The Roles of Moonlighting Nicotinamide Mononucleotide Adenylyl Transferases in Cell Physiology
Abstract
1. Introduction
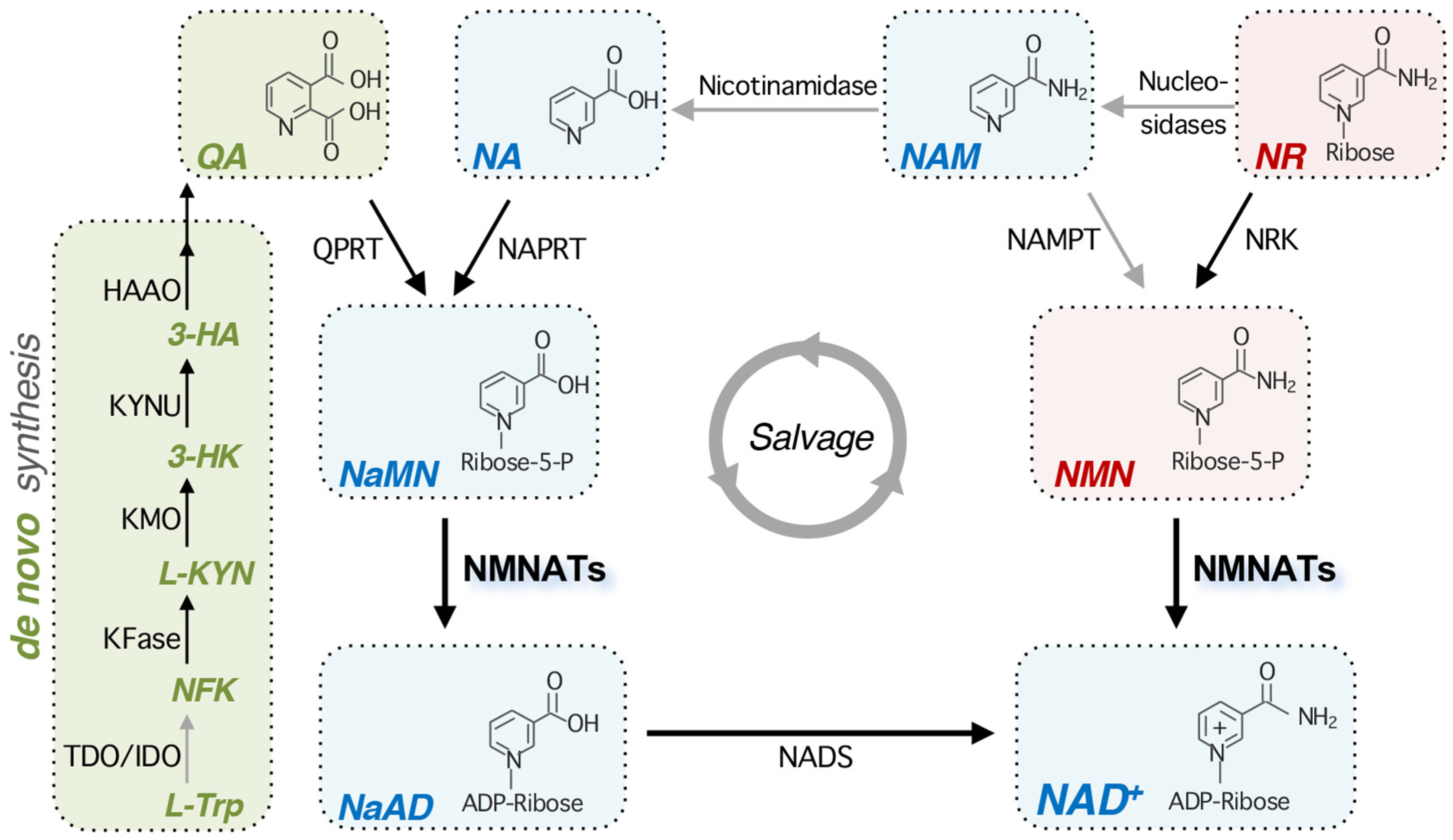
2. Overview of NAD+ Metabolism
3. Functional and Conserved Motifs in NMNATs
4. Subcellular Localization of NMNATs and NAD+ Pools
5. The Multifaceted Roles of NMNATs in Neuroprotection
5.1. NMNATs Exert Neuroprotection Through NAD+-Dependent Autophagy Boosting
| NMNAT Types | Mutations | Effects | Reference |
|---|---|---|---|
| hNMNAT2 | Δ1-100 (removes N-terminus) | Blocks NAD+ synthesis No effect on chaperone activity Reduces hAtx-1(82Q) aggregates | [35] |
| W92G (NMN binding site) | Blocks NAD+ synthesis No effect on chaperone activity Reduces hAtx-1(82Q)/pTau aggregates | [35] | |
| C164S, C165S (palmitoylation sites) | Increases hNMNAT2 protein stability Increases NAD+ synthesis Increases chaperone activity Reduces hAtx-1(82Q)/pTau aggregates | [35] | |
| Δ269-274 (removes C-terminal ATP binding site) | No effect on NAD+ synthesis Reduces chaperone activity (foldase) Fails to reduce hAtx-1(82Q)/pTau aggregates | [35] | |
| Δ200-303 (removes C-terminus) | No effect on NAD+ synthesis Reduces chaperone activity (foldase) Fails to reduce hAtx-1(82Q)/pTau aggregates | [35] | |
| mNMNAT1 | H24A (catalytic motif) | Blocks NAD+ synthesis No protection against Wallerian degeneration No protection against axotomy-induced Ca2+ spike | [89,90] |
| R213A, R215A (NLS) | NMNAT1 redistributes from nucleus to cytoplasm Enhances protection against Wallerian degeneration | [91] | |
| mNMNAT3 | H22A (catalytic motif) | Blocks NAD+ synthesis No effect on chaperone activity | [30] |
| KKRK (K55E, K56E, R205E, K206E) (removes “+” charged interface of substrate binding sites) | Blocks NAD+ synthesis Reduces chaperone activity | [30] | |
| dNMNAT | H30A (catalytic motif) | Blocks NAD+ synthesis Rescues neurodegeneration morphology defect | [34] |
| W98G (NMN binding site) | Reduces NAD+ synthesis | [34] | |
| R224A (C-terminal ATP Binding site) | Reduces NAD+ synthesis | [34] | |
| W98G, R224A | Blocks NAD+ synthesis Rescues neurodegeneration morphology defect | [34] | |
| H30A (catalytic motif) | No effect on chaperone activity Reduces hAtx-1(82Q) aggregates | [31] | |
| W98G, R224A | No effect on chaperone activity Reduces hAtx-1(82Q) aggregates Colocalizes with hAtx-1(82Q) aggregates | [31] | |
| Δ1-64 (removes N-terminus) | No effect on chaperone activity | [31] | |
| Δ244-297 (removes C-terminus) | Disrupts chaperone activity | [31] | |
| ΔCN (removes N- and C-terminus) | Disrupts chaperone activity | [31] | |
| yNMNAT1 (Nma1) | H181A (catalytic motif) | Blocks NAD+ synthesis No effect on chaperone activity Reduces polyQ-GFP aggregates Rescues proteotoxicity-caused growth defect | [36] |
| Δ375-394 (removes C-terminus; preserves the C-terminal ATP binding site) | No effect on NAD+ synthesis Reduces chaperone activity (foldase) Fails to rescue proteotoxicity-caused growth defect | [36] |
5.2. NMNATs Reduce Protein Aggregates Likely by Acting as Chaperones
5.3. NMNATs Enhance Mitochondrial Integrity and Protect Neurons from Injuries
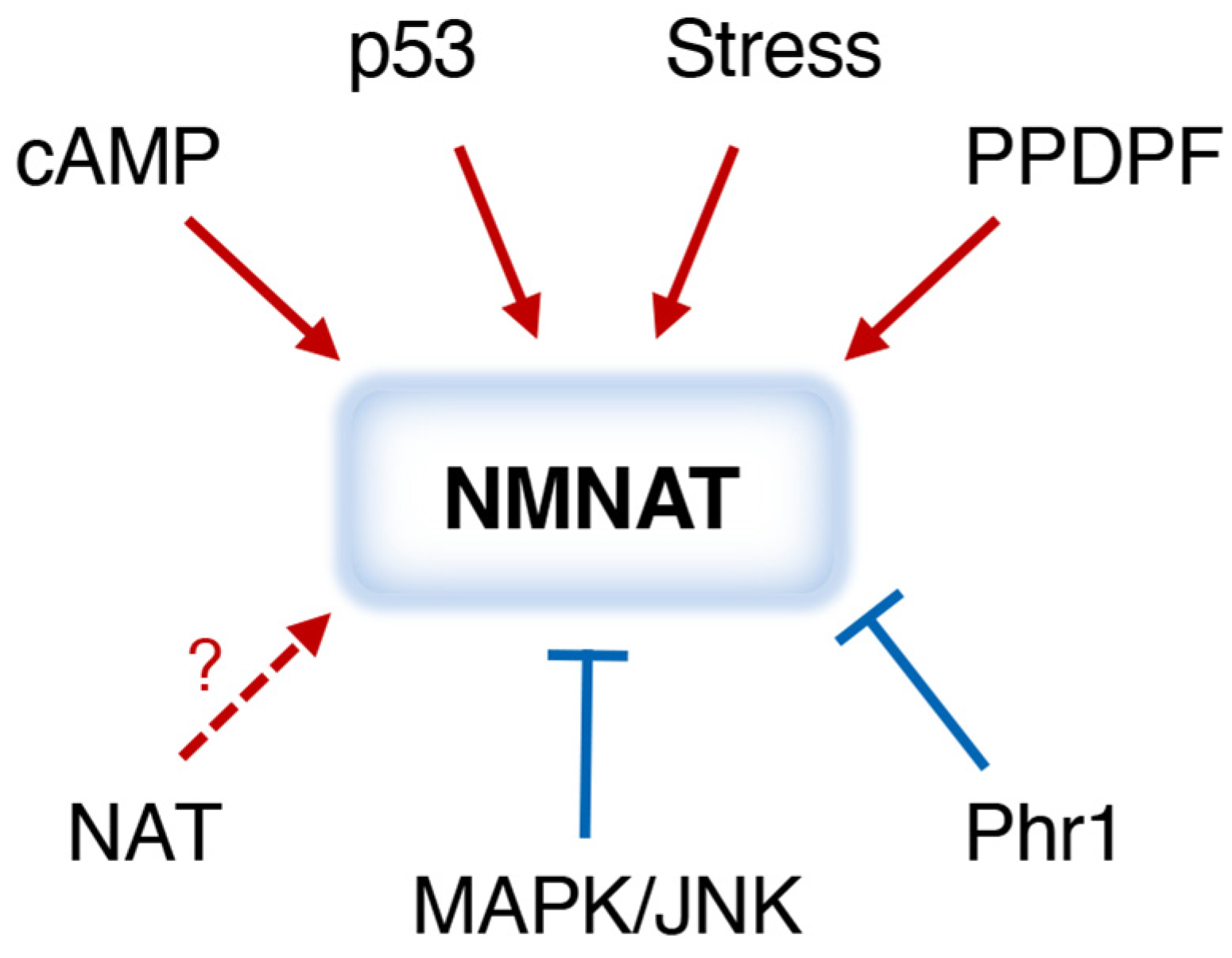
6. Regulation of NMNATs
7. NMNAT Activators and Inhibitors
7.1. NMNAT Activators
7.2. NMNAT Inhibitors
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-HA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxykynurenine |
| AMPK | AMP activated protein kinase |
| Aβ | amyloid beta-protein |
| ATG | Autophagy |
| cAMP | cyclic-AMP |
| CD157 | ADP-ribosyl cyclase |
| CD38 | NAD+ hydrolase |
| CHIP | C-terminus of Hsc70 interacting protein (STUB1); mediates Tau degradation |
| CREB | cAMP response element-binding protein |
| EGCG | epigallocatechin gallate |
| FK866 | NAMPT inhibitor |
| HAAO | 3-hydroxyanthranilic acid 3,4-dioxygenase |
| Htt | Huntingtin aggregates |
| HSF | Heat shock factor |
| IDO | indoleamine 2,3-dioxygenase |
| JNK | c-Jun N-terminal kinase |
| KFase | kynurenine formamidase |
| KMO | kynurenine 3-monooxygenase |
| KYNU | L-kynurenine hydrolase |
| L-KYN | L-kynurenine |
| L-Trp | L-tryptophan |
| MAPK | mitogen-activated protein kinase |
| NA | nicotinic acid, nicotinate |
| NaAD | deamido-NAD+ |
| NAD+ | nicotinamide adenine dinucleotide |
| NADS | NAD+ synthetase |
| NAM | nicotinamide |
| NaMN | nicotinic acid mononucleotide |
| NAMPT | nicotinamide phosphoribosyl transferase |
| NAPRT | nicotinic acid phosphoribosyl transferase |
| NAT | N-terminal acetyl transferase |
| NFK | N-formylkynurenine |
| NMN | nicotinamide mononucleotide |
| NMNAT | nicotinamide mononucleotide adenylyl transferase |
| NR | nicotinamide riboside |
| NRK | NR kinase; converts NR to NMN |
| PARP | poly (ADP-ribose) polymerase |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | protein kinase A; a nutrient sensing protein kinase |
