The Activity of Protectin DX, 17 HDHA and Leukotriene B4 Is Correlated with Interleukin-1β (IL-1β) and Interleukin-1 Receptor Antagonist (IL-1Ra) in the Early Subacute Phase of Stroke
Abstract
1. Introduction
2. Results
- -
- PC1 (23.4% variance)—eicosanoid metabolic network, top loadings: 15S HETE (0.377), Maresin 1 (0.353), 13S HODE (0.328), 5 HETE (0.322).
- -
- PC2 (12.5% variance) lipid-inflammatory axis—non-HDL (0.559), LDL (0.468), TG (0.435), CRP (0.356).
- -
- PC3 (9.4% variance)—IL-1β resolution axis: IL-1β (0.549), Protectin DX (0.426), Resolvin E1 (0.349).
- -
- PC1 (23.5% variance)—eicosanoid metabolic network, top loadings: 15S HETE (0.378), Maresin 1 (0.354), 13S HODE (0.328), 5 HETE (0.32).
- -
- PC2 (12.5% variance) lipid-inflammatory axis—non-HDL (0.559), LDL (0.467), TG (0.435), CRP (0.355).
- -
- PC3 (8.8% variance)—IL1-Ra primary axis, top loadings: IL1-Ra (0.546), Protectin DX (−0.528), Resolvin E1 (−0.282).
3. Discussion
4. Material and Methods
4.1. Subjects
4.2. Eicosanoids Analysis
4.3. Interleukin-1 Beta and Interleukin-1 Receptor Antagonist Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joynt Maddox, K.E.; Elkind, M.S.V.; Aparicio, H.J.; Commodore-Mensah, Y.; de Ferranti, S.D.; Dowd, W.N.; Hernandez, A.F.; Khavjou, O.; Michos, E.D.; Palaniappan, L.; et al. Forecasting the Burden of Cardiovascular Disease and Stroke in the United States through 2050—Prevalence of Risk Factors and Disease: A Presidential Advisory from the American Heart Association. Circulation 2024, 150, e65–e88. [Google Scholar] [CrossRef] [PubMed]
- Kotlęga, D.; Ciećwież, S.; Turowska-Kowalska, J.; Nowacki, P. Justification of Statin Use in Ischaemic Stroke Prevention According to Inflammatory Theory in Development of Atherosclerosis. Neurol. Neurochir. Pol. 2012, 46, 176–183. [Google Scholar] [CrossRef]
- Catană, M.G.; Popențiu, I.A.; Văleanu, M.; Roman-Filip, C.; Mihăilă, R.G. IL-1 Beta—A Biomarker for Ischemic Stroke Prognosis and Atherosclerotic Lesions of the Internal Carotid Artery. Medicina 2023, 59, 1790. [Google Scholar] [CrossRef]
