Fenofibrate as a PPARα Agonist Modulates Neuroinflammation and Glutamate Receptors in a Rat Model of Temporal Lobe Epilepsy: Region-Specific Effects and Behavioral Outcomes
Abstract
1. Introduction
2. Results
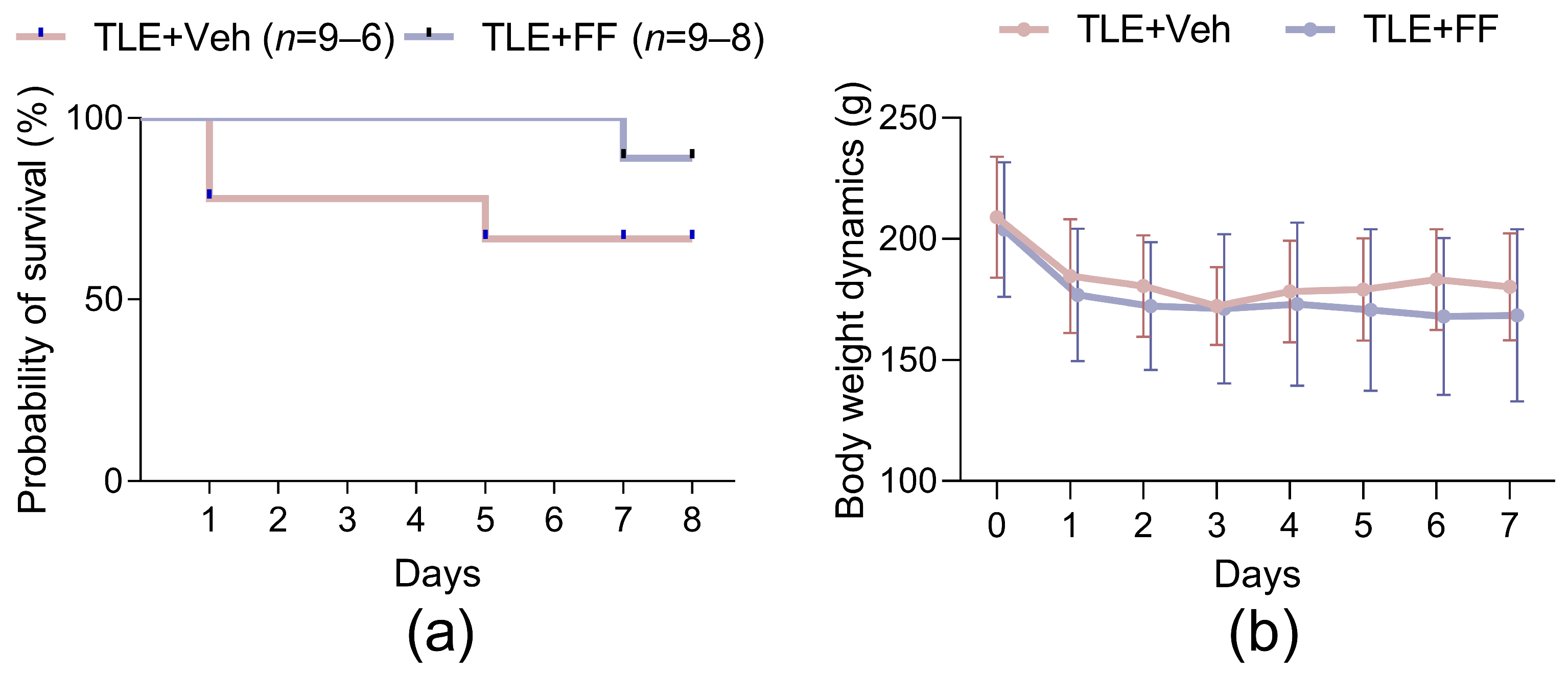
2.1. Fenofibrate Administration Has No Effect on Rat Survival or Body Weight in the Latent Phase of the Lithium-Pilocarpine Model
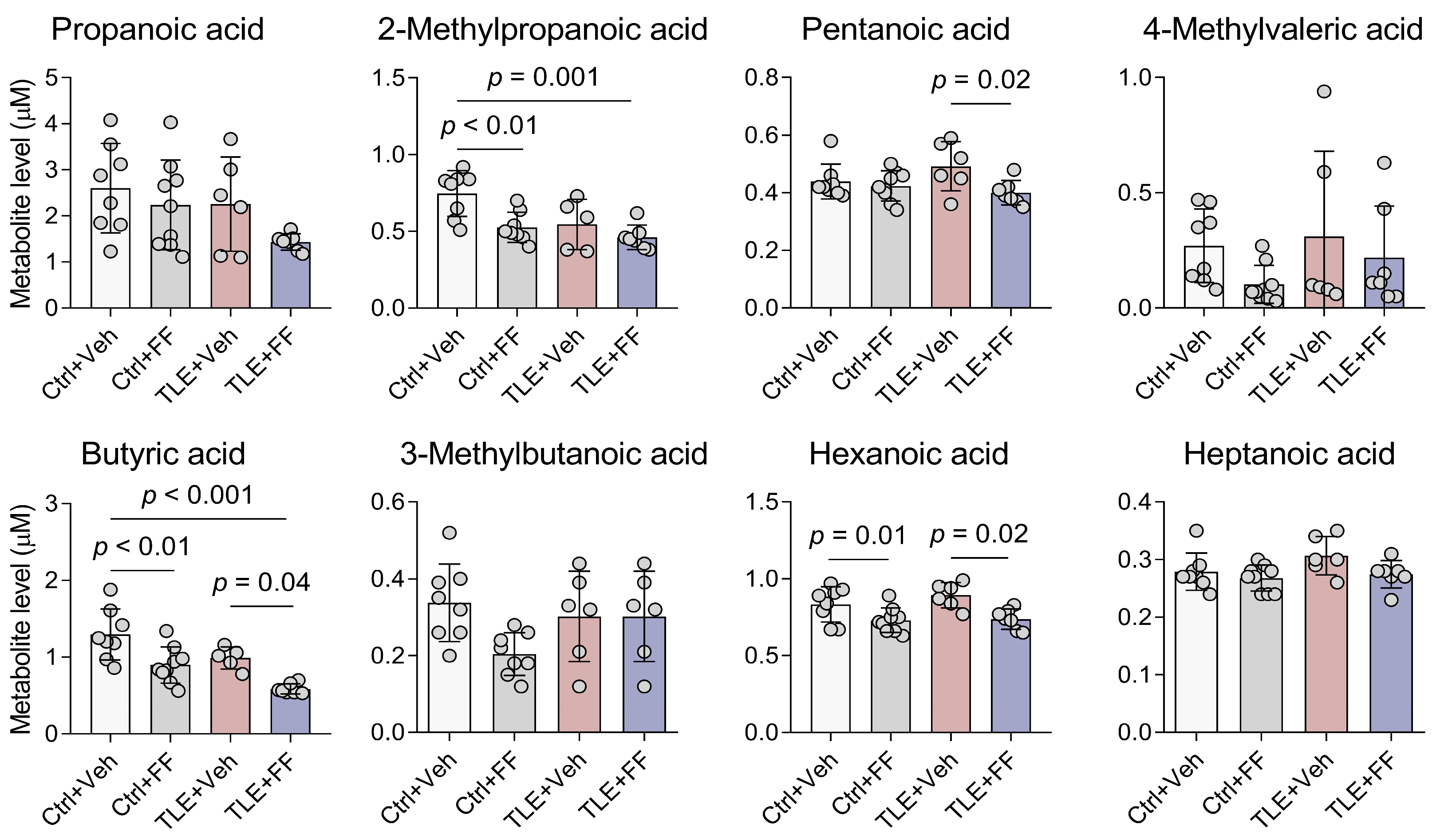
2.2. SCFA Content in Rat Blood Plasma in the Lithium-Pilocarpine Model During Fenofibrate Treatment
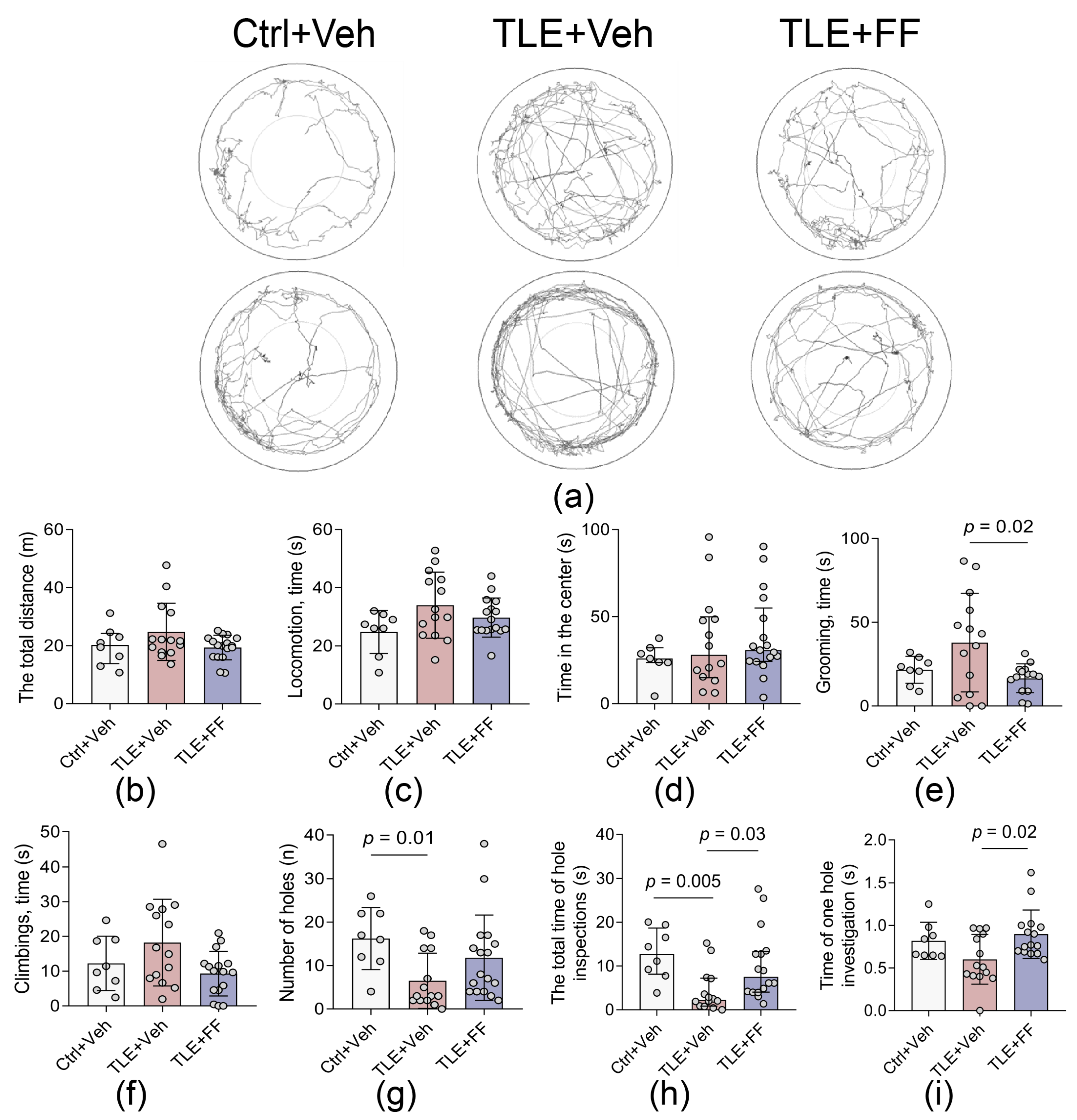
2.3. Fenofibrate Ameliorates Specific Exploratory Deficits and Reduces Anxiety-like Behaviors in the Open Field Test
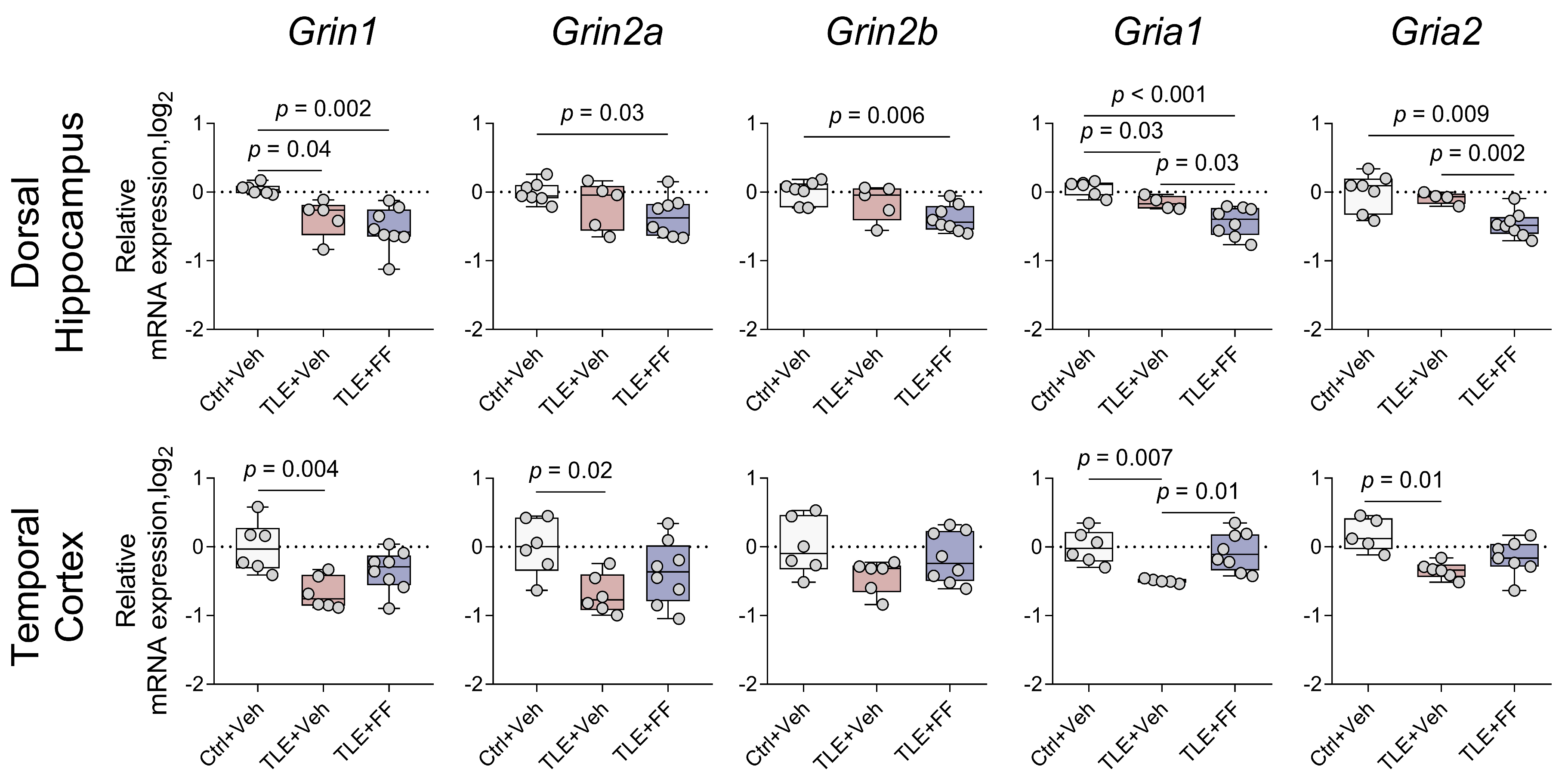
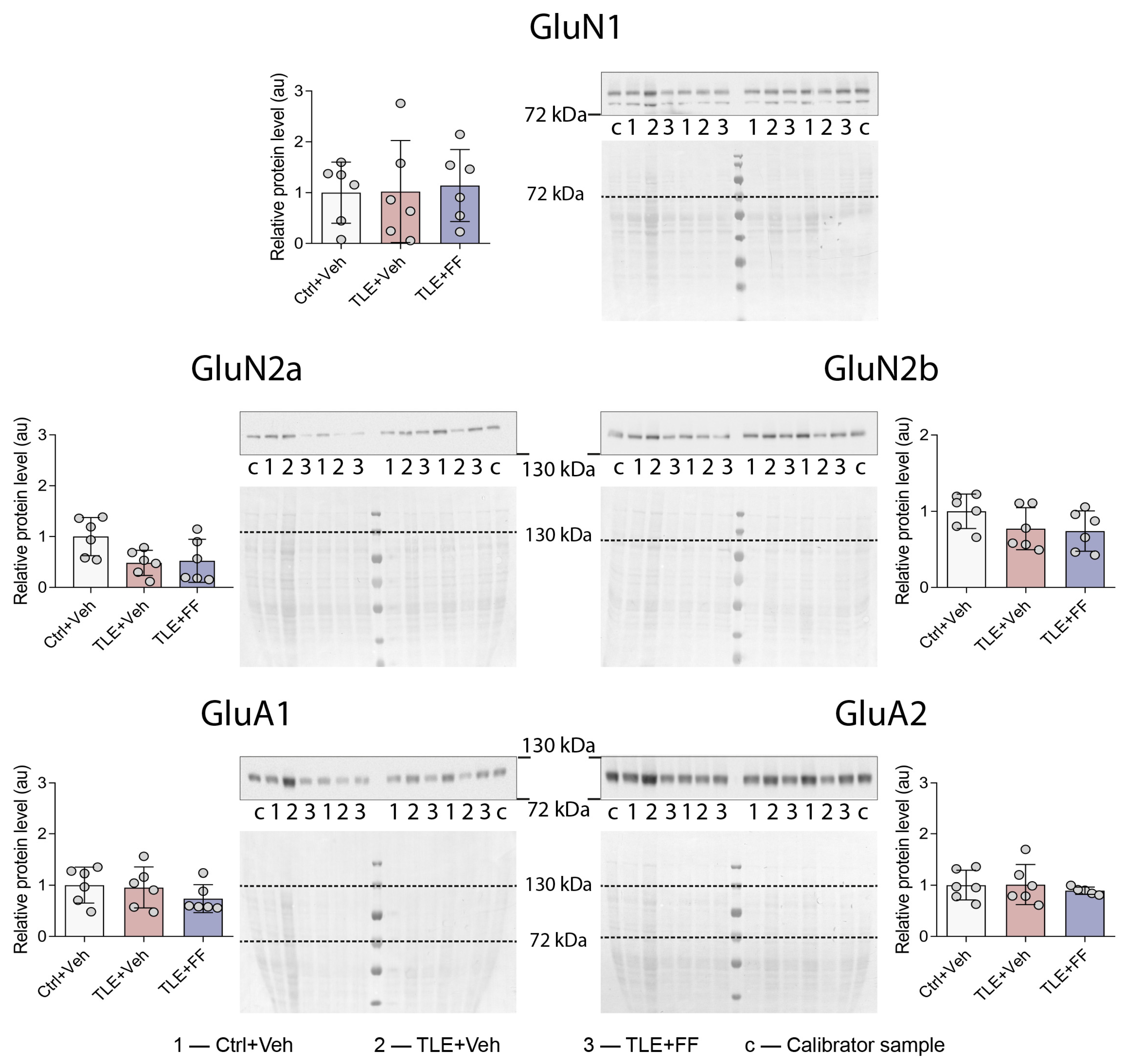
2.4. Region-Specific Modulation of Ionotropic Glutamate Receptor Gene Expression by Fenofibrate
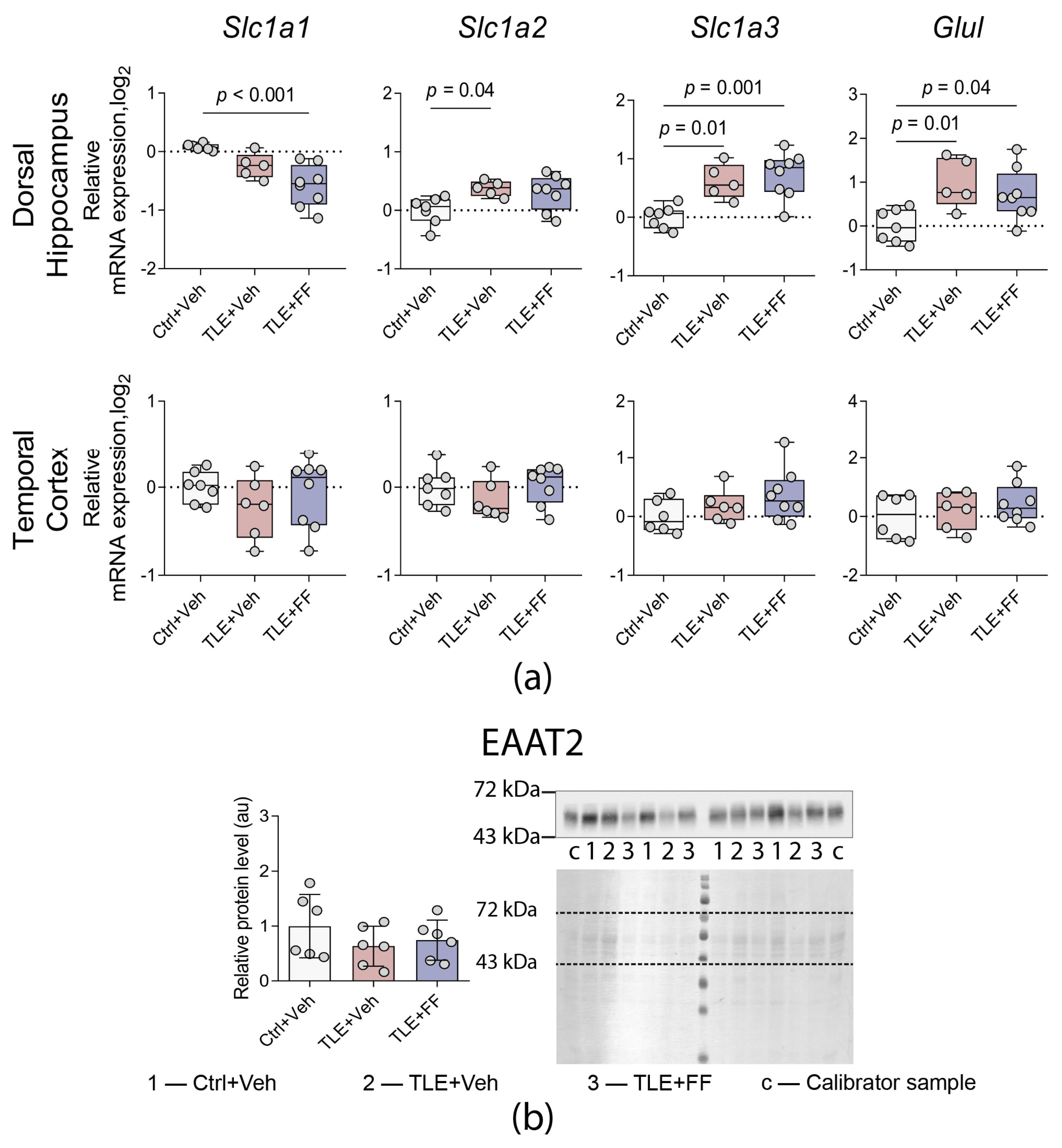
2.5. Fenofibrate Treatment Results in Decreased Expression of the Neuronal Glutamate Transporter Gene in the Dorsal Hippocampus
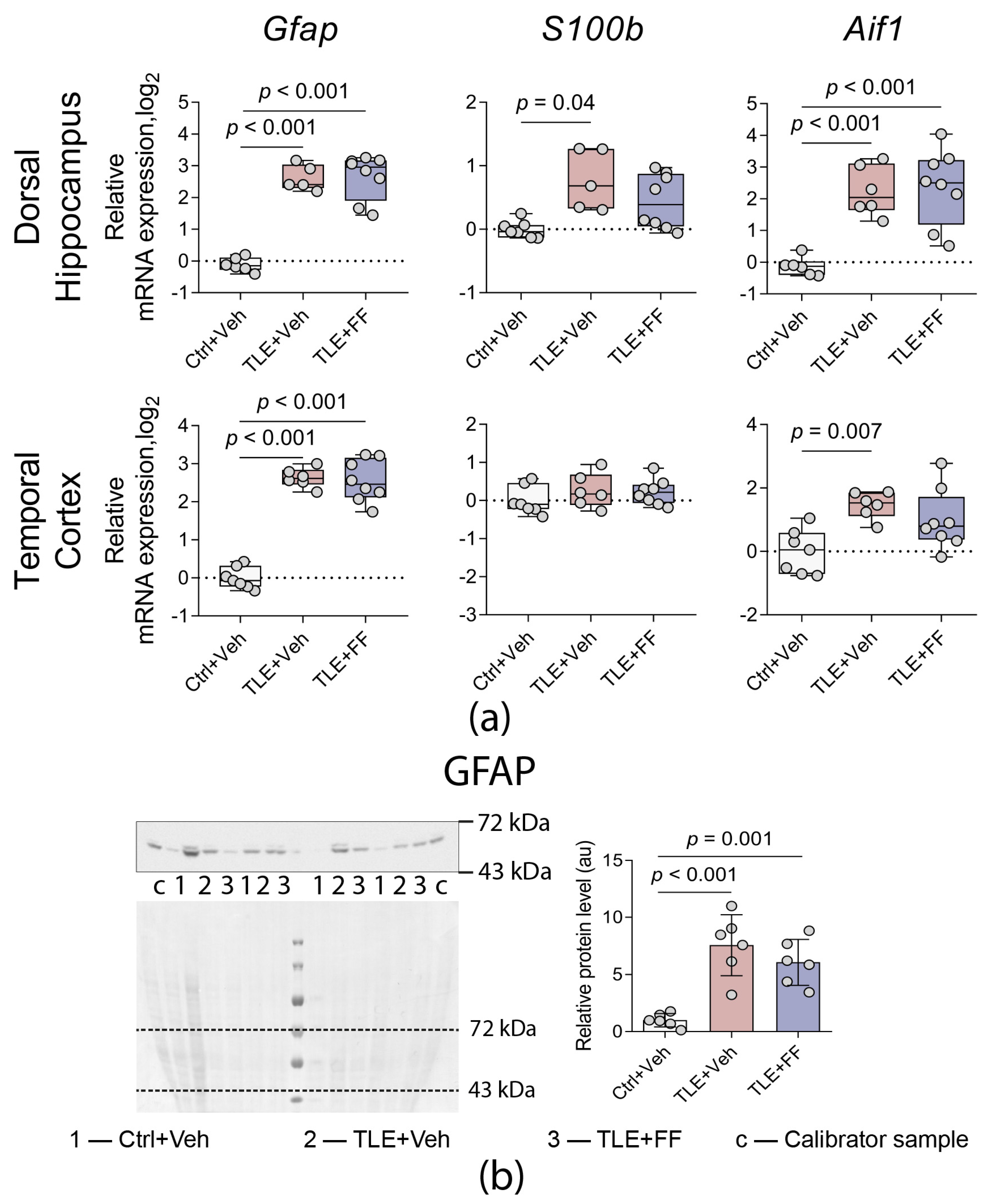
2.6. Administration of Fenofibrate Does Not Affect the Expression of Astroglial and Microglial Marker Genes
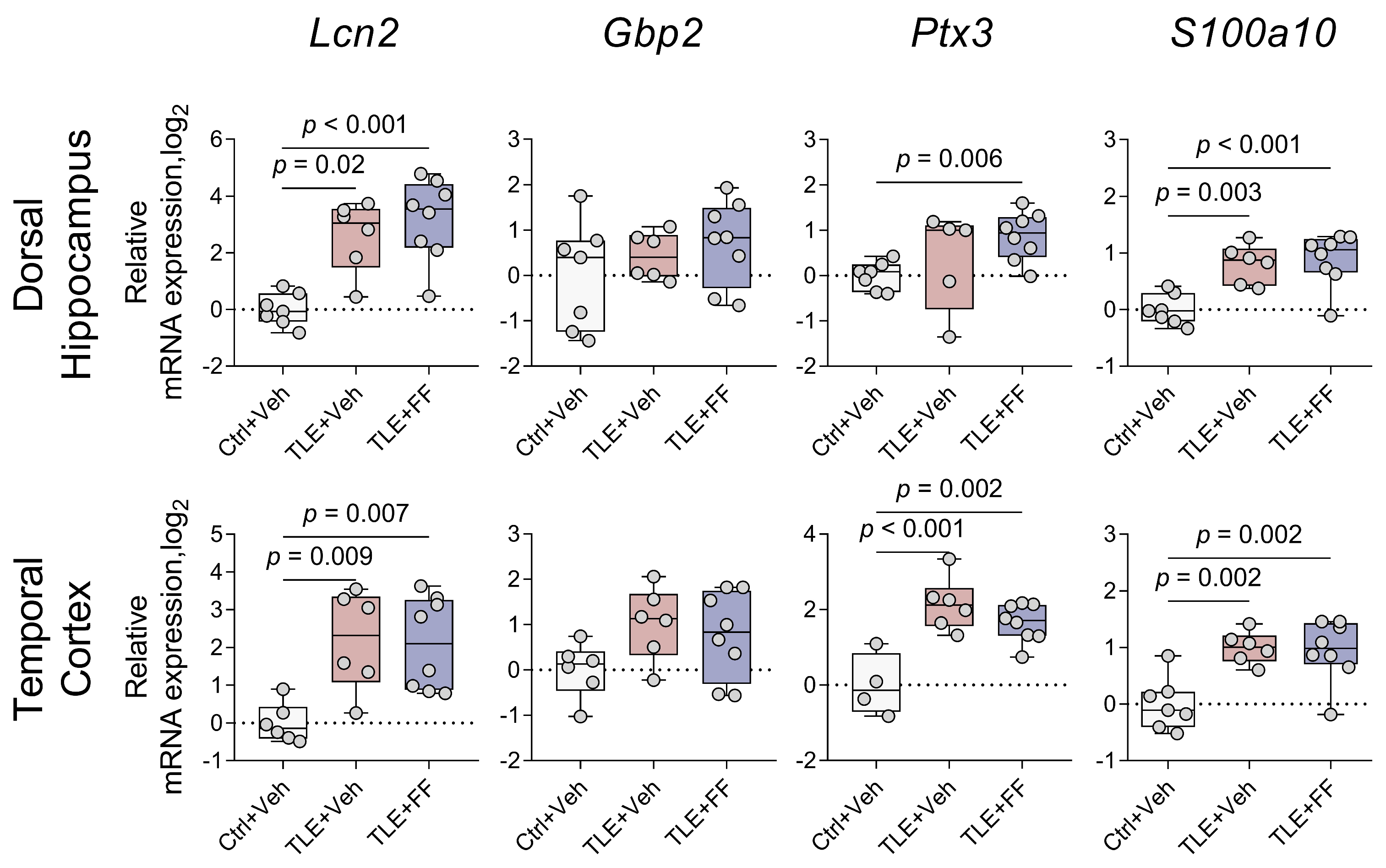
2.7. Fenofibrate Enhances the Expression of Neuroprotective A2 Phenotype Astrocyte Marker but Reduces the Expression of M2 Phenotype Microglia Marker
2.8. Fenofibrate Attenuates the Inflammatory Response in the Temporal Cortex but Not in the Dorsal Hippocampus
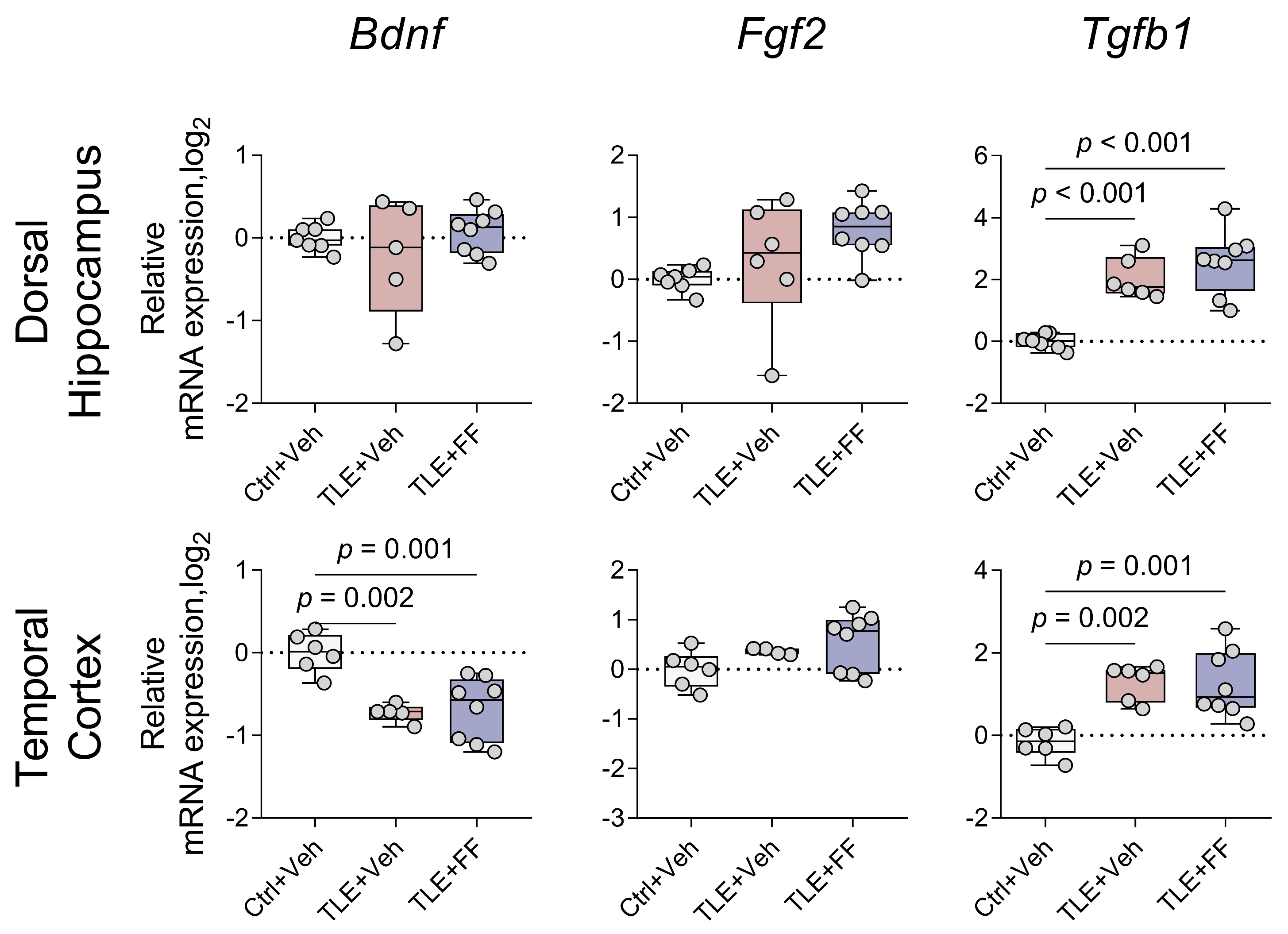
2.9. Fenofibrate Did Not Affect the Expression of Trophic Factor Genes
3. Discussion
3.1. Fenofibrate Decreased Blood Levels of Short-Chain Fatty Acids
3.2. Fenofibrate Exerts Region-Specific Effects on Gene Expression of Glutamate Receptors and Transporters
3.3. Effect of Fenofibrate on Gene Expression of Glial and Neuroinflammation Factors
3.4. Regional-Specific Effects of Fenofibrate on the Expression of the Investigated Genes
3.5. Attenuation of Epilepsy-Associated Behavioral Comorbidities by Fenofibrate
3.6. Limitations of the Study
4. Materials and Methods
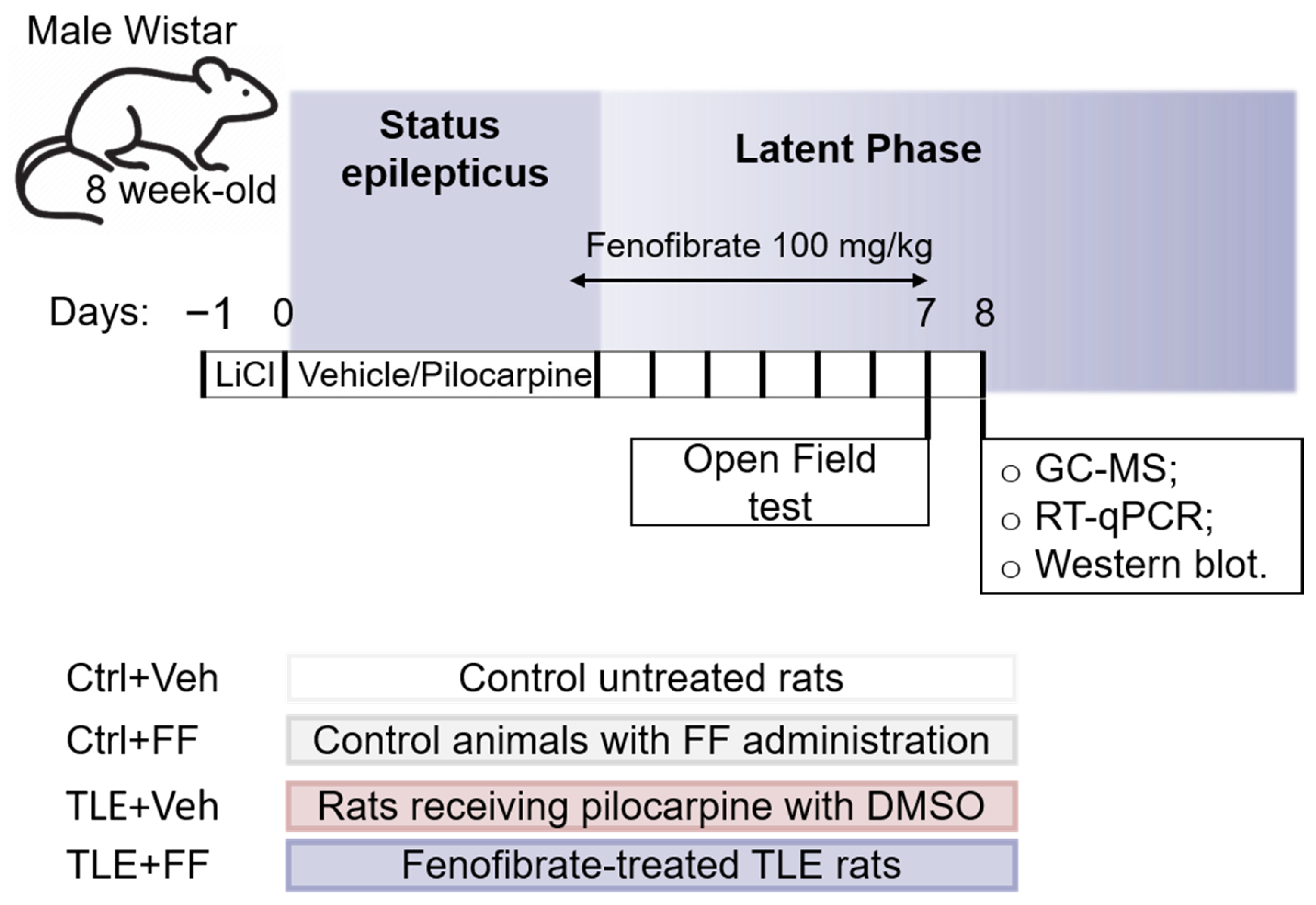
4.1. Experimental Design
4.2. Behavioral Testing
4.3. Biochemical Methods
4.3.1. GS-MS
4.3.2. RT-qPCR
4.3.3. Western Blot
4.4. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene Symbol RefSeq Accession Number | Nucleotide Sequences (Forward, Reverse, TaqMan-Probe) | Final Concentration nM | Reference |
|---|---|---|---|
| Housekeeping genes | |||
| Actb NM_031144 | TGTCACCAACTGGGACGATA GGGGTGTTGAAGGTCTCAAA FAM-CGTGTGGCCCCTGAGGAGCAC-BHQ1 | 200 200 | [119] [120] |
| Gapdh NM_017008 | TGCACCACCAACTGCTTAG GGATGCAGGGATGATGTTC R6G-ATCACGCCACAGCTTTCCAGAGGG-BHQ2 | 200 100 | [121] |
| B2m NM_012512 | TGCCATTCAGAAAACTCCCC GAGGAAGTTGGGCTTCCCATT ROX-ATTCAAGTGTACTCTCGCCATCCACCG-BHQ1 | 200 100 | [122] |
| Rpl13a NM_173340 | GGATCCCTCCACCCTATGACA CTGGTACTTCCACCCGACCTC FAM-CTGCCCTCAAGGTTGTGCGGCT-BHQ1 | 200 100 | [123] [120] |
| Sdha NM_130428 | AGACGTTTGACAGGGGAATG TCATCAATCCGCACCTTGTA R6G-ACCTGGTGGAGACGCTGGAGCT-BHQ2 | 200 100 | [124] [120] |
| Ppia NM_017101 | AGGATTCATGTGCCAGGGTG CTCAGTCTTGGCAGTGCAGA ROX-CACGCCATAATGGCACTGGTGGCA-BHQ1 | 200 100 | [125] |
| Hprt1 NM_012583 | TCCTCAGACCGCTTTTCCCGC TCATCATCACTAATCACGACGCTGG FAM-CCGACCGGTTCTGTCATGTCGACCCT-BHQ1 | 200 100 | [126] [120] |
| Pgk1 NM_053291 | ATGCAAAGACTGGCCAAGCTAC AGCCACAGCCTCAGCATATTTC R6G-TGCTGGCTGGATGGGCTTGGA-BHQ2 | 200 100 | [127] [120] |
| Ywhaz NM_013011 | GATGAAGCCATTGCTGAACTTG GTCTCCTTGGGTATCCGATGTC ROX-TGAAGAGTCGTACAAAGACAGCACGC-BHQ1 | 200 100 | [127] [120] |
| Ionotropic glutamate receptor subunit genes | |||
| Grin1 NM_017010 | GTTCTTCCGCTCAGGCTTTG AGGGAAACGTTCTGCTTCCA FAM-CGGCATGCGCAAGGACAGCC-BHQ1 | 200 100 | [128] |
| Grin2a NM_012573 | GCTACACACCCTGCACCAATT CACCTGGTAACCTTCCTCAGTGA FAM-TGGTCAATGTGACTTGGGATGGCAA-BHQ1 | 200 100 | [129] |
| Grin2b NM_012574 | CCCAACATGCTCTCTCCCTTAA CAGCTAGTCGGCTCTCTTGGTT FAM-GACGCCAAACCTCTAGGCGGACAG-BHQ1 | 200 100 | [129] |
| Gria1 NM_031608 | TCAGAACGCCTCAACGCC TGTAGTGGTACCCGATGCCA ROX-TCCTGGGCCAGATCGTGAAGCTAGAAAA-BHQ1 | 200 100 | [130] |
| Gria2 NM_017261 | CAGTGCATTTCGGGTAGGGA TGCGAAACTGTTGGCTACCT FAM-TCGGAGTTCAGACTGACACCCCA-BHQ1 | 200 100 | [130] |
| Astrocyte and microglial marker genes | |||
| Gfap NM_017009.2 | TGGCCACCAGTAACATGCAA CAGTTGGCGGCGATAGTCAT HEX-CGGTCCAAGTTTGCAGACCTCACAG-BHQ2 | 200 200 | [131] [45] |
| S100b NM_013191.2 | AAGTCCACACCCAGTCCTCT AGGCTCCTGGTCACCTTTTG HEX-ACACCGAAGCCAGAGAGGACTCCGG-BHQ2 | 200 100 | [44] |
| Aif1 NM_017196.3 | CAACACACTGCAGCCTCATC AAGCTTTTCCTCCCTGCAAA Cy5-CCCCACCTAAGGCCACCAGCGTCTGA-BHQ3 | 200 100 | [132] |
| Glutamate-glutamine cycle genes | |||
| Slc1a2 NM_001035233.1 | CCAGTGCTGGAACTTTGCCT TAAAGGGCTGTACCATCCAT FAM-AGCGTGTGACCAGATTCGTCCTCCCA-BHQ1 | 200 150 | [133] [45] |
| Slc1a3 NM_019225.2 | GCGCTGTCATTGTGGGTACA CAGAAGCTCCCCAGGAAAGG Cy5-CCTTGGATTTGCCCTCCGACCGT-BHQ3 | 200 100 | [134] |
| Slc1a1 NM_013032.3 | CCTGCATCCCTCATCCCAC CTCCTACCACGATGCCCAGTA HEX-CCGCCGCGCTCCCCGATTCC-BHQ2 | 200 100 | [134] |
| Glul NM_017073.4 | CCTTTCGGCTGGCCTTCTAA GCTCCCACACCGCAGTAATA ROX-TGGCTTCCCTGGACCCCAAGGACC-BHQ2 | 200 150 | [44] |