| Pnc1 | nicotinamidase, yeast |
| PncA | nicotinamidase, bacterial |
| PP2A | protein phosphatase 2A |
| PPDPF | progenitor cell differentiation and proliferation factor |
| QA | quinolinic acid, quinolinate |
| QPRT | quinolinate phosphoribosyl transferase |
| SAM | S-adenosylmethionine |
| SARM1 | NAD+ hydrolase |
| SIRT1 | sirtuin 1, NAD+-dependent protein deacetylase |
| SIRT3 | sirtuin 3, NAD+-dependent protein deacetylase |
| Tau | a microtubule-associated protein (MAP) |
| pTau | phosphorylated tau, tends to aggregate in degenerating neurons |
| TDO | tryptophan 2,3-dioxygenase |
| TOR | Target of rapamycin; a nutrient sensing protein kinase |
| Urh1 | nucleosidase; Meu1, Urh1, Pnp1 are nucleosidases that convert NR to NAM |
| Wlds | Ube4b-mNMNAT1 fusion protein; suppresses Wallerian degeneration |
References
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Landry, J.; Sutton, A.; Tafrov, S.T.; Heller, R.C.; Stebbins, J.; Pillus, L.; Sternglanz, R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA 2000, 97, 5807–5811. [Google Scholar] [CrossRef]
- Smith, J.S.; Brachmann, C.B.; Celic, I.; Kenna, M.A.; Muhammad, S.; Starai, V.J.; Avalos, J.L.; Escalante-Semerena, J.C.; Grubmeyer, C.; Wolberger, C.; et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. USA 2000, 97, 6658–6663. [Google Scholar] [CrossRef]
- Kraus, W.L. PARPs and ADP-Ribosylation: 50 Years … and Counting. Mol. Cell 2015, 58, 902–910. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, E.; Augeri, S.; Fissolo, G.; Musso, I.; Funaro, A. CD157: From immunoregulatory protein to potential therapeutic target. Immunol. Lett. 2019, 205, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.J.; Collins, C.A. Multifaceted roles of SARM1 in axon degeneration and signaling. Front. Cell. Neurosci. 2022, 16, 958900. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Lautrup, S.; Hou, Y.; Fang, E.F.; Bohr, V.A. Roles of NAD+ in Health and Aging. Cold Spring Harb. Perspect. Med. 2024, 14, a041193. [Google Scholar] [CrossRef]
- Migaud, M.E.; Ziegler, M.; Baur, J.A. Regulation of and challenges in targeting NAD+ metabolism. Nat. Rev. Mol. Cell Biol. 2024, 25, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Braidy, N. Supplementation with NAD+ Precursors for Treating Alzheimer’s Disease: A Metabolic Approach. J. Alzheimers Dis. 2024, 101, S467–S477. [Google Scholar] [CrossRef] [PubMed]
- Imai, S. Nicotinamide phosphoribosyltransferase (Nampt): A link between NAD biology, metabolism, and diseases. Curr. Pharm. Des. 2009, 15, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Bheda, P.; Revollo, J.R.; Imai, S.; Wolberger, C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat. Struct. Mol. Biol. 2006, 13, 661–662. [Google Scholar] [CrossRef]
- Hasmann, M.; Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003, 63, 7436–7442. [Google Scholar]
- Wosikowski, K.; Mattern, K.; Schemainda, I.; Hasmann, M.; Rattel, B.; Löser, R. WK175, a Novel Antitumor Agent, Decreases the Intracellular Nicotinamide Adenine Dinucleotide Concentration and Induces the Apoptotic Cascade in Human Leukemia Cells. Cancer Res. 2002, 62, 1057–1062. [Google Scholar]
- Nahimana, A.; Attinger, A.; Aubry, D.; Greaney, P.; Ireson, C.; Thougaard, A.V.; Tjørnelund, J.; Dawson, K.M.; Dupuis, M.; Duchosal, M.A. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood 2009, 113, 3276–3286. [Google Scholar] [CrossRef]
- Gibson, A.E.; Yeung, C.; Issaq, S.H.; Collins, V.J.; Gouzoulis, M.; Zhang, Y.; Ji, J.; Mendoza, A.; Heske, C.M. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) with OT-82 induces DNA damage, cell death, and suppression of tumor growth in preclinical models of Ewing sarcoma. Oncogenesis 2020, 9, 80. [Google Scholar] [CrossRef]
- Sharif, T.; Ahn, D.G.; Liu, R.Z.; Pringle, E.; Martell, E.; Dai, C.; Nunokawa, A.; Kwak, M.; Clements, D.; Murphy, J.P.; et al. The NAD+ salvage pathway modulates cancer cell viability via p73. Cell Death Differ. 2016, 23, 669–680. [Google Scholar] [CrossRef]
- Aksoy, S.; Szumlanski, C.L.; Weinshilboum, R.M. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J. Biol. Chem. 1994, 269, 14835–14840. [Google Scholar] [CrossRef]
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.D.; Zhu, X.J.; Li, J.J.; Mei, Y.Z.; Li, W.S.; Li, J.H. Nicotinamide N-methyltransferase (NNMT): A novel therapeutic target for metabolic syndrome. Front. Pharmacol. 2024, 15, 1410479. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Mateuszuk, Ł.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Shi, W.T.; Zhang, J.; Zhao, W.R.; Xiao, Y.; Zhang, K.Y.; Ma, J.; Tang, J.Y.; Wang, Y. Sodium tanshinone IIA sulfonate protects against hyperhomocysteine-induced vascular endothelial injury via activation of NNMT/SIRT1-mediated NRF2/HO-1 and AKT/MAPKs signaling in human umbilical vascular endothelial cells. Biomed. Pharmacother. 2023, 158, 114137. [Google Scholar] [CrossRef]
