The Right Approach: Power of Biomarkers in the Assessment and Management of Right Ventricular Dysfunction
Abstract
1. Introduction
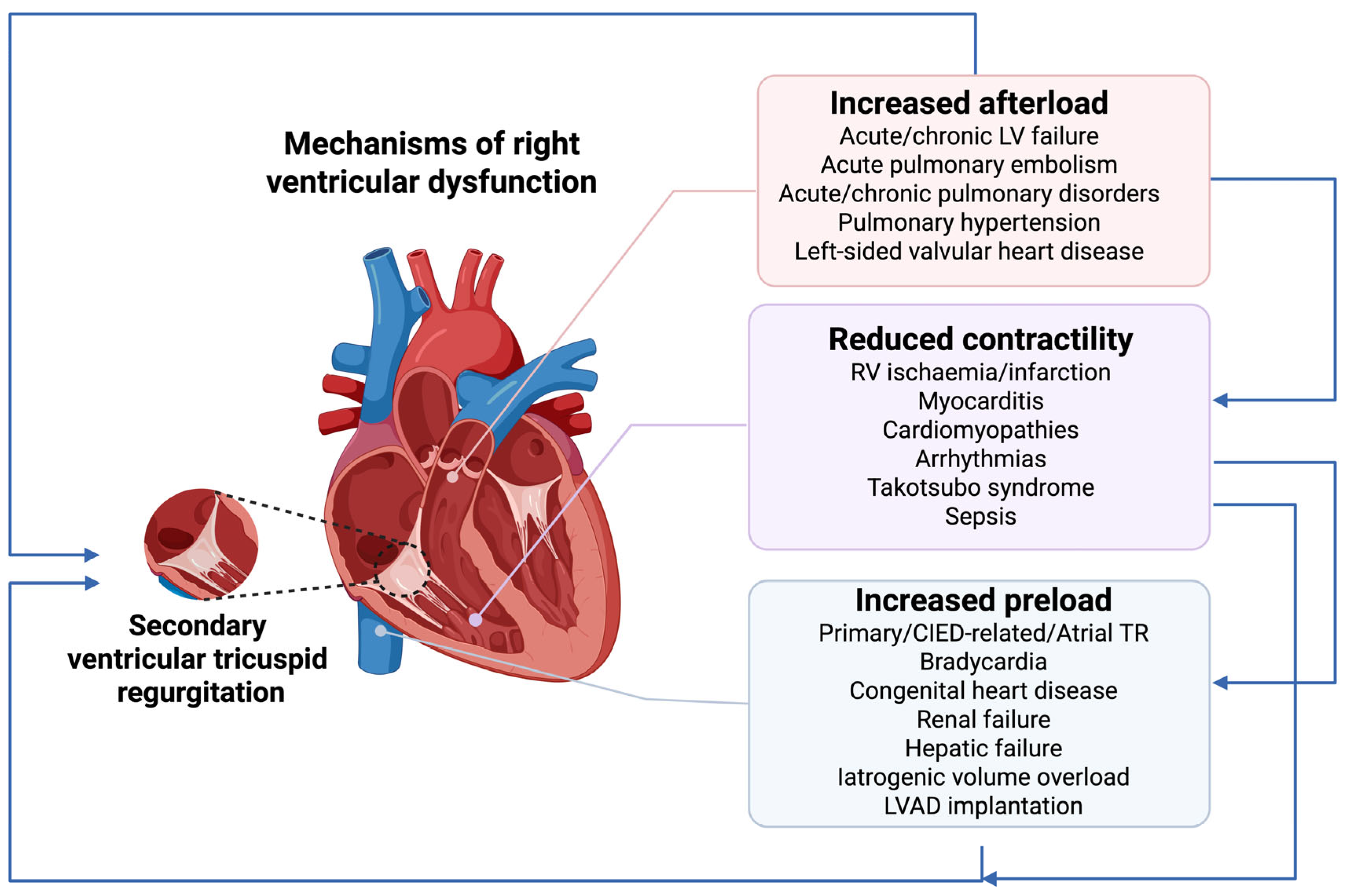
2. Epidemiology, Pathophysiology and Prognosis of Right Ventricular Dysfunction
2.1. Increased Afterload
2.2. Reduced Contractility
2.3. Increased Preload
3. Diagnostic Assessment of Right Ventricular Dysfunction
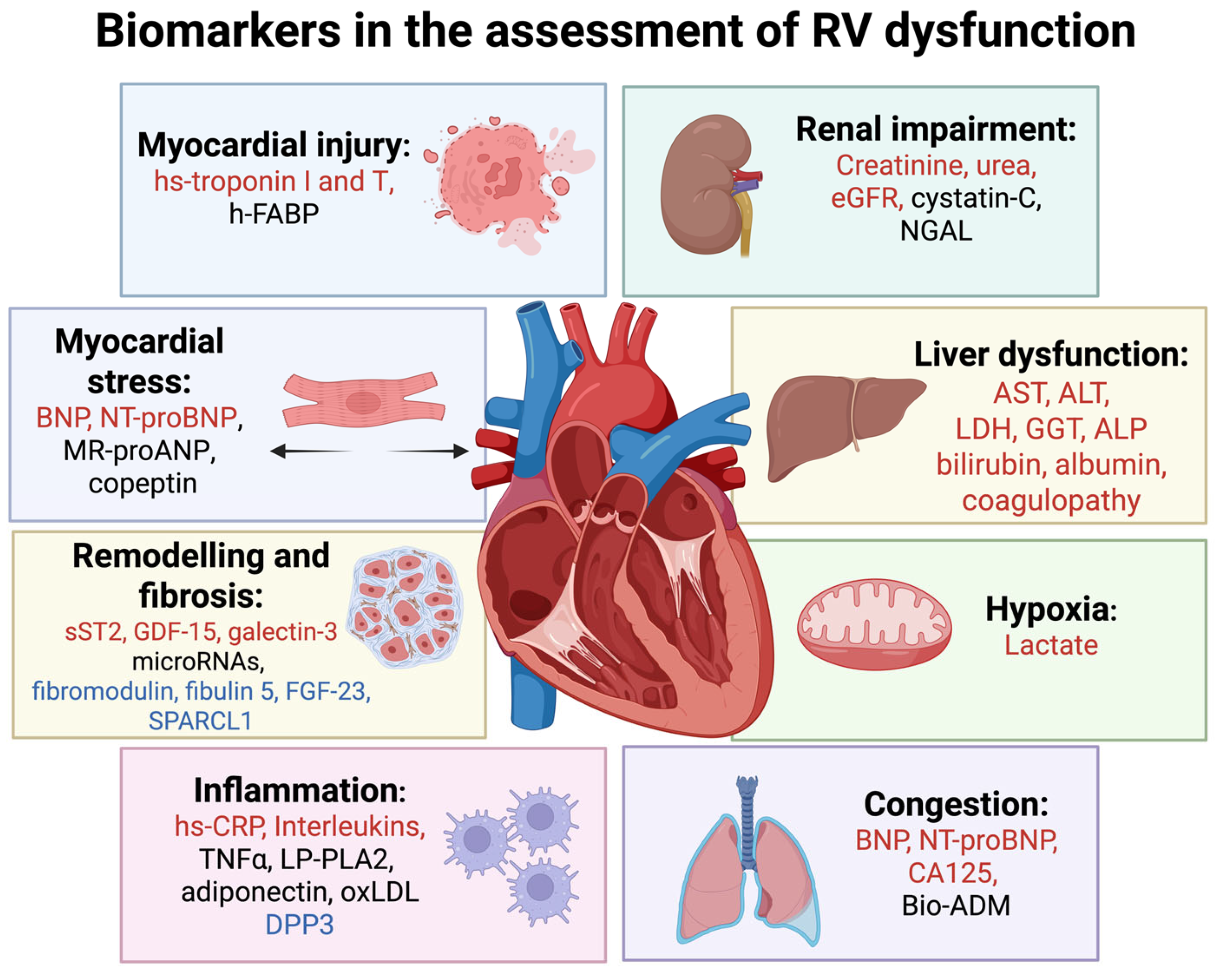
4. Biomarkers in Diagnostic and Prognostic Evaluation of Right Ventricular Disfunction
4.1. Biomarkers of Myocardial Injury
4.2. Biomarkers of Myocardial Stress
4.3. Biomarkers of Myocardial Remodelling and Fibrosis
4.4. Biomarkers of Congestion, Systemic Inflammation, and Hypoxia
4.5. Biomarkers of Renal Impairment
4.6. Biomarkers of Liver Dysfunction
4.7. Emerging Biomarkers
5. The Role of Biomarkers in the Management of Right Ventricular Dysfunction
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaker, M.M.; Taha, H.S.; Kandil, H.I.; Kamal, H.M.; Mahrous, H.A.; Elamragy, A.A. Prognostic significance of right ventricular dysfunction in patients presenting with acute left-sided heart failure. Egypt. Heart J. 2024, 76, 2. [Google Scholar] [CrossRef]
- Iglesias-Garriz, I.; Olalla-Gómez, C.; Garrote, C.; López-Benito, M.; Martín, J.; Alonso, D.; Rodríguez, M.A. Contribution of right ventricular dysfunction to heart failure mortality: A meta-analysis. Rev. Cardiovasc. Med. 2012, 13, e62–e69. [Google Scholar] [CrossRef]
- Adamo, M.; Maccagni, G.; Fiorina, C.; Giannini, C.; Angelillis, M.; Costa, G.; Trani, C.; Burzotta, F.; Bruschi, G.; Merlanti, B.; et al. Prognostic value of right ventricle to pulmonary artery coupling in transcatheter aortic valve implantation recipients. J. Cardiovasc. Med. 2022, 23, 615–622. [Google Scholar] [CrossRef]
- Polovina, M.; Chioncel, O.; Savarese, G.; Abdelhamid, M.; Krljanac, G.; Tschöpe, C.; Seferović, P. Predischarge and Postdischarge Heart Failure Management: Treatment Optimisation, Adherence, and Multidisciplinary Care. Med. Res. Arch. 2025, 13. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Ordonez-Llanos, J. Multiple biomarker strategies for risk stratifiction in heart failure. Clin. Chim. Acta 2015, 443, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Jacobs, A.K.; Leopold, J.A.; Bates, E.; Mendes, L.A.; Sleeper, L.A.; White, H.; Davidoff, R.; Boland, J.; Modur, S.; Forman, R.; et al. Cardiogenic shock caused by right ventricular infarction: A report from the SHOCK registry. J. Am. Coll. Cardiol. 2003, 41, 1273–1279. [Google Scholar] [CrossRef]
- ten Wolde, M.; Söhne, M.; Quak, E.; Mac Gillavry, M.R.; Büller, H.R. Prognostic Value of Echocardiographically Assessed Right Ventricular Dysfunction in Patients with Pulmonary Embolism. Arch. Intern. Med. 2004, 164, 1685–1689. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.-J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef]
- Nochioka, K.; Querejeta Roca, G.; Claggett, B.; Biering-Sørensen, T.; Matsushita, K.; Hung, C.-L.; Solomon, S.D.; Kitzman, D.; Shah, A.M. Right Ventricular Function, Right Ventricular–Pulmonary Artery Coupling, and Heart Failure Risk in 4 US Communities: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2018, 3, 939–948. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Noordegraaf, A.V.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.A.; Movahed, A. Right ventricular infarction--diagnosis and treatment. Clin. Cardiol. 2000, 23, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Copeland, V.J.; Fardman, A.; Furer, A. Keep the Right in Mind-A Focused Approach to Right Ventricle-Predominant Cardiogenic Shock. Life 2023, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Lanspa, M.J.; Cirulis, M.M.; Wiley, B.M.; Olsen, T.D.; Wilson, E.L.; Beesley, S.J.; Brown, S.M.; Hirshberg, E.L.; Grissom, C.K. Right Ventricular Dysfunction in Early Sepsis and Septic Shock. Chest 2021, 159, 1055–1063. [Google Scholar] [CrossRef]
- Agarwal, R.; Yakkali, S.; Shah, P.; Vyas, R.; Kushwaha, A.; Krishnan, A.; Nair, A.S.; Hanumanthu, B.K.J.; Faillace, R.T.; Gashi, E.; et al. Predictors and Outcomes of Right Ventricular Dysfunction in Patients Admitted to the Medical Intensive Care Unit for Sepsis—A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 5423. [Google Scholar] [CrossRef]
