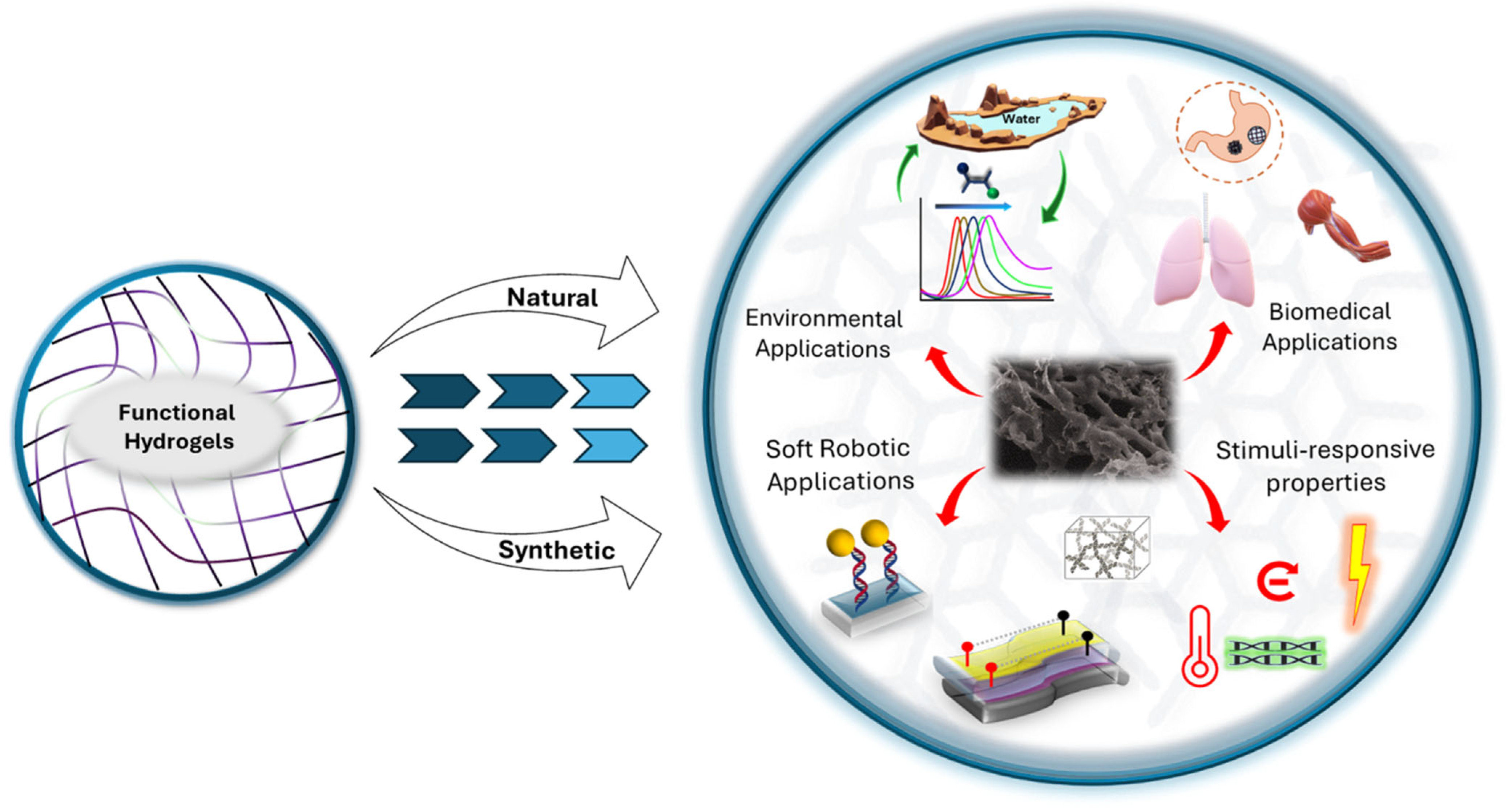
Functional Hydrogels: A Promising Platform for Biomedical and Environmental Applications
Abstract
1. Introduction
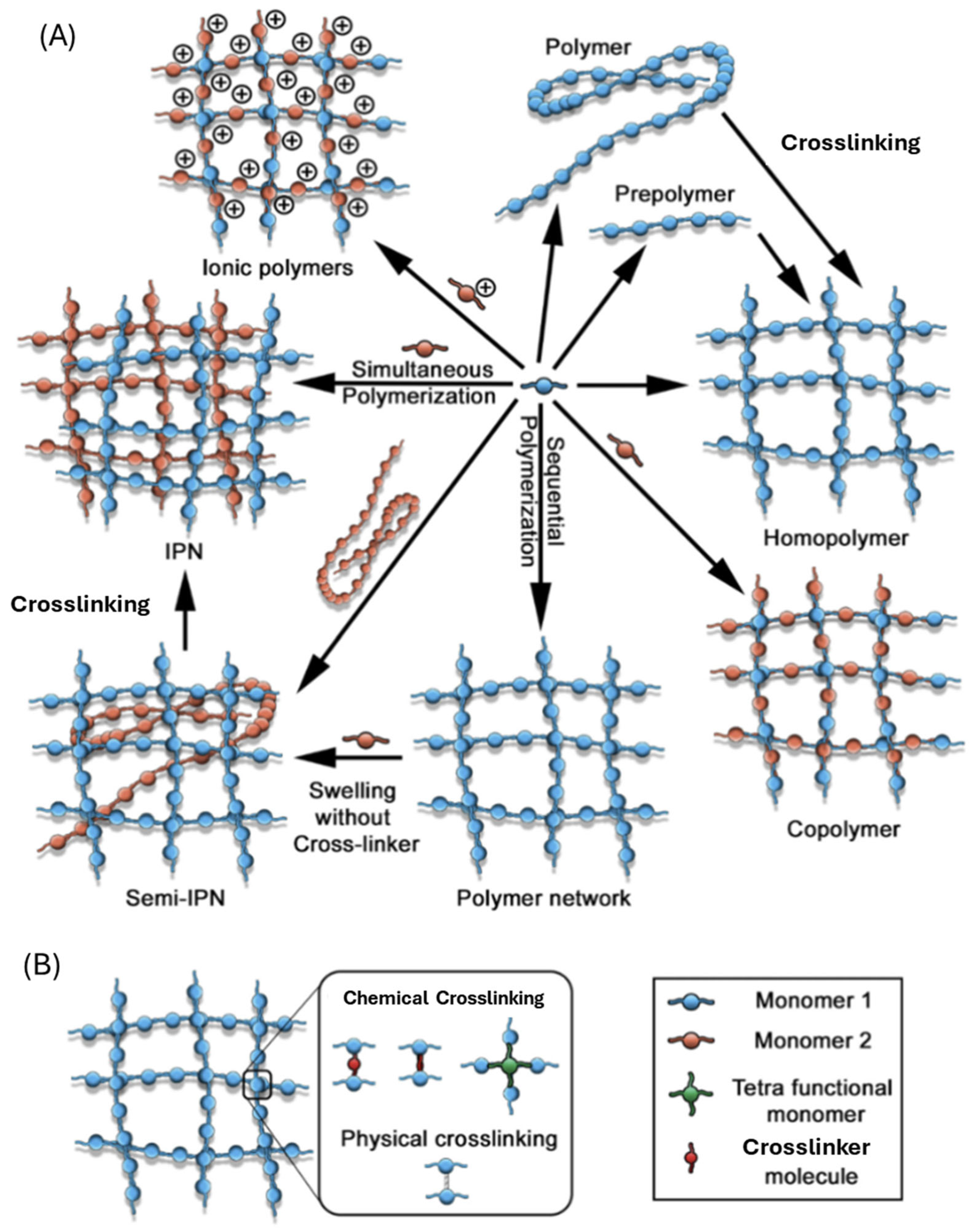
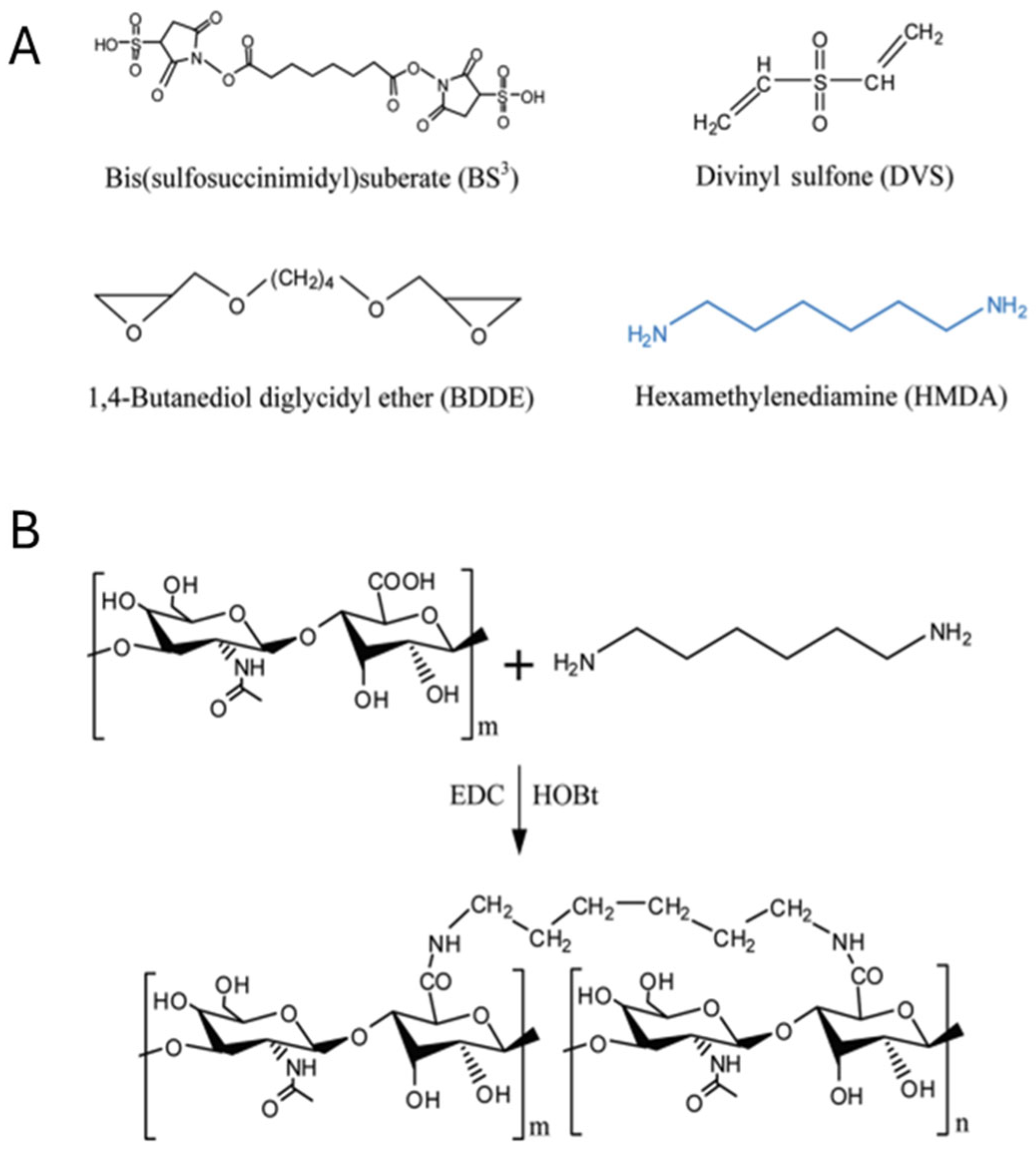
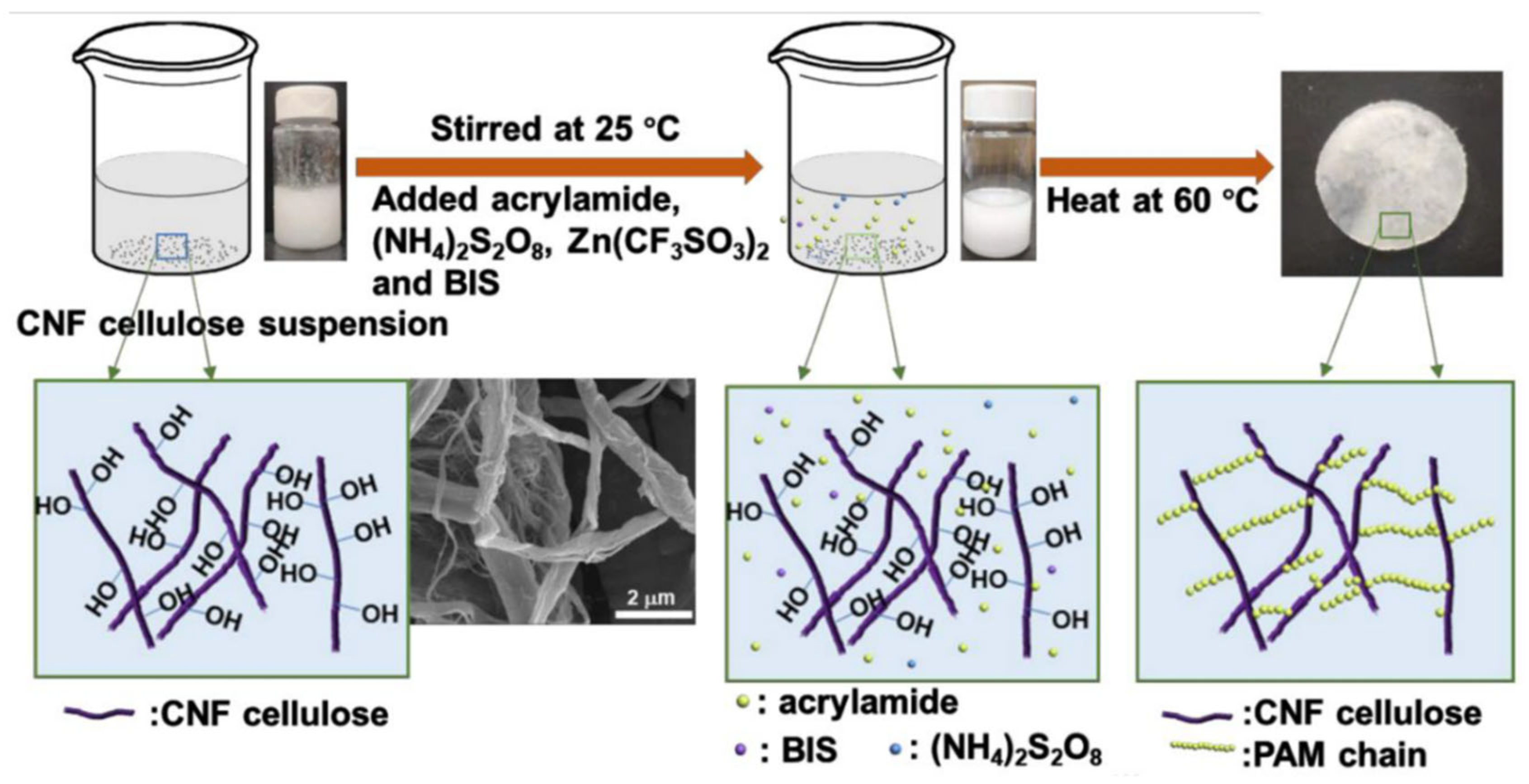
2. Rational Design Strategies for Functional Hydrogels
3. Biomedical Applications of Functional Hydrogels
4. Environmental and Soft Robotics Applications of Functional Hydrogels
5. The Role of Functional Hydrogels in Clinical Settings
6. Challenges, Limitations, and Safety Considerations
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FDA EPR | Food and Drug Administration Enhanced permeability and retention |
| MPS | Mononuclear phagocyte system |
| PEG | Polyethylene glycol |
| PA | Polyacrylamide |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PLGA | Poly(lactic-co-glycolic acid) |
| GMP | Good manufacturing product |
| Si-CaP | Silica calcium phosphate |
| PLLA | Poly-L-lactic acid |
| CaHA | Calcium hydroxylapatite |
| CMC | Carboxymethylcellulose |
| PHEMA | Poly 2-hydroxyethyl methacrylate |
| DBM | Demineralized bone matrix |
| HA | Hyaluronic acid |
| HSA | Human serum albumin |
| PMT | Pea metallothionein |
| DVS | Divinyl sulfone |
| BDDE | 1,4-butanediol diglycidyl ether |
References
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Tateishi-Karimata, H.; Sugimoto, N. Biological and Nanotechnological Applications Using Interactions between Ionic Liquids and Nucleic Acids. Biophys. Rev. 2018, 10, 931–940. [Google Scholar] [CrossRef]
- Dalwadi, C.; Patel, G. Application of Nanohydrogels in Drug Delivery Systems: Recent Patents Review. Recent. Pat. Nanotechnol. 2015, 9, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent Advance in Delivery System and Tissue Engineering Applications of Chondroitin Sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Dwivedi, S.; Purohit, P.; Misra, R.; Pareek, P.; Goel, A.; Khattri, S.; Pant, K.K.; Misra, S.; Sharma, P. Diseases and Molecular Diagnostics: A Step Closer to Precision Medicine. Indian. J. Clin. Biochem. 2017, 32, 374–398. [Google Scholar]
- Xie, S.; Ai, L.; Cui, C.; Fu, T.; Cheng, X.; Qu, F.; Tan, W. Functional Aptamer-Embedded Nanomaterials for Diagnostics and Therapeutics. ACS Appl. Mater. Interfaces 2021, 13, 9542–9560. [Google Scholar] [CrossRef]
- Ma, Y.; Song, M.; Li, L.; Lao, X.; Wong, M.; Hao, J. Advances in Upconversion Luminescence Nanomaterial-based Biosensor for Virus Diagnosis. Exploration 2022, 2, 20210216. [Google Scholar] [CrossRef]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart Gold Nanostructures for Light Mediated Cancer Theranostics: Combining Optical Diagnostics with Photothermal Therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Quazi, M.Z.; Kim, T.; Yang, J.; Park, N. Tuning Plasmonic Properties of Gold Nanoparticles by Employing Nanoscale DNA Hydrogel Scaffolds. Biosensors 2023, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Schwall, C.T.; Banerjee, I.A. Micro- and Nanoscale Hydrogel Systems for Drug Delivery and Tissue Engineering. Materials 2009, 2, 577–612. [Google Scholar] [CrossRef]
- Kambhampati, P. Nanoparticles, Nanocrystals, and Quantum Dots: What Are the Implications of Size in Colloidal Nanoscale Materials? J. Phys. Chem. Lett. 2021, 12, 4769–4779. [Google Scholar] [CrossRef]
- Teoh, J.Y.; Jeon, S.; Yim, B.; Yang, H.M.; Hwang, Y.; Kim, J.; Lee, S.K.; Park, E.; Kong, T.Y.; Kim, S.Y.; et al. Tuning Surface Plasmon Resonance Responses through Size and Crosslinking Control of Multivalent Protein Binding-Capable Nanoscale Hydrogels. ACS Biomater. Sci. Eng. 2022, 8, 2878–2889. [Google Scholar] [CrossRef]
- Samanta, A.; Medintz, I.L. Nanoparticles and DNA-a Powerful and Growing Functional Combination in Bionanotechnology. Nanoscale 2016, 8, 9037–9095. [Google Scholar] [CrossRef]
- Haraguchi, K. Nanocomposite Hydrogels. Curr. Opin. Solid. State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Xiang, M.; Zhu, M.; Yang, Z.; He, P.; Wei, J.; Gao, X.; Song, J. Dual-Functionalized Apatite Nanocomposites with Enhanced Cytocompatibility and Osteogenesis for Periodontal Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1704–1714. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite Hydrogels for Biomedical Applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired Nanocomposite Hydrogels with Highly Ordered Structures. Adv. Mater. 2017, 29, 1703045. [Google Scholar] [CrossRef]
- Su, C.H.; Cheng, F.Y. In Vitro and in Vivo Applications of Alginate/Iron Oxide Nanocomposites for Theranostic Molecular Imaging in a Brain Tumor Model. RSC Adv. 2015, 5, 90061–90064. [Google Scholar] [CrossRef]
- Kaur, K.; Paiva, S.S.; Caffrey, D.; Cavanagh, B.L.; Murphy, C.M. Injectable Chitosan/Collagen Hydrogels Nano-Engineered with Functionalized Single Wall Carbon Nanotubes for Minimally Invasive Applications in Bone. Mater. Sci. Eng. C 2021, 128, 112340. [Google Scholar] [CrossRef]