| Neuroinflammation genes | |||
| Nlrp3 NM_001191642 | CAGACCCTCATGTTGCCTGT AGACCTCGGCAGAAGCTAGA FAM-CCAGACTGGTGAACTGCTGCCTCA-BHQ1 | 200 100 | [134] |
| Il1b NM_031512 | CACCTCTCAAGCAGAGCACAG GGGTTCCATGGTGAAGTCAAC FAM-TGTCCCGACCATTGCTGTTTCCTAG-BHQ1 | 400 200 | [135] |
| Il1rn NM_022194.2 | GGGGACCTTACAGTCACCTAAT GGTTAGTATCCCAGATTCTGAAGG ROX-AGTCAGCTGGCCACCCTGCTGGGA-BHQ2 | 400 100 | [132] |
| Trophic factor genes | |||
| Bdnf NM_001270630.1 | CCATAAGGACGCGGACTTGTAC GAGGAGGCTCCAAAGGCACTT ROX-CTTCCCGGGTGATGCTCAGCAGT-BHQ2 | 400 200 | [134] |
| Fgf2 NM_019305.2 | AGCGGCTCTACTGCAAGAAC TGGAGCTGTAGTTTGACGTGT R6G-AGACGGCCGCGTGGACGGCGTCCG-BHQ2 | 400 200 | [134] |
| Tgfb1 NM_021578.2 | CTGCTGACCCCCACTGATAC AGCCCTGTATTCCGTCTCCT FAM-TGTCCGGCAGTGGCTGAACCA-BHQ1 | 200 100 | [134] |
| Genes encoding markers of A1/A2 astrocyte states | |||
| Lcn2 NM_130741.1 | AGCTACGATGTGCAAGTGGC CCCCTTGGTTCTTCCGTACA FAM-CGACACTGACTACGACCAGTTTGCCA-BHQ1 | 200 150 | [134] |
| Gbp2 NM_133624.2 | AGTCAATGGGCCACGTCTAA AGTGGGTGATGGCCTTTTGT HEX-AGCAGTGGGTCTCTCCCCTGCA-BHQ2 | 200 100 | [44] |
| Ptx3 NM_001109536.2 | AAACTTCGCCTCTCCAGCAA CATGGTGTGGGGTCCTCG HEX-TGCTCTCTGGTCTGCAGTGTTGGC- BHQ2 | 400 200 | [44] |
| S100a10 NM_031114.1 | CATTTCACAGGTTTGCAGGGG GCACTGGTCCAGGTCTTTCA Cy5-AGGACCCTCTGGCTGTGGACA-BHQ3 | 200 250 | [134] |
| Genes encoding markers of M1/M2 microglial states | |||
| Nos2 NM_012611.3 | CAGAAGCAGAATGTGACCATCAT CGGAGGGACCAGCCAAATC ROX-ACCACCACACAGCCTCAGAGTCCTT-BHQ2 | 200 200 | [136] |
| Arg1 NM_017134.3 | AGCTGGGAATTGGCAAAGTG AACTCAGGTGAATGGGCCTT HEX-TGGAAGAGACCTTCAGCTACCTGC-BHQ2 | 300 100 | [137] [134] |
| Antibody | Clonality | Host Species | Dilution | Manufacturer |
|---|---|---|---|---|
| Primary antibodies | ||||
| Anti-GFAP (ab7260) | Polyclonal | Rabbit | 1:20,000 | Abcam (Cambridge, UK) |
| Anti-EAAT2 (ab205248) | Monoclonal | Rabbit | 1:6000 | Abcam (Cambridge, UK) |
| Anti-NMDAR1 (ab13345) | Polyclonal | Rabbit | 1:1000 | Abcam (Cambridge, UK) |
| Anti-GluN2a (ab169873) | Polyclonal | Rabbit | 1:1000 | Abcam (Cambridge, UK) |
| Anti-GluN2b (ab65783) | Polyclonal | Rabbit | 1:1000 | Abcam (Cambridge, UK) |
| Anti-GluA1 (ab109450) | Monoclonal | Rabbit | 1:1000 | Abcam (Cambridge, UK) |
| Anti-GluA2 (MAB397) | Monoclonal | Mouse | 1:7500 | Merck Millipore (Burlington, MA, USA) |
| Secondary antibodies | ||||
| Anti-rabbit IgG-HRP (31460) | Goat | 1:20,000 | Thermo Fisher Scientific (Rockford, IL, USA) | |
| Anti-mouse IgG-HRP (31430) | Goat | 1:25,000 | Thermo Fisher Scientific (Rockford, IL, USA) | |
| Short-Chain Fatty Acids (Figure 2) | |
| Propanoic acid | F1,26 (Interaction) = 0.528, p = 0.474 F1,26 (TLE) = 3.213, p = 0.085 F1,26 (Treatment) = 3.422, p = 0.076 |
| 2-Methylpropanoic acid | F1,24 (Interaction) = 2.016, p = 0.169 F1,24 (TLE) = 7.721, p = 0.010 F1,24 (Treatment) = 10.190, p = 0.004 |
| Butyric acid | F1,25 (Interaction) = 0.002, p = 0.16 F1,25 (TLE) = 12.490, p = 0.002 F1,25 (Treatment) = 20.910, p < 0.001 |
| 3-Methylbutanoic acid | F1,24 (Interaction) = 3.174, p = 0.088 F1,24 (TLE) = 0.684, p = 0.416 F1,24 (Treatment) = 3.174, p = 0.088 |
| Pentanoic acid | F1,26 (Interaction) = 2.911, p = 0.099 F1,26 (TLE) = 0.438, p = 0.514 F1,26 (Treatment) = 5.741, p = 0.024 |
| 4-Methylvaleric acid | F1,26 (Interaction) = 0.229, p = 0.636 F1,26 (TLE) = 0.959, p = 0.336 F1,26 (Treatment) = 2.636, p = 0.116 |
| Hexanoic acid | F1,26 (Interaction) = 0.610, p = 0.442 F1,26 (TLE) = 1.088, p = 0.306 F1,26 (Treatment) = 15.660, p < 0.001 |
| Heptanoic acid | F1,26 (Interaction) = 1.073, p = 0.310 F1,26 (TLE) = 2.775, p = 0.108 F1,26 (Treatment) = 4.401, p = 0.046 |
| Open Field test (Figure 3) | |
| The total distance | F2,36 = 2.384, p = 0.107 |
| Locomotion | F2,35 = 2.788, p = 0.075 |
| Time in the center | H = 1.459, p = 0.482 |
| Grooming, time | F2,33 = 4.389, p = 0.02 |
| Climbings | F2,17,5 = 2.853, p = 0.085 |
| Number of holes | H = 8.377, p = 0.015 |
| The total time of hole inspections | H = 11.79, p = 0.003 |
| Time of one hole investigation | F2,34 = 4.343, p = 0.021 |
| RT-qPCR | |
| Ionotropic glutamate receptor subunit gene expression (Figure 4) | |
| Grin1 | DH: F(2,16) = 9.239, p = 0.002 TC: F(2,17) = 7.155, p = 0.006 |
| Grin2a | DH: F(2,8.676) = 4.410, p = 0.048 TC: F(2,17) = 4.370, p = 0.029 |
| Grin2b | DH: F(2,17) = 6.726, p = 0.007 TC: F(2,17) = 2.306, p = 0.130 |
| Gria1 | DH: F(2,9.257) = 15.942, p = 0.001 TC: F(2,8.050) = 19.612, p = 0.001 |
| Gria2 | DH: F(2,16) = 9.789, p = 0.002 TC: F(2,17) = 7.952, p = 0.004 |
| Glutamate-glutamine cycle gene expression (Figure 6a) | |
| Slc1a3 | DH: F(2,17) = 11.021, p = 0.001 TC: F(2,17) = 1.760, p = 0.202 |
| Slc1a2 | DH: F(2,17) = 4.280, p = 0.031 TC: F(2,18) = 1.187, p = 0.329 |
| Slc1a1 | DH: F(2,16) = 11.268, p = 0.001 TC: F(2,18) = 0.841, p = 0.448 |
| Glul | DH: F(2,17) = 5.958, p = 0.011 TC: F(2,17) = 0.709, p = 0.506 |
| Astrocyte and microglial marker gene expression (Figure 7a) | |
| Gfap | DH: F(2,8.883) = 118.217, p < 0.001 TC: F(2,18) = 96.363, p < 0.001 |
| Aif1 | DH: F(2,17) = 15.952, p < 0.001 TC: F(2,18) = 6.595, p = 0.007 |
| S100b | DH: F(2,7.691) = 8.731, p = 0.010 TC: F(2,18) = 0.928, p = 0.413 |