- Carreras-Mora, J.; Duran-Cambra, A.; Vilades-Medel, D.; Jimenez-Kockar, M.; Sole-Gonzalez, E.; Llao-Ferrando, I.; Sans-Rosello, J.; Vidal-Burdeus, M.; Vila-Perales, M.; Sionis, A. An Exceptional Cause of Acute Right Heart Failure: Isolated Right Ventricular Takotsubo Syndrome. JACC Case Rep. 2020, 2, 365–369. [Google Scholar] [CrossRef]
- Yalta, K.; Madias, J.E.; Kounis, N.G.; Y-Hassan, S.; Polovina, M.; Altay, S.; Mebazaa, A.; Yilmaz, M.B.; Lopatin, Y.; Mamas, M.A.; et al. Takotsubo Syndrome: An International Expert Consensus Report on Practical Challenges and Specific Conditions (Part-2: Specific Entities, Risk Stratification and Challenges After Recovery). Balk. Med. J. 2024, 41, 442–457. [Google Scholar] [CrossRef]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef]
- Adamo, M.; Chioncel, O.; Pagnesi, M.; Bayes-Genis, A.; Abdelhamid, M.; Anker, S.D.; Antohi, E.L.; Badano, L.; Ben Gal, T.; Böhm, M.; et al. Epidemiology, pathophysiology, diagnosis and management of chronic right-sided heart failure and tricuspid regurgitation. A clinical consensus statement of the Heart Failure Association (HFA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur. J. Heart Fail. 2024, 26, 18–33. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Everett, K.D.; Gulati, G.; Brener, M.I.; Kapur, N.K. Epidemiology and management of right ventricular-predominant heart failure and shock in the cardiac intensive care unit. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 584–594. [Google Scholar] [CrossRef]
- Muraru, D.; Haugaa, K.; Donal, E.; Stankovic, I.; Voigt, J.U.; Petersen, S.E.; Popescu, B.A.; Marwick, T. Right ventricular longitudinal strain in the clinical routine: A state-of-the-art review. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Negru, A.; Tarcău, B.M.; Agoston-Coldea, L. Cardiac Magnetic Resonance Imaging in the Evaluation of Functional Impairments in the Right Heart. Diagnostics 2024, 14, 2581. [Google Scholar] [CrossRef] [PubMed]
- Kanjanahattakij, N.; Sirinvaravong, N.; Aguilar, F.; Agrawal, A.; Krishnamoorthy, P.; Gupta, S. High Right Ventricular Stroke Work Index Is Associated with Worse Kidney Function in Patients with Heart Failure with Preserved Ejection Fraction. Cardiorenal Med. 2018, 8, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zern, E.K.; Wang, D.; Rambarat, P.; Bernard, S.; Paniagua, S.M.; Liu, E.E.; McNeill, J.; Wang, J.K.; Andrews, C.T.; Pomerantsev, E.V.; et al. Association of Pulmonary Artery Pulsatility Index With Adverse Cardiovascular Events Across a Hospital-Based Sample. Circ. Heart Fail. 2022, 15, e009085. [Google Scholar] [CrossRef]
- Seferović, P.M.; Tsutsui, H.; McNamara, D.M.; Ristić, A.D.; Basso, C.; Bozkurt, B.; Cooper, L.T., Jr.; Filippatos, G.; Ide, T.; Inomata, T.; et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur. J. Heart Fail. 2021, 23, 854–871. [Google Scholar] [CrossRef]
- Pitta, F.G.; Leme, A.C.; Gomes, S.R.; Mota, T.P.; Paladino, F.V.; de Souza Júnior, J.L.; de Paula Braz, R.; Lamounier, T.; Ferreira, J.B.G.; Dos Santos Ferreira, C.E. Clinical Laboratory Validation Study of a High Sensitivity Troponin I Assay on a POCT (Point of Care Testing) Device. Glob. Heart 2024, 19, 96. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Rubini Giménez, M.; Wildi, K.; Wussler, D.; Koechlin, L.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; Sedlmayer, R.; Puelacher, C.; Zimmermann, T.; et al. Early kinetics of cardiac troponin in suspected acute myocardial infarction. Rev. Española De Cardiol. (Engl. Ed.) 2021, 74, 480–564. [Google Scholar] [CrossRef]
- Stubbs, P.; Collinson, P.; Moseley, D.; Greenwood, T.; Noble, M. Prognostic Significance of Admission Troponin T Concentrations in Patients With Myocardial Infarction. Circulation 1996, 94, 1291–1297. [Google Scholar] [CrossRef]