- Tułowiecka, N.; Kotlęga, D.; Prowans, P.; Szczuko, M. The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7628. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Matys, P.; Mirończuk, A.; Starosz, A.; Grubczak, K.; Kochanowicz, J.; Kułakowska, A.; Kapica-Topczewska, K. Expanding Role of Interleukin-1 Family Cytokines in Acute Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 10515. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Sobowale, O.A.; Parry-Jones, A.R.; Smith, C.J.; Tyrrell, P.J.; Rothwell, N.J.; Allan, S.M. Interleukin-1 in Stroke: From Bench to Bedside. Stroke 2016, 47, 2160–2167. [Google Scholar] [CrossRef]
- Denes, A.; Pinteaux, E.; Rothwell, N.J.; Allan, S.M. Interleukin-1 and Stroke: Biomarker, Harbinger of Damage, and Therapeutic Target. Cerebrovasc. Dis. 2011, 32, 517–527. [Google Scholar] [CrossRef]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The Role of Interleukin-1β in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef]
- Joung, K.H.; Kim, J.M.; Choung, S.; Lee, J.H.; Kim, H.J.; Ku, B.J. Association Between IL-1β and Cardiovascular Disease Risk in Patients with Newly Diagnosed, Drug-Naive Type 2 Diabetes Mellitus: A Cross-Sectional Study. Ann. Transl. Med. 2020, 8, 5. [Google Scholar] [CrossRef]
- Ridker, P.M. Interleukin-1 Inhibition and Ischaemic Stroke: Has the Time for a Major Outcomes Trial Arrived? Eur. Heart J. 2018, 39, 3518–3520. [Google Scholar] [CrossRef]
- Dinarello, C.A.; van der Meer, J.W.M. Treating Inflammation by Blocking Interleukin-1 in Humans. Semin. Immunol. 2013, 25, 469–484. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Kazmi, S.; Salehi-Pourmehr, H.; Sadigh-Eteghad, S.; Farhoudi, M. The Efficacy and Safety of Interleukin-1 Receptor Antagonist in Stroke Patients: A Systematic Review. J. Clin. Neurosci. 2024, 120, 120–128. [Google Scholar] [CrossRef]
- Abbate, A.; Kontos, M.C.; Abouzaki, N.A.; Melchior, R.D.; Thomas, C.; Van Tassell, B.W.; Oddi, C.; Carbone, S.; Trankle, C.R.; Roberts, C.S.; et al. Comparative Safety of Interleukin-1 Blockade with Anakinra in Patients with ST-Segment Elevation Acute Myocardial Infarction. Am. J. Cardiol. 2015, 115, 288–292. [Google Scholar] [CrossRef]
- Sheppe, A.E.F.; Edelmann, M.J. Roles of Eicosanoids in Regulating Inflammation and Neutrophil Migration as an Innate Host Response to Bacterial Infections. Infect. Immun. 2021, 89, e00095-21. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- Barden, A.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Short-Term N-3 Fatty Acid Supplementation but Not Aspirin Increases Plasma Proresolving Mediators of Inflammation. J. Lipid Res. 2014, 55, 2401–2407. [Google Scholar] [CrossRef]