- Endres, T.K.; Beck-Broichsitter, M.; Samsonova, O.; Renette, T.; Kissel, T.H. Self-Assembled Biodegradable Amphiphilic PEG–PCL–LPEI Triblock Copolymers at the Borderline between Micelles and Nanoparticles Designed for Drug and Gene Delivery. Biomaterials 2011, 32, 7721–7731. [Google Scholar] [CrossRef]
- Fentahun Darge, H.; Yibru Hanurry, E.; Simegniew Birhan, Y.; Worku Mekonnen, T.; Tizazu Andrgie, A.; Chou, H.Y.; Lai, J.Y.; Tsai, H.C. Multifunctional Drug-Loaded Micelles Encapsulated in Thermo-Sensitive Hydrogel for in Vivo Local Cancer Treatment: Synergistic Effects of Anti-Vascular and Immuno-Chemotherapy. Chem. Eng. J. 2021, 406, 126879. [Google Scholar] [CrossRef]
- Yougen, L.I.; Tseng, Y.D.; Kwon, S.Y.; D’Espaux, L.; Bunch, J.S.; McEuen, P.L.; Luo, D. Controlled Assembly of Dendrimer-like DNA. Nat. Mater. 2004, 3, 38–42. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, T.; Peng, C.; Sheng, R.; Cao, A.; Cao, X.; Shen, M.; Guo, R.; Tomás, H.; Shi, X. Gene Delivery Using Dendrimer-Entrapped Gold Nanoparticles as Nonviral Vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.; Zhao, Q.; Wang, Z.; Xu, Z.; Jia, X. An Enzyme-Responsive Nanogel Carrier Based on PAMAM Dendrimers for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 19899–19906. [Google Scholar] [CrossRef]
- Luo, Y.; Ling, Y.; Guo, W.; Pang, J.; Liu, W.; Fang, Y.; Wen, X.; Wei, K.; Gao, X. Docetaxel Loaded Oleic Acid-Coated Hydroxyapatite Nanoparticles Enhance the Docetaxel-Induced Apoptosis through Activation of Caspase-2 in Androgen Independent Prostate Cancer Cells. J. Control. Release 2010, 147, 278–288. [Google Scholar] [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in Drug Delivery: Past, Present and Future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Pisal, D.S.; Kosloski, M.P.; Balu-Iyer, S.V. Delivery of Therapeutic Proteins. J. Pharm. Sci. 2010, 99, 2557–2575. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Frokjaer, S.; Otzen, D.E. Protein Drug Stability: A Formulation Challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, M.; Ji, C.; Tan, J.; Yuan, Q. Preparation, Applications, and Challenges of Functional DNA Nanomaterials. Nano Res. 2023, 16, 3895–3912. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Ma, W.; Zhan, Y.; Zhang, Y.; Mao, C.; Xie, X.; Lin, Y. The Biological Applications of DNA Nanomaterials: Current Challenges and Future Directions. Signal Transduct. Target. Ther. 2021, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Oh, J.K.; Siegwart, D.J.; Lee, H.; Sherwood, G.; Peteanu, L.; Hollinger, J.O.; Kataoka, K.; Matyjaszewski, K. Biodegradable Nanogels Prepared by Atom Transfer Radical Polymerization as Potential Drug Delivery Carriers: Synthesis, Biodegradation, In Vitro Release, and Bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of Engineered Nanomaterials: Focus on Biocompatibility, Biodistribution and Biodegradation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 361–373. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Li, M.; Yin, F.; Song, L.; Mao, X.; Li, F.; Fan, C.; Zuo, X.; Xia, Q. Nucleic Acid Tests for Clinical Translation. Chem. Rev. 2021, 121, 10469–10558. [Google Scholar] [CrossRef]
- Quazi, M.Z.; Park, N. DNA Hydrogel-Based Nanocomplexes with Cancer-Targeted Delivery and Light-Triggered Peptide Drug Release for Cancer-Specific Therapeutics. Biomacromolecules 2023, 24, 2127–2137. [Google Scholar] [CrossRef]
- Quazi, M.Z.; Lee, U.; Park, S.; Shin, S.; Sim, E.; Son, H.; Park, N. Cancer Cell-Specific Enhanced Raman Imaging and Photothermal Therapeutic Effect Based on Reversibly PH-Responsive Gold Nanoparticles. ACS Appl. Bio Mater. 2021, 4, 8377–8385. [Google Scholar] [CrossRef] [PubMed]
- Gładysz, M.Z.; Stevanoska, M.; Włodarczyk-Biegun, M.K.; Nagelkerke, A. Breaking through the Barrier: Modelling and Exploiting the Physical Microenvironment to Enhance Drug Transport and Efficacy. Adv. Drug Deliv. Rev. 2022, 184, 114183. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Betz, U.A.K.; Arora, L.; Assal, R.A.; Azevedo, H.; Baldwin, J.; Becker, M.S.; Bostock, S.; Cheng, V.; Egle, T.; Ferrari, N.; et al. Game Changers in Science and Technology—Now and Beyond. Technol. Forecast. Soc. Change 2023, 193, 122588. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (Epr) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Bhatti, R.; Shakeel, H.; Malik, K.; Qasim, M.; Khan, M.A.; Ahmed, N.; Jabeen, S. Inorganic Nanoparticles: Toxic Effects, Mechanisms of Cytotoxicity and Phytochemical Interactions. Adv. Pharm. Bull. 2022, 12, 757–762. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J. V Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A. Dos Strategic Role of Selected Noble Metal Nanoparticles in Medicine. Crit. Rev. Microbiol. 2015, 42, 696–719. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, B.R.; Lee, H.; Jo, S.D.; Kim, H.; Won, Y.Y.; Lee, J. Near-Infrared Plasmonic Assemblies of Gold Nanoparticles with Multimodal Function for Targeted Cancer Theragnosis. Sci. Rep. 2017, 7, 17327. [Google Scholar] [CrossRef]
