Roles of Neutrophils in Autoimmune Diseases and Cancers
Abstract
1. Introduction
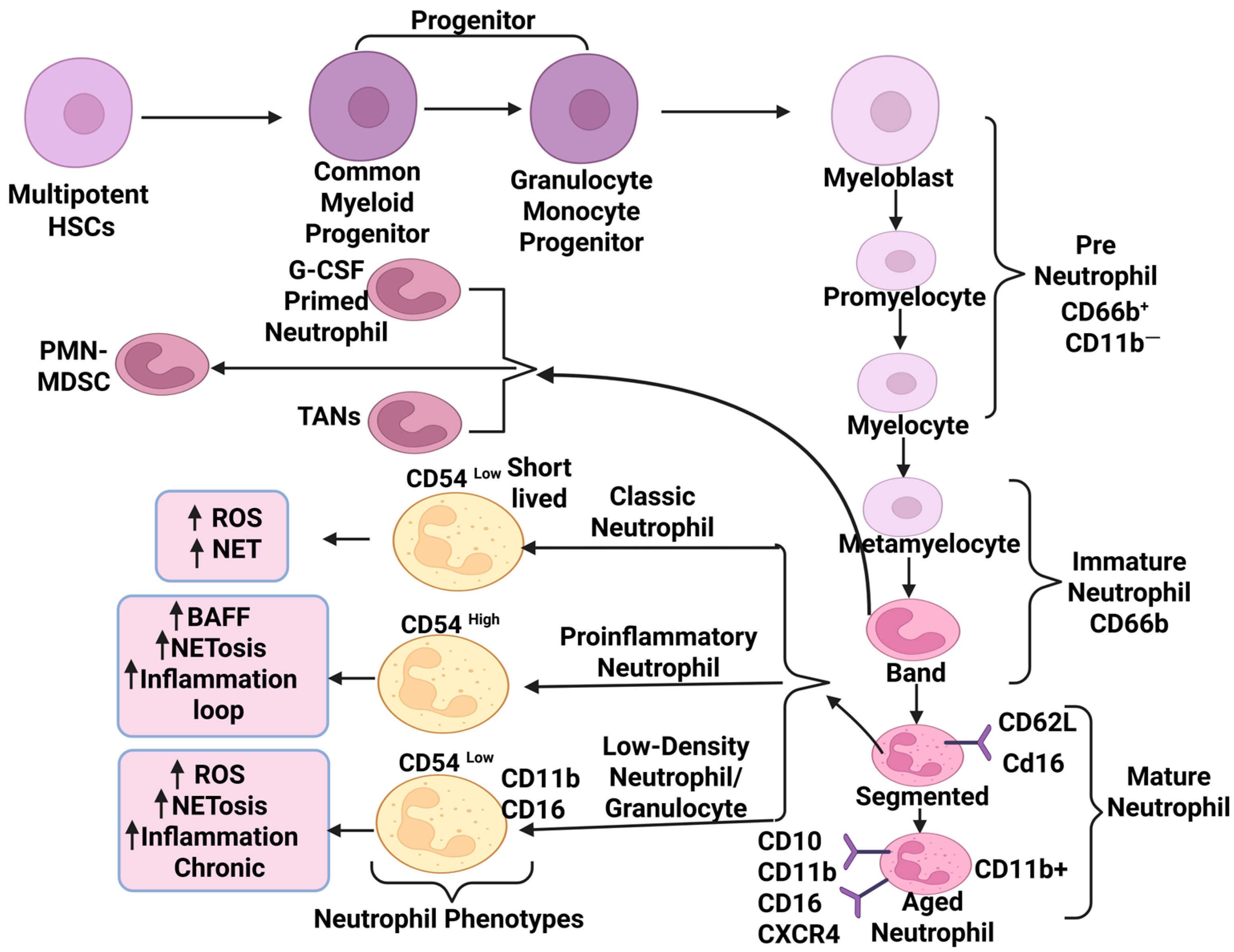
2. Neutrophil Biology: Maturation, Subtypes, and Functional Plasticity
3. Neutrophils in Autoimmune Diseases
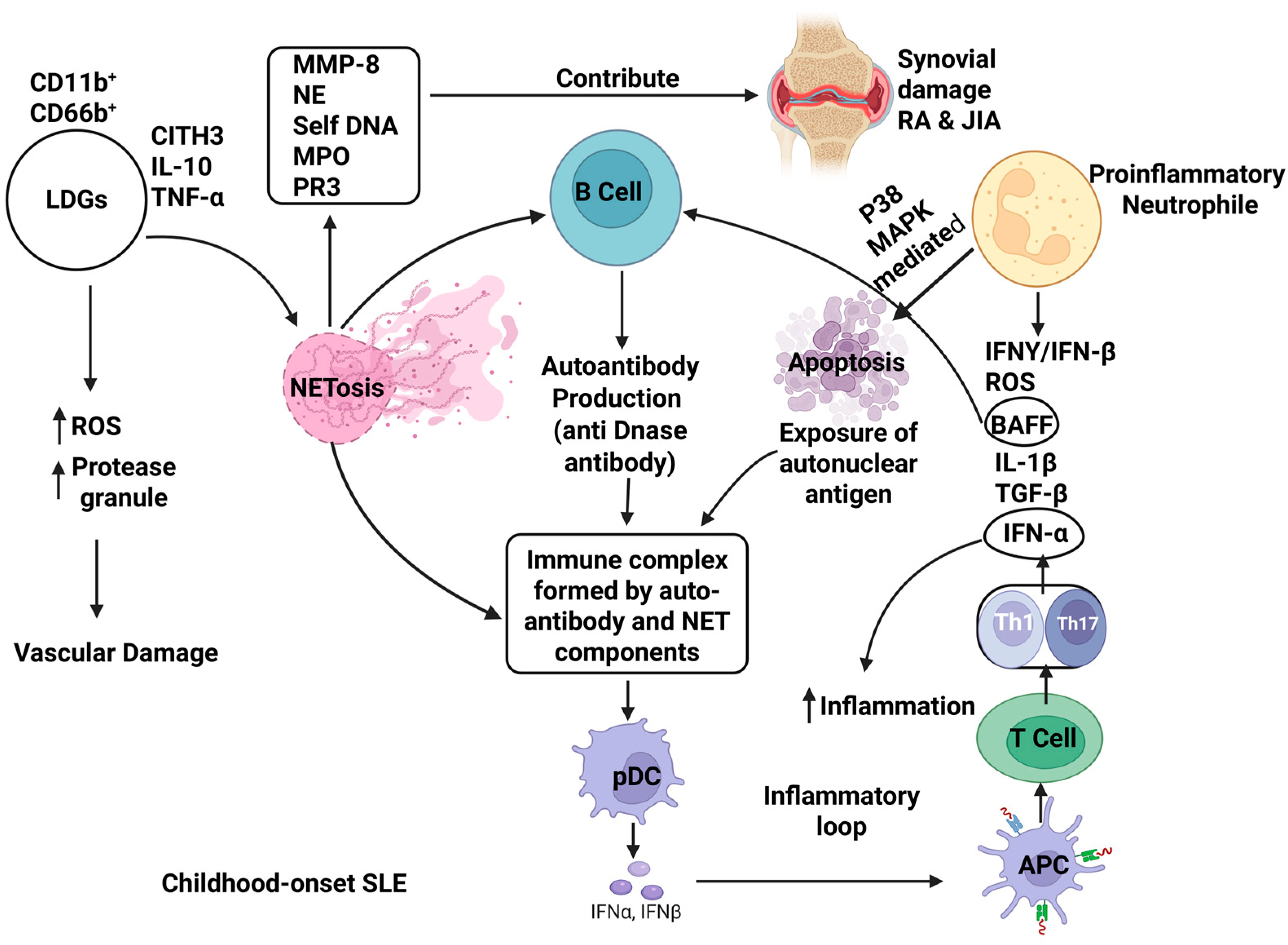
3.1. Systemic Lupus Erythematosus (SLE)
3.2. Rheumatoid Arthritis (RA)
3.3. Juvenile Idiopathic Arthritis (JIA)
4. Neutrophils Heterogeneity in Cancer
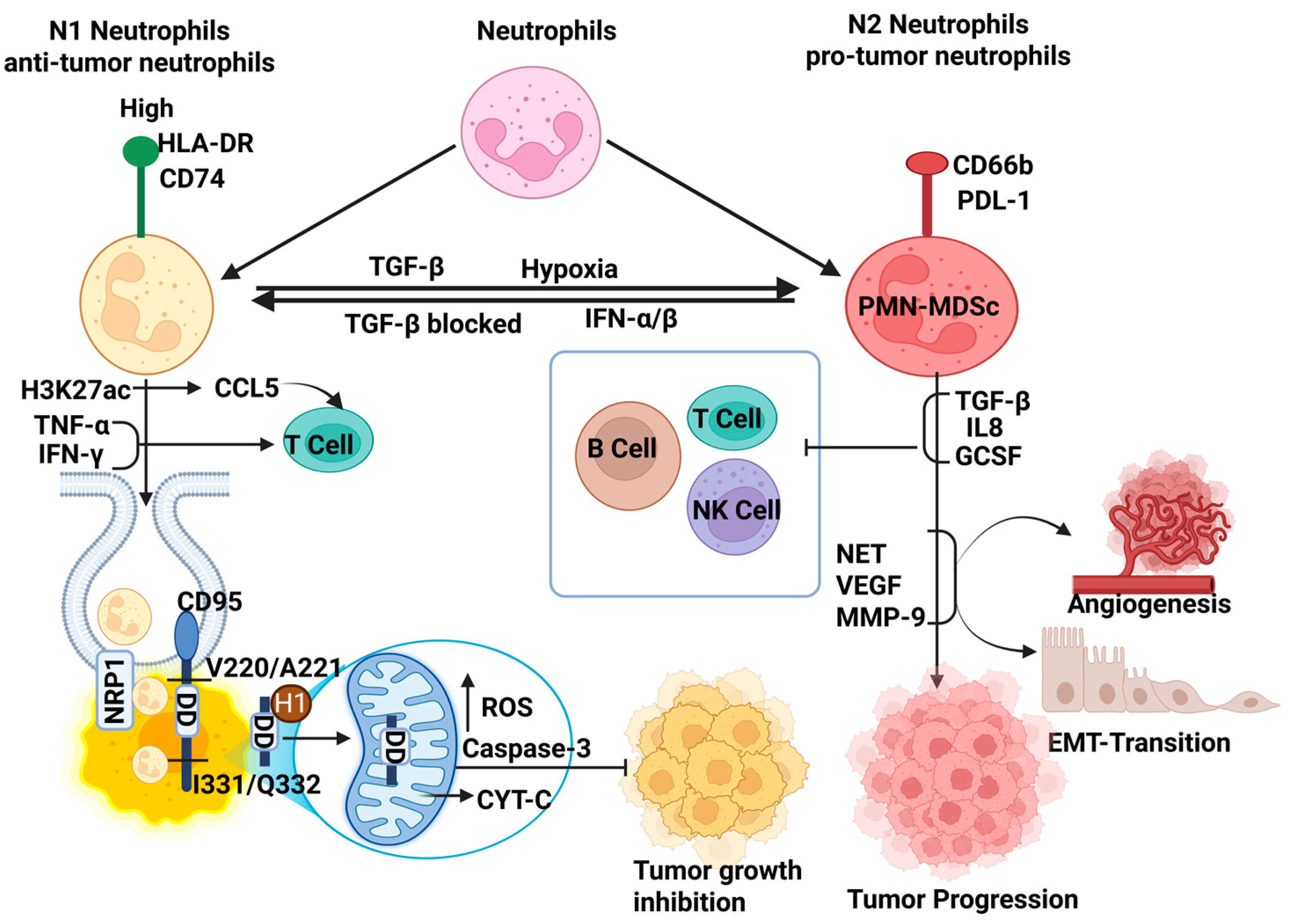
4.1. Anti-Tumorigenic Neutrophils
4.2. Pro-Tumorigenic Neutrophils
4.3. Phenotypes Transition in TME: Anti-Tumorigenic (N1) and Pro-Tumorigenic (N2)
5. Therapeutic Targeting of Neutrophils
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSCs | Hematopoietic stem cells |
| preNeus | Pre-neutrophils |
| NET | Neutrophil extracellular traps |
| NETosis | NET formation |
| ROS | Reactive oxygen species |
| pDC | Plasmacytoid dendritic cells |
| APC | Antigen-presenting cells |
| LDG | Low-density granulocytes |
| LDNs | Low-density neutrophils |
| SLE | Systemic lupus erythematosus |
| RA | Rheumatoid arthritis |
| T1D | Type 1 diabetes |
| TME | Tumor microenvironment |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| TANs | Tumor-associated neutrophils |
| EMT | Epithelial–mesenchymal transition |
| NK Cells | Natural killer cells |
| MDSCs | Myeloid-derived suppressor cells |
| NSCLC | Non-small-cell lung cancer |
| T reg | Regulatory T cell |
| H2O2 | Hydrogen peroxide |
| NO | Nitric oxide |
| NE | Neutrophil elastase |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| CTL | Cytotoxic T lymphocyte |
| G-CSF | Granulocyte colony-stimulating factor |
| BCR | B cell receptors |
| TCR | T cell receptors |
| BAFF | B cell-activating factor |
| IFNs | Interferons |
| ETs | Extracellular traps |
| CitH3 | Citrullinated histone H3 |
| MPO | Myeloperoxidase |
| MMP-9 | Matrix metalloproteinase-9 |
| PR3 | Proteinase 3 |
| JIA | Juvenile idiopathic arthritis |
| sJIA | Systemic JIA |
| TNBC | Triple-negative breast cancer |
| NPR1 | Neuropilin-1 |
| PMN-MDSCs | Polymorphonuclear myeloid-derived suppressor cells |
| N1 | Anti-tumorigenic Neutrophil |
| N2 | Pro-tumorigenic Neutrophil |
| VEGFA | Vascular endothelial growth factor |
| DCIS | Ductal carcinoma in situ |
| PD-L1 | Programmed death ligand-1 |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor Necrosis Factors-α |
References
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 2018, 48, 364–379.e8. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef]