- Pizzini, A.; Lunger, L.; Sonnweber, T.; Weiss, G.; Tancevski, I. The Role of Omega-3 Fatty Acids in the Setting of Coronary Artery Disease and COPD: A Review. Nutrients 2018, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Au Yeung, S.L.; Schooling, C.M. Associations of Arachidonic Acid Synthesis with Cardiovascular Risk Factors and Relation to Ischemic Heart Disease and Stroke: A Univariable and Multivariable Mendelian Randomization Study. Nutrients 2021, 13, 1489. [Google Scholar] [CrossRef]
- Valdes, A.M.; Ravipati, S.; Menni, C.; Abhishek, A.; Metrustry, S.; Harris, J.; Nessa, A.; Williams, F.M.K.; Spector, T.D.; Doherty, M.; et al. Association of the Resolvin Precursor 17-HDHA, but Not D- or E-Series Resolvins, with Heat Pain Sensitivity and Osteoarthritis Pain in Humans. Sci. Rep. 2017, 7, 10748. [Google Scholar] [CrossRef]
- Ramon, S.; Baker, S.F.; Sahler, J.M.; Kim, N.; Feldsott, E.A.; Serhan, C.N.; Topham, D.J.; Phipps, R.P. The Specialized Proresolving Mediator 17-HDHA Enhances the Antibody-Mediated Immune Response Against Influenza Virus: A New Class of Adjuvant? J. Immunol. 2014, 193, 6031–6040. [Google Scholar] [CrossRef]
- Neuhofer, A.; Zeyda, M.; Mascher, D.; Itariu, B.K.; Murano, I.; Leitner, L.; Hochbrugger, E.E.; Fraisl, P.; Cinti, S.; Strunk, D.; et al. Impaired Local Production of Proresolving Lipid Mediators in Obesity and 17-HDHA as a Potential Treatment for Obesity-Associated Inflammation. Diabetes 2013, 62, 1945–1956. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Nowacki, P.; Szczuko, U.; Szylińska, A. Lipoxins, RevD1 and 9,13 HODE as the Most Important Derivatives after an Early Incident of Ischemic Stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.F.; Claudino, R.F.; Dutra, R.C.; Marcon, R.; Calixto, J.B. Omega-3 Fatty Acid-Derived Mediators 17(R)-Hydroxy Docosahexaenoic Acid, Aspirin-Triggered Resolvin D1 and Resolvin D2 Prevent Experimental Colitis in Mice. J. Immunol. 2011, 187, 1957–1969. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and Protectin D1 Activate Inflammation-Resolution Programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Perazza, L.R.; Gower, A.C.; Brown-Borg, H.M.; Pajevic, P.D.; Thompson, L.V. Protectin DX as a Therapeutic Strategy Against Frailty in Mice. Geroscience 2023, 45, 2601–2627. [Google Scholar] [CrossRef]
