Translational Evaluation of an Intraparenchymal Collagen Matrix Tamponade: Initial Preclinical and Clinical Experiments to Prevent CSF Reflux Following Endoscopic Brain Surgery
Abstract
1. Introduction
2. Results
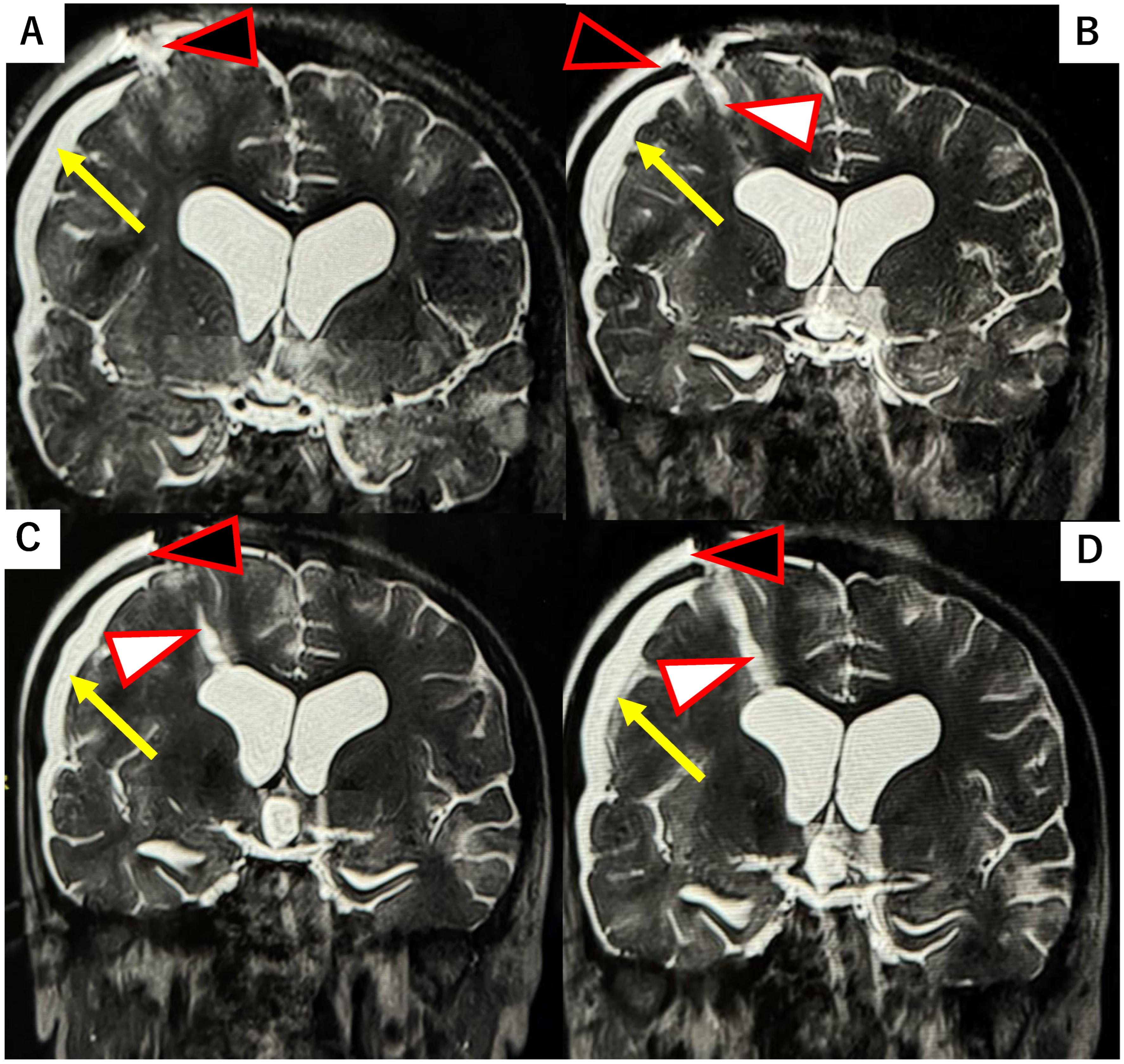
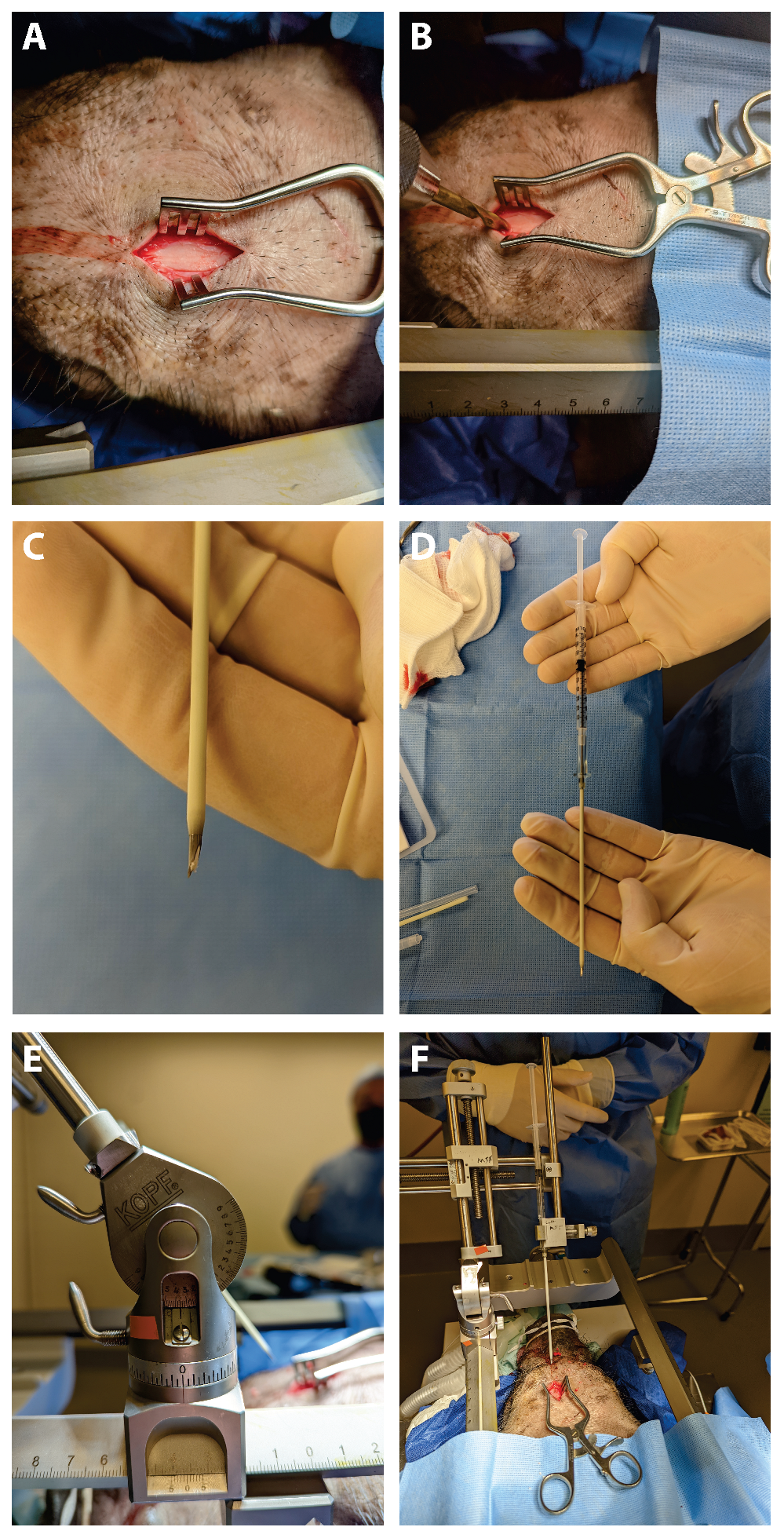
2.1. Case Report
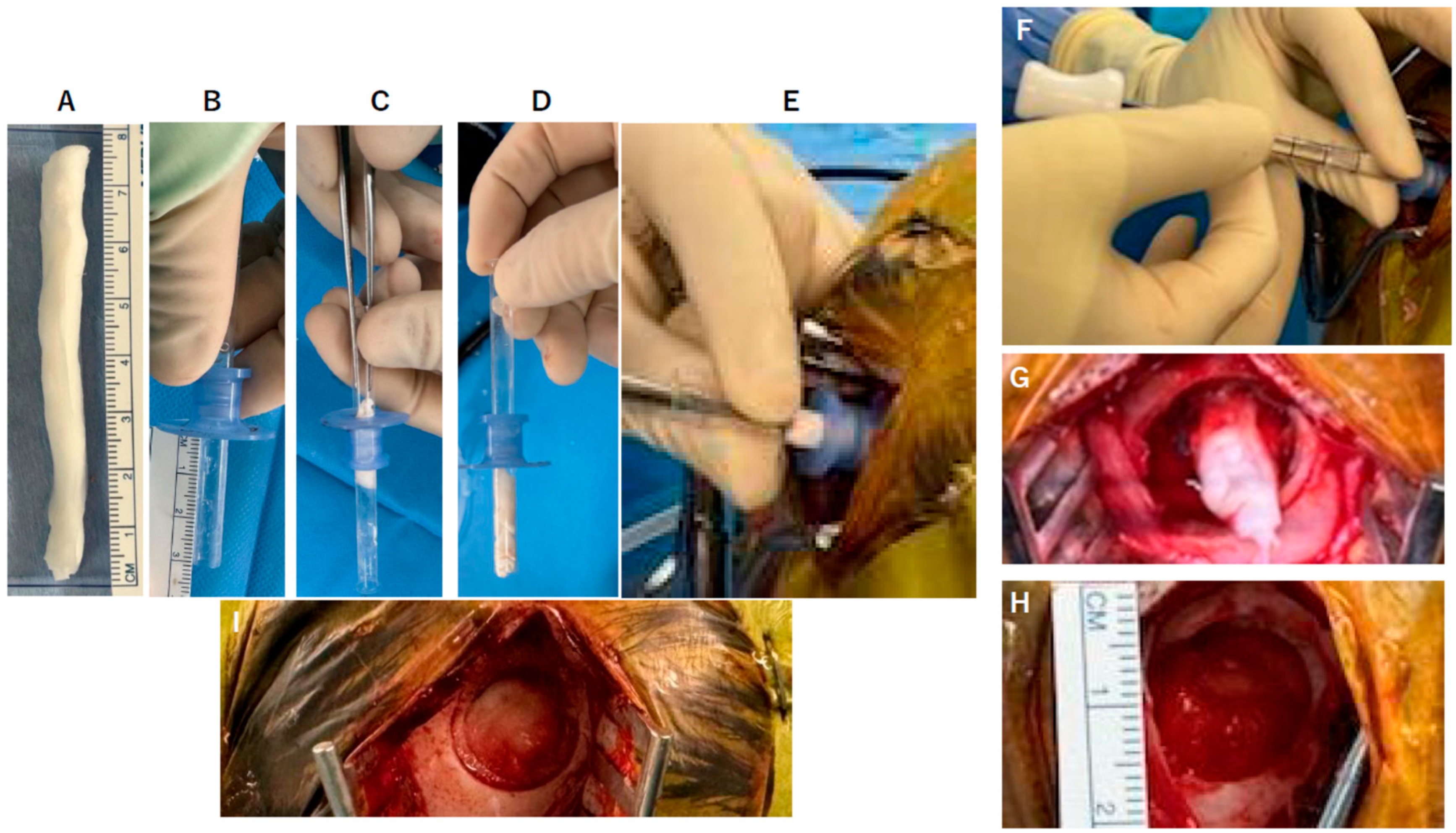
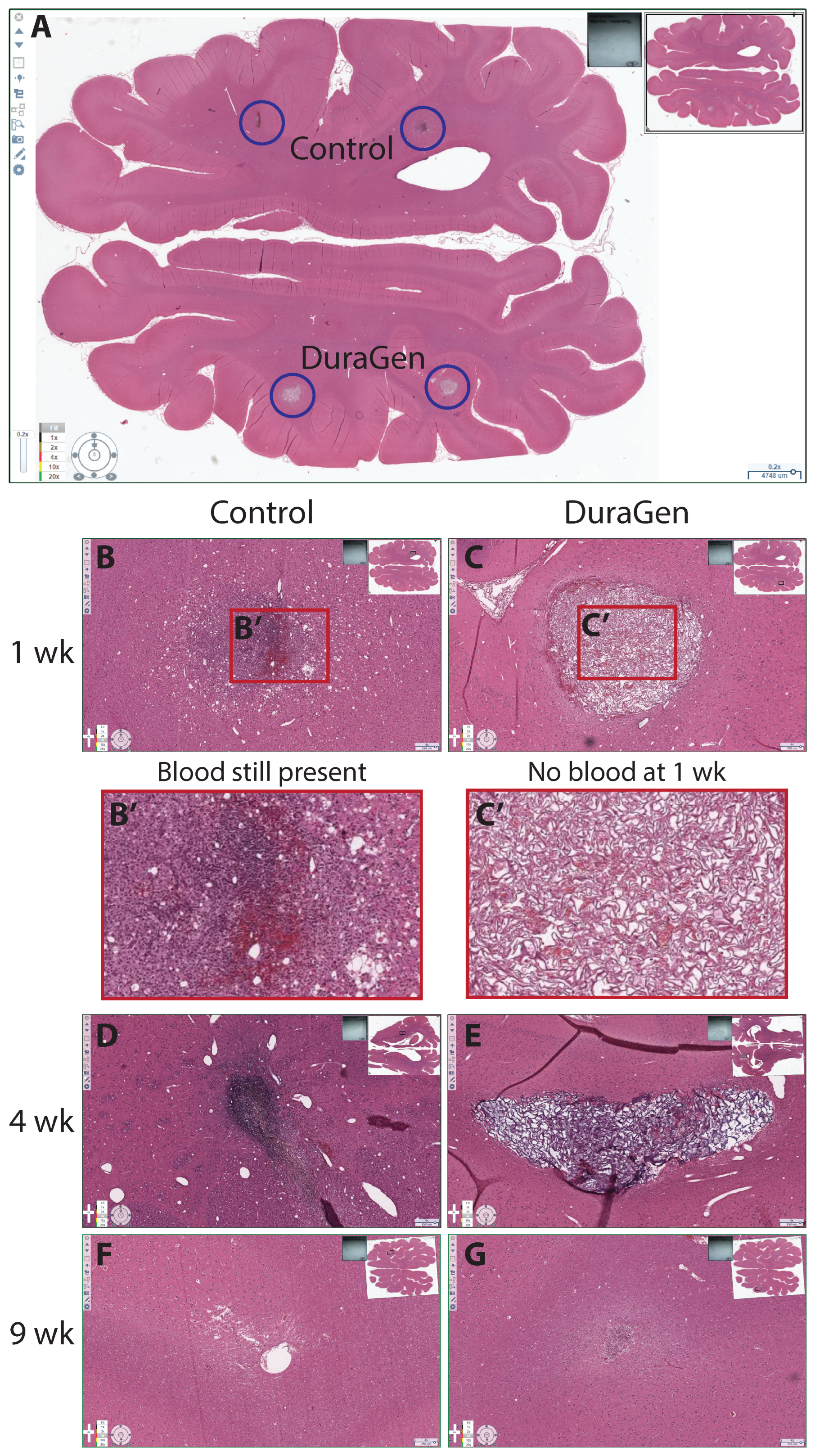
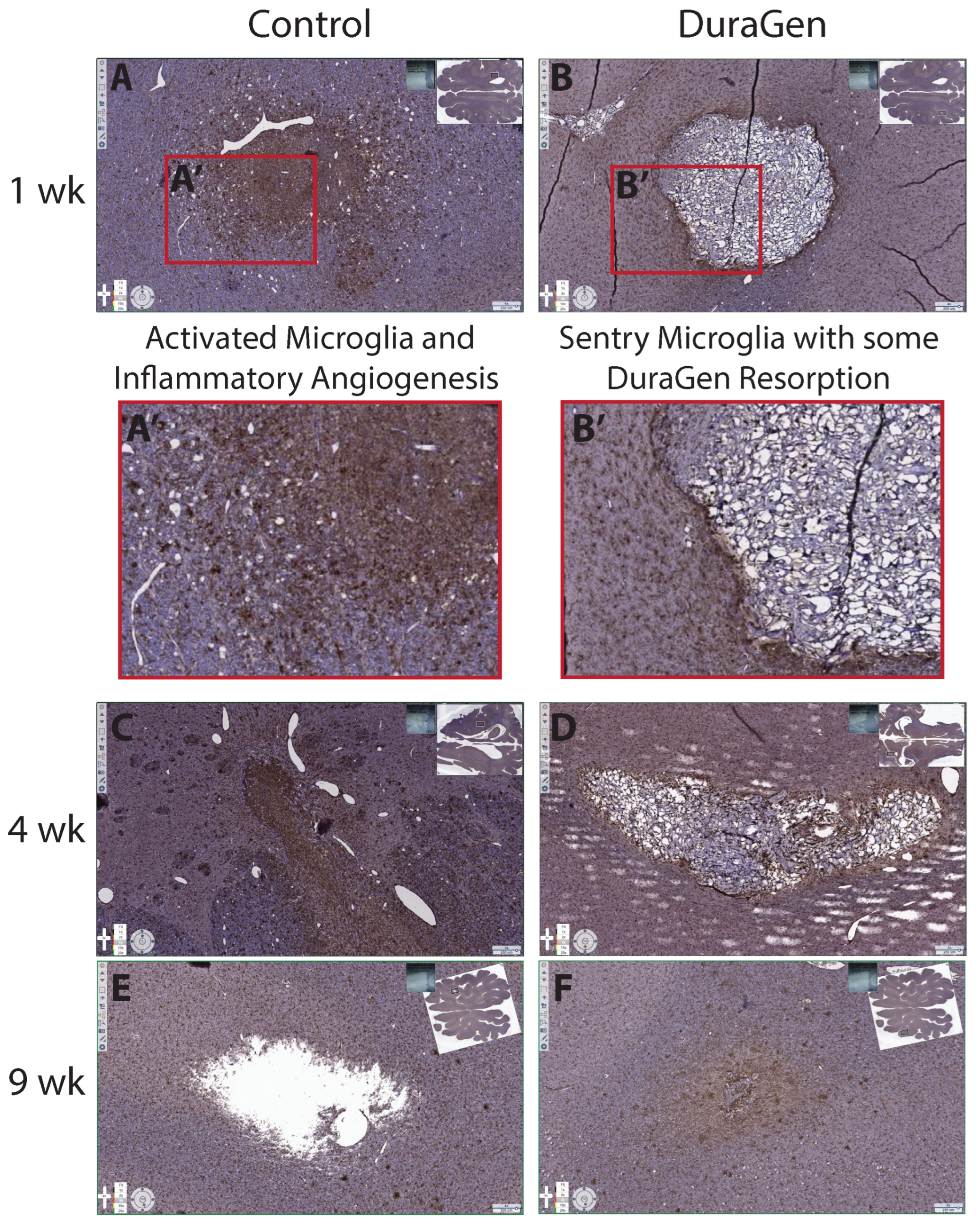
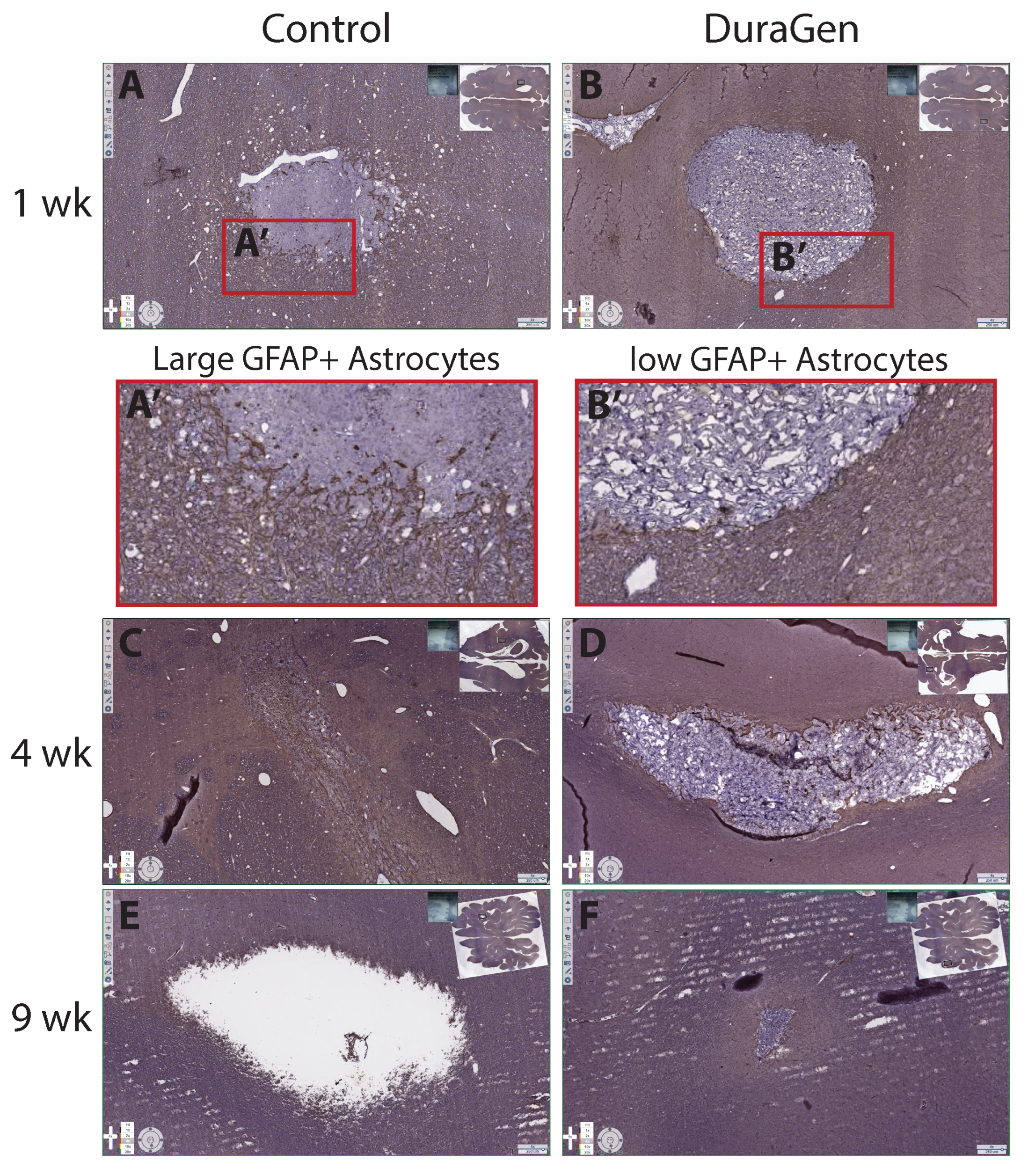
2.2. DuraGen Implant in Porcine Brain Parenchyma
3. Discussion
4. Materials and Methods
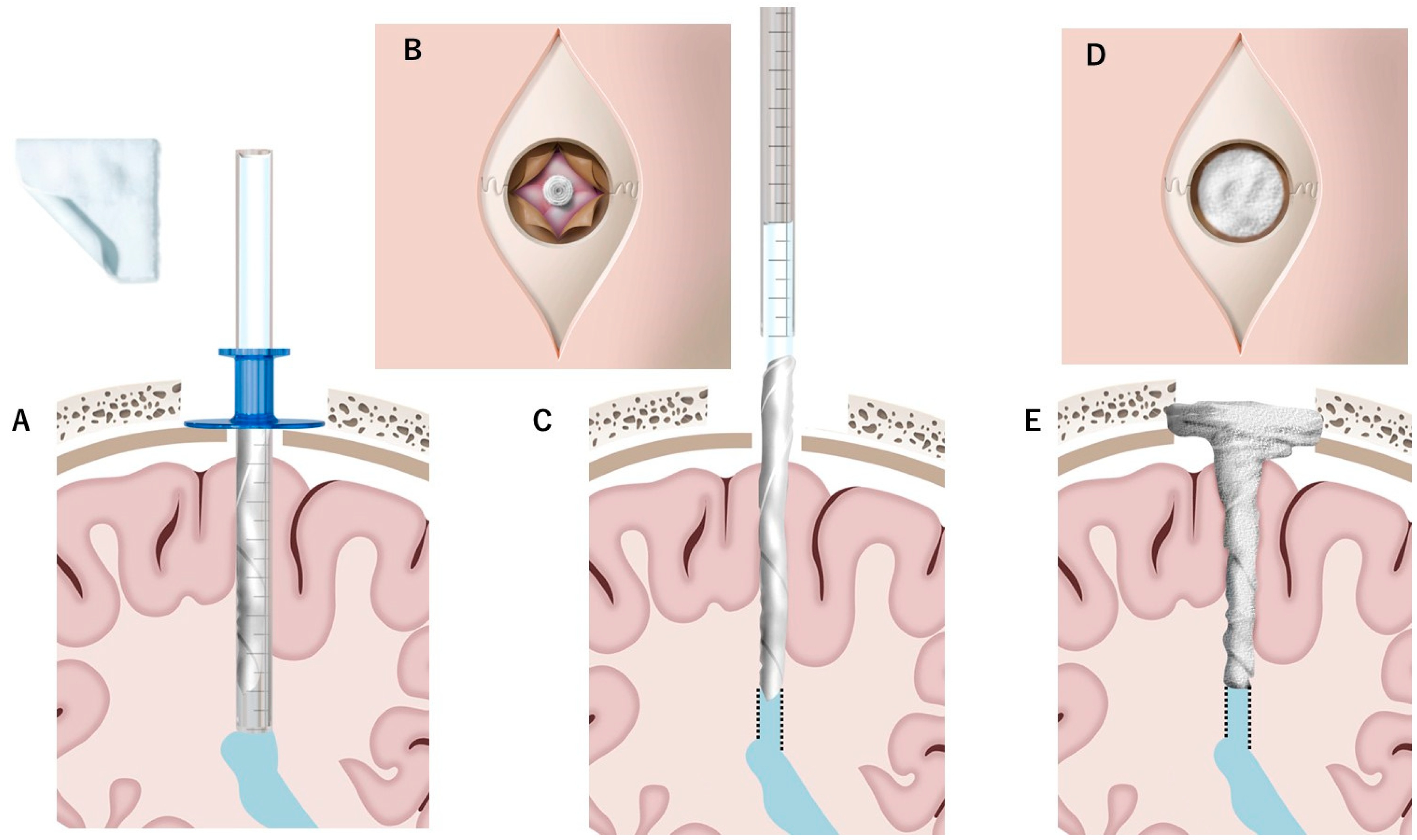