- Zeng, L.; Xiang, W.; Xiao, W.; Wu, Y.; Sun, L. The emerging role of neutrophil extracellular traps in autoimmune and autoinflammatory diseases. Medcomm 2025, 6, e70101. [Google Scholar] [CrossRef]
- Salemme, R.; Peralta, L.N.; Meka, S.H.; Pushpanathan, N.; Alexander, J.J. The Role of NETosis in Systemic Lupus Erythematosus. J. Cell Immunol. 2019, 1, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ye, J.; Pan, N.; Tan, C.; Herrmann, M. Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2020, 11, 578129. [Google Scholar] [CrossRef]
- Fu, X.; Liu, H.; Huang, G.; Dai, S.-S. The emerging role of neutrophils in autoimmune-associated disorders: Effector, predictor, and therapeutic targets. MedComm 2021, 2, 402–413. [Google Scholar] [CrossRef]
- Carnevale, S.; Di Ceglie, I.; Grieco, G.; Rigatelli, A.; Bonavita, E.; Jaillon, S. Neutrophil diversity in inflammation and cancer. Front. Immunol. 2023, 14, 1180810. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kloecker, G.; Fleming, C.; Bousamra, M.; Hansen, R.; Hu, X.; Ding, C.; Cai, Y.; Xiang, D.; Donninger, H.; et al. Human polymorphonuclear neutrophils specifically recognize and kill cancerous cells. OncoImmunology 2014, 3, e950163. [Google Scholar] [CrossRef]
- Kundu, M.; Greer, Y.E.; Lobanov, A.; Ridnour, L.; Donahue, R.N.; Ng, Y.; Ratnayake, S.; Voeller, D.; Weltz, S.; Chen, Q.; et al. TRAIL-induced cytokine production via NFKB2 pathway promotes neutrophil chemotaxis and immune suppression in triple negative breast cancers. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Zuo, J.; Feng, D. Tumor associated neutrophils governs tumor progression through an IL-10/STAT3/PD-L1 feedback signaling loop in lung cancer. Transl. Oncol. 2024, 40, 101866. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Kalafati, L.; Chavakis, T. Neutrophils are shaped by the tumor microenvironment: Novel possibilities for targeting neutrophils in cancer. Signal Transduct. Target. Ther. 2024, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Peng, H.; Gou, R.; Zhou, Y.; Ren, S.; Li, F. Exploring neutrophil extracellular traps: Mechanisms of immune regulation and future therapeutic potential. Exp. Hematol. Oncol. 2025, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, G.; Zhou, X.; Li, J. Therapeutic potential of tumor-associated neutrophils: Dual role and phenotypic plasticity. Signal Transduct. Target. Ther. 2025, 10, 178. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, H.; Wu, Y.; Gao, Q. Complex role of neutrophils in the tumor microenvironment: An avenue for novel immunotherapies. Cancer Biol. Med. 2024, 21, 849–863. [Google Scholar] [CrossRef]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: A transcriptomics analysis of pro- vs. antitumor TANs. OncoImmunology 2016, 5, e1232221. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wang, Z.; Guo, Y.; Zhang, X.; Wan, B. Targeting tumor-associated neutrophils: A promising strategy in lung cancer immunotherapy. Cancer Pathog. Ther. 2025, 3, E01–E07. Available online: https://mednexus.org/doi/10.1016/j.cpt.2025.02.004 (accessed on 15 August 2025). [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.-W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Sengupta, S.; Caldwell, C.C.; Nomellini, V. Distinct Neutrophil Populations in the Spleen During PICS. Front. Immunol. 2020, 11, 804. [Google Scholar] [CrossRef]
- Weirich, E.; Rabin, R.L.; Maldonado, Y.; Benitz, W.; Modler, S.; Herzenberg, L.A. Neutrophil CD11b expression as a diagnostic marker for early-onset neonatal infection. J. Pediatr. 1998, 132 Pt 1, 445–451. [Google Scholar] [CrossRef] [PubMed]
- O’DEa, M.I.; Kelly, L.; McKenna, E.; Melo, A.M.; Ni Bhroin, M.; Hurley, T.; Byrne, A.T.; Colleran, G.; Vavasseur, C.; El-Khuffash, A.; et al. Dysregulated Monocyte and Neutrophil Functional Phenotype in Infants with Neonatal Encephalopathy Requiring Therapeutic Hypothermia. Front. Pediatr. 2021, 8, 598724. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Maitz, K.; Kindler, O.; Raftopoulou, S.; Kienzl, M.; Santiso, A.; Mihalic, Z.N.; Brcic, L.; Lindenmann, J.; Fediuk, M.; et al. Identification of Novel Low-Density Neutrophil Markers Through Unbiased High-Dimensional Flow Cytometry Screening in Non-Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 703846. [Google Scholar] [CrossRef]
- Vanhaver, C.; Nana, F.A.; Delhez, N.; Luyckx, M.; Hirsch, T.; Bayard, A.; Houbion, C.; Dauguet, N.; Brochier, A.; van der Bruggen, P.; et al. Immunosuppressive low-density neutrophils in the blood of cancer patients display a mature phenotype. Life Sci. Alliance 2024, 7, e202302332. [Google Scholar] [CrossRef]
- Ning, X.; Wang, W.-M.; Jin, H.-Z.; Fang, W. Low-Density Granulocytes in Immune-Mediated Inflammatory Diseases. J. Immunol. Res. 2022, 2022, 1622160. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef]
- Ye, Z.; Cheng, P.; Huang, Q.; Hu, J.; Huang, L.; Hu, G. Immunocytes interact directly with cancer cells in the tumor microenvironment: One coin with two sides and future perspectives. Front. Immunol. 2024, 15, 1388176. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Su, J.; Quan, F.; Zhou, H.; Li, Q.; Feng, Q.; Lin, C.; Wang, D.; Jiang, Z. Neutrophil diversity and function in health and disease. Signal Transduct. Target. Ther. 2024, 9, 343. [Google Scholar] [CrossRef]