| Gene expression of markers of A1/A2 astrocyte states (Figure 8) | |
| Lcn2 | DH: F(2,18) = 15.239, p < 0.001 TC: F(2,9.810) = 13.625, p = 0.001 |
| S100a10 | DH: F(2,18) = 12.052, p < 0.001 TC: F(2,18) = 10.486, p = 0.001 |
| Gbp2 | DH: F(2,18) = 1.096, p = 0.355 TC: F(2,17) = 2.561, p = 0.107 |
| Ptx3 | DH: F(2,8.231) = 7.140, p = 0.016 TC: F(2,15) = 13.980, p < 0.001 |
| Gene expression of markers of M1/M2 microglial states (Figure 9) | |
| Nos2 | DH: F(2,16) = 0.228, p = 0.798 TC: F(2,13) = 6.170, p = 0.013 |
| Arg1 | DH: F(2,18) = 6.536, p = 0.007 TC: F(2,15) = 7.295, p = 0.006 |
| Gene expression of neuroinflammation factors (Figure 10) | |
| Nlrp3 | DH: F(2,18) = 17.826, p < 0.001 TC: F(2,16) = 7.153, p = 0.006 |
| Il1b | DH: F(2,17) = 3.675, p = 0.047 TC: F(2,17) = 0.174, p = 0.842 |
| Il1rn | DH: F(2,18) = 11.648, p = 0.001 TC: F(2,16) = 5.820, p = 0.013 |
| Trophic factor gene expression (Figure 11) | |
| Bdnf | DH: F(2,8.138) = 0.473, p = 0.639 TC: F(2,9.843) = 21.840, p < 0.001 |
| Fgf2 | DH: F(2,18) = 3.270, p = 0.061 TC: F(2,15) = 2.501, p = 0.115 |
| Tgfb1 | DH: F(2,18) = 23.978, p < 0.001 TC: F(2,11.162) = 22.629, p < 0.001 |
| Western blot (Figure 5, Figure 6b and Figure 7b) | |
| GluN1 | F2,15 = 0.054, p = 0.948 |
| GluN2a | F2,15 = 3.917, p = 0.043 |
| GluN2b | F2,15 = 1.837, p = 0.193 |
| GluA1 | F2,15 = 0.996, p = 0.392 |
| GluA2 | F2,14 = 0.262, p = 0.773 |
| GFAP | F2,15 = 18.620, p < 0.001 |
| EAAT2 | F2,14 = 2.526, p = 0.116 |
References
- Milligan, T.A. Epilepsy: A Clinical Overview. Am. J. Med. 2021, 134, 840–847. [Google Scholar] [CrossRef]
- Janmohamed, M.; Brodie, M.J.; Kwan, P. Pharmacoresistance—Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 2020, 168, 107790. [Google Scholar] [CrossRef] [PubMed]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Rubio, T.; Garcia-Gimeno, M.A. Neuroinflammation and Epilepsy: From Pathophysiology to Therapies Based on Repurposing Drugs. Int. J. Mol. Sci. 2024, 25, 4161. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Li, P.; Ji, X.; Shan, M.; Wang, Y.; Dai, X.; Yin, M.; Liu, Y.; Guan, L.; Ye, L.; Cheng, H. Melatonin regulates microglial polarization to M2 cell via RhoA/ROCK signaling pathway in epilepsy. Immunity Inflamm. Dis. 2023, 11, e900. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Melik-Kasumov, T.B. The Gut–Brain Axis and Peroxisome Proliferator-Activated Receptors in the Regulation of Epileptogenesis. J. Evol. Biochem. Physiol. 2021, 57, 743–760. [Google Scholar] [CrossRef]
- Simeone, T.A.; Matthews, S.A.; Samson, K.K.; Simeone, K.A. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp. Neurol. 2017, 287, 54–64. [Google Scholar] [CrossRef]
- Sagheddu, C.; Melis, M.; Muntoni, A.L.; Pistis, M. Repurposing Peroxisome Proliferator-Activated Receptor Agonists in Neurological and Psychiatric Disorders. Pharmaceuticals 2021, 14, 1025. [Google Scholar] [CrossRef]
- Hollis, A.; Lukens, J.R. Role of inflammasomes and neuroinflammation in epilepsy. Immunol. Rev. 2025, 329, e13421. [Google Scholar] [CrossRef]
- Crisafulli, C.; Cuzzocrea, S. The role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor alpha (PPAR-alpha) in the regulation of inflammation in macrophages. Shock 2009, 32, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, K.; Xiang, W.; Li, Y.; Hao, Y.; Guan, Y. Rosiglitazone polarizes microglia and protects against pilocarpine-induced status epilepticus. CNS Neurosci. Ther. 2019, 25, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, Y.; Yu, X.; Li, Y.; Yang, J.; Li, R.; Deng, Y.; Zhao, G. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. Int. J. Dev. Neurosci. 2008, 26, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shao, X.G.; Sun, H.; Li, Y.N.; Yang, J.; Deng, Y.C.; Huang, Y.G. Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus. Brain Res. 2008, 1200, 146–158. [Google Scholar] [CrossRef]
- Adabi Mohazab, R.; Javadi-Paydar, M.; Delfan, B.; Dehpour, A.R. Possible involvement of PPAR-gamma receptor and nitric oxide pathway in the anticonvulsant effect of acute pioglitazone on pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2012, 101, 28–35. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Qu, Y.; Wang, D.; Zhu, Y.; Zhu, Y. Rosiglitazone Prevents Autophagy by Regulating Nrf2-Antioxidant Response Element in a Rat Model of Lithium-pilocarpine-induced Status Epilepticus. Neuroscience 2021, 455, 212–222. [Google Scholar] [CrossRef]
- Porta, N.; Vallée, L.; Lecointe, C.; Bouchaert, E.; Staels, B.; Bordet, R.; Auvin, S. Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, exerts anticonvulsive properties. Epilepsia 2009, 50, 943–948. [Google Scholar] [CrossRef]
- Puligheddu, M.; Pillolla, G.; Melis, M.; Lecca, S.; Marrosu, F.; De Montis, M.G.; Scheggi, S.; Carta, G.; Murru, E.; Aroni, S.; et al. PPAR-Alpha Agonists as Novel Antiepileptic Drugs: Preclinical Findings. PLoS ONE 2013, 8, e64541. [Google Scholar] [CrossRef]