- Vicario-De-la-torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud. Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steiger, C.; Lin, S.; Parada, G.A.; Liu, J.; Chan, H.F.; Yuk, H.; Phan, N.V.; Collins, J.; Tamang, S.; et al. Ingestible Hydrogel Device. Nat. Commun. 2019, 10, 493. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Health Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef] [PubMed]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943. [Google Scholar] [CrossRef] [PubMed]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanemaru, K.; Lazarek, M.; et al. Hydrogel-Based Biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.O.; Kim, S.; Park, N. Light-Responsible DNA Hydrogel-Gold Nanoparticle Assembly for Synergistic Cancer Therapy. J. Mater. Chem. B 2015, 3, 1537–1543. [Google Scholar] [CrossRef]
- Du, X.; Zhai, J.; Li, X.; Zhang, Y.; Li, N.; Xie, X. Hydrogel-Based Optical Ion Sensors: Principles and Challenges for Point-of-Care Testing and Environmental Monitoring. ACS Sens. 2021, 6, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.; Walia, S.; Mandal, B.B.; Ghoroi, C.; Bhatia, D. Functional DNA Based Hydrogels: Development, Properties and Biological Applications. ACS Biomater. Sci. Eng. 2020, 6, 6021–6035. [Google Scholar] [CrossRef]
- Bush, J.; Hu, C.H.; Veneziano, R. Mechanical Properties of DNA Hydrogels: Towards Highly Programmable Biomaterials. Appl. Sci. 2021, 11, 1885. [Google Scholar] [CrossRef]
- Kahn, J.S.; Hu, Y.; Willner, I. Stimuli-Responsive DNA-Based Hydrogels: From Basic Principles to Applications. Acc. Chem. Res. 2017, 50, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O.; et al. A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics 2018, 10, 16. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart Polymeric Hydrogels for Cartilage Tissue Engineering: A Review on the Chemistry and Biological Functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Health Mater. 2023, 12, e2300105. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Ernst, A.U.; Wang, L.H.; Shariati, K.; Wang, X.; Liu, Q.; Ma, M. Hydrogels in Emerging Technologies for Type 1 Diabetes. Chem. Rev. 2021, 121, 11458–11526. [Google Scholar] [CrossRef]
- Zhang, L.; Abbaspourrad, A.; Parsa, S.; Tang, J.; Cassiola, F.; Zhang, M.; Tian, S.; Dai, C.; Xiao, L.; Weitz, D.A. Core-Shell Nanohydrogels with Programmable Swelling for Conformance Control in Porous Media. ACS Appl. Mater. Interfaces 2020, 12, 34217–34225. [Google Scholar] [CrossRef]
- Guerrero-Ramírez, L.G.; Nũo-Donlucas, S.M.; Cesteros, L.C.; Katime, I. Novel Functionalized Nanohydrogels, Synthesis and Some Applications. J. Phys. Conf. Ser. 2008, 127, 012010. [Google Scholar] [CrossRef]
- Setia, A.; Ahuja, P. Nanohydrogels; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128136638. [Google Scholar]
- Yang, S.; Li, Z.; Li, S.; Zhang, J.; Huang, J.; Ren, J.; Wu, X. Advances in Drug Delivery Systems Based on Liposome-Composite Hydrogel Microspheres. J. Mater. Chem. B 2025. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Hong, Y.; Krsko, P.; Libera, M. Protein Surface Patterning Using Nanoscale PEG Hydrogels. Langmuir 2004, 20, 11123–11126. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Reza Farajollahi, A.; Akbar Entezami, A. Synthesis of Fast Response Crosslinked PVA-g-NIPAAm Nanohydrogels by Very Low Radiation Dose in Dilute Aqueous Solution. Radiat. Phys. Chem. 2013, 86, 145–154. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Vandermeulen, G.W.M.; Klok, H.A. Peptide/Protein Hybrid Materials: Enhanced Control of Structure and Improved Performance through Conjugation of Biological and Synthetic Polymers. Macromol. Biosci. 2004, 4, 383–398. [Google Scholar] [CrossRef]
- Pei, M.; Jia, X.; Zhao, X.; Li, J.; Liu, P. Alginate-Based Cancer-Associated, Stimuli-Driven and Turn-on Theranostic Prodrug Nanogel for Cancer Detection and Treatment. Carbohydr. Polym. 2018, 183, 131–139. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.H.; Qi, X.J.; Orbach, R.; Fadeev, M.; Yang, H.H.; Willner, I. Switchable Bifunctional Stimuli-Triggered Poly-N-Isopropylacrylamide/DNA Hydrogels. Angew. Chem.—Int. Ed. 2014, 53, 10134–10138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kuckling, D.; Kretschmer, K.; Choudhary, V.; Adler, H.J. Synthesis and Characterization of Stimuli-Sensitive Micro- and Nanohydrogels Based on Photocrosslinkable Poly(Dimethylaminoethyl Methacrylate). J. Polym. Sci. A Polym. Chem. 2007, 45, 669–679. [Google Scholar] [CrossRef]
- Karnoosh-Yamchi, J.; Mobasseri, M.; Akbarzadeh, A.; Davaran, S.; Ostad-Rahimi, A.R.; Hamishehkar, H.; Salehi, R.; Bahmani, Z.; Nejati-Koshki, K.; Darbin, A.; et al. Preparation of PH Sensitive Insulin-Loaded Nano Hydrogels and Evaluation of Insulin Releasing in Different PH Conditions. Mol. Biol. Rep. 2014, 41, 6705–6712. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. PH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef]
