Antitumor and Antiangiogenic Effect of Tannic Acid in the Advanced Stage of Ehrlich Ascites Tumor in Mice
Abstract
1. Introduction
2. Results
2.1. Change in Tumor Weight of Animals During the Experiment
2.2. TA Inhibits Total Cell Number and Tumor Growth in Mice Bearing EAT
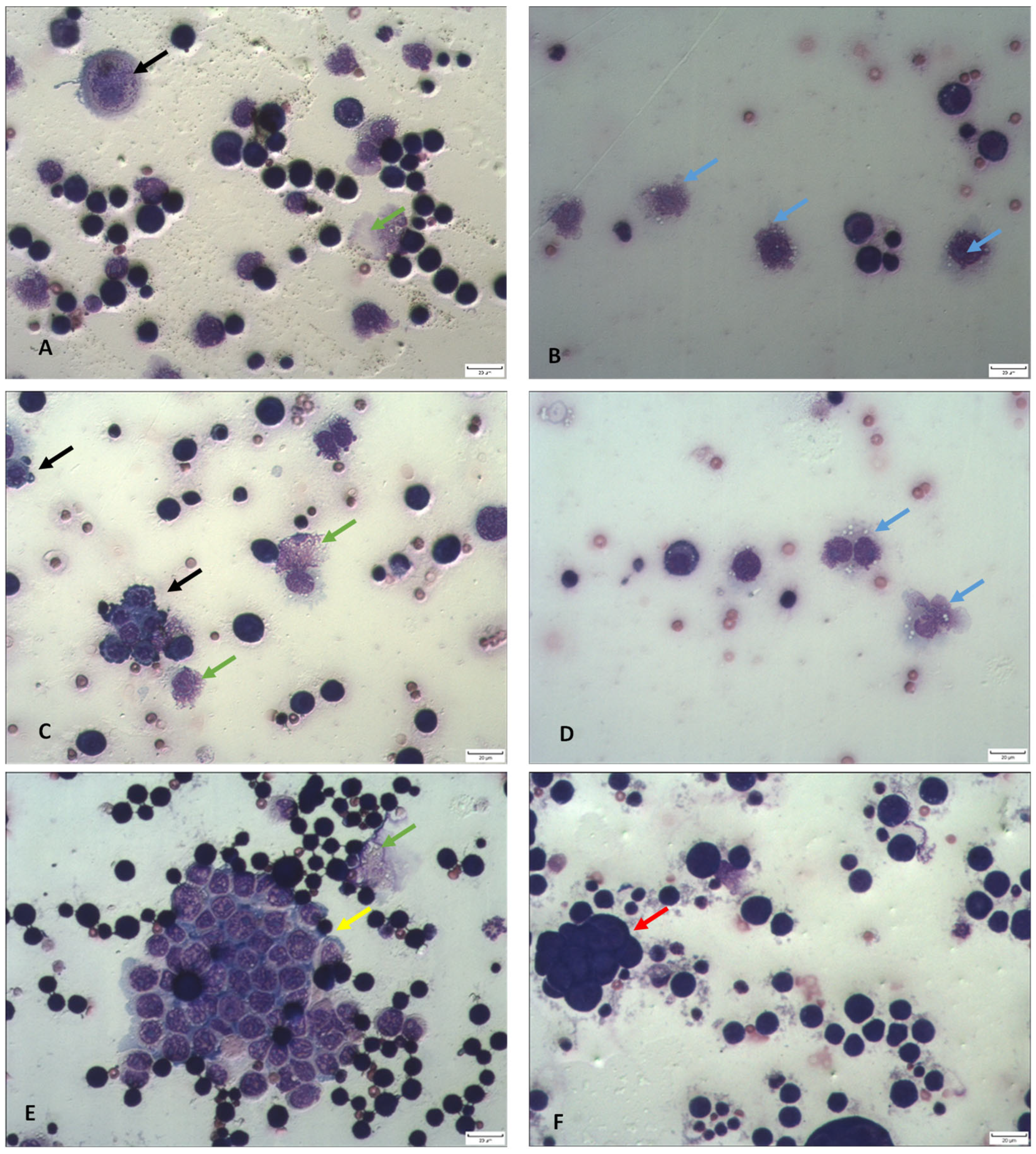
2.3. Differential Analysis of Ascites Cells in Peritoneal Lavage Fluid and Peripheral Blood Count
2.4. TA Affects Functional Activity and Macrophage Polarization
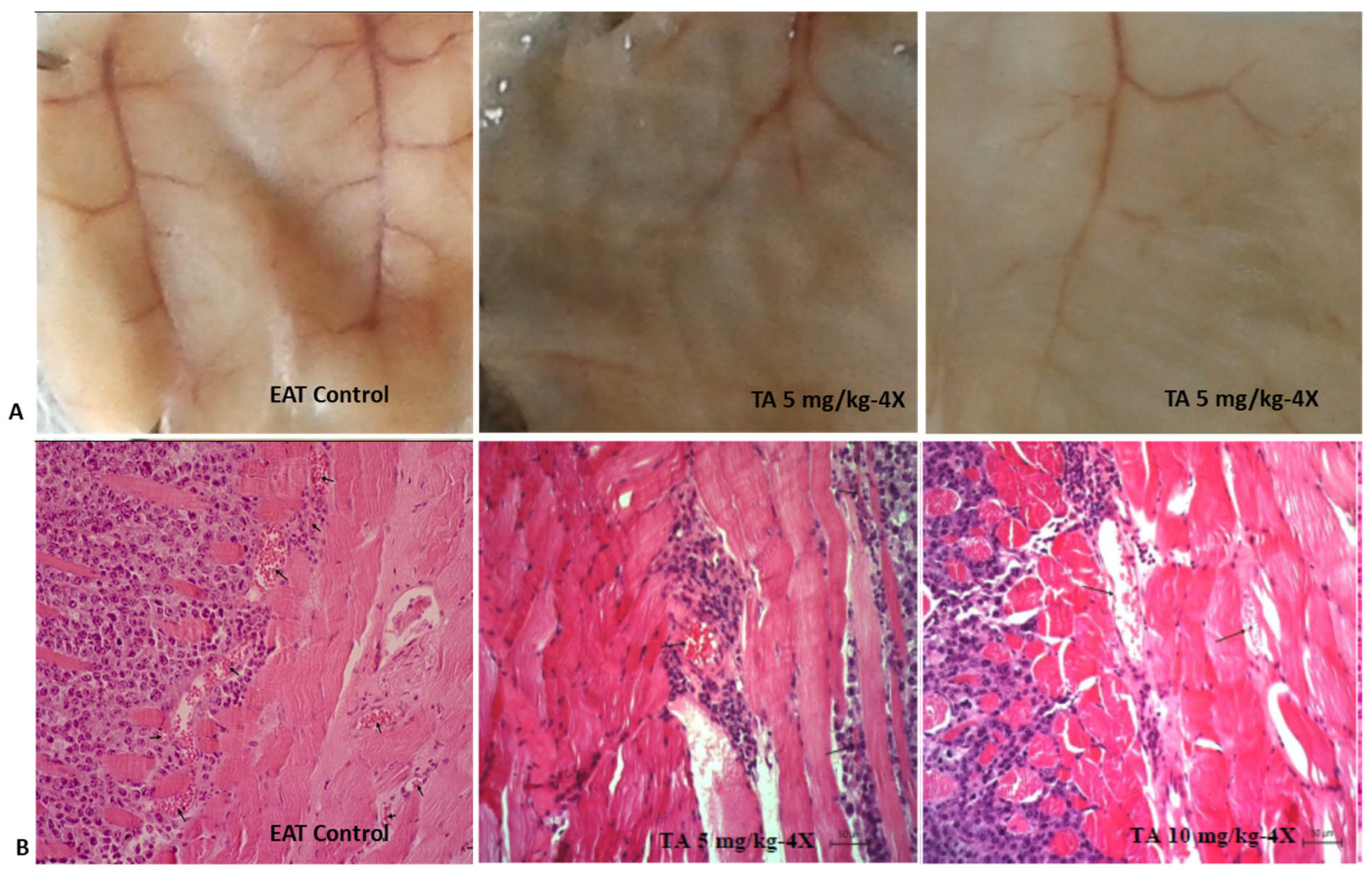
2.5. TA Exhibits Antiangiogenic Activity
2.6. Tannic Acid Reduces Levels of COX-2 in the Peritoneal Cavity of EAT-Bearing Mice
2.7. TA Reduces MMP-2 and MMP-9 Levels
2.8. TA Induces DNA Damage in Tumor and Whole Blood Cells
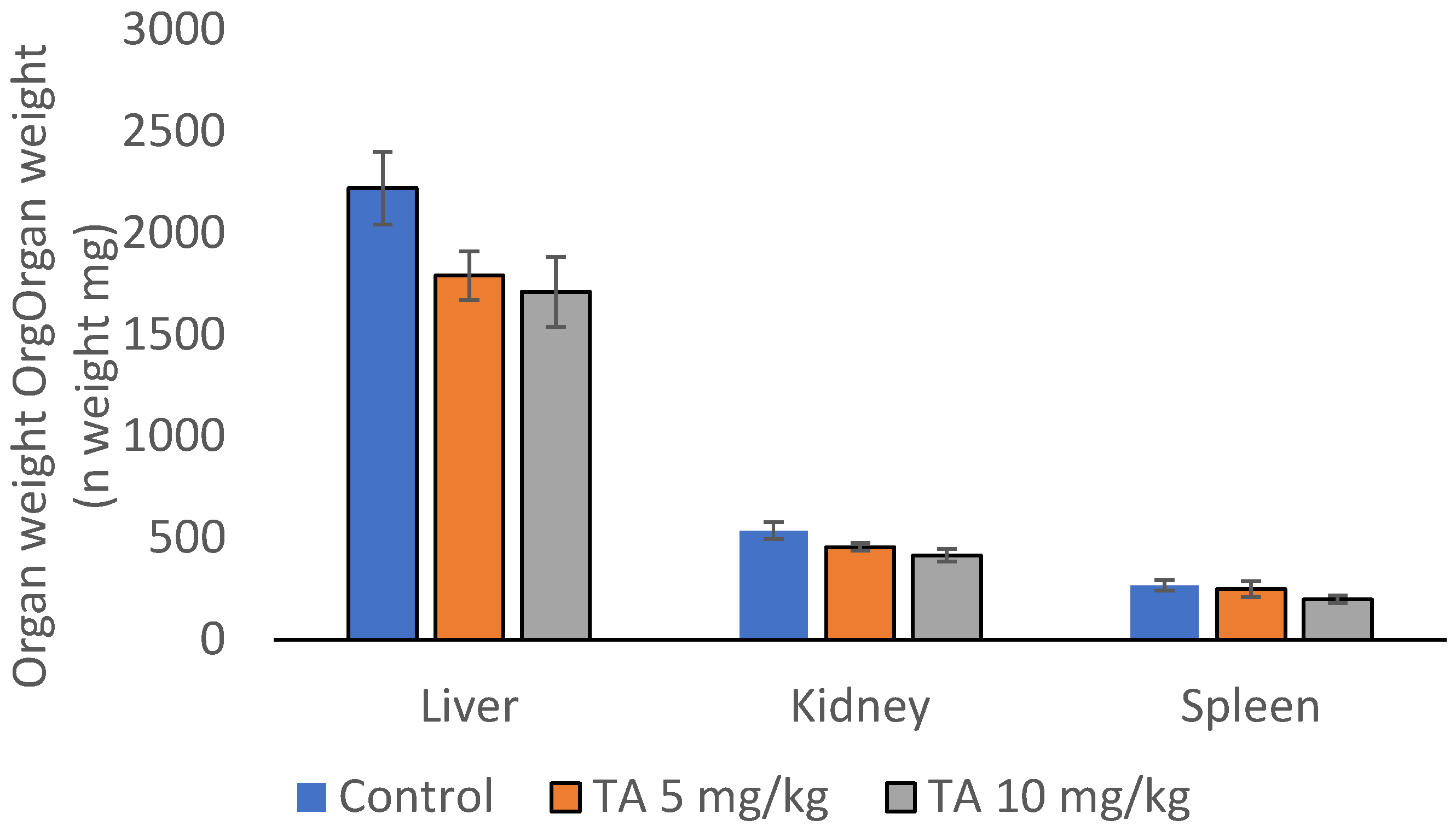
2.9. TA Effect on Hematological and Biochemical Determinants, Organ Weight and Function
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Ethics Statement
4.2. Tumor Cells
4.3. Tannic Acid
4.4. Experimental Design and Animal Treatment
4.5. Monitoring Changes in Body and Organs Weight
4.6. Antitumor Efficacy of TA
4.6.1. Determination of the Total Volume of Peritoneal Fluid, Tumor Cell Counts and Tumor Inhibition
4.6.2. Differential Cell Analysis in Peritoneal Fluid
4.6.3. Analysis of Hematological and Biochemical Parameters
4.7. Effect of TA on Angiogenesis
4.7.1. Histological Analysis of the Peritoneum and Micro Vessel Density (MVD)
4.7.2. Quantitative Measurement of Vascular Density
4.8. Isolation and Preparation of Macrophages from Ascites Fluid and Spleen
4.8.1. Determination of Functional Activity of Peritoneal Macrophages
4.8.2. Nitric Oxide Analyses by Griess Reaction
4.8.3. Arginase Activity (Arg)
4.9. Determination of Proangiogenic Factors: VEGF, MMP-2, MMP-9 and COX-2
4.9.1. VEGF Analysis
4.9.2. Determination of COX-2 Concentration
4.9.3. Determination of MMP-2 and MMP-9 Concentrations
4.9.4. Cytokine Analysis
4.10. Comet Assay
4.11. Statitical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Arg | Arginase |
| EAT | Ehrlich ascites tumor |
| COX-2 | Cyclooxygenase -2 |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FC | Fold change |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LMR | Lymphocyte-to-monocyte ratio |
| MMP-2 | Matrix metalloproteinase 2 |
| MMP-9 | Matrix metalloproteinase 9 |
| MVD | Microvessel density |
| NK | Natural killer |
| NLR | Neutrophil-to-lymphocyte ratio |
| NO | Nitric oxide |
| PD-L1 | Programmed death-ligand 1 |
| P/M | Polymorphonuclear to mononuclear ratio |
| ROS | Reactive oxygen species |
| TA | Tannic acid |
| TAMs | Tumor-associated macrophages |
| TANs | Tumor-associated neutrophils |
| TGF-β | Tumor growth factor β |
| TNFα | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Guleng, B.; Tateishi, K.; Kanai, F.; Jazag, A.; Ohta, M.; Asaoka, Y.; Ijichi, H.; Tanaka, Y.; Imamura, J.; Ikenoue, T.; et al. Cancer-derived VEGF plays no role in malignant ascites formation in the mouse. World J. Gastroenterol. 2005, 11, 5455–5459. [Google Scholar] [CrossRef]