- Watała, C.; Kaźmierczak, P.; Dobaczewski, M.; Przygodzki, T.; Bartuś, M.; Łomnicka, M.; Słomińska, E.M.; Durackova, Z.; Chłopicki, S. Anti-diabetic effects of 1-methylnicotinamide (MNA) in streptozocin-induced diabetes in rats. Pharmacol. Rep. 2009, 61, 86–98. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Tao, X.; Brazill, J.M.; Park, J.; Diaz-Perez, Z.; Zhai, R.G. Nmnat restores neuronal integrity by neutralizing mutant Huntingtin aggregate-induced progressive toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 19165–19175. [Google Scholar] [CrossRef]
- Ali, Y.O.; Ruan, K.; Zhai, R.G. NMNAT suppresses tau-induced neurodegeneration by promoting clearance of hyperphosphorylated tau oligomers in a Drosophila model of tauopathy. Hum. Mol. Genet. 2012, 21, 237–250. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Y.; Lu, J.; Xie, J.; Li, C.; Shin, W.S.; Qiang, J.; Liu, J.; Dou, S.; Xiao, Y.; et al. Nicotinamide mononucleotide adenylyltransferase uses its NAD+ substrate-binding site to chaperone phosphorylated Tau. eLife 2020, 9, e51859. [Google Scholar] [CrossRef]
- Zhai, R.G.; Zhang, F.; Hiesinger, P.R.; Cao, Y.; Haueter, C.M.; Bellen, H.J. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature 2008, 452, 887–891. [Google Scholar] [CrossRef]
- Ruan, K.; Zhu, Y.; Li, C.; Brazill, J.M.; Zhai, R.G. Alternative splicing of Drosophila Nmnat functions as a switch to enhance neuroprotection under stress. Nat. Commun. 2015, 6, 10057. [Google Scholar] [CrossRef]
- Gilley, J.; Coleman, M.P. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010, 8, e1000300. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.G.; Cao, Y.; Hiesinger, P.R.; Zhou, Y.; Mehta, S.Q.; Schulze, K.L.; Verstreken, P.; Bellen, H.J. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006, 4, e416. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.O.; Allen, H.M.; Yu, L.; Li-Kroeger, D.; Bakhshizadehmahmoudi, D.; Hatcher, A.; McCabe, C.; Xu, J.; Bjorklund, N.; Taglialatela, G.; et al. NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLoS Biol. 2016, 14, e1002472. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, M.; Ruetenik, A.; Bazylianska, V.; Nyvltova, E.; Barrientos, A. Salvage NAD+ biosynthetic pathway enzymes moonlight as molecular chaperones to protect against proteotoxicity. Hum. Mol. Genet. 2021, 30, 672–686. [Google Scholar] [CrossRef]
- Brazill, J.M.; Li, C.; Zhu, Y.; Zhai, R.G. NMNAT: It’s an NAD+ synthase… It’s a chaperone… It’s a neuroprotector. Curr. Opin. Genet. Dev. 2017, 44, 156–162. [Google Scholar] [CrossRef]
- Panozzo, C.; Nawara, M.; Suski, C.; Kucharczyk, R.; Skoneczny, M.; Bécam, A.-M.; Rytka, J.; Herbert, C.J. Aerobic and anaerobic NAD+ metabolism in Saccharomyces cerevisiae. FEBS Lett. 2002, 517, 97–102. [Google Scholar] [CrossRef]
- Preiss, J.; Handler, P. Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J. Biol. Chem. 1958, 233, 488–492. [Google Scholar] [CrossRef]
- Emanuelli, M.; Amici, A.; Carnevali, F.; Pierella, F.; Raffaelli, N.; Magni, G. Identification and characterization of a second NMN adenylyltransferase gene in Saccharomyces cerevisiae. Protein Expr. Purif. 2003, 27, 357–364. [Google Scholar] [CrossRef]
- Emanuelli, M.; Carnevali, F.; Lorenzi, M.; Raffaelli, N.; Amici, A.; Ruggieri, S.; Magni, G. Identification and characterization of YLR328W, the Saccharomyces cerevisiae structural gene encoding NMN adenylyltransferase. Expression and characterization of the recombinant enzyme. FEBS Lett. 1999, 455, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bieganowski, P.; Pace, H.C.; Brenner, C. Eukaryotic NAD+ synthetase Qns1 contains an essential, obligate intramolecular thiol glutamine amidotransferase domain related to nitrilase. J. Biol. Chem. 2003, 278, 33049–33055. [Google Scholar] [CrossRef] [PubMed]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020, 31, 564–579.e7. [Google Scholar] [CrossRef]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 2007, 129, 473–484. [Google Scholar] [CrossRef]
- Sorci, L.; Cimadamore, F.; Scotti, S.; Petrelli, R.; Cappellacci, L.; Franchetti, P.; Orsomando, G.; Magni, G. Initial-rate kinetics of human NMN-adenylyltransferases: Substrate and metal ion specificity, inhibition by products and multisubstrate analogues, and isozyme contributions to NAD+ biosynthesis. Biochemistry 2007, 46, 4912–4922. [Google Scholar] [CrossRef]
- Lau, C.; Niere, M.; Ziegler, M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front. Biosci. 2009, 14, 410–431. [Google Scholar] [CrossRef]
- Magni, G.; Amici, A.; Emanuelli, M.; Orsomando, G.; Raffaelli, N.; Ruggieri, S. Structure and function of nicotinamide mononucleotide adenylyltransferase. Curr. Med. Chem. 2004, 11, 873–885. [Google Scholar] [CrossRef]