- Ren, J.; Hu, Y.; Lu, C.H.; Guo, W.; Aleman-Garcia, M.A.; Ricci, F.; Willner, I. PH-Responsive and Switchable Triplex-Based DNA Hydrogels. Chem. Sci. 2015, 6, 4190–4195. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 Dual-Targeted Multifunctional Hyaluronic Acid Nanogels Boost Protein Delivery to Ovarian and Breast Cancers in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic Acid-Based Nanogel–Drug Conjugates with Enhanced Anticancer Activity Designed for the Targeting of CD44-Positive and Drug-Resistant Tumors. Bioconjug Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Jin, S.; Wan, J.; Meng, L.; Huang, X.; Guo, J.; Liu, L.; Wang, C. Biodegradation and Toxicity of Protease/Redox/PH Stimuli-Responsive PEGlated PMAA Nanohydrogels for Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 19843–19852. [Google Scholar] [CrossRef]
- Kettel, M.J.; Schaefer, K.; Pich, A.; Moeller, M. Functional PMMA Nanogels by Cross-Linking with Cyclodextrin Methacrylate. Polymer 2016, 86, 176–188. [Google Scholar] [CrossRef]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-Based Polymer Hybrids for Thermo-Responsive Nanogel Delivery of Curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-Based Hybrid Nanogels: A Synergistic Nanoplatform for Combined Biosensing, Bioimaging, and Responsive Drug Delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Kawamura, A. Design of Nano- and Micro-Structured Molecule-Responsive Hydrogels. Polym. J. 2017, 49, 751–757. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Bui, H.L.; Nguyen, H.N.; Lai, J.Y.; Huang, C.J. Engineering Principles of Zwitterionic Hydrogels: Molecular Architecture to Manufacturing Innovations for Advanced Healthcare Materials. Mater. Today Bio. 2025, 33, 102085. [Google Scholar] [CrossRef]
- He, H.; Tang, Y.; Zheng, M.; Chang, Y.; Chen, H.; Wei, J.; Wu, J.; Zheng, J. Zwitterionic Hydrogels from Material Design to Wound Dressing Applications. Supramol. Mater. 2025, 4, 100108. [Google Scholar] [CrossRef]
- Butcher, N.J.; Mortimer, G.M.; Minchin, R.F. Unravelling the Stealth Effect. Nat. Nanotechnol. 2016, 11, 310–311. [Google Scholar] [CrossRef]
- Lu, J.; Gao, X.; Wang, S.; He, Y.; Ma, X.; Zhang, T.; Liu, X. Advanced Strategies to Evade the Mononuclear Phagocyte System Clearance of Nanomaterials. Exploration 2023, 3, 20220045. [Google Scholar] [CrossRef]
- Cui, T.; Li, X.; He, S.; Xu, D.; Yin, L.; Huang, X.; Deng, S.; Yue, W.; Zhong, W. Instant Self-Assembly Peptide Hydrogel Encapsulation with Fibrous Alginate by Microfluidics for Infected Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and Controlled Release of Bioactive Compounds in Lactoferrin-Glycomacropeptide Nanohydrogels: Curcumin and Caffeine as Model Compounds. J. Food Eng. 2016, 180, 110–119. [Google Scholar] [CrossRef]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, T.; Fan, Q.; Liu, D. A PH-Triggered, Fast-Responding DNA Hydrogel. Angew. Chem.—Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef]
- De Coen, R.; Vanparijs, N.; Risseeuw, M.D.P.; Lybaert, L.; Louage, B.; De Koker, S.; Kumar, V.; Grooten, J.; Taylor, L.; Ayres, N.; et al. PH-Degradable Mannosylated Nanogels for Dendritic Cell Targeting. Biomacromolecules 2016, 17, 2479–2488. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Pradhan, A.; Banerjee, R.; Bahadur, D. Dual PH and Temperature Stimuli-Responsive Magnetic Nanohydrogels for Thermo-Chemotherapy. J. Nanosci. Nanotechnol. 2014, 14, 4082–4089. [Google Scholar] [CrossRef] [PubMed]
- Pitakchatwong, C.; Chirachanchai, S. Thermo-Magnetoresponsive Dual Function Nanoparticles: An Approach for Magnetic Entrapable-Releasable Chitosan. ACS Appl. Mater. Interfaces 2017, 9, 10398–10407. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent Advances in Smart Hydrogels for Biomedical Applications: From Self-Assembly to Functional Approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical Applications of Functional Hydrogels: Innovative Developments, Relevant Clinical Trials and Advanced Products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama-Elbert, S.E. Incorporation of Heparin into Biomaterials. Acta Biomater. 2014, 10, 1581–1587. [Google Scholar] [CrossRef]
- Mauri, E.; Perale, G.; Rossi, F. Nanogel Functionalization: A Versatile Approach to Meet the Challenges of Drug and Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 6525–6541. [Google Scholar] [CrossRef]
- Chiu, T.C.; Huang, C.C. Aptamer-Functionalized Nano-Biosensors. Sensors 2009, 9, 10356–10388. [Google Scholar] [CrossRef]
- Gelmi, A.; Schutt, C.E. Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control. Adv. Heal. Mater. 2021, 10, 2001125. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Zhang, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Qin, K.; Li, S.; Gao, Z. Stimuli-Responsive DNA-Based Hydrogels for Biosensing Applications. J. Nanobiotechnol. 2022, 20, 40. [Google Scholar] [CrossRef]