- Oršolić, N.; Kunštić, M.; Kukolj, M.; Gračan, R.; Nemrava, J. Oxidative stress, polarization of macrophages and tumour angiogenesis: Efficacy of caffeic acid. Chem.-Biol. Interact. 2016, 256, 111–124. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Cheng, G.; Gao, J.; Wang, L.; Ding, Y.; Wu, Q.; Wang, Q.; Xiao, J.; Wang, S. The TGF-β1/COX-2-dependant pathway serves a key role in the generation of OKC-induced M2-polarized macrophage-like cells and angiogenesis. Oncol. Lett. 2020, 20, 39. [Google Scholar] [CrossRef]
- Na, Y.R.; Yoon, Y.N.; Son, D.I.; Seok, S.H. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef] [PubMed]
- Baer-Dubowska, W.; Szaefer, H.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Tannic acid: Specific form of tannins in cancer chemoprevention and therapy-old and new applications. Curr. Pharmacol. Rep. 2020, 6, 28–37. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. Int. J. Mol. Sci. 2022, 23, 10479. [Google Scholar] [CrossRef]
- Majed, F.; Rashid, S.; Khan, A.Q.; Nafees, S.; Ali, N.; Ali, R.; Khan, R.; Hasan, S.K.; Mehdi, S.J.; Sultana, S. Tannic Acid Mitigates the DMBA/croton Oil-induced Skin Cancer Progression in Mice. Mol. Cell. Biochem. 2015, 399, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Mejia, J.A.A.; Rodrigues, D.M.; Alves, G.R.; de Freitas Pinheiro, A.M.; Tanimoto, M.H.; Bastos, J.K.; Ambrósio, S.R. Brazilian Brown Propolis: An Overview About Its Chemical Composition, Botanical Sources, Quality Control, and Pharmacological Properties. Rev. Bras. Farmacogn. 2023, 33, 288–299. [Google Scholar] [CrossRef]
- Sarapa, A.; Peter, A.; Buettner, A.; Loos, H.M. Organoleptic and chemical properties of propolis: A review. Eur. Food Res. Technol. 2025, 251, 1331–1352. [Google Scholar] [CrossRef]
- Kandir, S.; Karakurt, S.; Gökçek-Saraç, Ç.; Karakurt, S. Tannic acid elicits differential gene regulation in prostate cancer apoptosis. Acta Pharm. 2024, 74, 539–550. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Jo, E.S.; Rugamba, A.; Kim, W.S.; Park, Y.-M.; Hwang, D.-Y.; Yoo, J.-S.; Liu, Q.; Jang, K.-J.; et al. Tannic Acid Promotes TRAIL-Induced Extrinsic Apoptosis by Regulating Mitochondrial ROS in Human Embryonic Carcinoma Cells. Cells 2020, 9, 282. [Google Scholar] [CrossRef]
- Li, C.-C.; Tsai, B.C.-K.; Annseles Rajula, S.; Hsu, C.-H.; Chen, M.-C.; Kuo, C.-H.; Yeh, C.-M.; Hsieh, D.J.-Y.; Kuo, W.-W.; Huang, C.-Y. Tannic Acid Impedes the Proliferation of Bladder Cancer Cells by Elevating Mitochondrial Pathways of Apoptosis. Cell Biochem. Biophys. 2024, 82, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, D.; Han, D.M.; Cheng, Y.H.; Dai, C.; Wu, X.J.; Che, F.Y.; Heng, X.Y. Tannic acid mediated induction of apoptosis in human glioma Hs 683 cells. Oncol. Lett. 2018, 5, 6845–6850. [Google Scholar] [CrossRef]
- Wang, G.; Mu, M.; Zhang, Z.; Chen, Y.; Yang, N.; Zhong, K.; Li, Y.; Lu, F.; Guo, G.; Tong, A. Systemic delivery of tannic acid-ferric-masked oncolytic adenovirus reprograms tumor microenvironment for improved therapeutic efficacy in glioblastoma. Cancer Gene Ther. 2024, 31, 1804–1817. [Google Scholar] [CrossRef]
- Darvin, P.; Baeg, S.J.; Joung, Y.H.; Sp, N.; Kang, D.Y.; Byun, H.J.; Park, J.U.; Yang, Y.M. Tannic Acid Inhibits the Jak2/STAT3 Pathway and Induces G1/S Arrest and Mitochondrial Apoptosis in YD-38 Gingival Cancer Cells. Int. J. Oncol. 2015, 47, 1111–1120. [Google Scholar] [CrossRef]