- Henzler, T.; Roeger, S.; Meyer, M.; Schoepf, U.J.; Nance, J.W., Jr.; Haghi, D.; Kaminski, W.E.; Neumaier, M.; Schoenberg, S.O.; Fink, C. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur. Respir. J. 2012, 39, 919–926. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, K.H.; Yoon, H.J.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H.; Cho, J.G.; Park, J.C.; Kang, J.C. Usefulness of cardiac biomarkers in the prediction of right ventricular dysfunction before echocardiography in acute pulmonary embolism. J. Cardiol. 2012, 60, 508–513. [Google Scholar] [CrossRef]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef]
- Ng, A.C.C.; Yong, A.S.C.; Chow, V.; Chung, T.; Freedman, S.B.; Kritharides, L. Cardiac troponin-T and the prediction of acute and long-term mortality after acute pulmonary embolism. Int. J. Cardiol. 2013, 165, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 543–603. [Google Scholar] [CrossRef]
- Lankeit, M.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuss, G.; Pruszczyk, P.; Konstantinides, S. Predictive Value of the High-Sensitivity Troponin T Assay and the Simplified Pulmonary Embolism Severity Index in Hemodynamically Stable Patients With Acute Pulmonary Embolism. Circulation 2011, 124, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Kaeberich, A.; Seeber, V.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuß, G.; Giannitsis, E.; Pruszczyk, P.; Konstantinides, S.; Lankeit, M. Age-adjusted high-sensitivity troponin T cut-off value for risk stratification of pulmonary embolism. Eur. Respir. J. 2015, 45, 1323–1331. [Google Scholar] [CrossRef]
- Yalta, K.; Madias, J.E.; Kounis, N.G.; Y-Hassan, S.; Polovina, M.; Altay, S.; Mebazaa, A.; Yilmaz, M.B.; Lopatin, Y.; Mamas, M.A.; et al. Takotsubo Syndrome: An International Expert Consensus Report on Practical Challenges and Specific Conditions (Part-1: Diagnostic and Therapeutic Challenges). Balk. Med. J. 2024, 41, 421–441. [Google Scholar] [CrossRef]
- Ganipineni, V.D.P.; Jitta, S.R.; Gudiwada, M.; Jasti, J.R.; Janga, C.; Merugu, B.; Bandaru, R.R.; Puli, S.; Venkata, V.S.; Vasavada, A.; et al. High-Sensitivity Cardiac Troponin [hs-cTn] as a Valuable Biomarker for Pulmonary Hypertension Risk Stratification: A Contemporary Review of the Literature. Healthcare 2024, 12, 2037. [Google Scholar] [CrossRef]
- Gerull, B.; Brodehl, A. Insights Into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail. Rep. 2021, 18, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Hosbond, S.; Jangaard, N.; Diederichsen, A.C.P.; Thygesen, K.; Mickley, H. Prognostic Impact of Myocardial Injury Related to Various Cardiac and Noncardiac Conditions. Am. J. Med. 2016, 129, 506–514.e1. [Google Scholar] [CrossRef] [PubMed]
- Gunsolus, I.; Sandoval, Y.; Smith, S.W.; Sexter, A.; Schulz, K.; Herzog, C.A.; Apple, F.S. Renal Dysfunction Influences the Diagnostic and Prognostic Performance of High-Sensitivity Cardiac Troponin I. J. Am. Soc. Nephrol. 2018, 29, 636–643. [Google Scholar] [CrossRef]
- Goel, H.; Melot, J.; Krinock, M.D.; Kumar, A.; Nadar, S.K.; Lip, G.Y.H. Heart-type fatty acid-binding protein: An overlooked cardiac biomarker. Ann. Med. 2020, 52, 444–461. [Google Scholar] [CrossRef]
- Boscheri, A.; Wunderlich, C.; Langer, M.; Schoen, S.; Wiedemann, B.; Stolte, D.; Elmer, G.; Barthel, P.; Strasser, R.H. Correlation of heart-type fatty acid-binding protein with mortality and echocardiographic data in patients with pulmonary embolism at intermediate risk. Am. Heart J. 2010, 160, 294–300. [Google Scholar] [CrossRef]
- Wunderlich, C.; Langer, M.; Richter, U.; Tausche, A.K.; Sveric, K.; Boscheri, A.; Christoph, M.; Strasser, R.H.; Ibrahim, K. Combined value of heart-type fatty acid-binding protein and myocardial creatine kinase in risk stratification of normotensive patients with pulmonary embolism. Eur. Heart J. 2013, 34 (Suppl. S1), 761. [Google Scholar] [CrossRef][Green Version]
- Bajaj, A.; Rathor, P.; Sehgal, V.; Shetty, A.; Kabak, B.; Hosur, S. Risk stratification in acute pulmonary embolism with heart-type fatty acid-binding protein: A meta-analysis. J. Crit. Care 2015, 30, 1151.e1–e7. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takahashi, T.; Okuyama, H.; Takabatake, N.; Nozaki, N.; Hirono, O.; Tsunoda, Y.; Shishido, T.; et al. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ. J. 2005, 69, 922–927. [Google Scholar] [CrossRef]