- Keating, G.M.; Croom, K.F. Fenofibrate: A review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs 2007, 67, 121–153. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Liu, X.; Yang, J.; Feng, Y.; Wu, Y.; Xu, Y.; Zhu, Y.; Li, W. Fenofibrate Ameliorated Systemic and Retinal Inflammation and Modulated Gut Microbiota in High-Fat Diet-Induced Mice. Front. Cell. Infect. Microbiol. 2022, 12, 839592. [Google Scholar] [CrossRef]
- Liu, Q. Fenofibrate alleviates the composition and metabolic pathways of gut microbiota in high-fat diet treated hamsters. Ann. Microbiol. 2024, 74, 21. [Google Scholar] [CrossRef]
- Scheggi, S.; Pinna, G.; Braccagni, G.; De Montis, M.G.; Gambarana, C. PPARα Signaling: A Candidate Target in Psychiatric Disorder Management. Biomolecules 2022, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.G.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef]
- Rossi, A.R.; Angelo, M.F.; Villarreal, A.; Lukin, J.; Ramos, A.J. Gabapentin administration reduces reactive gliosis and neurodegeneration after pilocarpine-induced status epilepticus. PLoS ONE 2013, 8, e78516. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Kharisova, A.R.; Roginskaya, A.I.; Kovalenko, A.A.; Zakharova, M.V.; Schwarz, A.P.; Sinyak, D.S.; Zaitsev, A.V. PPARβ/δ Agonist GW0742 Modulates Microglial and Astroglial Gene Expression in a Rat Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2024, 25, 10015. [Google Scholar] [CrossRef]
- Mihály, A. The Reactive Plasticity of Hippocampal Ionotropic Glutamate Receptors in Animal Epilepsies. Int. J. Mol. Sci. 2019, 20, 1030. [Google Scholar] [CrossRef]
- Walker, M.C. Pathophysiology of status epilepticus. Neurosci. Lett. 2018, 667, 84–91. [Google Scholar] [CrossRef]
- Loddenkemper, T.; Talos, D.M.; Cleary, R.T.; Joseph, A.; Sánchez Fernández, I.; Alexopoulos, A.; Kotagal, P.; Najm, I.; Jensen, F.E. Subunit composition of glutamate and gamma-aminobutyric acid receptors in status epilepticus. Epilepsy Res. 2014, 108, 605–615. [Google Scholar] [CrossRef]
- Naylor, D.E.; Liu, H.; Niquet, J.; Wasterlain, C.G. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol. Dis. 2013, 54, 225–238. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, H.; Klein, P.M.; Jensen, F.E. Neonatal seizures alter NMDA glutamate receptor GluN2A and 3A subunit expression and function in hippocampal CA1 neurons. Front. Cell. Neurosci. 2015, 9, 362. [Google Scholar] [CrossRef]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-d-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef]
- Lee, K.; Goodman, L.; Fourie, C.; Schenk, S.; Leitch, B.; Montgomery, J.M. AMPA Receptors as Therapeutic Targets for Neurological Disorders. Adv. Protein Chem. Struct. Biol. 2016, 103, 203–261. [Google Scholar] [PubMed]
- Szczurowska, E.; Mareš, P. NMDA and AMPA receptors: Development and status epilepticus. Physiol. Res. 2013, 62 (Suppl. 1), S21–S38. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Gruenbaum, S.E.; Dhaher, R.; Lee, T.-S.W.; Zhou, Y.; Danbolt, N.C. The Glutamate-Glutamine Cycle in Epilepsy. Adv. Neurobiol. 2016, 13, 351–400. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V.; Smolensky, I.V.; Jorratt, P.; Ovsepian, S.V. Neurobiology, Functions, and Relevance of Excitatory Amino Acid Transporters (EAATs) to Treatment of Refractory Epilepsy. CNS Drugs 2020, 34, 1089–1103. [Google Scholar] [CrossRef]
- Eid, T.; Lee, T.S.W.; Patrylo, P.; Zaveri, H.P. Astrocytes and Glutamine Synthetase in Epileptogenesis. J. Neurosci. Res. 2019, 97, 1345–1362. [Google Scholar] [CrossRef]
- DiNuzzo, M.; Mangia, S.; Maraviglia, B.; Giove, F. Physiological bases of the K+ and the glutamate/GABA hypotheses of epilepsy. Epilepsy Res. 2014, 108, 995–1012. [Google Scholar] [CrossRef]
- Cavender, C.E.; Gottipati, M.K.; Parpura, V. Trafficking of excitatory amino acid transporter 2-laden vesicles in cultured astrocytes: A comparison between approximate and exact determination of trajectory angles. Amino Acids 2016, 47, 357–367. [Google Scholar] [CrossRef][Green Version]
- Bjørn-Yoshimoto, W.E.; Underhill, S.M. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem. Int. 2016, 98, 4–18. [Google Scholar] [CrossRef]
- Dyomina, A.V.; Kovalenko, A.A.; Zakharova, M.V.; Postnikova, T.Y.; Griflyuk, A.V.; Smolensky, I.V.; Antonova, I.V.; Zaitsev, A.V. MTEP, a Selective mGluR5 Antagonist, Had a Neuroprotective Effect but Did Not Prevent the Development of Spontaneous Recurrent Seizures and Behavioral Comorbidities in the Rat Lithium-Pilocarpine Model of Epilepsy. Int. J. Mol. Sci. 2022, 23, 497. [Google Scholar] [CrossRef]
- Kyriatzis, G.; Bernard, A.; Bôle, A.; Khrestchatisky, M.; Ferhat, L. In the Rat Hippocampus, Pilocarpine-Induced Status Epilepticus Is Associated with Reactive Glia and Concomitant Increased Expression of CD31, PDGFRβ, and Collagen IV in Endothelial Cells and Pericytes of the Blood–Brain Barrier. Int. J. Mol. Sci. 2024, 25, 1693. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Dyomina, A.V.; Kovalenko, A.A.; Zubareva, O.E.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Promotes M2 Microglia Activation during the Latent Phase of the Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. J. Evol. Biochem. Physiol. 2024, 60, 672–689. [Google Scholar] [CrossRef]