- Honda, Y.; Nomoto, T.; Matsui, M.; Takemoto, H.; Miura, Y.; Nishiyama, N. Sequentially Self-Assembled Nanoreactor Comprising Tannic Acid and Phenylboronic Acid-Conjugated Polymers Inducing Tumor-Selective Enzymatic Activity. ACS Appl. Mater. Interfaces 2021, 13, 54850–54859. [Google Scholar] [CrossRef]
- Molina-Ramírez, B.; Cabral-Hipólito, N.; Castillo-Maldonado, I.; Delgadillo-Guzmán, D.; Meza-Velázquez, R.; Ramírez-Moreno, A.; Flores-Loyola, E.; Ruíz-Flores, P.; Cruz, J.H.; Espino-Silva, P.K.; et al. Tannic Acid, as a Structural Moiety Coupled to a Protein Antigen, Exhibiting a Molecular-structure Adjuvant Activity for Antibody Specificity Enhancement. Protein Pept. Lett. 2022, 29, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Majchrzak-Celińska, A.; Baer-Dubowska, W. Tannins in cancer prevention and therapy. Br. J. Pharmacol. 2025, 182, 2075–2093. [Google Scholar] [CrossRef]
- Qu, W.F.; Zhu, G.Q.; Yang, R.; Chu, T.H.; Guan, Z.Q.; Huang, R.; Tian, M.X.; Jiang, X.F.; Tao, C.Y.; Fang, Y.; et al. Targeting HMGB2 acts as dual immunomodulator by bolstering CD8+ T cell function and inhibiting tumor growth in hepatocellular carcinoma. Sci. Adv. 2025, 11, eads8597. [Google Scholar] [CrossRef] [PubMed]
- Barboura, M.; Cornebise, C.; Hermetet, F.; Guerrache, A.; Selmi, M.; Salek, A.; Chekir-Ghedira, L.; Aires, V.; Delmas, D. Tannic Acid, A Hydrolysable Tannin, Prevents Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition to Counteract Colorectal Tumor Growth. Cells 2022, 11, 3645. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Yamout, S.Z.; Sidani, M.M. Tannins protect against skin tumor promotion induced by ultraviolet-B radiation in hairless mice. Nutr. Cancer 2000, 37, 73–77. [Google Scholar] [CrossRef]
- Nepka, C.; Sivridis, E.; Antonoglou, O.; Kortsaris, A.; Georgellis, A.; Taitzoglou, I.; Hytiroglou, P.; Papadimitriou, C.; Zintzaras, I.; Kouretas, D. Chemopreventive activity of very low dose dietary tannic acid administration in hepatoma bearing C3H male mice. Cancer Lett. 1999, 141, 57–62. [Google Scholar] [CrossRef]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Hasegawa, M. Tannic acid raises survival rate of mice bearing syngeneic tumors. Cancer Biother. Radiopharm. 1999, 14, 231–234. [Google Scholar] [CrossRef]
- Radulski, D.R.; Stipp, M.C.; Galindo, C.M.; Acco, A. Features and applications of Ehrlich tumor model in cancer studies: A literature review. Transl. Breast Cancer Res. 2023, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Odeh, D.; Jembrek, M.J.; Knežević, J.; Kučan, D. Interactions between Cisplatin and Quercetin at Physiological and Hyperthermic Conditions on Cancer Cells In Vitro and In Vivo. Molecules 2020, 25, 3271. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, P.; Szymczyk, A.; Podhorecka, M. The Neutrophil to Lymphocyte and Lymphocyte to Monocyte Ratios as New Prognostic Factors in Hematological Malignancies—A Narrative Review. Cancer Manag. Res. 2020, 12, 2961–2977. [Google Scholar] [CrossRef]
- Deng, L.; Qi, Y.; Liu, Z.; Xi, Y.; Xue, W. Effect of tannic acid on blood components and functions. Colloids Surf. B Biointerfaces 2019, 184, 110505. [Google Scholar] [CrossRef]