- Klok, F.A.; Mos, I.C.; Huisman, M.V. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: A systematic review and meta-analysis. Am. J. Respir. Crit. Care Med. 2008, 178, 425–430. [Google Scholar] [CrossRef]
- Bajaj, A.; Rathor, P.; Sehgal, V.; Kabak, B.; Shetty, A.; Al Masalmeh, O.; Hosur, S. Prognostic Value of Biomarkers in Acute Non-massive Pulmonary Embolism: A Systematic Review and Meta-analysis. Lung 2015, 193, 639–651. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- de la Espriella, R.; Núñez-Marín, G.; Codina, P.; Núñez, J.; Bayés-Genís, A. Biomarkers to Improve Decision-making in Acute Heart Failure. Card. Fail. Rev. 2023, 9, e13. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, O.; Gögebakan, Ö.; Klöting, N.; Sparwasser, A.; Weickert, M.O.; Haddad, I.; Nikiforova, V.J.; Bergmann, A.; Kruse, M.; Seltmann, A.-C.; et al. Insulin Up-Regulates Natriuretic Peptide Clearance Receptor Expression in the Subcutaneous Fat Depot in Obese Subjects: A Missing Link between CVD Risk and Obesity? J. Clin. Endocrinol. Metab. 2012, 97, E731–E739. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Keyes, M.J.; Levy, D.; Benjamin, E.J.; Vasan, R.S. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 2007, 115, 1345–1353. [Google Scholar] [CrossRef]
- Bishu, K.; Deswal, A.; Chen, H.H.; LeWinter, M.M.; Lewis, G.D.; Semigran, M.J.; Borlaug, B.A.; McNulty, S.; Hernandez, A.F.; Braunwald, E.; et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am. Heart J. 2012, 164, 763–770.e3. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Tada, A.; Obokata, M.; Carter, R.E.; Kaye, D.M.; Handoko, M.L.; Andersen, M.J.; Sharma, K.; Tedford, R.J.; Redfield, M.M.; et al. Evidence-Based Application of Natriuretic Peptides in the Evaluation of Chronic Heart Failure With Preserved Ejection Fraction in the Ambulatory Outpatient Setting. Circulation 2025, 151, 976–989. [Google Scholar] [CrossRef]
- Semalulu, T.; Rudski, L.; Huynh, T.; Langleben, D.; Wang, M.; Fritzler, M.J.; Pope, J.; Baron, M.; Hudson, M. An evidence-based strategy to screen for pulmonary arterial hypertension in systemic sclerosis. Semin. Arthritis Rheum. 2020, 50, 1421–1427. [Google Scholar] [CrossRef]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic peptides: Role in the diagnosis and management of heart failure: A scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur. J. Heart Fail. 2023, 25, 616–631. [Google Scholar] [CrossRef]
- Harbrücker, M.; Natale, M.; Kim, S.H.; Müller, J.; Ansari, U.; Huseynov, A.; Zworowsky, M.V.; Borggrefe, M.; Hoffmann, U.; Lang, S.; et al. Copeptin reliably reflects longitudinal right ventricular function. Ann. Clin. Biochem. 2021, 58, 270–279. [Google Scholar] [CrossRef]
- Usul, E.; Ozkan, S.; Höke, M.H.; Kaya, A.E.; Ucar, F.; Cimen, T. Relationship between right ventricular dilatation and blood copeptin levels in patients with acute pulmonary embolism. Clin. Respir. J. 2020, 14, 965–972. [Google Scholar] [CrossRef]
- Schimmel, K.; Ichimura, K.; Reddy, S.; Haddad, F.; Spiekerkoetter, E. Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 886553. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.W.; Wu, A.H.B.; Fang, J.C.; et al. High-Sensitivity ST2 for Prediction of Adverse Outcomes in Chronic Heart Failure. Circ. Heart Fail. 2011, 4, 180–187. [Google Scholar] [CrossRef]
- Riccardi, M.; Myhre, P.L.; Zelniker, T.A.; Metra, M.; Januzzi, J.L.; Inciardi, R.M. Soluble ST2 in Heart Failure: A Clinical Role beyond B-Type Natriuretic Peptide. J. Cardiovasc. Dev. Dis. 2023, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Monzo, L.; Jarolim, P.; Borlaug, B.A.; Benes, J.; Jurcova, I.; Jenca, D.; Kroupova, K.; Wohlfahrt, P.; Kotrc, M.; Melenovsky, V. Growth Differentiation Factor–15 Is Associated With Congestion-Related Anorexia and Weight Loss in Advanced Heart Failure. JACC Heart Fail. 2025, 13, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, B.; Sygitowicz, G.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A. Galectin-3 is related to right ventricular dysfunction in heart failure patients with reduced ejection fraction and may affect exercise capacity. Sci. Rep. 2020, 10, 16682. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Batkai, S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series). Pulm. Circ. 2014, 4, 185–190. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef]