- Lee, M.; Lee, M.; Kim, S.; Park, N. Stimuli-Responsive DNA Hydrogel Design Strategies for Biomedical Applications. Biosensors 2025, 15, 355. [Google Scholar] [CrossRef]
- Wang, D.; Duan, J.; Liu, J.; Yi, H.; Zhang, Z.; Song, H.; Li, Y.; Zhang, K. Stimuli-Responsive Self-Degradable DNA Hydrogels: Design, Synthesis, and Applications. Adv. Heal. Mater. 2023, 12, 2203031. [Google Scholar] [CrossRef]
- Yeom, J.; Bhang, S.H.; Kim, B.-S.; Seo, M.S.; Hwang, E.J.; Cho, I.H.; Park, J.K.; Hahn, S.K. Effect of Cross-Linking Reagents for Hyaluronic Acid Hydrogel Dermal Fillers on Tissue Augmentation and Regeneration. Bioconjug Chem. 2010, 21, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liang, X.; Wang, Q.; Wang, M.; Li, Z.; Sun, G. A Semi-Interpenetrating Network Ionic Composite Hydrogel with Low Modulus, Fast Self-Recoverability and High Conductivity as Flexible Sensor. Carbohydr. Polym. 2020, 248, 116797. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, C.; Wu, Q.; Xie, W.; Kim, W.-Y.; Lee, S.-Y.; Gwon, J. A Stretchable Solid-State Zinc Ion Battery Based on a Cellulose Nanofiber–Polyacrylamide Hydrogel Electrolyte and a Mg0.23V2O5·1.0H2O Cathode. J. Mater. Chem. A Mater. 2020, 8, 18327–18337. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids as a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Duan, H.; Yin, L.; Chen, T.; Qi, D.; Zhang, D. A “Metal Ions-Induced Poisoning Behavior of Biomolecules” Inspired Polymeric Probe for Cu2+ Selective Detection on Basis of Coil to Helix Conformation Transition. Eur. Polym. J. 2022, 167, 111070. [Google Scholar] [CrossRef]
- Esser-Kahn, A.P.; Francis, M.B. Protein-Cross-Linked Polymeric Materials through Site-Selective Bioconjugation. Angew. Chem.—Int. Ed. 2008, 47, 3751–3754. [Google Scholar] [CrossRef]
- Singh, A.; Shah, S.S.; Sharma, C.; Gupta, V.; Sundramoorthy, A.K.; Kumar, P.; Arya, S. An Approach towards Different Techniques for Detection of Heavy Metal Ions and Their Removal from Waste Water. J. Env. Chem. Eng. 2024, 12, 113032. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, M.; Chen, D. Electrosorption of Uranium(VI) by Highly Porous Phosphate-Functionalized Graphene Hydrogel. Appl. Surf. Sci. 2019, 484, 83–96. [Google Scholar] [CrossRef]
- Dave, N.; Chan, M.Y.; Huang, P.J.J.; Smith, B.D.; Liu, J. Regenerable DNA-Functionalized Hydrogels for Ultrasensitive, Instrument-Free Mercury(II) Detection and Removal in Water. J. Am. Chem. Soc. 2010, 132, 12668–12673. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, B.; Celikkol, A.N. Pullulan Based Hydrogels for the Removal of Various Metal Ions from Aqueous Solutions. J. Env. Chem. Eng. 2021, 9, 106188. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Hamada, S.; Yancey, K.G.; Pardo, Y.; Gan, M.; Vanatta, M.; An, D.; Hu, Y.; Derrien, T.L.; Ruiz, R.; Liu, P.; et al. Dynamic DNA Material with Emergent Locomotion Behavior Powered by Artificial Metabolism. Sci. Robot. 2019, 4, eaaw3512. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, X.; Yang, L.; Chang, Z.; Luo, S. Progress toward Hydrogels in Removing Heavy Metals from Water: Problems and Solutions—A Review. ACS EST Water 2021, 1, 1098–1116. [Google Scholar] [CrossRef]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic Skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef]
- Cheng, S.; Narang, Y.S.; Yang, C.; Suo, Z.; Howe, R.D. Stick-On Large-Strain Sensors for Soft Robots. Adv. Mater. Interfaces 2019, 6, 1900985. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.; Bang, J.; Jung, M.; Park, S.; Yun, H.; Kwak, H.W. PH-Sensitive Cellulose/Chitin Nanofibrillar Hydrogel for Dye Pollutant Removal. Carbohydr. Polym. 2023, 317, 121090. [Google Scholar] [CrossRef]
- Ma, J.X.; Gong, S.H.; Cheng, Y.J.; Cao, W.; Wei, X.H.; Wang, P.; Xia, D.Y. A pillararene-based supramolec-ular polymer hydrogel for removal of organic dyes from water. Mater. Chem. Front. 2025, 9, 451–459. [Google Scholar] [CrossRef]
- Fulya Taktak, F.; Gokce, S. Efficient Oxytetracycline Removal Using Poly(DMAEMA)@TiO2 Nanocomposite Hydrogel: Real Wastewater Application from a Cattle Farm and Water Quality Improvement. Int. J. Environ. Sci. Technol. 2025, 22, 13393–13412. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic Contamination of River Beds Significantly Reduced by Catchment-Wide Flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.; Park, S.; Bang, J.; Yun, H.; Won, S.; Kim, S.; Lim, H.; Kim, S.-G.; Kim, J.-C.; et al. Nature-Derived Hydrogel for Microplastic Removal. Adv. Compos. Hybrid. Mater. 2025, 8, 346. [Google Scholar] [CrossRef]
- Dutta, S.; Misra, A.; Bose, S. Polyoxometalate Nanocluster-Infused Triple IPN Hydrogels for Excellent Microplastic Removal from Contaminated Water: Detection, Photodegradation, and Upcycling. Nanoscale 2024, 16, 5188–5205. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the Clinic: An Update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels 2021, 7, 207. [Google Scholar] [CrossRef]