- Dyomina, A.V.; Zubareva, O.E.; Smolensky, I.V.; Vasilev, D.S.; Zakharova, M.V.; Kovalenko, A.A.; Schwarz, A.P.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Reduces Epileptogenesis, Provides Neuroprotection, and Attenuates Behavioral Impairments in Rats in the Lithium–Pilocarpine Model of Epilepsy. Pharmaceuticals 2020, 13, 340. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Guo, Y.; Wang, W. Bioinformatics analysis reveals multiple functional changes in astrocytes in temporal lobe epilepsy. Brain Res. 2024, 1831, 148820. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G.; Yan, Y.-Q.; Ma, C.-G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Puttachary, S.; Thippeswamy, T. Glial source of nitric oxide in epileptogenesis: A target for disease modification in epilepsy. J. Neurosci. Res. 2019, 97, 1363–1377. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine Metabolism: Boundaries of Our Knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef] [PubMed]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, X.; Wang, Y. Interleukins in Epilepsy: Friend or Foe. Neurosci. Bull. 2024, 40, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Iori, V.; Frigerio, F.; Vezzani, A. Modulation of neuronal excitability by immune mediators in epilepsy. Curr. Opin. Pharmacol. 2016, 26, 118–123. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zeng, Y.; Zheng, W. Neuroinflammation in epileptogenesis: From pathophysiology to therapeutic strategies. Front. Immunol. 2023, 14, 1269241. [Google Scholar] [CrossRef]
- Litovchenko, A.V.; Zabrodskaya, Y.M.; Sitovskaya, D.A.; Khuzhakhmetova, L.K.; Nezdorovina, V.G.; Bazhanova, E.D. Markers of Neuroinflammation and Apoptosis in the Temporal Lobe of Patients with Drug-Resistant Epilepsy. J. Evol. Biochem. Physiol. 2021, 57, 1040–1049, https://doi.org/10.1134/S0022093021050069; Erratum in J. Evol. Biochem. Phys. 2021, 57, 1533. [Google Scholar]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef]
- Gliwińska, A.; Czubilińska-Łada, J.; Więckiewicz, G.; Świętochowska, E.; Badeński, A.; Dworak, M.; Szczepańska, M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Diagnosis and Treatment of Epilepsy, Depression, Schizophrenia, Anorexia Nervosa and Alzheimer’s Disease as Highly Drug-Resistant Diseases: A Narrative Review. Brain Sci. 2023, 13, 163. [Google Scholar] [CrossRef]
- Simonato, M. Neurotrophic factors and status epilepticus. Epilepsia 2018, 59, 87–91. [Google Scholar] [CrossRef]
- Simonato, M.; Tongiorgi, E.; Kokaia, M. Angels and demons: Neurotrophic factors and epilepsy. Trends Pharmacol. Sci. 2006, 27, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lasoń, W.; Chlebicka, M.; Rejdak, K. Research advances in basic mechanisms of seizures and antiepileptic drug action. Pharmacol. Rep. 2013, 65, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Soltani Khaboushan, A.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Alsaleem, M.A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Abdel-Fattah, M.M.; Alrouji, M.; Al-Harchan, N.A.; Alruwaili, M.; Papadakis, M.; Alexiou, A.; Batiha, G.E.-S. Decrypting the Possible Mechanistic Role of Fenofibrate in Alzheimer’s Disease and Type 2 Diabetes: The Truth and Mystery. J. Cell. Mol. Med. 2025, 29, e70378. [Google Scholar] [CrossRef]
- Kopf, T.; Schaefer, H.-L.; Troetzmueller, M.; Koefeler, H.; Broenstrup, M.; Konovalova, T.; Schmitz, G. Influence of Fenofibrate Treatment on Triacylglycerides, Diacylglycerides and Fatty Acids in Fructose Fed Rats. PLoS ONE 2014, 9, e106849. [Google Scholar] [CrossRef][Green Version]
- Grabacka, M.; Płonka, P.M.; Pierzchalska, M. The PPARα Regulation of the Gut Physiology in Regard to Interaction with Microbiota, Intestinal Immunity, Metabolism, and Permeability. Int. J. Mol. Sci. 2022, 23, 14156. [Google Scholar] [CrossRef]
- Rho, J.M.; Boison, D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 2022, 18, 333–347. [Google Scholar] [CrossRef]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef]
- Zhong, H.; Yu, H.; Chen, J.; Mok, S.W.F.; Tan, X.; Zhao, B.; He, S.; Lan, L.; Fu, X.; Chen, G.; et al. The short-chain fatty acid butyrate accelerates vascular calcification via regulation of histone deacetylases and NF-κB signaling. Vasc. Pharmacol. 2022, 146, 107096. [Google Scholar] [CrossRef]
- Simeone, T.A.; Simeone, K.A.; Rho, J.M. Ketone Bodies as Anti-Seizure Agents. Neurochem. Res. 2017, 42, 2011–2018. [Google Scholar] [CrossRef]
- Chuang, D.-M.; Leng, Y.; Marinova, Z.; Kim, H.-J.; Chiu, C.-T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009, 32, 591–601. [Google Scholar] [CrossRef]
- Reddy, S.D.; Clossen, B.L.; Reddy, D.S. Epigenetic Histone Deacetylation Inhibition Prevents the Development and Persistence of Temporal Lobe Epilepsy. J. Pharmacol. Exp. Ther. 2018, 364, 97–109. [Google Scholar] [CrossRef]