- Wenisch, C.; Patruta, S.; Daxböck, F.; Krause, R.; Hörl, W. Effect of age on human neutrophil function. J. Leukoc. Biol. 2000, 67, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2022, 23, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Saisorn, W.; Santiworakul, C.; Phuengmaung, P.; Siripen, N.; Rianthavorn, P.; Leelahavanichkul, A. Extracellular traps in peripheral blood mononuclear cell fraction in childhood-onset systemic lupus erythematosus. Sci. Rep. 2024, 14, 23177. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.; Reimers, T.; Abdulahad, W.; Fierro, J.J.; der Meer, B.D.-V.; Bootsma, H.; Horvath, B.; de Leeuw, K.; Westra, J. Low-density granulocytes and neutrophil extracellular trap formation are increased in incomplete systemic lupus erythematosus. Rheumatology 2025, 64, 1234–1242. [Google Scholar] [CrossRef]
- Tay, S.H.; Celhar, T.; Fairhurst, A. Low-Density Neutrophils in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Bashant, K.R.; Aponte, A.M.; Randazzo, D.; Sangsari, P.R.; Wood, A.J.; A Bibby, J.; E West, E.; Vassallo, A.; Manna, Z.G.; Playford, M.P.; et al. Proteomic, biomechanical and functional analyses define neutrophil heterogeneity in systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 209–218. [Google Scholar] [CrossRef]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A Distinct Subset of Proinflammatory Neutrophils Isolated from Patients with Systemic Lupus Erythematosus Induces Vascular Damage and Synthesizes Type I IFNs. J. Immunol. 2010, 184, 3284–3297, Correction in J. Immunol. 2010, 185, 3779. [Google Scholar] [CrossRef]
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T.; et al. Mature CD10+ and immature CD10− neutrophils present in G-CSF–treated donors display opposite effects on T cells. Blood 2017, 129, 1343–1356. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Vasconcellos, A.; Marken, J.; Skopelja-Gardner, S.; Lood, C.; Giltiay, N.V. Immune complex–driven neutrophil activation and BAFF release: A link to B cell responses in SLE. Lupus Sci. Med. 2022, 9, e000709. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Liu, Y.; Xu, J.; Zhu, C.; Sun, R.; Hu, H.; Liu, Y.; Dai, L.; Holmdahl, R.; et al. Neutrophils with low production of reactive oxygen species are activated during immune priming and promote development of arthritis. Redox Biol. 2024, 78, 103401. [Google Scholar] [CrossRef]