- Garavaglia, S.; D’Angelo, I.; Emanuelli, M.; Carnevali, F.; Pierella, F.; Magni, G.; Rizzi, M. Structure of human NMN adenylyltransferase. A key nuclear enzyme for NAD homeostasis. J. Biol. Chem. 2002, 277, 8524–8530. [Google Scholar] [CrossRef]
- Zhai, R.G.; Rizzi, M.; Garavaglia, S. Nicotinamide/nicotinic acid mononucleotide adenylyltransferase, new insights into an ancient enzyme. Cell. Mol. Life Sci. 2009, 66, 2805–2818. [Google Scholar] [CrossRef]
- Saridakis, V.; Pai, E.F. Mutational, structural, and kinetic studies of the ATP-binding site of Methanobacterium thermoautotrophicum nicotinamide mononucleotide adenylyltransferase. J. Biol. Chem. 2003, 278, 34356–34363. [Google Scholar] [CrossRef]
- Yalowitz, J.A.; Xiao, S.; Biju, M.P.; Antony, A.C.; Cummings, O.W.; Deeg, M.A.; Jayaram, H.N. Characterization of human brain nicotinamide 5′-mononucleotide adenylyltransferase-2 and expression in human pancreas. Biochem. J. 2004, 377, 317–326. [Google Scholar] [CrossRef]
- Zhu, W.J.; Liu, J.; Li, W.H.; Zhao, Z.Y.; Huang, C.; Yang, J.Y.; Lee, H.C.; Zhao, Y.J. Gap junction intercellular communications regulates activation of SARM1 and protects against axonal degeneration. Cell Death Dis. 2025, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Conforti, L.; Wilbrey, A.; Morreale, G.; Janeckova, L.; Beirowski, B.; Adalbert, R.; Mazzola, F.; Di Stefano, M.; Hartley, R.; Babetto, E.; et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J. Cell Biol. 2009, 184, 491–500. [Google Scholar] [CrossRef]
- Conforti, L.; Fang, G.; Beirowski, B.; Wang, M.S.; Sorci, L.; Asress, S.; Adalbert, R.; Silva, A.; Bridge, K.; Huang, X.P.; et al. NAD+ and axon degeneration revisited: Nmnat1 cannot substitute for WldS to delay Wallerian degeneration. Cell Death Differ. 2007, 14, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.W.; Nandu, T.; Kim, J.; Challa, S.; DeBerardinis, R.J.; Kraus, W.L. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science 2018, 360, eaan5780. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Sasaki, Y.; Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004, 305, 1010–1013. [Google Scholar] [CrossRef]
- Cambronne, X.A.; Stewart, M.L.; Kim, D.; Jones-Brunette, A.M.; Morgan, R.K.; Farrens, D.L.; Cohen, M.S.; Goodman, R.H. Biosensor reveals multiple sources for mitochondrial NAD+. Science 2016, 352, 1474–1477. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, Y.; Kitano, A.; Hu, T.; Murdaugh, R.L.; Li, Y.; Hoegenauer, K.A.; Chen, R.; Takahashi, K.; Nakada, D. Nuclear NAD+ homeostasis governed by NMNAT1 prevents apoptosis of acute myeloid leukemia stem cells. Sci. Adv. 2021, 7, eabf3895. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hikosaka, K.; Mahmood, A.; Tobe, K.; Shojaku, H.; Inohara, H.; Nakagawa, T. Nmnat3 Is Dispensable in Mitochondrial NAD Level Maintenance In Vivo. PLoS ONE 2016, 11, e0147037. [Google Scholar] [CrossRef]
- Haferkamp, I.; Schmitz-Esser, S.; Linka, N.; Urbany, C.; Collingro, A.; Wagner, M.; Horn, M.; Neuhaus, H.E. A candidate NAD+ transporter in an intracellular bacterial symbiont related to Chlamydiae. Nature 2004, 432, 622–625. [Google Scholar] [CrossRef]
- Todisco, S.; Agrimi, G.; Castegna, A.; Palmieri, F. Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Rieder, B.; Ventrella, A.; Blanco, E.; Do, P.T.; Nunes-Nesi, A.; Trauth, A.U.; Fiermonte, G.; Tjaden, J.; Agrimi, G.; et al. Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. J. Biol. Chem. 2009, 284, 31249–31259. [Google Scholar] [CrossRef]
- Kory, N.; Uit de Bos, J.; van der Rijt, S.; Jankovic, N.; Gura, M.; Arp, N.; Pena, I.A.; Prakash, G.; Chan, S.H.; Kunchok, T.; et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci. Adv. 2020, 6, eabe5310. [Google Scholar] [CrossRef] [PubMed]
- Luongo, T.S.; Eller, J.M.; Lu, M.J.; Niere, M.; Raith, F.; Perry, C.; Bornstein, M.R.; Oliphint, P.; Wang, L.; McReynolds, M.R.; et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 2020, 588, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Girardi, E.; Agrimi, G.; Goldmann, U.; Fiume, G.; Lindinger, S.; Sedlyarov, V.; Srndic, I.; Gurtl, B.; Agerer, B.; Kartnig, F.; et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat. Commun. 2020, 11, 6145. [Google Scholar] [CrossRef]
- Hoyland, L.E.; VanLinden, M.R.; Niere, M.; Stromland, O.; Sharma, S.; Dietze, J.; Tolas, I.; Lucena, E.; Bifulco, E.; Sverkeli, L.J.; et al. Subcellular NAD+ pools are interconnected and buffered by mitochondrial NAD+. Nat. Metab. 2024, 6, 2319–2337. [Google Scholar] [CrossRef]
- Berger, F.; Lau, C.; Dahlmann, M.; Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005, 280, 36334–36341. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Baur, J.A.; Perez, E.; Matsui, T.; Carmona, J.J.; Lamming, D.W.; Souza-Pinto, N.C.; Bohr, V.A.; Rosenzweig, A.; et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 2007, 130, 1095–1107. [Google Scholar] [CrossRef]