- Aronica, E.; Gorter, J.A. Gene expression profile in temporal lobe epilepsy. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2007, 13, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Olney, J.W.; Maniotis, A.; Collins, R.C.; Zorumski, C.F. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience 1987, 23, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Alonso, A.; Avoli, M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience 1996, 72, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Smolders, I.; Khan, G.M.; Manil, J.; Ebinger, G.; Michotte, Y. NMDA receptor-mediated pilocarpine-induced seizures: Characterization in freely moving rats by microdialysis. Br. J. Pharmacol. 1997, 121, 1171–1179. [Google Scholar] [CrossRef]
- Celli, R.; Fornai, F. Targeting Ionotropic Glutamate Receptors in the Treatment of Epilepsy. Curr. Neuropharmacol. 2021, 19, 747–765. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Diespirov, G.P.; Amakhin, D.V.; Vylekzhanina, E.N.; Soboleva, E.B.; Zaitsev, A.V. Impairments of Long-Term Synaptic Plasticity in the Hippocampus of Young Rats during the Latent Phase of the Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2021, 22, 13355. [Google Scholar] [CrossRef]
- Eichelbaum, K.; Krijgsveld, J. Rapid temporal dynamics of transcription, protein synthesis, and secretion during macrophage activation. Mol. Cell. Proteom. 2014, 13, 792–810. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Ai, S.; Ouyang, W.; Le, Y.; Tong, J. Sepsis-induced selective loss of NMDA receptors modulates hippocampal neuropathology in surviving septic mice. PLoS ONE 2017, 12, e0188273. [Google Scholar] [CrossRef]
- Kovalenko, A.A.; Zakharova, M.V.; Zubareva, O.E.; Schwarz, A.P.; Postnikova, T.Y.; Zaitsev, A.V. Alterations in mRNA and Protein Expression of Glutamate Receptor Subunits Following Pentylenetetrazole-induced Acute Seizures in Young Rats. Neuroscience 2021, 468, 1–15. [Google Scholar] [CrossRef]
- Todd, A.C.; Hardingham, G.E. The Regulation of Astrocytic Glutamate Transporters in Health and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9607. [Google Scholar] [CrossRef] [PubMed]
- Rakhade, S.N.; Loeb, J.A. Focal reduction of neuronal glutamate transporters in human neocortical epilepsy. Epilepsia 2008, 49, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B.; Jin, H.; Shumate, M.D.; Robinson, M.B.; Coulter, D.A.; Brooks-Kayal, A.R. Increased Expression of the Neuronal Glutamate Transporter (EAAT3/EAAC1) in Hippocampal and Neocortical Epilepsy. Epilepsia 2002, 43, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Xin, Y.; HaiQin, W.; GuiLian, Z.; Ru, Z.; ShuQin, Z.; HuQing, W.; Li, Y.; Yun, D. The PPARγ agonist rosiglitazone prevents cognitive impairment by inhibiting astrocyte activation and oxidative stress following pilocarpine-induced status epilepticus. Neurol. Sci. 2012, 33, 559–566. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Peixoto-Santos, J.E.; Monteiro, M.R.; Scandiuzzi, R.C.; Carlotti, C.G.; Assirati, J.A.; Hallak, J.E.; Leite, J.P. Mesial temporal lobe epilepsy with psychiatric comorbidities: A place for differential neuroinflammatory interplay. J. Neuroinflamm. 2015, 12, 38. [Google Scholar] [CrossRef]
- Robel, S.; Sontheimer, H. Glia as drivers of abnormal neuronal activity. Nat. Neurosci. 2015, 19, 28–33. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Akbulut, M.C.; Altuntaş, İ.; Tomruk, C.; Uyanıkgil, Y.; Erbaş, O. Amelioration of propionic acid-induced autism-like behaviors in rats by fenofibrate: A focus on reduction of brain galectin-3 levels. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2024, 84, 977–990. [Google Scholar] [CrossRef]
- Villavicencio-Tejo, F.; Flores-Bastías, O.; Marambio-Ruiz, L.; Pérez-Reytor, D.; Karahanian, E. Fenofibrate (a PPAR-α Agonist) Administered During Ethanol Withdrawal Reverts Ethanol-Induced Astrogliosis and Restores the Levels of Glutamate Transporter in Ethanol-Administered Adolescent Rats. Front. Pharmacol. 2021, 12, 653175. [Google Scholar] [CrossRef]
- Fakan, B.; Szalardy, L.; Vecsei, L. Exploiting the Therapeutic Potential of Endogenous Immunomodulatory Systems in Multiple Sclerosis-Special Focus on the Peroxisome Proliferator-Activated Receptors (PPARs) and the Kynurenines. Int. J. Mol. Sci. 2019, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Yadav, S.; Gupta, R.K.; Waggoner, G.R.; Deloach, A.; Calingasan, N.Y.; Beal, M.F.; Kiaei, M. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Hum. Mol. Genet. 2016, 25, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Abulaban, A.A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Alanazi, A.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Role of fenofibrate in multiple sclerosis. Eur. J. Med. Res. 2024, 29, 113. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Y.; Liu, N.; Hai, D.; Wei, W.; Liu, Y.; Lan, X.; Jin, X.; Yu, J.; Ma, L. Mechanism of NLRP3 Inflammasome in Epilepsy and Related Therapeutic Agents. Neuroscience 2024, 546, 157–177. [Google Scholar] [CrossRef]
- Fernández-García, S.; Sancho-Balsells, A.; Longueville, S.; Hervé, D.; Gruart, A.; Delgado-García, J.M.; Alberch, J.; Giralt, A. Astrocytic BDNF and TrkB regulate severity and neuronal activity in mouse models of temporal lobe epilepsy. Cell Death Dis. 2020, 11, 411. [Google Scholar] [CrossRef]
- Iughetti, L.; Lucaccioni, L.; Fugetto, F.; Predieri, B.; Berardi, A.; Ferrari, F. Brain-derived neurotrophic factor and epilepsy: A systematic review. Neuropeptides 2018, 72, 23–29. [Google Scholar] [CrossRef]