- Fousert, E.; Toes, R.; Desai, J. Neutrophil Extracellular Traps (NETs) Take the Central Stage in Driving Autoimmune Responses. Cells 2020, 9, 915. [Google Scholar] [CrossRef]
- Yang, F.; Luo, X.; Luo, G.; Zhai, Z.; Zhuang, J.; He, J.; Han, J.; Zhang, Y.; Zhuang, L.; Sun, E.; et al. Inhibition of NET formation by polydatin protects against collagen-induced arthritis. Int. Immunopharmacol. 2019, 77, 105919. [Google Scholar] [CrossRef]
- Wright, H.L.; A Makki, F.; Moots, R.J.; Edwards, S.W. Low-density granulocytes: Functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J. Leukoc. Biol. 2017, 101, 599–611. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Moots, R.J.; Edwards, S.W.; Wright, H.L. Type I interferon regulates cytokine-delayed neutrophil apoptosis, reactive oxygen species production and chemokine expression. Clin. Exp. Immunol. 2021, 203, 151–159. [Google Scholar] [CrossRef]
- Alarcon, M.F.; Abdullah, G.A.; Beggs, J.A.; Kynoch, I.; Sellin, A.; Cross, A.; Haldenby, S.; Antczak, P.; Gutiérrez, E.C.; Wright, H.L. Complexity of the neutrophil transcriptome in early and severe rheumatoid arthritis: A role for microRNAs? J. Leukoc. Biol. 2025, 117, qiaf090. [Google Scholar] [CrossRef] [PubMed]
- Hasni, S.A.; Gupta, S.; Davis, M.; Poncio, E.; Temesgen-Oyelakin, Y.; Carlucci, P.M.; Wang, X.; Naqi, M.; Playford, M.P.; Goel, R.R.; et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 2021, 12, 3391. [Google Scholar] [CrossRef]
- E Rysenga, C.; May-Zhang, L.; Zahavi, M.; Knight, J.S.; Ali, R.A. Taxifolin inhibits NETosis through activation of Nrf2 and provides protective effects in models of lupus and antiphospholipid syndrome. Rheumatology 2023, 63, 2006–2015. [Google Scholar] [CrossRef]
- Stabach, P.R.; Sims, D.; Gomez-Bañuelos, E.; Zehentmeier, S.; Dammen-Brower, K.; Bernhisel, A.; Kujawski, S.; Lopez, S.G.; Petri, M.; Goldman, D.W.; et al. A dual-acting DNASE1/DNASE1L3 biologic prevents autoimmunity and death in genetic and induced lupus models. J. Clin. Investig. 2024, 9, e177003. [Google Scholar] [CrossRef]
- Sun, F.; Wang, H.J.; Liu, Z.; Geng, S.; Wang, H.T.; Wang, X.; Li, T.; Morel, L.; Wan, W.; Lu, L.; et al. Safety and efficacy of metformin in systemic lupus erythematosus: A multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2020, 2, e210–e216. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Planellas, S.; Katsifis-Nezis, D.; Fanouriakis, A. Clinical Trials of Interferon Inhibitors in Systemic Lupus Erythematosus and Preliminary Real-World Efficacy of Anifrolumab. Mediterr. J. Rheumatol. 