- Kechkeche, D.; El Mousli, S.; Poujouly, C.; Secret, E.; Dupuis, V.; Le Potier, I.; Goriot, M.E.; Siracusa, J.; Banzet, S.; Gamby, J.; et al. Strain Promoted Azide Alkyne Cycloaddition, an Efficient Surface Functionalization Strategy for MicroRNA Magnetic Separation. Next Mater. 2025, 6, 100409. [Google Scholar] [CrossRef]
- Avti, P.K.; Maysinger, D.; Kakkar, A. Alkyne-Azide “Click” Chemistry in Designing Nanocarriers for Applications in Biology. Molecules 2013, 18, 9531–9549. [Google Scholar] [CrossRef]
- Alli, Y.A.; Anuar, H.; Bamisaye, A.; Manshor, M.R.; Etafo, N.O.; Bamidele, M.O.; Rasheed, M.A.; Olatunde, S.K.; Akinfenwa, A.S.; Lawal, A. The Appealing Prospect of Hydrogel in 3D/4D Printing Technology: Overview and Opportunities. Polymer 2024, 315, 127823. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI Energized Hydrogel Design, Optimization and Application in Biomedicine. Mater. Today Bio. 2024, 25, 101014. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.E.; Hindley, J.W.; Baxani, D.K.; Ces, O.; Elani, Y. Hydrogels as Functional Components in Artificial Cell Systems. Nat. Rev. Chem. 2022, 6, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B. Exploring the Potential of Artificial Intelligence for Hydrogel Development—A Short Review. Gels 2023, 9, 845. [Google Scholar] [CrossRef] [PubMed]

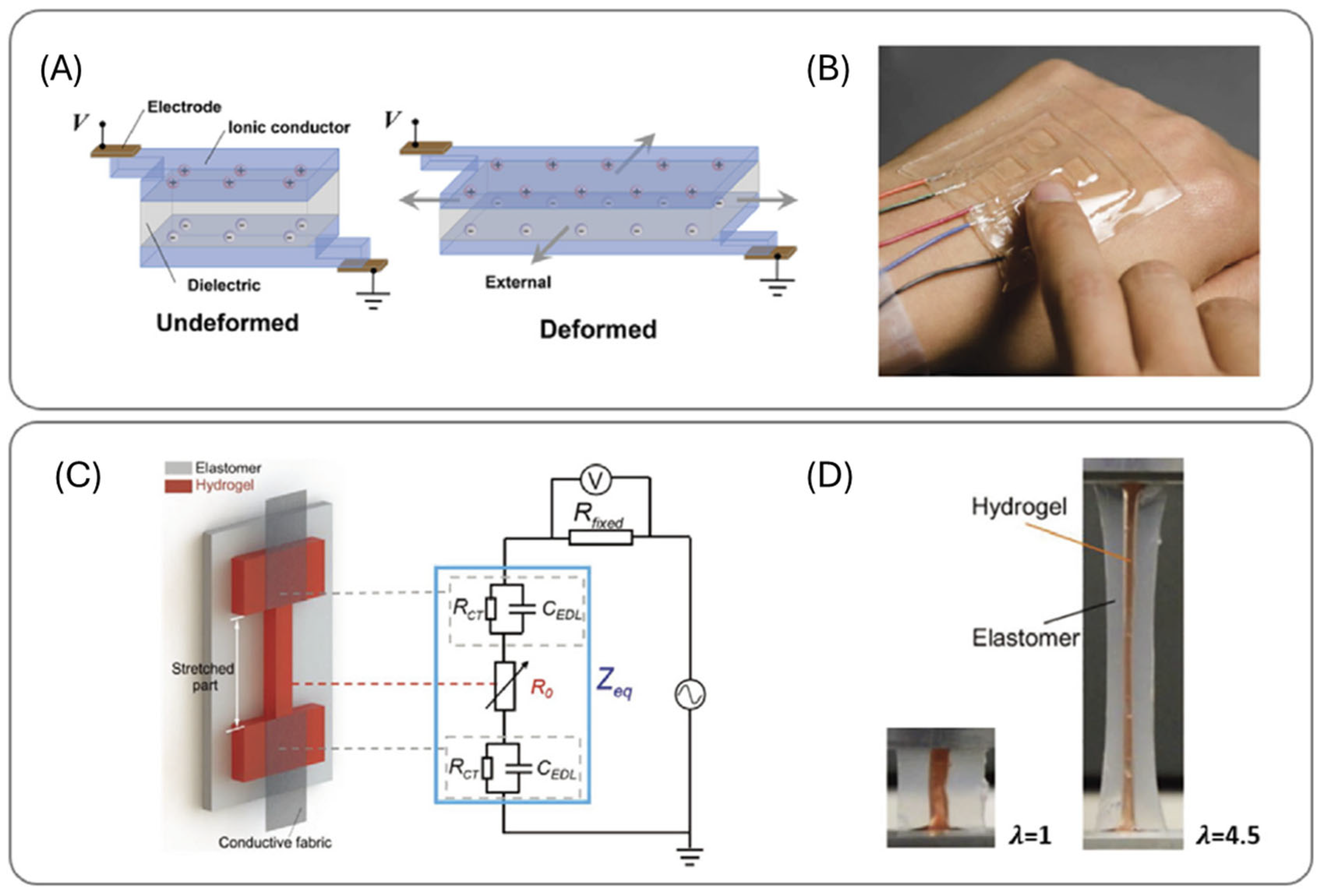
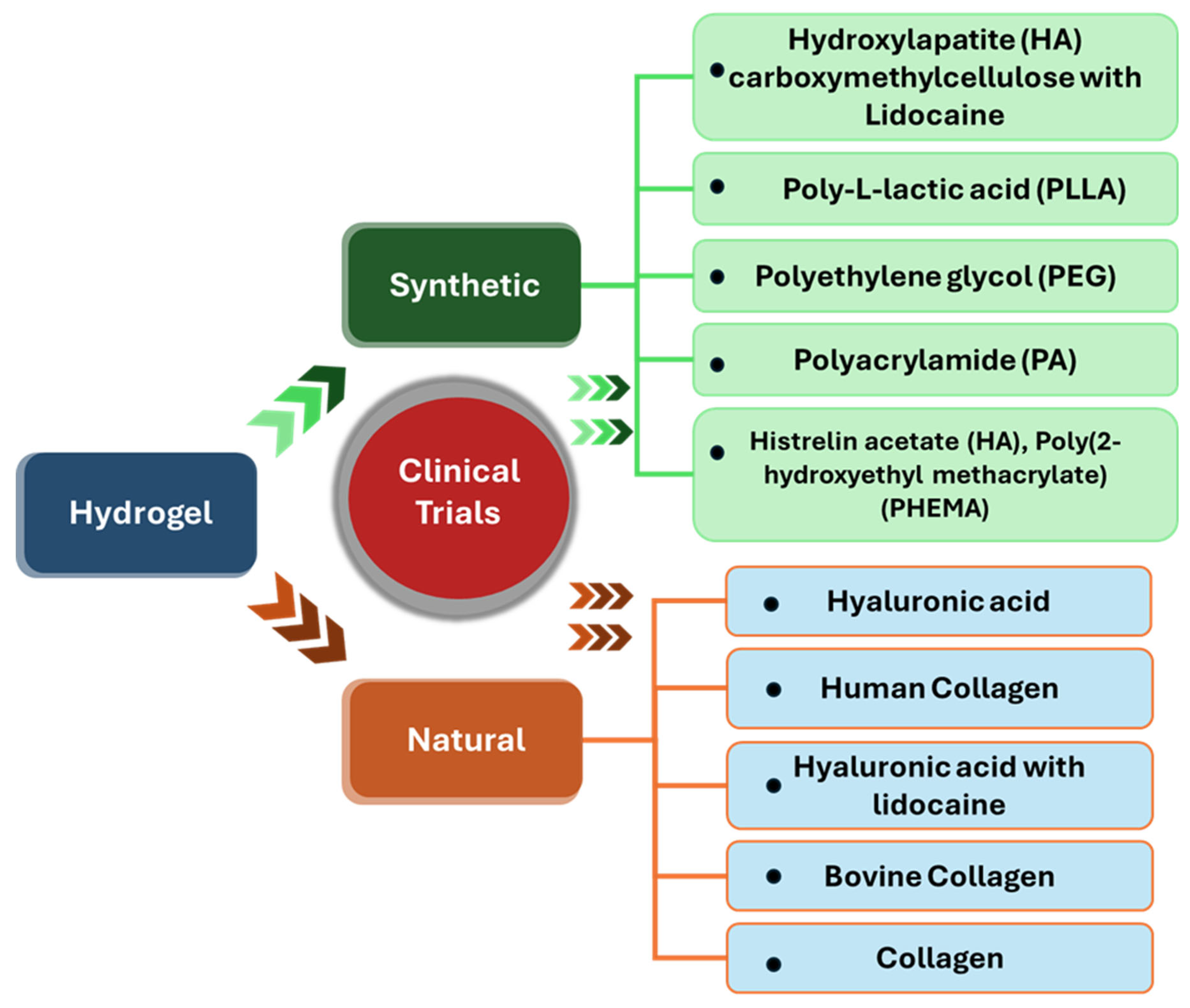
| Sr No. | Hydrogel Type and Building Components | Mechanism of Action | Applications | Clinical Status |
|---|---|---|---|---|
| 1 | Synthetic Histrelin acetate, poly (2-hydroxyethyl methacrylate), poly(2-hydroxypropyl methacrylate), and gonadotropin-releasing hormone | The chemical reaction involves the interaction of histrelin with these polymers to form a hydrogel matrix, allowing for the controlled diffusion of the drug over time. | Palliative treatment of prostate cancer. | Approved—FDA 2004 |
| 2 | Synthetic Polyethylene glycol (PEG) | (i) The hydrogel solidifies after its injection and creates a temporary spacer, creating a protective buffer zone to shield the rectum from the harmful effects of radiation while allowing the radiation to target the prostate cancer cells effectively. (ii) In cataract surgery it works as a sealant by creating a temporary, soft, and lubricious surface barrier to prevent fluid leakage from incisions following cataract surgery. (iii) The iodine from the gel provides a radiopaque property. The hydrogel functions primarily as a radiopaque tissue marker; visible hydrogel markers help guide radiation delivery, ensuring accurate targeting. | Prevention of tissue damage from prostate cancer radiotherapy. Sealant for wound healing and to prevent fluid leakage during surgeries. Assists with better soft tissue alignment for image-guided therapy. | Approved—FDA 2015 Approved—FDA 2014 Approved—FDA 2013 |
| 3 | Synthetic Hydroxylapatite carboxymethylcellulose with lidocaine | The hydroxylapatite carboxymethylcellulose (CaHA) microspheres, once injected, act as a scaffold, encouraging fibroblasts (cells that produce collagen) to produce new collagen and elastin. | (i) Correction of wrinkles. (ii) Correction of wrinkles and folds; stimulation of natural collagen production. | Approved—FDA 2006 Approved—FDA 2015 |
| 4 | Synthetic Polymethylmethacrylate beads, collagen, and lidocaine | Provides a long-lasting augmentation effect by stimulating the body’s collagen production. | Correction of nasolabial fold (smile lines). | Approved—FDA 2006 |
| 5 | Synthetic Histrelin acetate and poly(2-hydroxyethyl methacrylate) (PHEMA) | The hydrogel allows for the controlled release of histrelin acetate (gonadotropin-releasing hormone). It helps to reduce sex hormones and delays the onset of puberty. | Central precocious puberty. | Approved—FDA 2007 |
| 6 | Synthetic Poly-L-lactic acid (PLLA) | PLLA microparticles are recognized by the body as a foreign substance, triggering a controlled inflammatory response. This response activates fibroblasts, which are the cells responsible for producing collagen and elastin. | Restores facial volume and fat loss sign correction. | Approved—FDA 2004 |
| 7 | Synthetic Polyacrylamide (PA) | PAHG works by creating a bulking effect within the urethra to improve urethral coaptation and reduce urine leakage associated with stress urinary incontinence. (ii) PA integrates with the synovium (joint lining) and forms a hydrogel matrix that provides long-term mechanical support and reduces friction by creating a cushioning and lubricating effect within a joint. | Improvement in Female Stress Urinary Incontinence (SUI). Osteoarthritis. | Approved—FDA 2006 Approved—FDA 2014 |
| 8 | Synthetic Calcium hydroxylapatite (CaHA), sodium carboxymethylcellulose (CMC), and glycerine | CaHA particles provide immediate soft tissue augmentation. CaHA creates a bulking effect and helps to close the urethra. The increased bulk effect helps to reduce or eliminate SUI. | Improvement in Female Stress Urinary Incontinence (SUI). | Approved—FDA 2005 |