4.1. DuraGen Insertion Technique
- Determining the Insertion Depth: Estimate the required length of the DuraGen roll based on the measured distance from the ventricular wall to the dural surface. This ensures that the DuraGen plug reaches the dural defect without protruding into the ventricular cavity (Figure 1A).
- Preparation of Furled DuraGen: Cut a 7 × 7 cm2 DuraGen sheet and roll it diagonally into a cylindrical shape to facilitate insertion into the outer sheath of the NeuroPort endoscopic system and ultimately into the parenchymal tract (Figure 1B–D).
- Deploying DuraGen at the Target Site: With the DuraGen positioned inside the outer sheath, advance the NeuroPort’s inner obturator (or stylet) to the target depth while gradually retracting the outer sheath, leaving the DuraGen securely deployed within the parenchymal defect (Figure 1F,G and Figure 2B,C).
4.2. DuraGen Implantation into Pig Brain Parenchyma
4.3. Histology
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishihara, T.; Teraoka, A.; Morita, A.; Ueki, K.; Kirino, T. Transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas (technical note). J. Neurosurg. 2000, 92, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Morita, A.; Teraoka, A.; Kirino, T. Endoscopy-guided removal of spontaneous intracerebral hemorrhage: Comparison with CT-guided stereotactic evacuation. Childs Nerv. Syst. 2007, 23, 677–683. [Google Scholar] [CrossRef]
- Oi, S.; Abdullah, S.H. New transparent peel-away sheath with neuroendoscopic orientation markers (technical note). J. Neurosurg. 2007, 107, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Selesnick, S.H.; Liu, J.C.; Jen, A.; Newman, J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol. Neurotol. 2004, 25, 387–393. [Google Scholar] [CrossRef]