- Hosseini, M.; Moghaddam, L.; Barner, L.; Cometta, S.; Hutmacher, D.W.; Medeiros Savi, F. The Multifaceted Role of Tannic Acid: From Its Extraction and Structure to Antibacterial Properties and Applications. Prog. Polym. Sci. 2025, 160, 101908. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, X.; Wang, L.; Jiang, S.; Luo, H.; Chen, Y.; Peng, C. The pathogenesis of liver cancer and the therapeutic potential of bioactive substances. Front. Pharmacol. 2022, 13, 1029601. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.A.; Choi, H.S.; Ryu, E.S.; Ko, J.; Shin, H.S.; Lee, J.M.; Chung, H.; Jun, E.; Oh, E.S.; Kang, D.H. Tannic acid attenuates the formation of cancer stem cells by inhibiting NF-kappaB-mediated phenotype transition of breast cancer cells. Am. J. Cancer Res. 2019, 9, 1664–1681. [Google Scholar]
- Chen, M.C.; Annseles Rajula, S.; Bharath Kumar, V.; Hsu, C.H.; Day, C.H.; Chen, R.J.; Wang, T.F.; Viswanadha, V.P.; Li, C.C.; Huang, C.Y. Tannic acid attenuate AKT phosphorylation to inhibit UMUC3 bladder cancer cell proliferation. Mol. Cell. Biochem. 2022, 477, 2863–2869. [Google Scholar] [CrossRef]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, C.R.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging 2011, 3, 192–222. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic acid Modulates NFκB Signaling Pathway and Skin Inflammation in NC/Nga Mice Through PPARγ Expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef]
- Toraman, E.; Ceylan, H. Investigation of the effect of tannic acid on doxorubicin-ınduced testicular damage and functions in a rat model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025; advance online publication. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, L.; Zhai, W.; Geng, N.; Zhang, Z.; Li, X.; Wu, M. Synergistic anticancer activity of cisplatin combined with tannic acid enhances apoptosis in lung cancer through the PERK-ATF4 pathway. Eur. J. Med. Res. 2023, 28, 462. [Google Scholar] [CrossRef]
- Ghasemian, M.; Kazeminava, F.; Naseri, A.; Mohebzadeh, S.; Abbaszadeh, M.; Kafil, H.S.; Ahmadian, Z. Recent progress in tannic acid based approaches as a natural polyphenolic biomaterial for cancer therapy: A review. Biomed. Pharmacother. 2023, 166, 115328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, S.J.; Wang, D.; Wei, Y.J.; Hu, S.S. Intramyocardial Injection of Tannic Acid Attenuates Postinfarction Remodeling: A Novel Approach to Stabilize the Breaking Extracellular Matrix. J. Thorac. Cardiovasc. Surg. 2009, 137, 216–222.e2. [Google Scholar] [CrossRef]
- Tanimura, S.; Kadomoto, R.; Tanaka, T.; Zhang, Y.J.; Kouno, I.; Kohno, M. Suppression of tumor cell invasiveness by hydrolyzable tannins (plant polyphenols) via the inhibition of matrix metalloproteinase-2/-9 activity. Biochem. Biophys. Res. Commun. 2005, 330, 1306–1313. [Google Scholar] [CrossRef]