- Hopp, A.K.; Teloni, F.; Bisceglie, L.; Gondrand, C.; Raith, F.; Nowak, K.; Muskalla, L.; Howald, A.; Pedrioli, P.G.A.; Johnsson, K.; et al. Mitochondrial NAD+ Controls Nuclear ARTD1-Induced ADP-Ribosylation. Mol. Cell 2021, 81, 340–354.e345. [Google Scholar] [CrossRef]
- Gerdts, J.; Summers, D.W.; Milbrandt, J.; DiAntonio, A. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 2016, 89, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Loreto, A.; Antoniou, C.; Merlini, E.; Gilley, J.; Coleman, M.P. NMN: The NAD precursor at the intersection between axon degeneration and anti-ageing therapies. Neurosci. Res. 2023, 197, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.Y.; Huang, W.P.; Liou, H.C.; Fu, W.M. Autophagy protects neuron from Abeta-induced cytotoxicity. Autophagy 2009, 5, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lobato, A.G.; Zhai, R.G.; Pinto, M. Human Nmnat1 Promotes Autophagic Clearance of Amyloid Plaques in a Drosophila Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 852972. [Google Scholar] [CrossRef]
- Rodriguez-Vargas, J.M.; Rodriguez, M.I.; Majuelos-Melguizo, J.; Garcia-Diaz, A.; Gonzalez-Flores, A.; Lopez-Rivas, A.; Virag, L.; Illuzzi, G.; Schreiber, V.; Dantzer, F.; et al. Autophagy requires poly(adp-ribosyl)ation-dependent AMPK nuclear export. Cell Death Differ. 2016, 23, 2007–2018. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; Zhang, Y.; Zhang, F.; Huang, K. PARP1 promote autophagy in cardiomyocytes via modulating FoxO3a transcription. Cell Death Dis. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. SIRT1: Regulation of longevity via autophagy. Cell. Signal. 2009, 21, 1356–1360. [Google Scholar] [CrossRef]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; Malik, S.A.; Vitale, I.; et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010, 1, e10. [Google Scholar] [CrossRef]
- Berger, F.; Lau, C.; Ziegler, M. Regulation of poly(ADP-ribose) polymerase 1 activity by the phosphorylation state of the nuclear NAD biosynthetic enzyme NMN adenylyl transferase 1. Proc. Natl. Acad. Sci. USA 2007, 104, 3765–3770. [Google Scholar] [CrossRef]
- Kim, S.; Lee, W.; Cho, K. P62 Links the Autophagy Pathway and the Ubiquitin-Proteasome System in Endothelial Cells during Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 7791. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Bjorkoy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Overvatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Munemasa, Y.; Kojima, K.; Hirano, A.; Ueno, S.; Takagi, H. Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration. Cell Death Dis. 2013, 4, e860. [Google Scholar] [CrossRef] [PubMed]
- Mehmel, M.; Jovanovic, N.; Spitz, U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Sase, K.; Tsukahara, C.; Fujita, N.; Arizono, I.; Takagi, H. Axonal Protection by Nicotinamide Riboside via SIRT1-Autophagy Pathway in TNF-Induced Optic Nerve Degeneration. Mol. Neurobiol. 2020, 57, 4952–4960. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Sase, K.; Tsukahara, C.; Fujita, N.; Tokuda, N.; Kogo, J.; Takagi, H. Axonal protection by a small molecule SIRT1 activator, SRT2104, with alteration of autophagy in TNF-induced optic nerve degeneration. Jpn. J. Ophthalmol. 2020, 64, 298–303. [Google Scholar] [CrossRef]
- Avery, M.A.; Rooney, T.M.; Pandya, J.D.; Wishart, T.M.; Gillingwater, T.H.; Geddes, J.W.; Sullivan, P.G.; Freeman, M.R. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr. Biol. 2012, 22, 596–600. [Google Scholar] [CrossRef]
- Avery, M.A.; Sheehan, A.E.; Kerr, K.S.; Wang, J.; Freeman, M.R. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J. Cell Biol. 2009, 184, 501–513. [Google Scholar] [CrossRef]
- Beirowski, B.; Babetto, E.; Gilley, J.; Mazzola, F.; Conforti, L.; Janeckova, L.; Magni, G.; Ribchester, R.R.; Coleman, M.P. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J. Neurosci. 2009, 29, 653–668. [Google Scholar] [CrossRef]
- Soto, C.; Estrada, L.D. Protein misfolding and neurodegeneration. Arch. Neurol. 2008, 65, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, J.; Chen, T.; Cai, J.; Ren, R. Protein aggregation and its affecting mechanisms in neurodegenerative diseases. Neurochem. Int. 2024, 180, 105880. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.M.; Nasser, T.H.; Demasi, M.; Nascimento, R.M.; Netto, L.E.; Miyamoto, S.; Prado, F.M.; Monteiro, G. The promoter of filamentation (POF1) protein from Saccharomyces cerevisiae is an ATPase involved in the protein quality control process. BMC Microbiol. 2011, 11, 268. [Google Scholar] [CrossRef]
- Ocampo, A.; Liu, J.; Barrientos, A. NAD+ salvage pathway proteins suppress proteotoxicity in yeast models of neurodegeneration by promoting the clearance of misfolded/oligomerized proteins. Hum. Mol. Genet. 2013, 22, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Stoothoff, W.H.; Johnson, G.V. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta 2005, 1739, 280–297. [Google Scholar] [CrossRef]