- Graupman, P.; Nussbaum, E.S.; Patel, P.D. Preventing cerebrospinal fluid leakage following endoscopy through a burr hole using a novel watertight closure (technical note). Br. J. Neurosurg. 2023, 37, 1915–1917. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.Y.; Chong, S.; Kim, I.Y.; Lee, J.Y.; Phi, J.H.; Kim, S.K. Prevention of complications in endoscopic third ventriculostomy. J. Korean Neurosurg. Soc. 2017, 60, 282–288. [Google Scholar] [CrossRef]
- Lo, W.B.; Afshari, F.T.; Rodrigues, D.; Kulkami, A.V. The “mushroom”: A simple and safe technique to avoid cerebrospinal fluid leak after endoscopic third ventriculostomy. Ann. R Coll. Surg. Engl. 2020, 102, 312–319. [Google Scholar] [CrossRef]
- Oi, S.; Samii, A.; Samii, M. Frameless free-hand maneuvering of a small-diameter rigid-rod neuroendoscope with a working channel used during high-resolution imaging (technical note). J. Neurosurg. Pediatr. 2005, 102, 113–118. [Google Scholar] [CrossRef]
- Integra LifeSciences Corporation. DuraGen® Collagen Matrix: Technical Information. Integra LifeSciences. 2019. Available online: https://www.cardion.cz/file/90/duragen-ultrapure-collagen.pdf (accessed on 1 January 2019).