- Unsain, N.; Montroull, L.E.; Mascó, D.H. Brain-derived neurotrophic factor facilitates TrkB down-regulation and neuronal injury after status epilepticus in the rat hippocampus. J. Neurochem. 2009, 111, 428–440. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef]
- Holmes, G.L. Drug Treatment of Epilepsy Neuropsychiatric Comorbidities in Children. Paediatr. Drugs 2021, 23, 55–73. [Google Scholar] [CrossRef]
- Hermann, B.P.; Struck, A.F.; Busch, R.M.; Reyes, A.; Kaestner, E.; McDonald, C.R. Neurobehavioural comorbidities of epilepsy: Towards a network-based precision taxonomy. Nat. Rev. Neurol. 2021, 17, 731–746. [Google Scholar] [CrossRef]
- Inostroza, M.; Cid, E.; de la Prida, L.M.; Sandi, C. Different emotional disturbances in two experimental models of temporal Lobe Epilepsy in rats. PLoS ONE 2012, 7, e38959. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, I.V.; Zubareva, O.E.; Kalemenev, S.V.; Lavrentyeva, V.V.; Dyomina, A.V.; Karepanov, A.A.; Zaitsev, A.V. Impairments in cognitive functions and emotional and social behaviors in a rat lithium-pilocarpine model of temporal lobe epilepsy. Behav. Brain Res. 2019, 372, 112044. [Google Scholar] [CrossRef] [PubMed]
- Suleymanova, E.M.; Gulyaev, M.V.; Abbasova, K.R. Structural alterations in the rat brain and behavioral impairment after status epilepticus: An MRI study. Neuroscience 2016, 315, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Wulsin, A.C.; Franco-Villanueva, A.; Romancheck, C.; Morano, R.L.; Smith, B.L.; Packard, B.A.; Danzer, S.C.; Herman, J.P. Functional disruption of stress modulatory circuits in a model of temporal lobe epilepsy. PLoS ONE 2018, 13, e0197955. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Sinyak, D.S.; Kalita, A.D.; Griflyuk, A.V.; Diespirov, G.P.; Postnikova, T.Y.; Zaitsev, A.V. Antiepileptogenic Effects of Anakinra, Lamotrigine and Their Combination in a Lithium–Pilocarpine Model of Temporal Lobe Epilepsy in Rats. Int. J. Mol. Sci. 2023, 24, 15400. [Google Scholar] [CrossRef]
- Matovu, D.; Cavalheiro, E.A. Differences in Evolution of Epileptic Seizures and Topographical Distribution of Tissue Damage in Selected Limbic Structures Between Male and Female Rats Submitted to the Pilocarpine Model. Front. Neurol. 2022, 13, 802587. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Mohammadi, M.T.; Rezaee, R.; Sahebkar, A. Fenofibrate improves renal function by amelioration of NOX-4, IL-18, and p53 expression in an experimental model of diabetic nephropathy. J. Cell. Biochem. 2018, 119, 7458–7469. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schwarz, A.P.; Zakharova, M.V.; Kovalenko, A.A.; Dyomina, A.V.; Zubareva, O.E.; Zaitsev, A.V. Time- and Region-Specific Selection of Reference Genes in the Rat Brain in the Lithium–Pilocarpine Model of Acquired Temporal Lobe Epilepsy. Biomedicines 2024, 12, 1100. [Google Scholar] [CrossRef]
- Kopec, A.M.; Rivera, P.D.; Lacagnina, M.J.; Hanamsagar, R.; Bilbo, S.D. Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue. J. Neurosci. Methods 2017, 280, 64–76. [Google Scholar] [CrossRef]
- Harrington, C.R. Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determinations on fractions from brain tissue. Anal. Biochem. 1990, 186, 285–287. [Google Scholar] [CrossRef]
- Thacker, J.S.; Yeung, D.H.; Staines, W.R.; Mielke, J.G. Total protein or high-abundance protein: Which offers the best loading control for Western blotting? Anal. Biochem. 2016, 496, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar]
- Gilda, J.E.; Ghosh, R.; Cheah, J.X.; West, T.M.; Bodine, S.C.; Gomes, A.V. Western blotting inaccuracies with unverified antibodies: Need for a Western Blotting Minimal Reporting Standard (WBMRS). PLoS ONE 2015, 10, e0135392. [Google Scholar] [CrossRef] [PubMed]
- Pillai-Kastoori, L.; Schutz-Geschwender, A.R.; Harford, J.A. A systematic approach to quantitative Western blot analysis. Anal. Biochem. 2020, 593, 113608. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]
- Bonefeld, B.E.; Elfving, B.; Wegener, G. Reference genes for normalization: A study of rat brain tissue. Synapse 2008, 62, 302–309. [Google Scholar] [CrossRef]
- Schwarz, A.P.; Malygina, D.A.; Kovalenko, A.A.; Trofimov, A.N.; Zaitsev, A.V. Multiplex qPCR assay for assessment of reference gene expression stability in rat tissues/samples. Mol. Cell. Probes 2020, 53, 101611. [Google Scholar] [CrossRef]
- Lin, W.; Burks, C.A.; Hansen, D.R.; Kinnamon, S.C.; Gilbertson, T.A. Taste Receptor Cells Express pH-Sensitive Leak K+ Channels. J. Neurophysiol. 2004, 92, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yamauchi, A.; Nishimura, M.; Ueda, N.; Naito, S. Soybean Oil Fat Emulsion Prevents Cytochrome P450 mRNA Down-Regulation Induced by Fat-Free Overdose Total Parenteral Nutrition in Infant Rats. Biol. Pharm. Bull. 2005, 28, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Swijsen, A.; Nelissen, K.; Janssen, D.; Rigo, J.-M.; Hoogland, G. Validation of reference genes for quantitative real-time PCR studies in the dentate gyrus after experimental febrile seizures. BMC Res. Notes 2012, 5, 685. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R.; Niittynen, M.; Lindén, J.; Boutros, P.C.; Moffat, I.D.; Okey, A.B. Evaluation of various housekeeping genes for their applicability for normalization of mRNA expression in dioxin-treated rats. Chem. Biol. Interact. 2006, 160, 134–149. [Google Scholar] [CrossRef]