- Piao, A.; Du, W.; Wei, Y.; Yang, Y.; Feng, X.; Bai, L. Protectin DX Attenuates IL-1β-Induced Inflammation via the AMPK/NF-κB Pathway in Chondrocytes and Ameliorates Osteoarthritis Progression in a Rat Model. Int. Immunopharmacol. 2020, 78, 106043. [Google Scholar] [CrossRef]
- Shin, K.-C.; Lee, T.-E.; Kim, S.-E.; Ko, Y.-J.; Seo, M.-J.; Oh, D.-K. Enzymatic Formation of Protectin Dx and Its Production by Whole-Cell Reaction Using Recombinant Lipoxygenases. Catalysts 2022, 12, 1145. [Google Scholar] [CrossRef]
- Hansen, T.V.; Serhan, C.N. Protectins: Their Biosynthesis, Metabolism and Structure–Functions. Biochem. Pharmacol. 2022, 206, 115330. [Google Scholar] [CrossRef]
- Le Bel, M.; Brunet, A.; Gosselin, J. Leukotriene B4, an Endogenous Stimulator of the Innate Immune Response Against Pathogens. J. Innate Immun. 2014, 6, 159–168. [Google Scholar] [CrossRef]
- Chan, S.J.; Ng, M.P.E.; Zhao, H.; Ng, G.J.L.; De Foo, C.; Wong, P.T.-H.; Seet, R.C.S. Early and Sustained Increases in Leukotriene B4 Levels Are Associated with Poor Clinical Outcome in Ischemic Stroke Patients. Neurotherapeutics 2020, 17, 282–293. [Google Scholar] [CrossRef]
- Bevan, S.; Dichgans, M.; Wiechmann, H.E.; Gschwendtner, A.; Meitinger, T.; Markus, H.S. Genetic Variation in Members of the Leukotriene Biosynthesis Pathway Confers an Increased Risk of Ischemic Stroke: A Replication Study in Two Independent Populations. Stroke 2008, 39, 1109–1114. [Google Scholar] [CrossRef]
- Conti, P.; Panara, M.R.; Barbacane, R.C.; Placido, F.C.; Bongrazio, M.; Reale, M.; Dempsey, R.A.; Fiore, S. Blocking the Interleukin-1 Receptor Inhibits Leukotriene B4 and Prostaglandin E2 Generation in Human Monocyte Cultures. Cell. Immunol. 1992, 145, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.W.; Park, D.; Kim, J.H. Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1β Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation. Biomedicines 2021, 9, 535. [Google Scholar] [CrossRef]
- Grant, G.E.; Gravel, S.; Guay, J.; Patel, P.; Mazer, B.D.; Rokach, J.; Powell, W.S. 5-Oxo-ETE Is a Major Oxidative Stress-Induced Arachidonate Metabolite in B Lymphocytes. Free Radic. Biol. Med. 2011, 50, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Yuan, G.; Shen, L.; Zhang, L.; Fu, F.; Liu, Z.; Zhang, Y.; Kou, J.; Liu, S.; Yu, B.; et al. Oxoeicosanoid Receptor Inhibition Alleviates Acute Myocardial Infarction Through Activation of BCAT1. Basic Res. Cardiol. 2021, 116, 3. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.; Moore, K.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-Mediated Induction of COX-2 in the CNS Contributes to Inflammatory Pain Hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef]