- Eguchi, S.; Aihara, Y.; Hori, T.; Okada, Y. Postoperative Extra-Axial Cerebrospinal Fluid Collection—Its Pathophysiology and Clinical Management. Pediatr. Neurosurg. 2011, 47, 125–132. [Google Scholar] [CrossRef]
- Peretta, P.; Ragazzi, P.; Galarza, M.; Genitori, L.; Giordano, F.; Mussa, F.; Cinalli, G. Complications and pitfalls of neuroendoscopic surgery in children. J. Neurosurgery: Pediatr. 2006, 105, 187–193. [Google Scholar] [CrossRef]
- Cohen, A.R. Endoscopic Ventricular Surgery. Pediatr. Neurosurg. 1993, 19, 127–134. [Google Scholar] [CrossRef]
- Fries, G.; Perneczky, A. Endoscope-assisted brain surgery: Part 2—Analysis of 380 procedures. Neurosurgery 1998, 42, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Bouras, T.; Sgouros, S. Complications of endoscopic third ventriculostomy. J. Neurosurg. Pediatr. 2011, 7, 643–649. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Riva-Cambrin, J.; Holubkov, R.; Browd, S.R.; Cochrane, D.D.; Drake, J.M.; Limbrick, D.D.; Rozzelle, C.J.; Simon, T.D.; Tamber, M.S.; et al. Endoscopic third ventriculostomy in children: Prospective, multicenter results from the Hydrocephalus Clinical Research Network. J. Neurosurg. Pediatr. 2016, 18, 423–429. [Google Scholar] [CrossRef]
- Lin, B.; Yang, H.; Cui, M.; Li, Y.; Yu, J. Surgicel™ application in intracranial hemorrhage surgery contributed to giant-cell granuloma in a patient with hypertension: Case report and review of the literature. World J. Surg. Oncol. 2014, 12, 101. [Google Scholar] [CrossRef]
- Lei, T.; Liu, X.; Cao, J.; Sun, Y.; Li, G.; Huang, H. Intracranial foreign body granuloma caused by gelatin sponge: A case report and literature review. Int. J. Clin. Exp. Med. 2017, 10, 3996–4000. [Google Scholar]
- Nagata, Y.; Takeuchi, K.; Sasaki, H.; Mizuno, A.; Harada, H.; Tanahashi, K.; Saito, R. Modified shoelace dural closure with collagen matrix in extended transsphenoidal surgery. Neurol. Med. Chir. 2022, 62, 203–208. [Google Scholar] [CrossRef]
- Inoue, T.; Shitara, S.; Shima, A.; Goto, Y.; Fukushima, T. Double collagen matrix grafting for dural closure in microvascular decompression: An alternative use of autologous fascial grafting. Acta Neurochir. 2021, 163, 2395–2401. [Google Scholar] [CrossRef]
- Koyama, J.; Akutsu, N.; Kawamura, A. Duraplasty using a combination of a pedicled dural flap and collagen matrix in posterior fossa decompression for pediatric Chiari malformation type 1 with syrinx. Acta Neurochir. 2024, 166, 70. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; Reddy, K.; Fewer, D.; Qiao, F.; Nathoo, N. Collagen matrix duraplasty for cranial and spinal surgery: A clinical and imaging study. J. Neurosurg. 2007, 106, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; Qiao, F.; Nathoo, N. Collagen matrix duraplasty for posterior fossa surgery: Evaluation of surgical technique in 52 adult patients. J. Neurosurg. 2009, 111, 380–386. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef]
- Narotam, P.K.; Van Dellen, J.R.; Bhoola, K.; Raidoo, D. Experimental evaluation of collagen sponge as a dural graft. Br. J. Neurosurg. 1993, 7, 635–641. [Google Scholar] [CrossRef]
- Narotam, P.K.; van Dellen, J.R.; Bhoola, K.D. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J. Neurosurg. 1995, 82, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Shaffrey, C.I.; Spotnitz, W.D.; Shaffrey, M.E. Neurosurgical applications of fibrin glue: Augmentation of dural closure in 134 pa-tients. Neurosurgery 1990, 26, 207–210. [Google Scholar] [CrossRef]
- Stendel, R.; Danne, M.; Fiss, I.; Klein, I.; Schilling, A.; Hammersen, S.; Pietilae, T.; Jänisch, W.; Hopfenmüller, W. Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J. Neurosurg. 2008, 109, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Kuranari, Y.; Orikasa, H.; Katayama, M. Meningioma Cell Invasion into DuraGen-Derived Dura Mater: A Case Report. Medicines 2022, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Danish, S.F.; Samdani, A.; Hanna, A.; Storm, P.; Sutton, L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J. Neurosurg. Pediatr. 2006, 104, 16–20. [Google Scholar] [CrossRef]
- Neulen, A.; Gutenberg, A.; Takács, I.; Wéber, G.; Wegmann, J.; Schulz-Schaeffer, W.; Giese, A. Evaluation of efficacy and biocompatibility of a novel semisynthetic collagen matrix as a dural onlay graft in a large animal model. Acta Neurochir. 2011, 153, 2241–2250. [Google Scholar] [CrossRef]
- Ellingsworth, L.R.; DeLustro, F.; Brennan, J.E.; Sawamura, S.; McPherson, J. The human immune response to reconstituted bovine collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar] [CrossRef]
- Jinnin, M. An experimental study on the prevention of postlaminectomy scar formation: Efficacy of interpositional collagen-sponge. Nippon Seikeigeka Gakkai Zasshi 1995, 69, 198–208. [Google Scholar]