- Petrucelli, L.; Dickson, D.; Kehoe, K.; Taylor, J.; Snyder, H.; Grover, A.; De Lucia, M.; McGowan, E.; Lewis, J.; Prihar, G.; et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004, 13, 703–714. [Google Scholar] [CrossRef]
- Sahara, N.; Murayama, M.; Mizoroki, T.; Urushitani, M.; Imai, Y.; Takahashi, R.; Murata, S.; Tanaka, K.; Takashima, A. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J. Neurochem. 2005, 94, 1254–1263. [Google Scholar] [CrossRef]
- Nadel, C.M.; Pokhrel, S.; Wucherer, K.; Oehler, A.; Thwin, A.C.; Basu, K.; Callahan, M.D.; Southworth, D.R.; Mordes, D.A.; Craik, C.S.; et al. Phosphorylation of tau at a single residue inhibits binding to the E3 ubiquitin ligase, CHIP. Nat. Commun. 2024, 15, 7972. [Google Scholar] [CrossRef]
- Stein, L.R.; Imai, S.I. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Raff, M.C.; Whitmore, A.V.; Finn, J.T. Axonal self-destruction and neurodegeneration. Science 2002, 296, 868–871. [Google Scholar] [CrossRef]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef]
- Lunn, E.R.; Perry, V.H.; Brown, M.C.; Rosen, H.; Gordon, S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur. J. Neurosci. 1989, 1, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Lunn, E.R.; Brown, M.C.; Cahusac, S.; Gordon, S. Evidence that the Rate of Wallerian Degeneration is Controlled by a Single Autosomal Dominant Gene. Eur. J. Neurosci. 1990, 2, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Brown, M.C.; Lunn, E.R. Very Slow Retrograde and Wallerian Degeneration in the CNS of C57BL/Ola Mice. Eur. J. Neurosci. 1991, 3, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Mack, T.G.; Reiner, M.; Beirowski, B.; Mi, W.; Emanuelli, M.; Wagner, D.; Thomson, D.; Gillingwater, T.; Court, F.; Conforti, L.; et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 2001, 4, 1199–1206. [Google Scholar] [CrossRef]
- Yahata, N.; Yuasa, S.; Araki, T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 2009, 29, 6276–6284. [Google Scholar] [CrossRef]
- George, E.B.; Glass, J.D.; Griffin, J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 1995, 15, 6445–6452. [Google Scholar] [CrossRef]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Zundorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Ludhiadch, A.; Sharma, R.; Muriki, A.; Munshi, A. Role of Calcium Homeostasis in Ischemic Stroke: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 52–61. [Google Scholar] [CrossRef]
- Rahi, V.; Kaundal, R.K. Exploring the intricacies of calcium dysregulation in ischemic stroke: Insights into neuronal cell death and therapeutic strategies. Life Sci. 2024, 347, 122651. [Google Scholar] [CrossRef]
- Metwally, E.; Zhao, G.; Zhang, Y.Q. The calcium-dependent protease calpain in neuronal remodeling and neurodegeneration. Trends Neurosci. 2021, 44, 741–752. [Google Scholar] [CrossRef]
- Budd, S.L.; Nicholls, D.G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J. Neurochem. 1996, 66, 403–411. [Google Scholar] [CrossRef]
- Dridi, H.; Santulli, G.; Bahlouli, L.; Miotto, M.C.; Weninger, G.; Marks, A.R. Mitochondrial Calcium Overload Plays a Causal Role in Oxidative Stress in the Failing Heart. Biomolecules 2023, 13, 1409. [Google Scholar] [CrossRef]
- Verghese, P.B.; Sasaki, Y.; Yang, D.; Stewart, F.; Sabar, F.; Finn, M.B.; Wroge, C.M.; Mennerick, S.; Neil, J.J.; Milbrandt, J.; et al. Nicotinamide mononucleotide adenylyl transferase 1 protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death. Proc. Natl. Acad. Sci. USA 2011, 108, 19054–19059. [Google Scholar] [CrossRef]
- Galindo, R.; Banks Greenberg, M.; Araki, T.; Sasaki, Y.; Mehta, N.; Milbrandt, J.; Holtzman, D.M. NMNAT3 is protective against the effects of neonatal cerebral hypoxia-ischemia. Ann. Clin. Transl. Neurol. 2017, 4, 722–738. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Peng, W.; Wang, L.; Zhang, J.; Dong, W.; Tian, X.; Ye, C.; Li, Y.; Gong, Y. Overexpression of NMNAT3 improves mitochondrial function and enhances antioxidative stress capacity of bone marrow mesenchymal stem cells via the NAD+-Sirt3 pathway. Biosci. Rep. 2022, 42, BSR20211005. [Google Scholar] [CrossRef]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef]
- Cheng, H.; Perkins, G.A.; Ju, S.; Kim, K.; Ellisman, M.H.; Pamenter, M.E. Enhanced mitochondrial buffering prevents Ca2+ overload in naked mole-rat brain. J. Physiol. 2024, 602, 5685–5698. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, M.C.; Ali, Y.O.; Zhu, J.; Wu, C.S.; Oka, K.; Zhai, R.G.; Lu, H.C. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum. Mol. Genet. 2012, 21, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.O.; Bradley, G.; Lu, H.C. Screening with an NMNAT2-MSD platform identifies small molecules that modulate NMNAT2 levels in cortical neurons. Sci. Rep. 2017, 7, 43846. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Z.; Ahn, D.G.; Sharif, T.; Clements, D.; Gujar, S.A.; Lee, P.W. The NAD+ synthesizing enzyme nicotinamide mononucleotide adenylyltransferase 2 (NMNAT-2) is a p53 downstream target. Cell Cycle 2014, 13, 1041–1048. [Google Scholar] [CrossRef][Green Version]