- Di Mari, J.F.; Saada, J.I.; Mifflin, R.C.; Valentich, J.D.; Powell, D.W. HETEs Enhance IL-1-Mediated COX-2 Expression via Augmentation of Message Stability in Human Colonic Myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G719–G728. [Google Scholar] [CrossRef]
- Lee, Y.A.; Choi, H.; Lee, S.H.; Lee, Y.S.; Song, Y.W. Synergy Between Adiponectin and Interleukin-1β on the Expression of Interleukin-6, Interleukin-8, and Cyclooxygenase-2 in Fibroblast-Like Synoviocytes. Exp. Mol. Med. 2012, 44, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, J.C.; DeMars, K.M.; Ahmad, A.S.; Hawkins, K.E.; Yang, C.; Leclerc, J.L.; Doré, S.; Candelario-Jalil, E. Detrimental role of the EP1 prostanoid receptor in blood–brain barrier damage following experimental ischemic stroke. Sci. Rep. 2015, 5, 17956. [Google Scholar] [CrossRef]
- Ahmad, M.; Graham, S.H. Inflammation After Stroke: Mechanisms and Therapeutic Approaches. Transl. Stroke Res. 2010, 1, 74–84. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Ortiz, S.; Molina-Holgado, F.; Guaza, C. Induction of COX-2 and PGE2 Biosynthesis by IL-1β Is Mediated by PKC and Mitogen-Activated Protein Kinases in Murine Astrocytes. Br. J. Pharmacol. 2000, 131, 152–159. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Song, S.W.; Min, Y.; Zhong, Y.; Sheng, Y.C.; Li, R.P.; Liu, Q.H. The Effects of Anakinra on Focal Cerebral Ischemic Injury in Rats. CNS Neurosci. Ther. 2014, 20, 879–881. [Google Scholar] [CrossRef]
- Parry-Jones, A.R.; Stocking, K.; MacLeod, M.J.; Clarke, B.; Werring, D.J.; Muir, K.W.; Vail, A. Phase II Randomized, Placebo-Controlled, Clinical Trial of Interleukin-1 Receptor Antagonist in Intracerebral Hemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH). Eur. Stroke J. 2023, 8, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Cliteur, M.P.; van der Kolk, A.G.; Hannink, G.; Hofmeijer, J.; Jolink, W.; Klijn, C.; Schreuder, F. Anakinra in Cerebral Hemorrhage to Target Secondary Injury Resulting from Neuroinflammation (ACTION): Study Protocol of a Phase II Randomized Clinical Trial. Eur. Stroke J. 2024, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Chicheportiche, R.; Giri, J.G.; Dayer, J.M. The Inhibitory Activity of Human Interleukin-1 Receptor Antagonist Is Enhanced by Type II Interleukin-1 Soluble Receptor and Hindered by Type I Interleukin-1 Soluble Receptor. J. Clin. Investig. 