- Oršolić, N.; Kunštić, M.; Kukolj, M.; Odeh, D.; Ančić, D. Natural Phenolic Acid, Product of the Honey Bee, for the Control of Oxidative Stress, Peritoneal Angiogenesis, and Tumor Growth in Mice. Molecules 2020, 25, 5583. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-T.; Xiao, Y.-B.; Hsu, S.-H.; Chang, S.-W.; Chou, C.-C. Molecular Interactions of Tannic Acid and Matrix Metalloproteinases 2 and 9. Comput. Struct. Biotechnol. J. 2023, 21, 2792–2800. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Potential Strategies for Overcoming Drug Resistance Pathways Using Propolis and Its Polyphenolic/Flavonoid Compounds in Combination with Chemotherapy and Radiotherapy. Nutrients 2024, 16, 3741. [Google Scholar] [CrossRef]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.-D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Wahyuningtyas, R.; Aui, S.P.; Chang, K.T. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J. Cell. Mol. Med. 2019, 23, 1257–1267. [Google Scholar] [CrossRef]
- Iwata, C.; Kano, M.R.; Komuro, A.; Oka, M.; Kiyono, K.; Johansson, E.; Morishita, Y.; Yashiro, M.; Hirakawa, K.; Kaminishi, M.; et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007, 67, 10181–10189. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Pinto, S.; Pahl, J.; Schottelius, A.; Carter, P.J.; Koch, J. Reimagining antibody-dependent cellular cytotoxicity in cancer: The potential of natural killer cell engagers. Trends Immunol. 2022, 43, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Q.; Hu, Q.; Liu, Y.; Zhang, L.; Liu, R. Tannins in Terminalia bellirica inhibits hepatocellular carcinoma growth via re-educating tumor-associated macrophages and restoring CD8+T cell function. Biomed. Pharmacother. 2022, 154, 113543. [Google Scholar] [CrossRef]
- Li, D.; Fan, Y.; He, B.; Tang, Y.; Deng, G.; Guo, R.; Deng, M.; Tang, D. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-Monocyte Ratio in Patients with Hepatocellular Carcinoma Receiving HAIC-Based Conversion Hepatectomy: A Dual-Center Retrospective Cohort Study. J Inflamm Res. 2025, 18, 8675–8688. [Google Scholar] [CrossRef]
- Heshmat-Ghahdarijani, K.; Sarmadi, V.; Heidari, A.; Marvasti, A.F.; Neshat, S.; Raeisi, S. The neutrophil-to-lymphocyte ratio as a new prognostic factor in cancers: A narrative review. Front. Oncol. 2023, 13, 1228076. [Google Scholar] [CrossRef]
- Sun, X.; Gui, Y.; Yang, T.; Chen, L.; Zhang, Y.; Yan, L.; Chen, W.; Wang, B. PD-L1+ neutrophils induced NETs in malignant ascites is a potential biomarker in HCC. Cancer Immunol. Immunother. 2024, 73, 254. [Google Scholar] [CrossRef]
- Oršolić, N.; Car, N. Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo. Tumor Biol. 2014, 35, 6445–6454. [Google Scholar] [CrossRef]
- Mišík, M.; Staudinger, M.; Kundi, M.; Worel, N.; Nersesyan, A.; Ferk, F.; Dusinska, M.; Azqueta, A.; Møller, P.; Knasmueller, S. Use of the single cell gel electrophoresis assay for the detection of DNA-protective dietary factors: Results of human intervention studies. Mutat. Res.-Rev. Mutat. Res. 2023, 791, 108458. [Google Scholar] [CrossRef] [PubMed]
- Daza, P.; Torreblanca, J.; Moreno, F.J. The comet assay differentiates efficiently and rapidly between genotoxins and cytotoxins in quiescent cells. Cell Biol. Int. 2004, 28, 497–502. [Google Scholar] [CrossRef]