| 9 | Synthetic Porcine enamel matrix derivative in propylene glycol alginate gel | It attracts mesenchymal cells to the root surface, stimulating the formation of new cementum, periodontal ligament, and bone. Promotes angiogenesis. | Periodontal tissue regeneration. Periodontal ligament reattachment. | Approved—FDA 1996 |
| 10 | Synthetic Calcium phosphosilicate particles, a PEG, and glycerine gel-like binder | Calcium phosphosilicate stimulates bone growth, while the PEG and glycerine help maintain the material’s structure and facilitate its delivery. | Dental bone regeneration. | Approved—FDA 2005 |
| 11 | Synthetic Phase-pure silicon-substituted calcium phosphate in poloxamer 407 (Si-CaP) | A synthetic triblock copolymer, poloxamer 407, creates a composite material that combines the bone-forming potential of Si-CaP. | Bone regeneration; bone void filler in orthopedic conditions. | Approved—FDA 2018 |
| 12 | Synthetic Demineralized bone matrix in poloxamer | A bone graft material where (demineralized bone matrix) DBM, a collagen matrix with growth factors derived from human bone, is combined with a poloxamer 407 carrier. These components help provide the osteoconductive and osteoinductive signals to promote bone regeneration. | Bone regeneration; bone void filler in orthopedic conditions. | Approved—FDA 2005 |
| 13 | Synthetic Allographic demineralized bone matrix in polyethylene oxide polypropylene oxide block copolymer | A carrier material made of a polyethylene oxide (PEO) and polypropylene oxide (PPO) block copolymer combined with DBM; this combination creates osteoconductive signals for bone regeneration in spinal fusion. | Void filler and graft regeneration. | Approved—FDA 2011 |
| 14 | Synthetic Allographic demineralized bone matrix in glycerol | The DBM is processed to expose natural growth factors like BMPs (Bone Morphogenetic Proteins). The DBM provides a scaffold for new bone formation and releases growth factors that stimulate bone-forming cells to differentiate and build new bones. | Promoting bone health; bone graft extender and void filler. | Approved—FDA 2005 |
| 15 | Synthetic PEG 8000, dicyclohexyl methane-4, 40- diisocyanate, and 1,2,6-hexanetriol/ dinoprostone | Dinoprostone stimulates the uterine muscles, leading to contractions similar to those in labor. It also promotes cervical ripening by activating the enzyme collagenase, which breaks down the collagen fibers in the cervix, causing it to soften, efface, and dilate, preparing it for delivery. | Cervical ripening and labor induction. | Approved—FDA 1993 |
| 16 | Synthetic PEG 1000, polyvinyl alcohol, and rice starch/ Ondansetron | Blocks the action of serotonin (5-HT) at the 5-HT3 receptors located in the chemoreceptor trigger zone (CTZ) of the brain and peripherally on the vagal nerve terminals in the gastrointestinal tract. | Nausea and vomiting control caused due to chemotherapy and radiation. | Approved—FDA 1991 |
| 17 | Synthetic PEG ester, trilysine amine, and decahydrated sodium borate | The PEG ester and trilysine amine are the precursors, and the sodium borate acts as an accelerator, facilitating the crosslinking process. The hydrogel utilizes the crosslinking of PEG ester and trilysine amine triggered by the decahydrated sodium borate. | To seal the dura mater, the membrane surrounding the brain and spinal cord, during surgeries. | Approved—FDA 2005 |
| 18 | Synthetic Silicone | (i) The silicon-made hydrogel lenses flatten the central corneal tissue and create a mid-peripheral steepening. That helps to slow the axial length elongation of the eye. (ii) The incorporation of phosphorylcholine (PC), a biomimetic molecule, creates a uniform physiological surface that attracts and binds water molecules, mimicking the natural tear film. This resists protein and lipid deposition, reducing eye dehydration. (iii) A viscous, cohesive gel that mimics the texture of natural breast tissue and maintains its shape even if the shell is ruptured. | Hyperopia/ myopia management. Myopia. Breast implants. | Approved—FDA 2018 and 2021 Approved—FDA 2019 Approved—FDA 2013 and 2014 |
| 19 | Synthetic 2-Hydroxyethylmethacrylate, 2-methacryloxyethyl phosphorylcholine, and ethylene glycol dimethacrylate | It works by mimicking the properties of natural tears, specifically by phosphorylcholine (PC). This helps to maintain hydration and comfort. | Visual acuity. | Approved—FDA 2013 |
| 20 | Synthetic Cellulose and citric acid | The expanded hydrogel particles, mixed with food, increase the volume of the stomach contents, creating a sense of fullness. | Obesity. | Approved—FDA 2019 |
| 21 | Synthetic Human serum albumin (HSA) and polyethylene glycol (PEG) | The combination of PEG and HSA allows for the hydrogel to effectively seal or reduce air leaks in the lungs. Its elasticity enables it to move with the lungs during breathing, while its adhesiveness ensures a secure seal. | Pleural air leak sealant. | Approved—FDA 2010 |