- Xiong, X.; Hao, Y.; Sun, K.; Li, J.; Li, X.; Mishra, B.; Soppina, P.; Wu, C.; Hume, R.I.; Collins, C.A. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012, 10, e1001440. [Google Scholar] [CrossRef]
- Babetto, E.; Beirowski, B.; Russler, E.V.; Milbrandt, J.; DiAntonio, A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 2013, 3, 1422–1429. [Google Scholar] [CrossRef]
- Walker, L.J.; Summers, D.W.; Sasaki, Y.; Brace, E.J.; Milbrandt, J.; DiAntonio, A. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. eLife 2017, 6, e22540. [Google Scholar] [CrossRef]
- Lau, C.; Dolle, C.; Gossmann, T.I.; Agledal, L.; Niere, M.; Ziegler, M. Isoform-specific targeting and interaction domains in human nicotinamide mononucleotide adenylyltransferases. J. Biol. Chem. 2010, 285, 18868–18876. [Google Scholar] [CrossRef]
- Milde, S.; Gilley, J.; Coleman, M.P. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol. 2013, 11, e1001539. [Google Scholar] [CrossRef] [PubMed]
- Milde, S.; Fox, A.N.; Freeman, M.R.; Coleman, M.P. Deletions within its subcellular targeting domain enhance the axon protective capacity of Nmnat2 in vivo. Sci. Rep. 2013, 3, 2567. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.O.; McCormack, R.; Darr, A.; Zhai, R.G. Nicotinamide mononucleotide adenylyltransferase is a stress response protein regulated by the heat shock factor/hypoxia-inducible factor 1alpha pathway. J. Biol. Chem. 2011, 286, 19089–19099. [Google Scholar] [CrossRef] [PubMed]
- Bruder, E.D.; Lee, J.J.; Widmaier, E.P.; Raff, H. Microarray and real-time PCR analysis of adrenal gland gene expression in the 7-day-old rat: Effects of hypoxia from birth. Physiol. Genom. 2007, 29, 193–200. [Google Scholar] [CrossRef]
- Laifenfeld, D.; Gilchrist, A.; Drubin, D.; Jorge, M.; Eddy, S.F.; Frushour, B.P.; Ladd, B.; Obert, L.A.; Gosink, M.M.; Cook, J.C.; et al. The role of hypoxia in 2-butoxyethanol-induced hemangiosarcoma. Toxicol. Sci. 2010, 113, 254–266. [Google Scholar] [CrossRef]
- Paiva, B.S.; Neves, D.; Tomé, D.; Costa, F.J.; Bruno, I.C.; Trigo, D.; Silva, R.M.; Almeida, R.D. Neuroprotection by Mitochondrial NAD Against Glutamate-Induced Excitotoxicity. Cells 2025, 14, 582. [Google Scholar] [CrossRef]
- Tsang, F.; Lin, S.-J. Less is more: Nutrient limitation induces cross-talk of nutrient sensing pathways with NAD+ homeostasis and contributes to longevity. Front. Biol. 2015, 10, 333–357. [Google Scholar] [CrossRef][Green Version]
- James Theoga Raj, C.; Lin, S.J. Cross-talk in NAD+ metabolism: Insights from Saccharomyces cerevisiae. Curr. Genet. 2019, 65, 1113–1119. [Google Scholar] [CrossRef]
- Croft, T.; Venkatakrishnan, P.; Lin, S.J. NAD+ Metabolism and Regulation: Lessons From Yeast. Biomolecules 2020, 10, 330. [Google Scholar] [CrossRef]
- Lu, S.P.; Lin, S.J. Phosphate-responsive signaling pathway is a novel component of NAD+ metabolism in Saccharomyces cerevisiae. J. Biol. Chem. 2011, 286, 14271–14281. [Google Scholar] [CrossRef]
- James Theoga Raj, C.; Croft, T.; Venkatakrishnan, P.; Groth, B.; Dhugga, G.; Cater, T.; Lin, S.J. The copper-sensing transcription factor Mac1, the histone deacetylase Hst1, and nicotinic acid regulate de novo NAD+ biosynthesis in budding yeast. J. Biol. Chem. 2019, 294, 5562–5575. [Google Scholar] [CrossRef]
- Croft, T.; Raj, C.J.T.; Salemi, M.; Phinney, B.S.; Lin, S.-J. A functional link between NAD+ homeostasis and N-terminal protein acetylation in Saccharomyces cerevisiae. J. Biol. Chem. 2018, 293, 2927–2938. [Google Scholar] [CrossRef]
- Croft, T.; Venkatakrishnan, P.; James Theoga Raj, C.; Groth, B.; Cater, T.; Salemi, M.R.; Phinney, B.; Lin, S.J. N-terminal protein acetylation by NatB modulates the levels of Nmnats, the NAD+ biosynthetic enzymes in Saccharomyces cerevisiae. J. Biol. Chem. 2020, 295, 7362–7375. [Google Scholar] [CrossRef]
- Arnesen, T.; Van Damme, P.; Polevoda, B.; Helsens, K.; Evjenth, R.; Colaert, N.; Varhaug, J.E.; Vandekerckhove, J.; Lillehaug, J.R.; Sherman, F.; et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8157–8162. [Google Scholar] [CrossRef]
- Gottlieb, L.; Marmorstein, R. Biochemical and structural analysis of N-terminal acetyltransferases. Methods Enzymol. 2019, 626, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhong, Y.; Zheng, R.; Wu, Q.; Liu, Y.; Zhang, D.; Wang, Y.; Ding, W.; Wang, K.; Zhong, F.; et al. PPDPF preserves integrity of proximal tubule by modulating NMNAT activity in chronic kidney diseases. Sci. Adv. 2025, 11, eadr8648. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, N.; Sharma, S.; Kumar, R. Neuroprotective insights into epigallocatechin gallate (EGCG) for neurodegenerative disorders. Explor. Neurosci. 2025, 4, 100673. [Google Scholar] [CrossRef]