2024, 35 (Suppl. S2), 381–391. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, C.; Fukui, S.; Sadhu, N.M.; Zainuddin, M.; Rajagopal, S.; Gosu, R.; Gutch, S.; Fukui, S.; Sheehy, C.E.; Chu, L.; et al. Alleviation of arthritis through prevention of neutrophil extracellular traps by an orally available inhibitor of protein arginine deiminase 4. Sci. Rep. 2023, 13, 3189. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamanaka, H.; Toyoizumi, S.; Hirose, T.; Takeuchi, T. Laboratory markers predicting tofacitinib efficacy in Japanese patients with rheumatoid arthritis: A pooled analysis of Phase 2/3 randomised controlled clinical trials. Mod. Rheumatol. 2024, 35, 417–424. [Google Scholar] [CrossRef]
- Guo, C.; Sharp, A.; Gurel, B.; Crespo, M.; Figueiredo, I.; Jain, S.; Vogl, U.; Rekowski, J.; Rouhifard, M.; Gallagher, L.; et al. Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature 2023, 623, 1053–1061. [Google Scholar] [CrossRef]
- Lazennec, G.; Rajarathnam, K.; Richmond, A. CXCR2 chemokine receptor—A master regulator in cancer and physiology. Trends Mol. Med. 2024, 30, 37–55. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Pilarczyk, M.; Kot, M.; Defort, P.; Walaszek, I.; Chlubek, D.; Baranowska-Bosiacka, I. The CXCL1-CXCR2 Axis as a Component of Therapy Resistance, a Source of Side Effects in Cancer Treatment, and a Therapeutic Target. Cancers 2025, 17, 1674. [Google Scholar] [CrossRef]
- Parackova, Z.; Zentsova, I.; Bloomfield, M.; Klocperk, A.; Horvath, R.; Malcova, H.; Cebecauerova, D.; Sediva, A. Expanded population of low-density neutrophils in juvenile idiopathic arthritis. Front. Immunol. 2023, 14, 1229520. [Google Scholar] [CrossRef]
- Ramanathan, K.; Glaser, A.; Lythgoe, H.; Ong, J.; Beresford, M.W.; Midgley, A.; Wright, H.L. Neutrophil activation signature in juvenile idiopathic arthritis indicates the presence of low-density granulocytes. Rheumatology 2017, 57, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Prada-Medina, C.A.; Peron, J.P.S.; I Nakaya, H. Immature neutrophil signature associated with the sexual dimorphism of systemic juvenile idiopathic arthritis. J. Leukoc. Biol. 2020, 108, 1319–1327. [Google Scholar] [CrossRef]