| 22 | Natural Collagen | A denatured porcine collagen implant works by stimulating the body’s own collagen production and promoting an inflammatory response to fill in depressed areas like scars and wrinkles. It achieves this through soft tissue augmentation, effectively lifting the depressed area. | Treatment of depressed areas like scars and wrinkles. | Approved—FDA 1988 |
| 23 | Natural Collagen, carboxymethylcellulose, and recombinant OP-1 | Collagen provides a scaffold, CMC enhances handling and may improve bone formation, and rhOP-1 is a protein that stimulates bone growth. This combination is used in devices, like “OP-1 putty”, for bone grafting and spinal fusion. | Bone regeneration and spinal fusion. | Approved—FDA 2001 |
| 24 | Natural Bovine collagen | Collagen provides a scaffold for cell adhesion and tissue ingrowth, ultimately leading to correction of soft tissue defects. The crosslinking of the collagen with glutaraldehyde makes it more resistant to immediate degradation than non-crosslinked collagen, allowing for a longer-lasting correction. | Facial volume restoration; correction of contour deficiencies. | Approved—FDA 1981 |
| 25 | Natural Human collagen | It works by directly replenishing lost collagen in the skin, which helps to fill in wrinkles and other depressions caused by aging or other factors. It does this by integrating into the skin’s structure and providing structural support, similar to the body’s own collagen. | For wrinkles and acne scars correction; correction of soft tissue contour deficiencies. | Approved—FDA 2003 |
| 26 | Natural Hyaluronic acid with lidocaine | The crosslinked hyaluronic acid fills moderate/severe facial wrinkles and folds. The Cohesive Polydensified Matrix (CPM) technology allows for even distribution and integration into the skin, resulting in a natural-looking and smooth correction. | (i) Facial wrinkles and folds corrections. (ii) For the treatment of tear troughs. | Approved—FDA 2006, 2012, 2018 and 2019 Approved—FDA 2023 |
| 27 | Natural Hyaluronic acid | It works by replenishing lost volume and smoothing wrinkles through its HA component. The HA binds with water molecules, hydrating the skin and creating a plumping effect. (ii) Restoration of face volume by forming a structural support due to crosslinking of HA. (iii) The HA administered along the jawline and chin provides immediate structural support, lifting the jawline and improving definition. This also helps smooth out wrinkles and fine lines, creating a more sculpted look. | (i) Facial wrinkles and folds corrections. (ii) Lip filler; facial volume corrections. (iii) Jawline definition. | Approved—FDA 2017 Approved—FDA 2020 Approved—FDA 2022 |
| 28 | Natural Hyaluronic acid and 1,4-butanediol diglycidyl ether with lidocaine | 1,4-butanediol diglycidyl ether is a crosslinking agent used to bind HA molecules together, creating a highly stable and durable gel. HA crosslinked with 1,4-butanediol diglycidyl ether (BDDE) provides smoothness and hydration without adding significant volume. The 0.3% lidocaine component reduces pain during the injection. | (i) Improves the smoothness and hydration of the skin. (ii) For the correction of moderate-to-severe dynamic perioral rhytids. | Approved—FDA 2023 Approved—FDA 2017 and 2021 |
| 29 | Natural Hyaluronic acid with calcium phosphate (CaP) | HA’s ability to attract water creates a favorable microenvironment for cell attachment and proliferation, and CaP’s role promotes bone formation and mineralization. | Bone void filler for orthopedic applications. | Approved—FDA 2019 |
| 30 | Natural Cinnamic acid-functionalized hyaluronic acid | It acts as a viscoelastic supplement to the synovial fluid, aiming to restore the natural lubricating and shock-absorbing properties of a joint, reduce pain and inflammation, and potentially offer some level of cartilage protection. | For treatment of osteoarthritis. | Approved—FDA 2011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quazi, M.Z.; Quazi, A.S.; Song, Y.; Park, N. Functional Hydrogels: A Promising Platform for Biomedical and Environmental Applications. Int. J. Mol. Sci. 2025, 26, 9066. https://doi.org/10.3390/ijms26189066
Quazi MZ, Quazi AS, Song Y, Park N. Functional Hydrogels: A Promising Platform for Biomedical and Environmental Applications. International Journal of Molecular Sciences. 2025; 26(18):9066. https://doi.org/10.3390/ijms26189066
Chicago/Turabian StyleQuazi, Mohzibudin Z., Aaquib Saeed Quazi, Youngseo Song, and Nokyoung Park. 2025. "Functional Hydrogels: A Promising Platform for Biomedical and Environmental Applications" International Journal of Molecular Sciences 26, no. 18: 9066. https://doi.org/10.3390/ijms26189066
APA StyleQuazi, M. Z., Quazi, A. S., Song, Y., & Park, N. (2025). Functional Hydrogels: A Promising Platform for Biomedical and Environmental Applications. International Journal of Molecular Sciences, 26(18), 9066. https://doi.org/10.3390/ijms26189066