- Calikoglu, C.; Cakir, M.; Tuzun, Y. Histopathological investigation of the effectiveness of collagen matrix in the repair of experimental spinal dura mater defects. Eurasian J. Med. 2019, 51, 133–138. [Google Scholar] [CrossRef]
- Narotam, P.K.; José, S.; Nathoo, N.; Taylon, C.; Vora, Y. Collagen matrix (DuraGen) in dural repair: Analysis of a new modified technique. Spine 2004, 29, 2861–2867; discussion 2868–2869. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Cruz-Almeida, Y.; Siqueira, E.B.; Norenberg, M.; Green, B.A.; Levi, A.D. Postoperative fibrosis after surgical treatment of the porcine spinal cord: A comparison of dural substitutes. J. Neurosurg. Spine 2005, 2, 50–54. [Google Scholar] [CrossRef]
- Khorasani, L.; Kapur, R.; Lee, C.; Avellino, A. Histological analysis of DuraGen in a human subject: Case report. Clin. Neuropathol. 2008, 27, 361–364. [Google Scholar] [CrossRef]
- Esposito, F.; Grimod, G.; Cavallo, L.M.; Lanterna, L.; Biroli, F.; Cappabianca, P. Collagen-only biomatrix as dural substitute: Results at 5-year follow-up. Clin. Neurol. Neurosurg. 2013, 115, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Sade, B.; Oya, S.; Lee, J.H. Non-watertight dural reconstruction in meningioma surgery: Results in 439 consecutive patients and a review of the literature. J. Neurosurg. 2011, 114, 714–718. [Google Scholar] [CrossRef]
- Zerris, V.A.; James, K.S.; Roberts, J.B.; Bell, E.; Heilman, C.B. Repair of the dura mater with processed collagen devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 580–588. [Google Scholar] [CrossRef]
- Aksekili, M.A.E.; Ateş, O.; Yüksel, K. The comparison of histopathological results of repairing dural defects in rats using different collagen-based grafts. Electron. J. Biol. 2017, 13, 188–194. [Google Scholar]
- Petrov, D.; Katiyar, K.S.; Struzyna, L.A.; Harris, J.P.; Cullen, D.K. Extracellular matrix-derived tissues for neurological applications. In Extracellular Matrix–Derived Implants in Clinical Medicine; Mooradian, D.L., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 83–118. [Google Scholar] [CrossRef]
- Rabinowitz, L.; Monnerie, H.; Shashidhara, S.; Le Roux, P.D. Growth of rat cortical neurons on DuraGen, a collagen-based dural graft matrix. Neurol. Res. 2005, 27, 887–894. [Google Scholar] [CrossRef]
- Finch, L.; Harris, S.; Solomou, G.; Sen, J.; Tzerakis, N.; Emes, R.D.; Lane, C.S.; Hart, S.R.; Adams, C.F.; Chari, D.M. Safe nanoengineering and incorporation of transplant populations in a neurosurgical grade biomaterial, DuraGen PlusTM, for protected cell therapy applications. J. Control. Release 2020, 321, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kameno, K.; Kaku, Y.; Ohmori, Y.; Takemoto, Y.; Uekawa, K.; Mukasa, A. Artificial dural regeneration matrix as a substitute for autologous tissue in indirect bypass in Moyamoya disease: Investigation of a rat model of chronic cerebral hypoperfusion. Neurosurg. Rev. 2025, 48, 48. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Suzuki, K.; Nisson, P.L.; Mochizuki, Y.; Mizuno, R.; Teranishi, A.; Take, Y.; Kayahara, T.; Kurita, H. Histopathological analysis of Duragen collagen matrix over time in humans. Sci. Rep. 2025, 15, 11119. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aihara, Y.; Chiba, K.; Oda, Y.; Browne, K.; Petrov, D.; Kawamata, T.; O’Donnell, J.C. Translational Evaluation of an Intraparenchymal Collagen Matrix Tamponade: Initial Preclinical and Clinical Experiments to Prevent CSF Reflux Following Endoscopic Brain Surgery. Int. J. Mol. Sci. 2025, 26, 9081. https://doi.org/10.3390/ijms26189081
Aihara Y, Chiba K, Oda Y, Browne K, Petrov D, Kawamata T, O’Donnell JC. Translational Evaluation of an Intraparenchymal Collagen Matrix Tamponade: Initial Preclinical and Clinical Experiments to Prevent CSF Reflux Following Endoscopic Brain Surgery. International Journal of Molecular Sciences. 2025; 26(18):9081. https://doi.org/10.3390/ijms26189081
Chicago/Turabian StyleAihara, Yasuo, Kentaro Chiba, Yuichi Oda, Kevin Browne, Dmitriy Petrov, Takakazu Kawamata, and John C. O’Donnell. 2025. "Translational Evaluation of an Intraparenchymal Collagen Matrix Tamponade: Initial Preclinical and Clinical Experiments to Prevent CSF Reflux Following Endoscopic Brain Surgery" International Journal of Molecular Sciences 26, no. 18: 9081. https://doi.org/10.3390/ijms26189081
APA StyleAihara, Y., Chiba, K., Oda, Y., Browne, K., Petrov, D., Kawamata, T., & O’Donnell, J. C. (2025). Translational Evaluation of an Intraparenchymal Collagen Matrix Tamponade: Initial Preclinical and Clinical Experiments to Prevent CSF Reflux Following Endoscopic Brain Surgery. International Journal of Molecular Sciences, 26(18), 9081. https://doi.org/10.3390/ijms26189081