- Ančić, D.; Oršolić, N.; Odeh, D.; Tomašević, M.; Pepić, I.; Ramić, S. Resveratrol and Its Nanocrystals: A Promising Approach for Cancer Therapy? Toxicol. Appl. Pharmacol. 2022, 435, 115851. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V.K.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef]
- Bergami-Santos, P.C.; Mariano, M.; Barbuto, J.A. Dual role of polymorphonuclear neutrophils on the growth of Ehrlich ascites tumor (EAT) in mice. Life Sci. 2004, 75, 245–255. [Google Scholar] [CrossRef]
- Feitosa, I.B.; Mori, B.; Teles, C.B.G.; da Costa, A.G. What are the immune responses during the growth of Ehrlich’s tumor in ascitic and solid form? Life Sci. 2021, 264, 118578. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Cho, J.Y. Comparative oncology: Overcoming human cancer through companion animal studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Yoshida, K.; Hasegawa, S.; Wada, H.; Yasui, M.; Tahara, H. Significance of mouse xenograft tumor model using patient-derived cancer organoids for clinical drug development. Front. Oncol. 2025, 15, 1485886. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 40, 432–444. [Google Scholar] [CrossRef]
- European Parliament and of the Council. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 13 July 2025).
- European Union Commission. Commission Implementing Decision (EU) 2020/569 of 16 April 2020 Establishing a Common Format and Information Content for the Submission of the Information to Be Reported by Member States Pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes and Repealing Commission Implementing Decision 2012/707/EU, Official Journal of the European Union, 2020.Annex I of the Commission Implementing Decision (EU) 2020/569 of 16 April 2020 Establishing a Common Format and Information Content for the Submission of the Information to be Reported by the Member States Pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes Repealing Commission Implementing Decision 2012/707/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020D0569 (accessed on 13 July 2025).
- Narodne Novine. Zakon o zaštiti životinja, Narodne novine (Law on Animal Welfare), Zagreb, Croatia, Narodne Novine, 102/17. 2017. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2017_10_102_2342.html (accessed on 13 July 2025).
- Narodne Novine. Zakon o Izmjenama i Dopunama Zakona o Zaštiti Životinja (Law on Amendments to the Law on Animal Welfare), Zagreb, Croatia, Narodne Novine, 32/19. 2019. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_03_32_656.html (accessed on 13 July 2025).
- Narodne Novine. Pravilnik o Zaštiti Životnja Koje se Koriste u Znanstvene Svrhe (Regulation on the Protection of Animals Used for Scientific Purposes), Zagreb, Croatia, Narodne Novine, 55/13. 2013. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2013_05_55_1129.html (accessed on 13 July 2025).
- Narodne Novine. Pravilnik o izmjenama Pravilnika o zaštiti Životnja Koje se Koriste u Znanstvene Svrhe (Law on Amendments to Regulation on the Protection of Animals Used for Scientific Purposes), Zagreb, Croatia, Narodne Novine, 116/19. 2019. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_11_116_2320.html (accessed on 13 July 2025).
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 13 July 2025).