- Arve-Butler, S.; Schmidt, T.; Mossberg, A.; Berthold, E.; Gullstrand, B.; Bengtsson, A.A.; Kahn, F.; Kahn, R. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res. Ther. 2021, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.-W.; Rhim, J.W.; Park, K.-S.; Kim, K.-J. Blood molecular subtypes to guide precision treatment strategies in systemic juvenile idiopathic arthritis. Arthritis Res. Ther. 2025, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil pheno-type by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Yang, X.; Nan, F.; Zhang, T.; Ji, S.; Rao, D.; Feng, H.; Gao, K.; Gu, X.; et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell 2024, 187, 1422–1439.e24. [Google Scholar] [CrossRef]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffman, A.; Chang, Y.-F.; Blank, A.; Reardon, C.A.; et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell 2021, 184, 3163–3177.e21. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zheng, M.; Niu, R.; Yang, X.; Tian, S.; Fan, L.; Li, Y.; Zhang, S. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front. Cell Dev. Biol. 2022, 10, 938289. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Hashimoto, A.; Dweep, H.; Sanseviero, E.; De Leo, A.; Tcyganov, E.; Kossenkov, A.; Mulligan, C.; Nam, B.; Masters, G.; et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 2021, 218, e20201803. [Google Scholar] [CrossRef]
- Gil Del Alcazar, C.R.; Huh, S.J.; Ekram, M.B.; Trinh, A.; Liu, L.L.; Beca, F.; Zi, X.; Kwak, M.; Bergholtz, H.; Su, Y.; et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017, 7, 1098–1115. [Google Scholar] [CrossRef]
- Pylaeva, E.; Korschunow, G.; Spyra, I.; Bordbari, S.; Siakaeva, E.; Ozel, I.; Domnich, M.; Squire, A.; Hasenberg, A.; Thangavelu, K.; et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022, 40, 111171. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef] [PubMed]
| S. No. | Transition of TANs | Key Factors | Phenotypic Markers | References |
|---|---|---|---|---|
| 1. | Pro-tumor (N2) to Anti-tumor (N1) | Microbial signals, TGF-β blockade, IFN-α/β, acute inflammation | Increased ROS, HLA-DRhigh, CD74high, CD80, CD86, high chemokines recruiting T/NK cells | [15,16] |
| 2. | Anti-tumor (N1) to Pro-tumor (N2) | TGF-β, chronic inflammation, hypoxia | Increased VEGF, PD-L1, ARG1, HLA-DRlow and CD74low | [16,17] |
| Neutrophil-Targeted Therapeutics in Autoimmune Diseases and Cancer (Clinical Trials) | ||||
|---|---|---|---|---|
| Drugs and Therapeutic Agents | Mechanism of Action | Phase/Status of Clinical Trial | Outcome of Therapeutics | References |
| Baricitinib, Tofacitinib, Upadacitinib | Modulate neutrophil activation and NETosis targeting JAK/STAT pathway | Phase 1–3 trials | Reduces NETs, LDNs | [45] ClinicalTrials.gov ID NCT02535689 ClinicalTrials.gov ID NCT05843643 |