- Wolfram, S. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 26, 373s–388s. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, S.S.; He, Y.; Bi, X.Y.; Gao, S.; Yan, T.D.; Zheng, G.D.; Chen, T.T.; Ye, J.T.; Liu, P.Q. EGCG inhibits pressure overload-induced cardiac hypertrophy via the PSMB5/Nmnat2/SIRT6-dependent signalling pathways. Acta Physiol. 2021, 231, e13602. [Google Scholar] [CrossRef]
- Tribble, J.R.; Joe, M.; Varricchio, C.; Otmani, A.; Canovai, A.; Habchi, B.; Daskalakis, E.; Chaleckis, R.; Loreto, A.; Gilley, J.; et al. NMNAT2 is a druggable target to drive neuronal NAD production. Nat. Commun. 2024, 15, 6256. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.J.; Hong, J.; Li, C.; Smith, T.J.; Yang, G.Y.; Seril, D.N.; Yang, C.S. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer 2000, 37, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Fang, F.; Zhuang, P.; Feng, X.; Liu, P.; Liu, D.; Huang, H.; Li, L.; Chen, W.; Liu, L.; Sun, Y.; et al. NMNAT2 is downregulated in glaucomatous RGCs, and RGC-specific gene therapy rescues neurodegeneration and visual function. Mol. Ther. 2022, 30, 1421–1431. [Google Scholar] [CrossRef]
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Müller, C.E.; Buée, L.; et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging 2014, 35, 2079–2090. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Barrow, C.A.; Davis, A.J.; Mumby, M.C. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl. Acad. Sci. USA 2002, 99, 4221–4226. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Feng, C.; Alkon, D.L. Impairment of phosphatase 2A contributes to the prolonged MAP kinase phosphorylation in Alzheimer’s disease fibroblasts. Neurobiol. Dis. 2003, 14, 458–469. [Google Scholar] [CrossRef]
- Dennie, T.W.; Kolesar, J.M. Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma. Clin. Ther. 2009, 31 Pt 2, 2290–2311. [Google Scholar] [CrossRef]
- Hunsu, V.O.; Facey, C.O.B.; Fields, J.Z.; Boman, B.M. Retinoids as Chemo-Preventive and Molecular-Targeted Anti-Cancer Therapies. Int. J. Mol. Sci. 2021, 22, 7731. [Google Scholar] [CrossRef]
- Danciu, O.C.; Holdhoff, M.; Peterson, R.A.; Fischer, J.H.; Liu, L.C.; Wang, H.; Venepalli, N.K.; Chowdhery, R.; Nicholas, M.K.; Russell, M.J.; et al. Phase I study of procaspase-activating compound-1 (PAC-1) in the treatment of advanced malignancies. Br. J. Cancer 2023, 128, 783–792. [Google Scholar] [CrossRef]
- Li, W.; Gao, M.; Hu, C.; Chen, X.; Zhou, Y. NMNAT2: An important metabolic enzyme affecting the disease progression. Biomed. Pharmacother. 2023, 158, 114143. [Google Scholar] [CrossRef] [PubMed]
- Haubrich, B.A.; Ramesha, C.; Swinney, D.C. Development of a Bioluminescent High-Throughput Screening Assay for Nicotinamide Mononucleotide Adenylyltransferase (NMNAT). SLAS Discov. 2020, 25, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, F.; Ferrati, M.; Spinozzi, E.; Piergentili, A.; Del Bello, F.; Giorgioni, G.; Sorci, L.; Petrelli, R.; Cappellacci, L. Synthesis, Biological, and Computational Evaluations of Conformationally Restricted NAD-Mimics as Discriminant Inhibitors of Human NMN-Adenylyltransferase Isozymes. Pharmaceuticals 2024, 17, 739. [Google Scholar] [CrossRef]
- Buonvicino, D.; Mazzola, F.; Zamporlini, F.; Resta, F.; Ranieri, G.; Camaioni, E.; Muzzi, M.; Zecchi, R.; Pieraccini, G.; Dölle, C.; et al. Identification of the Nicotinamide Salvage Pathway as a New Toxification Route for Antimetabolites. Cell Chem. Biol. 2018, 25, 471–482.e477. [Google Scholar] [CrossRef]
- Sampath, D.; Zabka, T.S.; Misner, D.L.; O’Brien, T.; Dragovich, P.S. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol. Ther. 2015, 151, 16–31. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Lin, S.-J. The Roles of Moonlighting Nicotinamide Mononucleotide Adenylyl Transferases in Cell Physiology. Int. J. Mol. Sci. 2025, 26, 9098. https://doi.org/10.3390/ijms26189098
Lee Y-C, Lin S-J. The Roles of Moonlighting Nicotinamide Mononucleotide Adenylyl Transferases in Cell Physiology. International Journal of Molecular Sciences. 2025; 26(18):9098. https://doi.org/10.3390/ijms26189098
Chicago/Turabian StyleLee, Yi-Ching, and Su-Ju Lin. 2025. "The Roles of Moonlighting Nicotinamide Mononucleotide Adenylyl Transferases in Cell Physiology" International Journal of Molecular Sciences 26, no. 18: 9098. https://doi.org/10.3390/ijms26189098
APA StyleLee, Y.-C., & Lin, S.-J. (2025). The Roles of Moonlighting Nicotinamide Mononucleotide Adenylyl Transferases in Cell Physiology. International Journal of Molecular Sciences, 26(18), 9098. https://doi.org/10.3390/ijms26189098