1995, 96, 38–41. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of stroke subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| Age (years) | 60.38 ± 11.69 |
| Sex (Male) | 33 (44.6%) |
| BMI (kg/m2) | 28.75 ± 5.03 |
| Hypertension | 60 (82.2%) |
| Diabetes or IFG | 34 (46.6%) |
| Current smoking | 26 (35.6%) |
| Dyslipidemia | 41 (56.2%) |
| Ischaemic heart disease | 8 (11%) |
| Previous stroke | 5 (6.8%) |
| CRP (mg/L) | 2.79 ± 3.93 |
| Total Cholesterol (mg/dL) | 195.82 ± 52.86 |
| LDL (mg/dL) | 114.36 ± 44.83 |
| HDL (mg/dL) | 52.16 ± 15.23 |
| Non-HDL (mg/dL) | 143.06 ± 50.56 |
| Triglycerides (mg/dL) | 154.6 ± 75.78 |
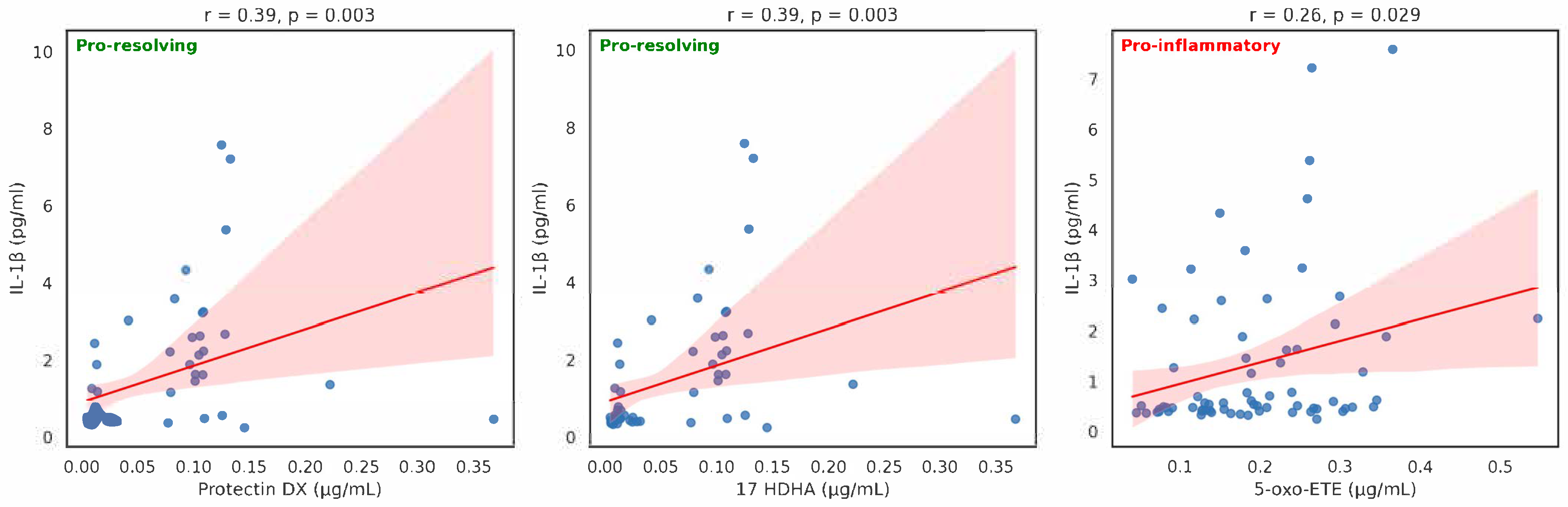
| Eicosanoid (µg/mL) | Mean | n | SD | Spearman Correlation | p-Value |
|---|---|---|---|---|---|
| Resolvin E1 | 0.059114 | 73 | 0.091654 | 0.153809 | 0.197064 |
| Prostaglandin E2 | 3.39386 | 72 | 4.261734 | 0.229074 | 0.054658 |
| Resolvin D1 | 0.173401 | 72 | 0.255647 | 0.131424 | 0.274619 |
| LTXA4 5S, 6R, 15R (Lipoxin A4 5S, 6R, 15R) | 0.06695 | 25 | 0.046868 | 0.302867 | 0.141122 |
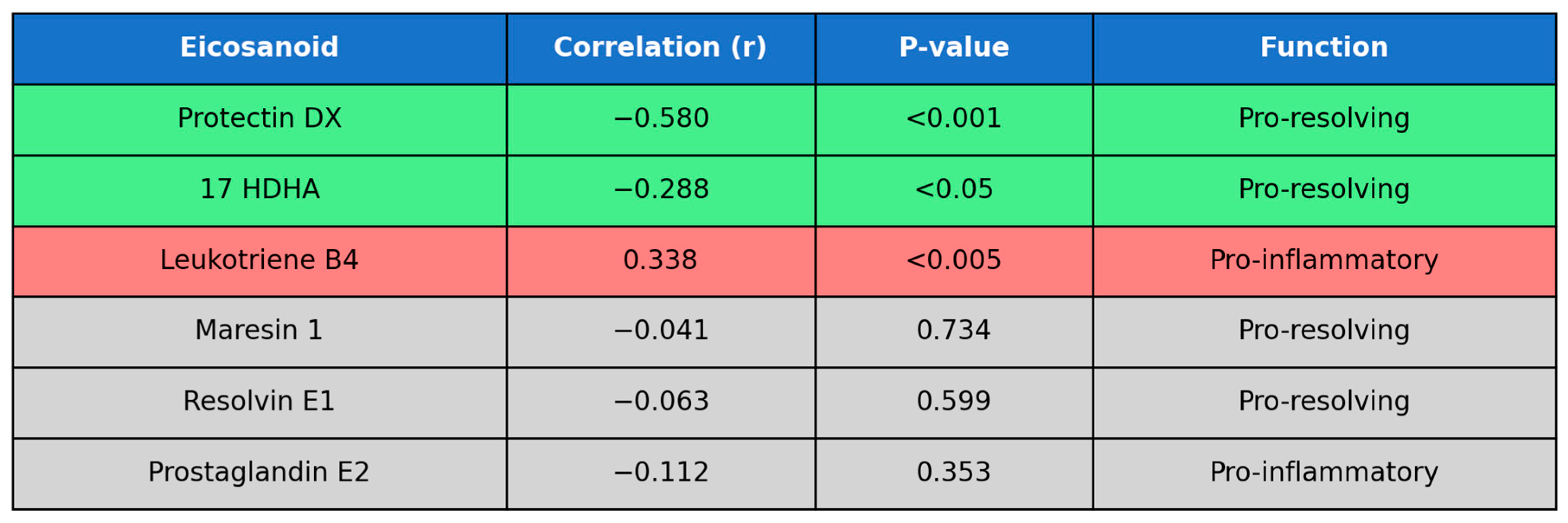
| 10S17R DiHDHA (protectin DX) | 0.059298 | 58 | 0.06683 | 0.559357 | <0.001 |
| Maresin 1 | 0.031977 | 71 | 0.014459 | 0.114281 | 0.346182 |
| Leukotriene B4 | 0.027423 | 71 | 0.012905 | −0.21579 | 0.072793 |
| 18RS HEPE | 0.108684 | 73 | 0.037517 | 0.038849 | 0.744184 |
| 13S HODE | 0.033289 | 73 | 0.029106 | −0.03504 | 0.770133 |
| 9S HODE | 0.03399 | 73 | 0.027604 | −0.06963 | 0.561119 |
| 15S HETE | 0.295675 | 73 | 0.204213 | 0.016145 | 0.892926 |
| 17 HDHA | 0.122553 | 73 | 0.085322 | 0.257562 | <0.05 |
| 12S HETE | 1.783897 | 73 | 1.137498 | −0.00318 | 0.97871 |
| 5-oxo-ETE | 0.193363 | 71 | 0.095486 | 0.266243 | <0.05 |
| 5 HETE | 0.025014 | 73 | 0.012951 | 0.092138 | 0.438165 |
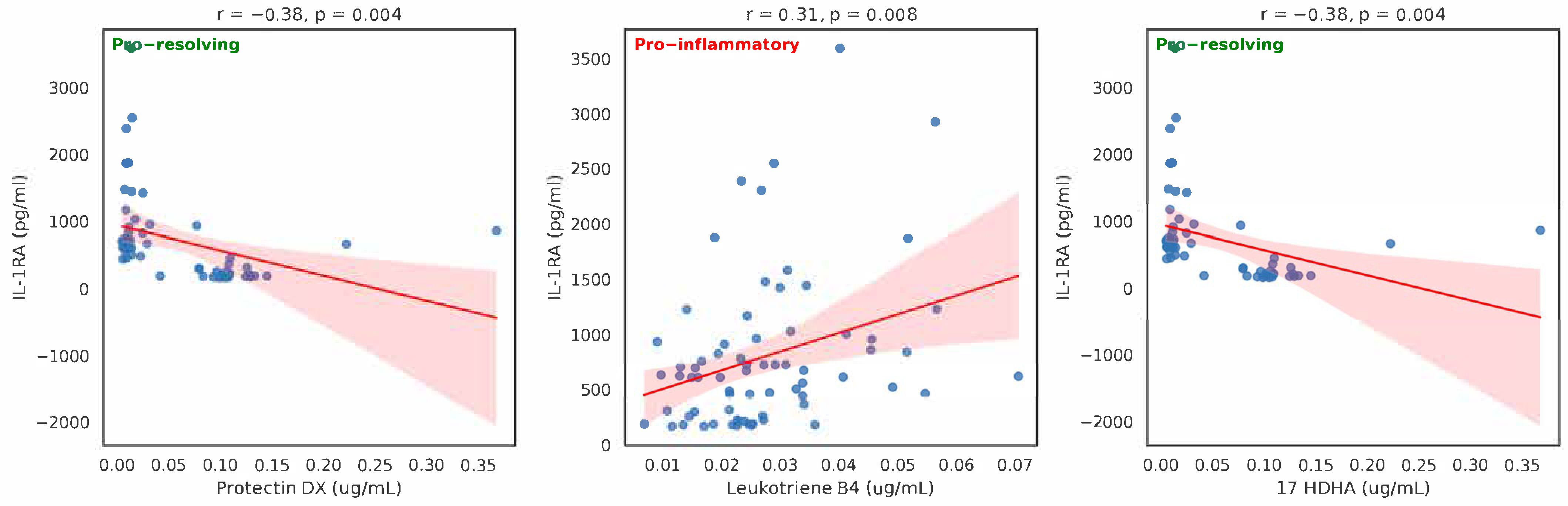
| Eicosanoid (µg/mL) | Mean | n | SD | Spearman Correlation | p-Value |
|---|---|---|---|---|---|
| Resolvin E1 | 0.059114 | 73 | 0.091654 | −0.06302 | 0.598982 |
| Prostaglandin E2 | 3.39386 | 72 | 4.261734 | −0.11198 | 0.352504 |
| Resolvin D1 | 0.173401 | 72 | 0.255647 | −0.0605 | 0.61623 |
