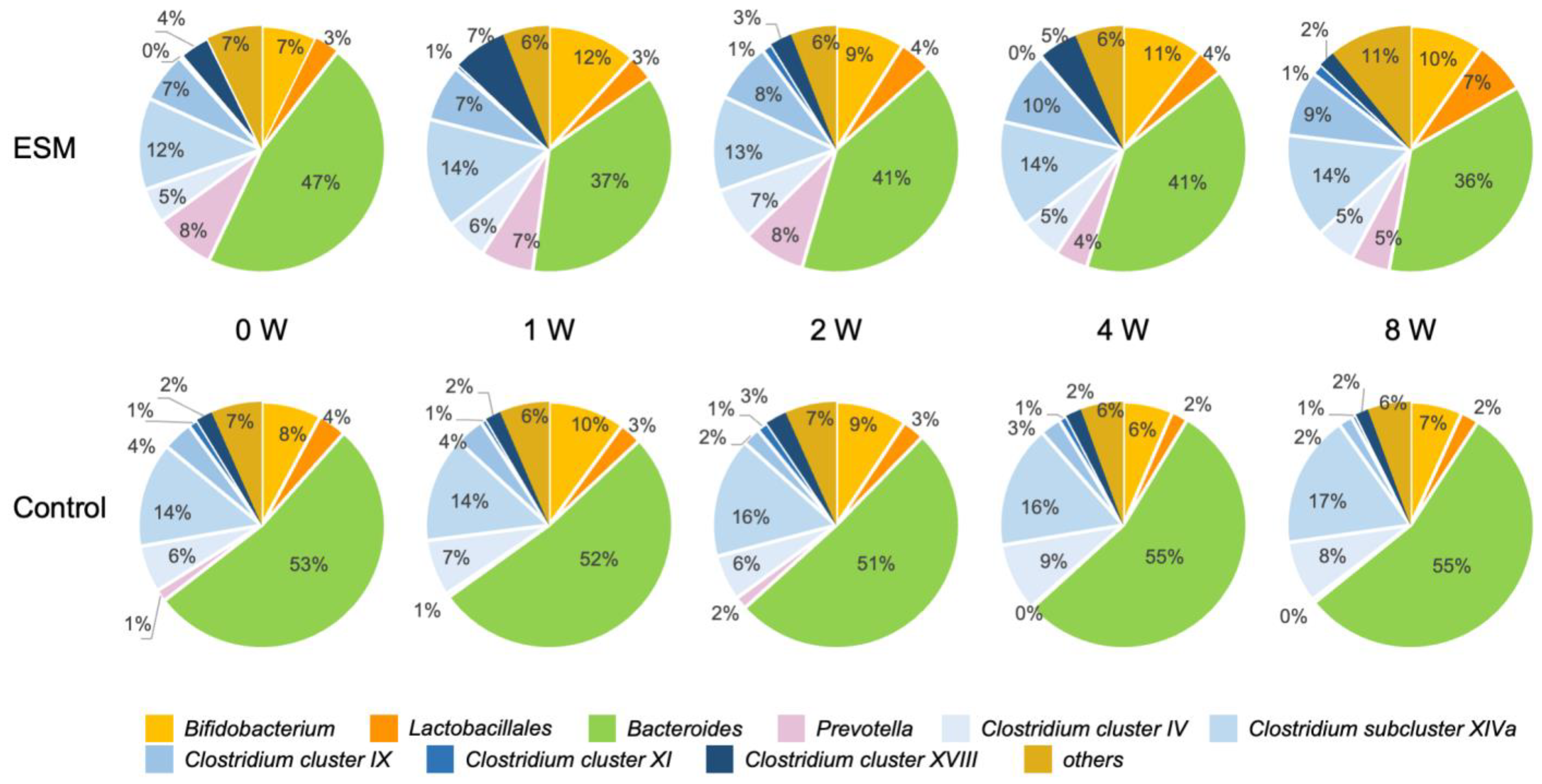
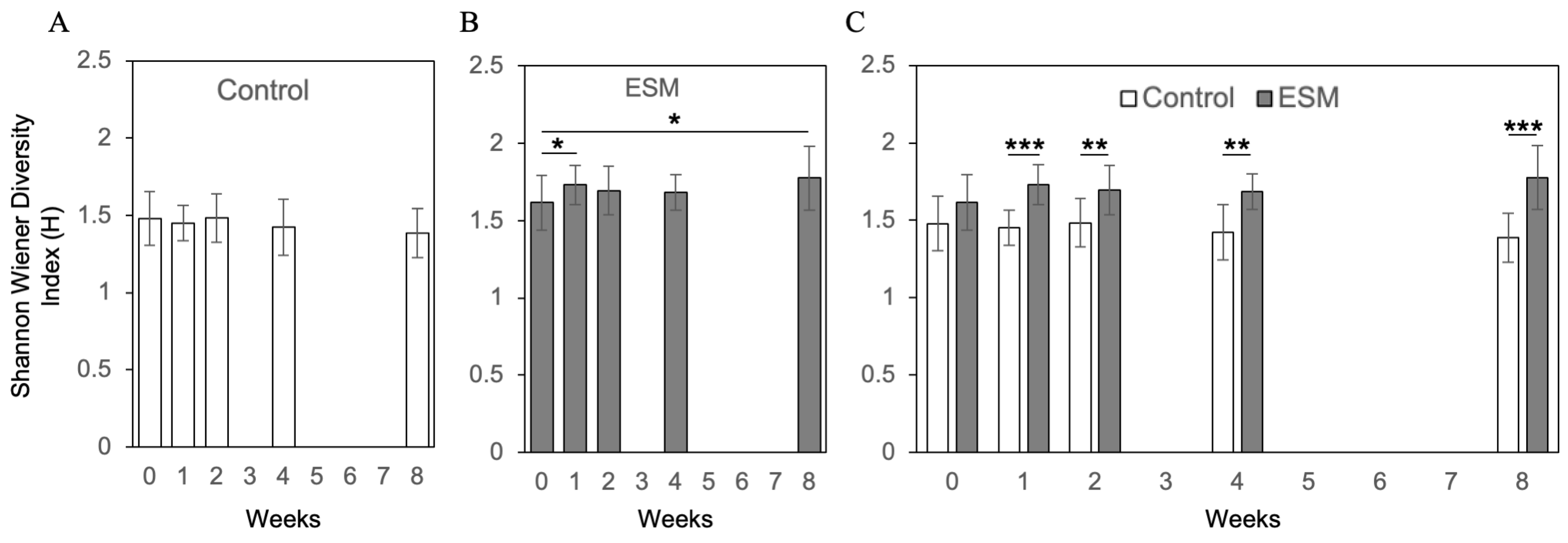
2.4. Randomized Controlled Trial Assessing Gut Microbiota Response to Eggshell Membrane
3. Discussion
4. Materials and Methods
4.1. Eggshell Membrane Powder for Oral Administration
4.2. Proteomics Analysis
4.3. Tritium Labeling of Eggshell Membrane (ESM)
4.4. In Vivo Distribution of Tritium-Labeled Eggshell Membrane Powder in Mice
4.5. Oral Administration of Eggshell Membrane in DSS-Induced Colitis Model
- Body weight loss (relative to initial weight): 0 = no loss; 1 = 1–10%; 2 = 10–20%; 3 = >20%.
- Stool consistency: 0 = normal; 1 = soft; 2 = diarrhea; 3 = watery diarrhea.
- Fecal blood: 0 = none; 1 = partial bleeding; 2 = extensive bleeding; 3 = blood around the anus.