- Malkin, S.L.; Amakhin, D.V.; Veniaminova, E.A.; Kim, K.K.; Zubareva, O.E.; Magazanik, L.G.; Zaitsev, A.V. Changes of AMPA receptor properties in the neocortex and hippocampus following pilocarpine-induced status epilepticus in rats. Neuroscience 2016, 327, 146–155. [Google Scholar] [CrossRef]
- Cook, N.L.; Vink, R.; Donkin, J.J.; van den Heuvel, C. Validation of reference genes for normalization of real-time quantitative RT-PCR data in traumatic brain injury. J. Neurosci. Res. 2009, 87, 34–41. [Google Scholar] [CrossRef]
- Langnaese, K.; John, R.; Schweizer, H.; Ebmeyer, U.; Keilhoff, G. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol. Biol. 2008, 9, 53. [Google Scholar] [CrossRef]
- Giza, C.C.; Maria, N.S.S.; Hovda, D.A. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J. Neurotrauma 2006, 23, 950–961. [Google Scholar] [CrossRef]
- Floyd, D.W.; Jung, K.-Y.; McCool, B.A. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J. Pharmacol. Exp. Ther. 2003, 307, 1020–1029. [Google Scholar] [CrossRef]
- Proudnikov, D.; Yuferov, V.; Zhou, Y.; LaForge, K.S.; Ho, A.; Kreek, M.J. Optimizing primer--probe design for fluorescent PCR. J. Neurosci. Methods 2003, 123, 31–45. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Attenuation of Morphine Tolerance, Withdrawal-Induced Hyperalgesia, and Associated Spinal Inflammatory Immune Responses by Propentofylline in Rats. Neuropsychopharmacology 2004, 29, 327–334. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Dyomina, A.V.; Kovalenko, A.A.; Roginskaya, A.I.; Melik-Kasumov, T.B.; Korneeva, M.A.; Chuprina, A.V.; Zhabinskaya, A.A.; Kolyhan, S.A.; Zakharova, M.V.; et al. Beneficial Effects of Probiotic Bifidobacterium longum in a Lithium-Pilocarpine Model of Temporal Lobe Epilepsy in Rats. Int. J. Mol. Sci. 2023, 24, 8451. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Hasselfeld, K.; Bauer, D.; Simmons, M.; Roussos, P.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Glutamate transporter splice variant expression in an enriched pyramidal cell population in schizophrenia. Transl. Psychiatry 2015, 5, e579. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, M.V.; Kovalenko, A.A.; Zubareva, O.E.; Schwarz, A.P.; Postnikova, T.Y.; Trofimova, A.M.; Zaitsev, A.V. Pentylenetetrazole-Induced Seizures Cause Short-Term Changes in the Phenotype of Microglial and Astroglial Cells in the Hippocampus and Temporal Cortex of Young Male Wistar Rats. J. Neurosci. Res. 2024, 102, e25385. [Google Scholar] [CrossRef] [PubMed]
- Rioja, I.; Bush, K.A.; Buckton, J.B.; Dickson, M.C.; Life, P.F. Joint cytokine quantification in two rodent arthritis models: Kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatment. Clin. Exp. Immunol. 2004, 137, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Yun, Y.; Li, H.; Hou, L.; Han, M.; Li, G. SO2 inhalation contributes to the development and progression of ischemic stroke in the brain. Toxicol. Sci. 2010, 114, 226–236. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Y.; Cheng, C.; Zhu, Y.; Ye, Y.; Sun, Y.; Xiang, S.; Wang, Y.; Liu, Z.; Zhang, X. Hydrogen regulates the M1/M2 polarization of alveolar macrophages in a rat model of chronic obstructive pulmonary disease. Exp. Lung Res. 2021, 47, 301–310. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalenko, A.A.; Zakharova, M.V.; Zubareva, O.E.; Schwarz, A.P.; Skorik, Y.A.; Zaitsev, A.V. Fenofibrate as a PPARα Agonist Modulates Neuroinflammation and Glutamate Receptors in a Rat Model of Temporal Lobe Epilepsy: Region-Specific Effects and Behavioral Outcomes. Int. J. Mol. Sci. 2025, 26, 9054. https://doi.org/10.3390/ijms26189054
Kovalenko AA, Zakharova MV, Zubareva OE, Schwarz AP, Skorik YA, Zaitsev AV. Fenofibrate as a PPARα Agonist Modulates Neuroinflammation and Glutamate Receptors in a Rat Model of Temporal Lobe Epilepsy: Region-Specific Effects and Behavioral Outcomes. International Journal of Molecular Sciences. 2025; 26(18):9054. https://doi.org/10.3390/ijms26189054
Chicago/Turabian StyleKovalenko, Anna A., Maria V. Zakharova, Olga E. Zubareva, Alexander P. Schwarz, Yury A. Skorik, and Aleksey V. Zaitsev. 2025. "Fenofibrate as a PPARα Agonist Modulates Neuroinflammation and Glutamate Receptors in a Rat Model of Temporal Lobe Epilepsy: Region-Specific Effects and Behavioral Outcomes" International Journal of Molecular Sciences 26, no. 18: 9054. https://doi.org/10.3390/ijms26189054
APA StyleKovalenko, A. A., Zakharova, M. V., Zubareva, O. E., Schwarz, A. P., Skorik, Y. A., & Zaitsev, A. V. (2025). Fenofibrate as a PPARα Agonist Modulates Neuroinflammation and Glutamate Receptors in a Rat Model of Temporal Lobe Epilepsy: Region-Specific Effects and Behavioral Outcomes. International Journal of Molecular Sciences, 26(18), 9054. https://doi.org/10.3390/ijms26189054