| Taxifolin | Inhibits NETosis (Nrf2) | Preclinical/ex vivo | Protective in lupus models | [46] |
| Dual-acting DNase1/DNase1L3 | Degrades NET DNA | Preclinical | Promising in SLE models | [47] |
| Metformin | Inhibits NETosis | Add-on trial (completed) | Reduced flares, indirect NETosis effect | [48] |
| Anifrolumab | Type I Interferon Inhibitor | Approved, real-world/clinical | Reduces NETs and LDNs; rapid efficacy in SLE | [49] |
| JBI-589 Peptidylargininedeiminase 4 (PAD4) inhibitor | Inhibits PAD4; blocks NETosis | Preclinical (animal models, in vitro human/mouse neutrophils) | Highly selective PAD4 inhibitor; blocks NET formation and reduces arthritis severity in mouse models; confirmed NET inhibition in vito | [50] |
| Tocilizumab | IL-6R blockade; modulates neutrophil function/NETs | Phase 3, approved | Reduces NETosis, neutrophil infiltration, and improves neutrophil function in RA, systemic JIA | [51] REC reference 10/H0904/14 |
| AZD5069 | Inhibits CXCR2 | Phase 1/2 (combo with immunotherapy) | Inhibits neutrophil recruitment to tumors; reduces intratumoral neutrophil and MDSC infiltration, shown to decrease neutrophil levels and improve response in Prostate, NSCLC, solid tumors | [52] ClinicalTrials.gov ID NCT02583477 |
| Navarixin (SCH-527123, MK-7123) | Inhibits CXCR2 | Phase 1b/2 | Inhibits CXCR2-mediated neutrophil chemotaxis and tumor infiltration; reduces tumor-promoting neutrophils in NSCLC, solid tumors | [53,54] ClinicalTrials.gov ID NCT03473925 |
| Reparixin | Inhibits CXCR1/2 | Phase 2 | Blocks neutrophil recruitment and MDSC infiltration by antagonizing CXCR1/2 in breast and pancreatic cancer | [53,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhargav, A.; Kumar, V.; Rai, N.K. Roles of Neutrophils in Autoimmune Diseases and Cancers. Int. J. Mol. Sci. 2025, 26, 9040. https://doi.org/10.3390/ijms26189040
Bhargav A, Kumar V, Rai NK. Roles of Neutrophils in Autoimmune Diseases and Cancers. International Journal of Molecular Sciences. 2025; 26(18):9040. https://doi.org/10.3390/ijms26189040
Chicago/Turabian StyleBhargav, Anjali, Vinay Kumar, and Neeraj Kumar Rai. 2025. "Roles of Neutrophils in Autoimmune Diseases and Cancers" International Journal of Molecular Sciences 26, no. 18: 9040. https://doi.org/10.3390/ijms26189040
APA StyleBhargav, A., Kumar, V., & Rai, N. K. (2025). Roles of Neutrophils in Autoimmune Diseases and Cancers. International Journal of Molecular Sciences, 26(18), 9040. https://doi.org/10.3390/ijms26189040