The Average Daily DAI Score for Each Group Was Calculated
4.6. Evaluation of the Effects of Oral Administration of Eggshell Membrane Fine Powder and Lysozyme on the Expression of Genes Related to the Intestinal ECM in Mice
4.7. Human Study
4.8. Fecal Sample Collection and Gut Microbiota Analysis by T-RFLP
4.9. Assessment of α-Diversity of Gut Microbiota
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESM | Eggshell membrane |
| LYZ | Lysozyme |
| DSS | Dextran sulfate sodium |
| ECM | Extracellular Matrix |
| IBD | Inflammatory bowel disease |
| DAI | Disease activity index |
References
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef] [PubMed]
- Alyami, A.S.; Madkhali, Y.; Majrashi, N.A.; Alwadani, B.; Elbashir, M.; Ali, S.; Ageeli, W.; El-Bahkiry, H.S.; Althobity, A.A.; Refaee, T. The role of molecular imaging in detecting fibrosis in Crohn’s disease. Ann. Med. 2024, 56, 2313676. [Google Scholar] [CrossRef]
- Lin, S.N.; Wang, J.; Mukherjee, P.K.; Veisman, I.; Massey, W.J.; Mao, R.; Chandra, J.; Fiocchi, C.; Rieder, F. The functional role of the extracellular matrix in inflammatory bowel disease associated gut fibrosis. Matrix Biol. 2025, 139, 29–48. [Google Scholar] [CrossRef]
- Grames, C.; Berry-Caban, C.S. Ischemic colitis in an endurance runner. Case Rep. Gastrointest. Med. 2012, 2012, 356895. [Google Scholar] [CrossRef]
- Haydont, V.; Vozenin-Brotons, M.C. Maintenance of radiation-induced intestinal fibrosis: Cellular and molecular features. World J. Gastroenterol. 2007, 13, 2675–2683. [Google Scholar] [CrossRef]
- Latella, G.; Sferra, R.; Speca, S.; Vetuschi, A.; Gaudio, E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1283–1304. [Google Scholar] [PubMed]
- Mignini, I.; Blasi, V.; Termite, F.; Esposto, G.; Borriello, R.; Laterza, L.; Scaldaferri, F.; Ainora, M.E.; Gasbarrini, A.; Zocco, M.A. Fibrostenosing Crohn’s Disease: Pathogenetic Mechanisms and New Therapeutic Horizons. Int. J. Mol. Sci. 2024, 25, 6326. [Google Scholar] [CrossRef]
- Bellone, F.; Morace, C.; Impala, G.; Viola, A.; Lo Gullo, A.; Cinquegrani, M.; Fries, W.; Sardella, A.; Scolaro, M.; Basile, G.; et al. Quality of Life (QoL) in Patients with Chronic Inflammatory Bowel Diseases: How Much Better with Biological Drugs? J. Pers. Med. 2023, 13, 947. [Google Scholar] [CrossRef]
- Latella, G.; Rieder, F. Intestinal fibrosis: Ready to be reversed. Curr. Opin. Gastroenterol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial Translocation in Gastrointestinal Cancers and Cancer Treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development. Front. Oncol. 2021, 11, 626349. [Google Scholar] [CrossRef] [PubMed]
- Mayorca-Guiliani, A.E.; Leeming, D.J.; Henriksen, K.; Mortensen, J.H.; Nielsen, S.H.; Anstee, Q.M.; Sanyal, A.J.; Karsdal, M.A.; Schuppan, D. ECM formation and degradation during fibrosis, repair, and regeneration. npj Metab. Health Dis. 2025, 3, 25. [Google Scholar] [CrossRef]
- Ruff, K.J.; DeVore, D.P.; Leu, M.D.; Robinson, M.A. Eggshell membrane: A possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin. Interv. Aging 2009, 4, 235–240. [Google Scholar] [CrossRef]
- Canovas, F.; Abellan-Ruiz, M.S.; Garcia-Munoz, A.M.; Luque-Rubia, A.J.; Victoria-Montesinos, D.; Perez-Pinero, S.; Sanchez-Macarro, M.; Lopez-Roman, F.J. Randomised Clinical Trial to Analyse the Efficacy of Eggshell Membrane to Improve Joint Functionality in Knee Osteoarthritis. Nutrients 2022, 14, 2340. [Google Scholar] [CrossRef]
- Garcia-Munoz, A.M.; Abellan-Ruiz, M.S.; Garcia-Guillen, A.I.; Victoria-Montesinos, D. Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2640. [Google Scholar] [CrossRef] [PubMed]
- Ohto-Fujita, E.; Hatakeyama, N.; Atomi, A.; Yasuda, S.; Kodama, S.; Atomi, T.; Tanaka, K.; Noboru, H.; Harada, K.; Asano, Y.; et al. Effect of Eggshell Membrane Powder Intake on the Body Function of Healthy Individuals. J. Fiber Sci. Technol. 2021, 77, 258–265. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Shimizu, M.; Atomi, A.; Hiruta, H.; Hosoda, R.; Horinouchi, S.; Miyazaki, S.; Murakami, T.; Asano, Y.; Hasebe, Y.; et al. Eggshell membrane and its major component lysozyme and ovotransferrin enhance the secretion of decorin as an endogenous antifibrotic mediator from lung fibroblasts and ameliorate bleomycin-induced pulmonary fibrosis. Biochem. Biophys. Rep. 2024, 39, 101806. [Google Scholar] [CrossRef]