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]




| Experimental Group a | Total Number of Cells (×106) | Min–Max Value (×106) | Inhibition of Tumor Growth (%) | Volume of Ascitic Fluid | Min–Max Value | Reduction in Ascitic Fluid Volume (%) | Animal Weight Change (%) |
|---|---|---|---|---|---|---|---|
| Control | 891.78 ± 76.32 | 672.00–1192.90 | 14.00 ± 0.43 | 12.10–15.60 | 24.40 ± 1.08 | ||
| TA 5 mg/kg | 223.98 ± 27.73 ** | 145.60–321.20 | 74.89 | 13.10 ± 0.45 | 11.20–14.60 | 6.43 | 22.17 ± 0.73 |
| TA 10 mg/kg | 223.14 ± 28.40 ** | 156.00–310.80 | 74.98 | 12.57 ± 0.85 | 11.00–14.80 | 10.2 | 21.30 ± 3.45 |
| Experimental Group a | Differential Analysis of Ascites Cells in Peritoneal Lavage Fluid (Mean ± SE) | ||||||
|---|---|---|---|---|---|---|---|
| Tumor Cells (%) | Lymphocytes (%) | Macrophages (%) | Neutrophils (%) | Basophils (%) | Eosinophils (%) | P/M | |
| Control | 73.75 ± 3.57 | 0.38 ± 0.24 | 11.00 ± 1.79 | 12.50 ± 3.34 | 2.00 ± 0.54 | 0.38 ± 0.24 | 1.31 |
| TA 5 mg/kg | 56.81 ± 4.12 * | 0.44 ± 0.20 | 11.37 ± 1.92 | 31.31 ± 2.92 * | 0.25 ± 0.13 * | 0.25 ± 0.19 | 2.80 |
| TA 10 mg/kg | 55.31 ± 3.61 * | 0.69 ± 0.38 | 21.38 ± 3.28 ** □ | 21.44 ± 4.13 | 0.56 ± 0.33 | 0.63 ± 0.63 | 1.02 |
| Experimental Group a | Absolute Number of Differential Blood Count (Mean ± SE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Leucocyte Number (×109) | Lymphocytes (×109) | Macrophages (×109) | Neutrophils (×109) | Basophils (×109) | Eosinophils (×109) | NLR | LMR | P/M | |
| Control | 5.48 ± 2.95 | 0.76 ± 0.15 (36.80%) | 0.31 ± 0.09 (10.33%) | 4.27 ± 2.92 (47.11%) | 0.07 ± 0.01 (2.94%) | 0.07 ± 0.02 (2.79%) | 5.63 | 2.41 | 4.09 |
| TA 5 mg/kg | 4.79 ± 1.42 | 1.34 ± 0.23 (34.10%) | 0.64 ± 0.26 (15.35%) | 2.75 ± 1.32 (49.06%) | 0.06 ± 0.01 (0.15%) | 0.01 ± 0.00 (1.34%) | 2.05 | 2.10 | 1.42 |
| TA 10 mg/kg | 9.17 ± 2.62 ** | 2.88 ± 0.53 ** □ (42.23%) | 0.37 ± 0.08 (5.54%) | 5.78 ± 2.14 (50.40%) | 0.06 ± 0.01 (0.74%) | 0.07 ± 0.03 (1.08%) | 2.00 | 7.82 | 1.82 |
| Experimental Group a | NO Levels in Supernatant of Macrophages or Ascitic Fluid (µM) (Mean ± SE) | Arg 1 Levels in Supernatant of Macrophages or Ascitic Fluid (µM) (Mean ± SE) | ||||
|---|---|---|---|---|---|---|
| Spleen Macrophages | Ascites Macrophages | Ascites Fluid | Spleen Macrophages | Ascites Macrophages | Ascites Fluid | |
| Control | 15.87 ± 0.91 | 13.48 ± 0.75 | 20.26 ± 1.22 | 1027.41 ± 82.44 | 1048.57 ± 74.43 | 38,576.78 ± 2375.677 |
| TA 5 mg/kg | 15.86 ± 1.37 | 19.29 ± 2.32 | 33.33 ± 1.61 ** | 446.85 ± 72.64 ** | 590.19 ± 85.39 * | 32,022.47 ± 874.395 * |
| TA 10 mg/kg | 22.06 ± 0.96 ** □□ | 18.71 ± 1.79 | 21.81 ± 2.65 | 273.10 ± 4.13 *** | 767.65 ± 74.35 * | 26,392.79 ± 482.421 ** |
| Experimental Group a | Ascites Macrophages (pg/mL) | Ascites Fluid (pg/mL) | Tumor Cells (pg/mL) |
|---|---|---|---|
| Control | 30.22 ± 3.58 | 1631.31 ± 39.7592 | 918.92 ± 110.247 |
| TA 5 mg/kg | 5.08 ± 1.02 **** | 1430.44 ± 50.8062 * | 344.44 ± 44.08 ** |
| TA 10 mg/kg | 7.66 ± 1.00 **** | 1358.08 ± 128.439 | 453.50 ± 18.02 ** |
| Experimental Group a | Microvessel Density | Minimum | Maximum |
|---|---|---|---|
| Control | 16.33 ± 4.14 | 9.00 | 23.33 |
| TA 5 mg/kg | 3.55 ± 0.48 * | 2.66 | 4.33 |
| TA 10 mg/kg | 3.00 ± 0.51 * | 2.33 | 4.00 |
| Experimental Group a | Ascites Macrophages (pg/mL) | Tumor Cells (pg/mL) |
|---|---|---|
| Control | 124.66 ± 1.92 | 1873.00 ± 17.77 |
| TA 5 mg/kg | 18.00 ± 2.35 **** | 851.00 ± 83.81 *** |
| TA 10 mg/kg | 28.31 ± 5.49 **** | 896.00 ± 176.57 ** |
| Experimental Group a | Concentration of MMP-2 (ng/mL) | |