- Miñana, G.; de la Espriella, R.; Mollar, A.; Santas, E.; Núñez, E.; Valero, E.; Bodí, V.; Chorro, F.J.; Fernández-Cisnal, A.; Martí-Cervera, J.; et al. Factors associated with plasma antigen carbohydrate 125 and amino-terminal pro-B-type natriuretic peptide concentrations in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 437–447. [Google Scholar] [CrossRef]
- Yilmaz, M.B.; Zorlu, A.; Dogan, O.T.; Karahan, O.; Tandogan, I.; Akkurt, I. Role of CA-125 in identification of right ventricular failure in chronic obstructive pulmonary disease. Clin. Cardiol. 2011, 34, 244–248. [Google Scholar] [CrossRef]
- Núñez, J.; Núñez, E.; Bayés-Genís, A.; Fonarow, G.C.; Miñana, G.; Bodí, V.; Pascual-Figal, D.; Santas, E.; Garcia-Blas, S.; Chorro, F.J.; et al. Long-term serial kinetics of N-terminal pro B-type natriuretic peptide and carbohydrate antigen 125 for mortality risk prediction following acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 685–696. [Google Scholar] [CrossRef]
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.D.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Kremer, D.; Demissei, B.G.; Struck, J.; Bergmann, A.; Anker, S.D.; Ng, L.L.; Dickstein, K.; Metra, M.; Samani, N.J.; et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur. J. Heart Fail. 2019, 21, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Ter Maaten, J.M.; Voors, A.A. Bio-adrenomedullin as a potential quick, reliable, and objective marker of congestion in heart failure. Eur. J. Heart Fail. 2018, 20, 1363–1365. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Balling, L.; Deis, T.; Struck, J.; Bergmann, A.; Gustafsson, F. Bioactive adrenomedullin in plasma is associated with biventricular filling pressures in patients with advanced heart failure. Eur. J. Heart Fail. 2021, 23, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Khan, M.A.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e003989. [Google Scholar] [CrossRef]
- Vittos, O.; Toana, B.; Vittos, A. Biomarkers and their involvement in the early diagnosis of right ventricular dysfunction in type 2 Diabetes Mellitus. J. Med. Life 2012, 5, 74–78. [Google Scholar]
- Sommariva, E.; Stadiotti, I.; Casella, M.; Catto, V.; Dello Russo, A.; Carbucicchio, C.; Arnaboldi, L.; De Metrio, S.; Milano, G.; Scopece, A.; et al. Oxidized LDL-dependent pathway as new pathogenic trigger in arrhythmogenic cardiomyopathy. EMBO Mol. Med. 2021, 13, e14365. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar] [CrossRef]
- Kumar, A.; Chidambaram, V.; Geetha, H.S.; Majella, M.G.; Bavineni, M.; Pona, P.K.; Jain, N.; Sharalaya, Z.; Al’Aref, S.J.; Asnani, A.; et al. Renal Biomarkers in Heart Failure: Systematic Review and Meta-Analysis. JACC Adv. 2024, 3, 100765. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Katz, R.; Fried, L.F.; Jenny, N.S.; Stehman-Breen, C.; Newman, A.B.; Siscovick, D.; Psaty, B.M.; Sarnak, M.J. Cystatin-C and mortality in elderly persons with heart failure. J. Am. Coll. Cardiol. 2005, 45, 268–271, Correction in J. Am. Coll. Cardiol. 2005, 45, 811. [Google Scholar] [CrossRef]
- Fenster, B.; Smyser, J.; Schroeder, J.; Buckner, J.K.; Lasalvia, L. CYSTATIN C: A NOVEL BIOMARKER FOR PULMONARY ARTERIAL HYPERTENSION. JACC 2013, 61 (Suppl. S10), E1300-E1300. [Google Scholar] [CrossRef]
- Duan, A.; Huang, Z.; Zhao, Z.; Zhao, Q.; Jin, Q.; Yan, L.; Zhang, Y.; Li, X.; Zhang, S.; Hu, M.; et al. The potential of cystatin C as a predictive biomarker in pulmonary hypertension. BMC Pulm. Med. 2023, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.E.; Solomon, S.D.; Gerstein, H.C.; Zetterstrand, S.; Olofsson, B.; Michelson, E.L.; Granger, C.B.; Swedberg, K.; Pfeffer, M.A.; Yusuf, S.; et al. Albuminuria in chronic heart failure: Prevalence and prognostic importance. Lancet 2009, 374, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Nickel, N.P.; de Jesus Perez, V.A.; Zamanian, R.T.; Fessel, J.P.; Cogan, J.D.; Hamid, R.; West, J.D.; de Caestecker, M.P.; Yang, H.; Austin, E.D. Low-grade albuminuria in pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894018824564. [Google Scholar] [CrossRef]