- Papavassiliou, K.A.; Sofianidi, A.A.; Spiliopoulos, F.G.; Gogou, V.A.; Gargalionis, A.N.; Papavassiliou, A.G. YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis. Cells 2024, 13, 1519. [Google Scholar] [CrossRef]
- King’Ori, A. A review of the uses of poultry eggshells and shell membranes. Int. J. Poult. Sci. 2011, 10, 908–912. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Suso, H.P.; Hincke, M.T. In-depth comparative analysis of the chicken eggshell membrane proteome. J. Proteomics 2017, 155, 49–62. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Nogawa, N.; Shimizu, M.; Ijiri, K.-i.; Hasebe, Y.; Atomi, Y. Application of Neutron-Irradiated 6Li (n, α) 3H Reaction to a Protein-Based Fibrous Non-Woven Fabric Biopolymer: Radiolabeling of Cross-Linked Natural Fibrous Chicken Eggshell Membranes and Their Whole-Body Tissue Distribution after Oral Ingestion in Mice. J. Fiber Sci. Technol. 2021, 77, 182–187. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Koyama, Y.; Nishikawa, H.; Murakami, T.; Yumoto, R. Segment-selective absorption of lysozyme in the intestine. Eur. J. Pharmacol. 2004, 502, 149–155. [Google Scholar] [CrossRef]
- Mathias, N.R.; Hussain, M.A. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Thallmair, M.; Jirkof, P. A scoping review on reporting of methods in DSS colitis mouse models. Lab. Anim. 2025, 236772251331677. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Whitby, D.J.; Ferguson, M.W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 1991, 112, 651–668. [Google Scholar] [CrossRef]
- Seifert, A.W.; Kiama, S.G.; Seifert, M.G.; Goheen, J.R.; Palmer, T.M.; Maden, M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012, 489, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, A.G.W.; Maden, M. Regeneration in the spiny mouse, Acomys, a new mammalian model. Curr. Opin. Genet. Dev. 2020, 64, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Brant, J.O.; Lopez, M.C.; Baker, H.V.; Barbazuk, W.B.; Maden, M. A Comparative Analysis of Gene Expression Profiles during Skin Regeneration in Mus and Acomys. PLoS ONE 2015, 10, e0142931. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Shimizu, M.; Sano, S.; Kurimoto, M.; Yamazawa, K.; Atomi, T.; Sakurai, T.; Murakami, Y.; Takami, T.; Murakami, T.; et al. Solubilized eggshell membrane supplies a type III collagen-rich elastic dermal papilla. Cell Tissue Res. 2019, 376, 123–135. [Google Scholar] [CrossRef]
- Nagashima, K.; Hisada, T.; Sato, M.; Mochizuki, J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl. Environ. Microbiol. 2003, 69, 1251–1262. [Google Scholar] [CrossRef]
- Jia, H.; Aw, W.; Saito, K.; Hanate, M.; Hasebe, Y.; Kato, H. Eggshell membrane ameliorates hepatic fibrogenesis in human C3A cells and rats through changes in PPARgamma-Endothelin 1 signaling. Sci. Rep. 2014, 4, 7473. [Google Scholar] [CrossRef]
- Jia, H.; Hanate, M.; Aw, W.; Itoh, H.; Saito, K.; Kobayashi, S.; Hachimura, S.; Fukuda, S.; Tomita, M.; Hasebe, Y.; et al. Eggshell membrane powder ameliorates intestinal inflammation by facilitating the restitution of epithelial injury and alleviating microbial dysbiosis. Sci. Rep. 2017, 7, 43993. [Google Scholar] [CrossRef]