| LTXA4 5S, 6R, 15R (Lipoxin A4 5S, 6R, 15R) | 0.06695 | 25 | 0.046868 | −0.12118 | 0.56394 |
| 10S17R DiHDHA (protectin DX) | 0.059298 | 58 | 0.06683 | −0.57978 | <0.001 |
| Maresin 1 | 0.031977 | 71 | 0.014459 | −0.04142 | 0.733536 |
| Leukotriene B4 | 0.027423 | 71 | 0.012905 | 0.338177 | <0.005 |
| 18RS HEPE | 0.108684 | 73 | 0.037517 | 0.104043 | 0.381041 |
| 13S HODE | 0.033289 | 73 | 0.029106 | 0.035021 | 0.770248 |
| 9S HODE | 0.03399 | 73 | 0.027604 | 0.034812 | 0.771582 |
| 15S HETE | 0.295675 | 73 | 0.204213 | 0.079416 | 0.507252 |
| 17 HDHA | 0.122553 | 73 | 0.085322 | −0.28791 | <0.05 |
| 12S HETE | 1.783897 | 73 | 1.137498 | −0.05227 | 0.660537 |
| 5-oxo-ETE | 0.193363 | 71 | 0.095486 | −0.10192 | 0.401138 |
| 5 HETE | 0.025014 | 73 | 0.012951 | −0.09634 | 0.417453 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlega, D.; Drozd, A.; Zembron-Lacny, A.; Morawin, B.; Ryterska, K.; Szczuko, M. The Activity of Protectin DX, 17 HDHA and Leukotriene B4 Is Correlated with Interleukin-1β (IL-1β) and Interleukin-1 Receptor Antagonist (IL-1Ra) in the Early Subacute Phase of Stroke. Int. J. Mol. Sci. 2025, 26, 9088. https://doi.org/10.3390/ijms26189088
Kotlega D, Drozd A, Zembron-Lacny A, Morawin B, Ryterska K, Szczuko M. The Activity of Protectin DX, 17 HDHA and Leukotriene B4 Is Correlated with Interleukin-1β (IL-1β) and Interleukin-1 Receptor Antagonist (IL-1Ra) in the Early Subacute Phase of Stroke. International Journal of Molecular Sciences. 2025; 26(18):9088. https://doi.org/10.3390/ijms26189088
Chicago/Turabian StyleKotlega, Dariusz, Arleta Drozd, Agnieszka Zembron-Lacny, Barbara Morawin, Karina Ryterska, and Malgorzata Szczuko. 2025. "The Activity of Protectin DX, 17 HDHA and Leukotriene B4 Is Correlated with Interleukin-1β (IL-1β) and Interleukin-1 Receptor Antagonist (IL-1Ra) in the Early Subacute Phase of Stroke" International Journal of Molecular Sciences 26, no. 18: 9088. https://doi.org/10.3390/ijms26189088
APA StyleKotlega, D., Drozd, A., Zembron-Lacny, A., Morawin, B., Ryterska, K., & Szczuko, M. (2025). The Activity of Protectin DX, 17 HDHA and Leukotriene B4 Is Correlated with Interleukin-1β (IL-1β) and Interleukin-1 Receptor Antagonist (IL-1Ra) in the Early Subacute Phase of Stroke. International Journal of Molecular Sciences, 26(18), 9088. https://doi.org/10.3390/ijms26189088