- Phan Thai, H.; Hoang Bui, B.; Hoang Anh, T.; Huynh Van, M. Value of Plasma NGAL and Creatinine on First Day of Admission in the Diagnosis of Cardiorenal Syndrome Type 1. Cardiol. Res. Pract. 2020, 2020, 2789410. [Google Scholar] [CrossRef]
- Poelzl, G.; Ess, M.; Mussner-Seeber, C.; Pachinger, O.; Frick, M.; Ulmer, H. Liver dysfunction in chronic heart failure: Prevalence, characteristics and prognostic significance. Eur. J. Clin. Investig. 2012, 42, 153–163. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Kitzberger, R.; Warszawska, J.; et al. Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009, 35, 1397–1405. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC: Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Gheorghiade, M.; Bubenek, S.; Vinereanu, D.; Vaduganathan, M.; Macarie, C.; Chioncel, O. The predictive value of transaminases at admission in patients hospitalized for heart failure: Findings from the RO-AHFS registry. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Sugano, Y.; Nagai, T.; Honda, Y.; Iwakami, N.; Nakano, H.; Takashio, S.; Honda, S.; Asaumi, Y.; Aiba, T.; et al. Usefulness of the Direct and/or Total Bilirubin to Predict Adverse Outcomes in Patients With Acute Decompensated Heart Failure. Am. J. Cardiol. 2017, 119, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Vaduganathan, M.; Huffman, M.D.; Khan, S.; Kwasny, M.J.; Fought, A.J.; Maggioni, A.P.; Swedberg, K.; Konstam, M.A.; Zannad, F.; et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: An analysis of the EVEREST trial. Eur. J. Heart Fail. 2012, 14, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Dunning, A.; DeVore, A.D.; Schulte, P.J.; Starling, R.C.; Tang, W.H.; Armstrong, P.W.; Ezekowitz, J.A.; Butler, J.; McMurray, J.J.; et al. Liver function tests in patients with acute heart failure and associated outcomes: Insights from ASCEND-HF. Eur. J. Heart Fail. 2016, 18, 424–432. [Google Scholar] [CrossRef]
- Myhre, P.L.; Grupper, A.; Mebazaa, A.; Davison, B.; Edwards, C.; Takagi, K.; Adamo, M.; Arrigo, M.; Barros, M.; Biegus, J.; et al. Changes in Liver Function Tests, Congestion, and Prognosis After Acute Heart Failure: The STRONG-HF Trial. JACC Adv. 2025, 4, 101607. [Google Scholar] [CrossRef]
- Biegus, J.; Hillege, H.L.; Postmus, D.; Valente, M.A.; Bloomfield, D.M.; Cleland, J.G.; Cotter, G.; Davison, B.A.; Dittrich, H.C.; Fiuzat, M.; et al. Abnormal liver function tests in acute heart failure: Relationship with clinical characteristics and outcome in the PROTECT study. Eur. J. Heart Fail. 2016, 18, 830–839. [Google Scholar] [CrossRef]
- Běhounek, M.; Lipcseyová, D.; Vít, O.; Žáček, P.; Talacko, P.; Husková, Z.; Kikerlová, S.; Tykvartová, T.; Wohlfahrt, P.; Melenovský, V.; et al. Biomarkers of RV Dysfunction in HFrEF Identified by Direct Tissue Proteomics: Extracellular Proteins Fibromodulin and Fibulin-5. Circ. Heart Fail. 2025, 18, e011984. [Google Scholar] [CrossRef]
- Benes, J.; Kroupova, K.; Kotrc, M.; Petrak, J.; Jarolim, P.; Novosadova, V.; Kautzner, J.; Melenovsky, V. FGF-23 is a biomarker of RV dysfunction and congestion in patients with HFrEF. Sci. Rep. 2023, 13, 16004. [Google Scholar] [CrossRef]
- Widmann, L.; Keranov, S.; Jafari, L.; Liebetrau, C.; Keller, T.; Troidl, C.; Kriechbaum, S.; Voss, S.; Arsalan, M.; Richter, M.J.; et al. Fibroblast growth factor 23 as a biomarker of right ventricular dysfunction in pulmonary hypertension. Clin. Res. Cardiol. 2023, 112, 1382–1393. [Google Scholar] [CrossRef]
- Keranov, S.; Dörr, O.; Jafari, L.; Liebetrau, C.; Keller, T.; Troidl, C.; Kriechbaum, S.; Voss, S.; Richter, M.; Tello, K.; et al. SPARCL1 as a biomarker of maladaptive right ventricular remodelling in pulmonary hypertension. Biomarkers 2020, 25, 290–295. [Google Scholar] [CrossRef]
- Ansari Ramandi, M.M.; van Melle, J.P.; Gorter, T.M.; Hoendermis, E.S.; van Veldhuisen, D.J.; Nauta, J.F.; van der Wal, M.H.L.; Warink-Riemersma, J.; Voors, A.A.; Dickinson, M.G. Right ventricular dysfunction in patients with new-onset heart failure: Longitudinal follow-up during guideline-directed medical therapy. Eur. J. Heart Fail. 2022, 24, 2226–2234. [Google Scholar] [CrossRef]