- Larsen, I.S.; Jensen, B.A.H.; Bonazzi, E.; Choi, B.S.Y.; Kristensen, N.N.; Schmidt, E.G.W.; Suenderhauf, A.; Morin, L.; Olsen, P.B.; Hansen, L.B.S.; et al. Fungal lysozyme leverages the gut microbiota to curb DSS-induced colitis. Gut Microbes 2021, 13, 1988836. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; El-Far, A.H.; Elbestawy, A.R.; Ghanem, R.; Mousa, S.A.; Abd El-Hamid, H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS ONE 2017, 12, e0185153. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Szakadati, H.; Kovalszky, I. Decorin the antifibrotic proteoglycan and its progression in therapy. Am. J. Physiol. Cell Physiol. 2025, 328, C1853–C1865. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Konno, T.; Shimizu, M.; Ishihara, K.; Sugitate, T.; Miyake, J.; Yoshimura, K.; Taniwaki, K.; Sakurai, T.; Hasebe, Y.; et al. Hydrolyzed eggshell membrane immobilized on phosphorylcholine polymer supplies extracellular matrix environment for human dermal fibroblasts. Cell Tissue Res. 2011, 345, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordonez-Moran, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49.e7. [Google Scholar] [CrossRef]
- Swain, S.M.; Liddle, R.A. Mechanosensing Piezo channels in gastrointestinal disorders. J. Clin. Investig. 2023, 133, e171955. [Google Scholar] [CrossRef]
- Alcaino, C.; Farrugia, G.; Beyder, A. Mechanosensitive Piezo Channels in the Gastrointestinal Tract. Curr. Top. Membr. 2017, 79, 219–244. [Google Scholar] [CrossRef]
- Ellison, R.T., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Chipman, D.M.; Sharon, N. Mechanism of lysozyme action. Science 1969, 165, 454–465. [Google Scholar] [CrossRef] [PubMed]
- de Wouters, T.; Jans, C.; Niederberger, T.; Fischer, P.; Ruhs, P.A. Adhesion Potential of Intestinal Microbes Predicted by Physico-Chemical Characterization Methods. PLoS ONE 2015, 10, e0136437. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Miyata, J.; Watanabe, T.; Shioya, J.; Arita, M.; Ohara, O. Proteogenomic Analyses of Cellular Lysates Using a Phenol-Guanidinium Thiocyanate Reagent. J. Proteome Res. 2019, 18, 301–308. [Google Scholar] [CrossRef]
- Nagashima, K.; Mochizuki, J.; Hisada, T.; Suzuki, S.; Shimomura, K. Phylogenetic Analysis of 16S Ribosomal RNA Gene Sequences from Human Fecal Microbiota and Improved Utility of Terminal Restriction Fragment Length Polymorphism Profiling. Biosci. Microflora 2006, 25, 99–107. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180, Erratum in Nature 2011, 474, 666. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated guideline for reporting randomised trials. BMJ 2025, 388, e081123. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, M.; Sugai, W.; Ohto-Fujita, E.; Atomi, A.; Nogawa, N.; Takamiya, K.; Yoshinaga, H.; Asano, Y.; Yamashita, T.; Sato, S.; et al. Pharmacokinetic Profiling Using 3H-Labeled Eggshell Membrane and Effects of Eggshell Membrane and Lysozyme Oral Supplementation on DSS-Induced Colitis and Human Gut Microbiota. Int. J. Mol. Sci. 2025, 26, 9102. https://doi.org/10.3390/ijms26189102
Shimizu M, Sugai W, Ohto-Fujita E, Atomi A, Nogawa N, Takamiya K, Yoshinaga H, Asano Y, Yamashita T, Sato S, et al. Pharmacokinetic Profiling Using 3H-Labeled Eggshell Membrane and Effects of Eggshell Membrane and Lysozyme Oral Supplementation on DSS-Induced Colitis and Human Gut Microbiota. International Journal of Molecular Sciences. 2025; 26(18):9102. https://doi.org/10.3390/ijms26189102
Chicago/Turabian StyleShimizu, Miho, Wataru Sugai, Eri Ohto-Fujita, Aya Atomi, Norio Nogawa, Koichi Takamiya, Hisao Yoshinaga, Yoshihide Asano, Takashi Yamashita, Shinichi Sato, and et al. 2025. "Pharmacokinetic Profiling Using 3H-Labeled Eggshell Membrane and Effects of Eggshell Membrane and Lysozyme Oral Supplementation on DSS-Induced Colitis and Human Gut Microbiota" International Journal of Molecular Sciences 26, no. 18: 9102. https://doi.org/10.3390/ijms26189102
APA StyleShimizu, M., Sugai, W., Ohto-Fujita, E., Atomi, A., Nogawa, N., Takamiya, K., Yoshinaga, H., Asano, Y., Yamashita, T., Sato, S., Enomoto, A., Hatakeyama, N., Yasuda, S., Tanaka, K., Atomi, T., Harada, K., Hasebe, Y., Watanabe, T., & Atomi, Y. (2025). Pharmacokinetic Profiling Using 3H-Labeled Eggshell Membrane and Effects of Eggshell Membrane and Lysozyme Oral Supplementation on DSS-Induced Colitis and Human Gut Microbiota. International Journal of Molecular Sciences, 26(18), 9102. https://doi.org/10.3390/ijms26189102