|---|---|---|
| Ascites Macrophages | Tumor Cells | |
| Control | 5.13 ± 0.40 | 68.06 ± 4.46 |
| TA 5 mg/kg | 1.47 ± 0.25 *** | 55.60 ± 2.35 * |
| TA 10 mg/kg | 0.81 ± 0.27 *** | 54.28 ± 3.48 * |
| Concentration of MMP-9 (ng/mL) | ||
| Control | 6.97 ± 1.61 | 48.77 ± 3.7 |
| TA 5 mg/kg | 2.15 ± 1.83 | 40.31 ± 0.36 * |
| TA 10 mg/kg | 3.58 ± 2.06 | 39.97 ± 0.78 * |
| Experimental Group a | Comet Assay Parameters of Whole Blood Cells (Mean ± SE) | |||||
|---|---|---|---|---|---|---|
| Tail Length (µm) | FC | Tail DNA % | FC | Tail Moment | FC | |
| Control | 15.83 ± 017 | 2.94 ± 0.33 | 0.38 ± 0.04 | |||
| TA 5 mg/kg | 15.37 ± 1.42 | 0.97 | 8.15 ± 0.44 *** | 2.77 | 0.87 ± 0.05 *** | 2.29 |
| TA 10 mg/kg | 15.56 ± 2.62 | 0.98 | 5.6 ± 0.26 *** □□□ | 1.90 | 0.67 ± 0.03 *** □□ | 1.76 |
| Comet assay parameters of tumor cells (Mean ± SE) | ||||||
| Control | 17.60 ± 0.27 | 6.60 ± 0.57 | 0.97 ± 0.08 | |||
| TA 5 mg/kg | 19.07 ± 0.23 *** | 1.08 | 9.75 ± 0.51 *** | 1.48 | 1.33 ± 0.07 ** | 1.37 |
| TA 10 mg/kg | 18.04 ± 0.24 | 1.02 | 9.94 ± 0.47 *** | 1.51 | 1.28 ± 0.06 * | 1.32 |
| Groups a | Hematological Parameters (Mean ± SE) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L (109/L) | E (1012/L) | Hgb (g/L) | Hct (L/L) | MCV (fL) | MCH (pg) | MCHC (g/L) | RDW (%) | Plt (109/L) | MPV (fL) | |
| EAT Control | 5.48 ± 2.95 | 7.08 ± 0.39 | 112.95 ± 5.20 | 0.37 ± 0.02 | 104.75 ± 0.46 | 31.95 ± 0.34 | 610.50 ± 5.32 | 14.23 ± 0.56 | 1262.50 ± 240.70 | 8.70 ± 3.33 |
| TA 5 mg/kg | 4.79 ± 1.42 | 7.47 ± 0.48 | 120.80 ± 7.46 | 0.40 ± 0.02 | 107.40 ± 2.16 | 32.40 ± 0.31 | 602.67 ± 7.51 | 14.70 ± 0.81 | 1676.00 ± 64.84 | 9.48 ± 3.91 |
| TA 10 mg/kg | 9.17 ± 2.62 | 8.42 ± 0.23 | 134.45 ± 4.15 | 0.44 ± 0.01 | 104.40 ± 0.47 | 31.95 ± 0.22 | 611.50 ± 2.50 | 14.95 ± 0.41 | 1325.50 ± 193.10 | 9.20 ± 3.94 |
| Groups a | Biochemical Parameters (Mean ± SE) | |||||||
|---|---|---|---|---|---|---|---|---|
| AST (U/L) | ALT (U/L) | LDH (U/L) | AMY (U/L) | CRP (mg/L) | GLU (mmol/L) | Urea (mmol/L) | TBIL (mmol/L) | |
| EAT Control | 421.25 ± 43.27 | 381.25 ± 109.02 | 12,672.50 ± 1865.48 | 3077.50 ± 142.16 | 0.38 ± 0.13 | 4.00 ± 0.58 | 5.50 ± 0.35 | 1.67 ± 1.25 |
| TA 5 mg/kg | 593.75 ± 68.29 | 665.25 ± 157.99 | 14,648.75 ± 1457.53 | 2516.25 ± 301.26 | 0.25 ± 0.14 | 4.25 ± 0.95 | 7.50 ± 0.74 | 2.31 ± 0.72 |
| TA 10 mg/kg | 475.00 ± 78.61 | 683.75 ± 193.20 | 10,385.00 ± 1787.02 | 3335.00 ± 223.55 | 0.38 ± 0.13 | 3.25 ± 0.25 | 5.75 ± 0.92 | 2.50 ± 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oršolić, N.; Kunštić, M.; Jazvinšćak Jembrek, M. Antitumor and Antiangiogenic Effect of Tannic Acid in the Advanced Stage of Ehrlich Ascites Tumor in Mice. Int. J. Mol. Sci. 2025, 26, 9070. https://doi.org/10.3390/ijms26189070
Oršolić N, Kunštić M, Jazvinšćak Jembrek M. Antitumor and Antiangiogenic Effect of Tannic Acid in the Advanced Stage of Ehrlich Ascites Tumor in Mice. International Journal of Molecular Sciences. 2025; 26(18):9070. https://doi.org/10.3390/ijms26189070
Chicago/Turabian StyleOršolić, Nada, Martina Kunštić, and Maja Jazvinšćak Jembrek. 2025. "Antitumor and Antiangiogenic Effect of Tannic Acid in the Advanced Stage of Ehrlich Ascites Tumor in Mice" International Journal of Molecular Sciences 26, no. 18: 9070. https://doi.org/10.3390/ijms26189070
APA StyleOršolić, N., Kunštić, M., & Jazvinšćak Jembrek, M. (2025). Antitumor and Antiangiogenic Effect of Tannic Acid in the Advanced Stage of Ehrlich Ascites Tumor in Mice. International Journal of Molecular Sciences, 26(18), 9070. https://doi.org/10.3390/ijms26189070