- Cotter, G.; Davison, B.A.; Freund, Y.; Voors, A.A.; Edwards, C.; Novosadova, M.; Takagi, K.; Hayrapetyan, H.; Mshetsyan, A.; Mayranush, D.; et al. Burst steroid therapy for acute heart failure: The CORTAHF randomized, open-label, pilot trial. Eur. J. Heart Fail. 2024, 26, 2282–2292. [Google Scholar] [CrossRef]
- Metra, M.; Adamo, M.; Tomasoni, D.; Mebazaa, A.; Bayes-Genis, A.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Bauersachs, J.; Belenkov, Y.; et al. Pre-discharge and early post-discharge management of patients hospitalized for acute heart failure: A scientific statement by the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Salah, K.; Kok, W.E.; Eurlings, L.W.; Bettencourt, P.; Pimenta, J.M.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Bettari, L.; Lazzarini, V.; et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: A European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014, 100, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Chen, J.; McCauley, B.D.; Parikh, C.R.; Tang, W.H. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: Importance of sustained decongestion. J. Am. Coll. Cardiol. 2013, 62, 516–524. [Google Scholar] [CrossRef]
- Fuernau, G.; Desch, S.; de Waha-Thiele, S.; Eitel, I.; Neumann, F.J.; Hennersdorf, M.; Felix, S.B.; Fach, A.; Böhm, M.; Pöss, J.; et al. Arterial Lactate in Cardiogenic Shock: Prognostic Value of Clearance Versus Single Values. JACC Cardiovasc. Interv. 2020, 13, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Lim, S.Y.; Si, X.; Monnet, X. Biomarkers in cardiogenic shock: Old pals, new friends. Ann. Intensive Care 2024, 14, 157. [Google Scholar] [CrossRef]
- Karakas, M.; Akin, I.; Burdelski, C.; Clemmensen, P.; Grahn, H.; Jarczak, D.; Keßler, M.; Kirchhof, P.; Landmesser, U.; Lezius, S.; et al. Single-dose of adrecizumab versus placebo in acute cardiogenic shock (ACCOST-HH): An investigator-initiated, randomised, double-blinded, placebo-controlled, multicentre trial. Lancet Respir. Med. 2022, 10, 247–254. [Google Scholar] [CrossRef]
- Jung, C.; Bruno, R.R.; Jumean, M.; Price, S.; Krychtiuk, K.A.; Ramanathan, K.; Dankiewicz, J.; French, J.; Delmas, C.; Mendoza, A.A.; et al. Management of cardiogenic shock: State-of-the-art. Intensive Care Med. 2024, 50, 1814–1829. [Google Scholar] [CrossRef]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef]
- Núñez, J.; Llàcer, P.; Bertomeu-González, V.; Bosch, M.J.; Merlos, P.; García-Blas, S.; Montagud, V.; Bodí, V.; Bertomeu-Martínez, V.; Pedrosa, V.; et al. Carbohydrate Antigen-125-Guided Therapy in Acute Heart Failure: CHANCE-HF: A Randomized Study. JACC Heart Fail. 2016, 4, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Pagnesi, M.; Mebazaa, A.; Davison, B.; Edwards, C.; Tomasoni, D.; Arrigo, M.; Barros, M.; Biegus, J.; Celutkiene, J.; et al. NT-proBNP and high intensity care for acute heart failure: The STRONG-HF trial. Eur. Heart J. 2023, 44, 2947–2962. [Google Scholar] [CrossRef] [PubMed]
- Ben Gal, T.; Ben Avraham, B.; Milicic, D.; Crespo-Leiro, M.G.; Coats, A.J.S.; Rosano, G.; Seferovic, P.; Ruschitzka, F.; Metra, M.; Anker, S.; et al. Guidance on the management of left ventricular assist device (LVAD) supported patients for the non-LVAD specialist healthcare provider: Executive summary. Eur. J. Heart Fail. 2021, 23, 1597–1609, Erratum in Eur. J. Heart Fail. 2022, 24, 733. [Google Scholar] [CrossRef]
- Geavlete, O.; Antohi, L.; Ben-Avraham, B.; Ben Gal, T. Advanced heart failure—Current strategy of management. Heart Fail. J. India 2024, 2, 43–49. [Google Scholar] [CrossRef]
- Woolley, R.J.; Ceelen, D.; Ouwerkerk, W.; Tromp, J.; Figarska, S.M.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Zannad, F.; Metra, M.; et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 983–991. [Google Scholar] [CrossRef] [PubMed]
| Clinical Symptoms and Signs | ECG Findings |
|---|---|
Symptoms:
| P-pulmonale |
Systemic venous congestion:
|
|
| Hypotension and hypoperfusion | Cardiac rhythm and conduction abnormalities:
|
| Cyanosis | Low voltage QRS in the limb leads (pericardial/pleural effusion, amyloidosis) |
| Tachycardia | ST elevation and/or negative T wave in the precordial leads |
| Kussmaul’s sign (increased jugular venous pressure on inspiration) | Tall R waves in V1 and V2 |
| Systolic heart murmur over tricuspid valve | Epsilon wave (ARVC) |
| Echocardiographic Findings |
|---|
| RVEDD/LVEDD > 1 |
| RV basal diameter > 41 mm, mid-ventricular diameter > 35 mm, longitudinal diameter < 86 mm (measured from apical 4-chamber view) |
| RV free wall thickness > 5 mm |
| TAPSE < 17 mm |
| Systolic S′ velocity of the tricuspid valve annulus < 9.5 cm/s |
| RV fractional area change < 35% |
| RV index of myocardial performance < 0.54 |
| Increased estimated RVSP |
| Significant TR |
| TR-V > 2.8 m/s |
| Abnormal IVC diameter and collapsibility (>21 mm diameter, <50% inspiratory collapsibility), VExUS |
| RVEF < 45% * |
| Impaired RV longitudinal strain by 2D speckle-tracking |
| Biomarkers | Specificity for RV Dysfunction | Diagnostic Utility | Prognostic Utility | Utility in Treatment Guidance |
|---|---|---|---|---|
| 1. Myocardial injury | ||||
| Cardiac troponins | Low | +++ | +++ | +++ |
| hFABP | Moderate | +++ | +++ | To be defined |
| 2. Myocardial stress | ||||
| B-type natriuretic peptides (BNP and NT-proBNP) | Low | +++ | +++ | +++ |
| MR-proANP | Low | + | + | To be defined |
| Copeptin | Modest | ++ | ++ | To be defined |
| 3. Remodelling and fibrosis | ||||
| sST2 | Modest | + | + | To be defined |
| GDF-15 | Low | + | + | To be defined |
| Galectin-3 | Low | + | + | To be defined |
| Micro-RNAs | Low | + | + | To be defined |
| 4. Inflammation | ||||
| hsCRP | Low | +++ | +++ | +++ |
| Interleukins | Low | +++ | +++ | +++ |
| TNFα | Low | + | + | + |
| PL-PLA2 | Low | + | + | To be defined |
| Adiponectin | Low | + | + | To be defined |
| oxLDL | Low | + | + | To be defined |
| 5. Congestion | ||||
| CA125 | High (RV dysfunction > LV dysfunction) | +++ | +++ | +++ |
| Bio-ADM | Modest | ++ | ++ | To be defined |
| 6. Hypoxia | ||||
| Lactate | Low | +++ | +++ | To be defined |
| 7. Liver dysfunction | ||||
| Hepatocellular damage/cholestasis (AST, ALT, ALP, GGT, bilirubin) | Modest | +++ | +++ | To be defined |
| Synthetic function (albumin, prothrombin time) | Modest | +++ | +++ | +++ |
| 8. Renal impairment | ||||
| Blood urea nitrogen, serum creatinine, eGFR | Low | +++ | +++ | +++ |
| Cystatin-C | Modest (better than natriuretic peptides) | +++ | +++ | ++ |
| NGAL | Modest | ++ | ++ | To be defined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viduljević, M.; Polovina, M.; Geavlete, O.; Adamo, M.; Hadžibegović, A.; Ašanin, M.; Stanković, S.; Ben Gal, T.; Abdelwahab, M.A.; Abdelhamid, M.; et al. The Right Approach: Power of Biomarkers in the Assessment and Management of Right Ventricular Dysfunction. Int. J. Mol. Sci. 2025, 26, 9064. https://doi.org/10.3390/ijms26189064
Viduljević M, Polovina M, Geavlete O, Adamo M, Hadžibegović A, Ašanin M, Stanković S, Ben Gal T, Abdelwahab MA, Abdelhamid M, et al. The Right Approach: Power of Biomarkers in the Assessment and Management of Right Ventricular Dysfunction. International Journal of Molecular Sciences. 2025; 26(18):9064. https://doi.org/10.3390/ijms26189064
Chicago/Turabian StyleViduljević, Mihajlo, Marija Polovina, Oliviana Geavlete, Marianna Adamo, Adi Hadžibegović, Milika Ašanin, Sanja Stanković, Tuvia Ben Gal, Mohamed A. Abdelwahab, Magdy Abdelhamid, and et al. 2025. "The Right Approach: Power of Biomarkers in the Assessment and Management of Right Ventricular Dysfunction" International Journal of Molecular Sciences 26, no. 18: 9064. https://doi.org/10.3390/ijms26189064
APA StyleViduljević, M., Polovina, M., Geavlete, O., Adamo, M., Hadžibegović, A., Ašanin, M., Stanković, S., Ben Gal, T., Abdelwahab, M. A., Abdelhamid, M., Ambrosy, A. P., Chioncel, O., & Seferović, P. M. (2025). The Right Approach: Power of Biomarkers in the Assessment and Management of Right Ventricular Dysfunction. International Journal of Molecular Sciences, 26(18), 9064. https://doi.org/10.3390/ijms26189064







