Redox Regulation of Endogenous Gasotransmitters in Vascular Health and Disease
Abstract
1. Introduction
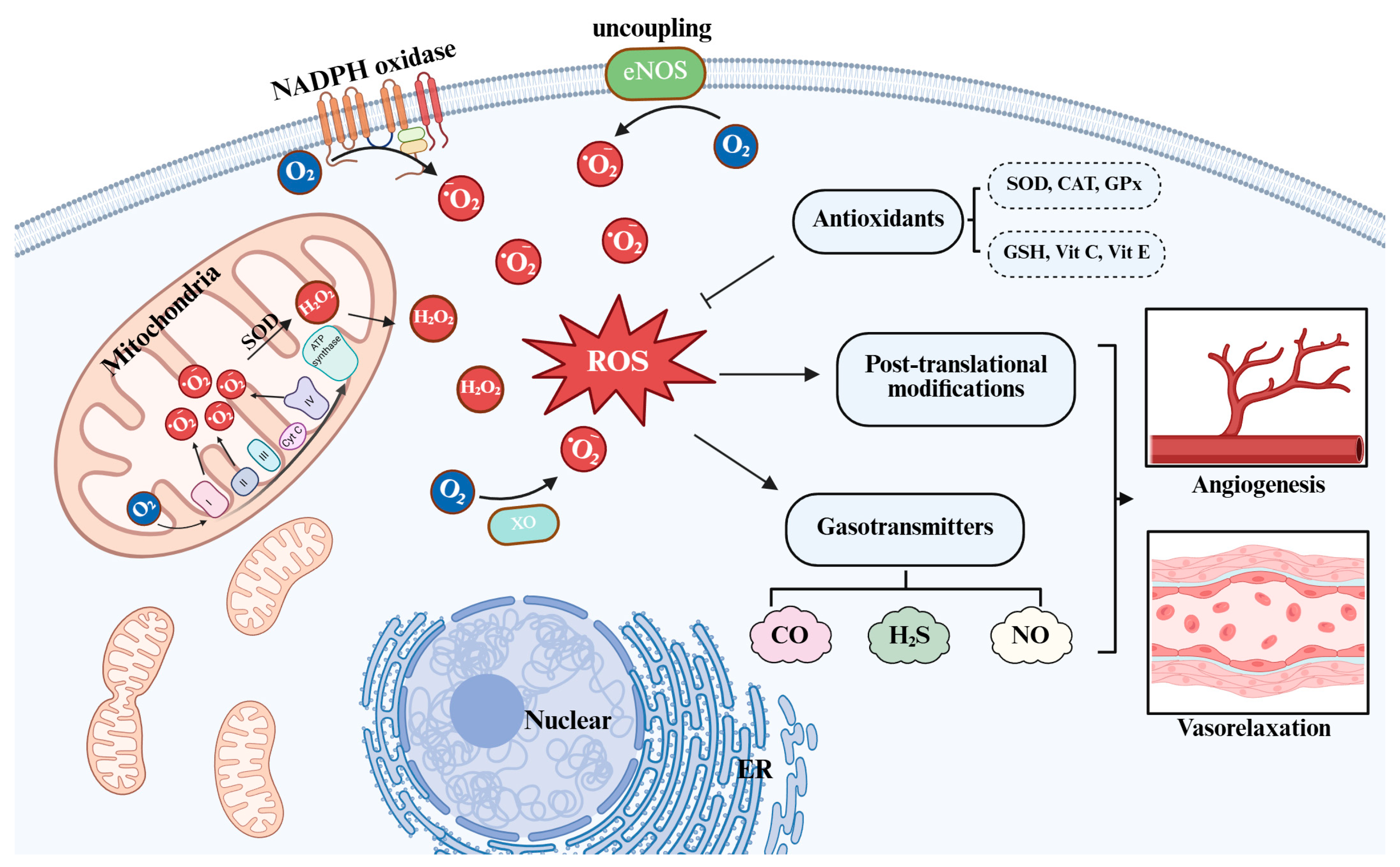
2. Reactive Oxygen Species and Redox-Signaling
3. Gasotransmitters
3.1. Biochemical Characteristics of Gasotransmitters
3.2. Functional Properties and Redox Sensitivity
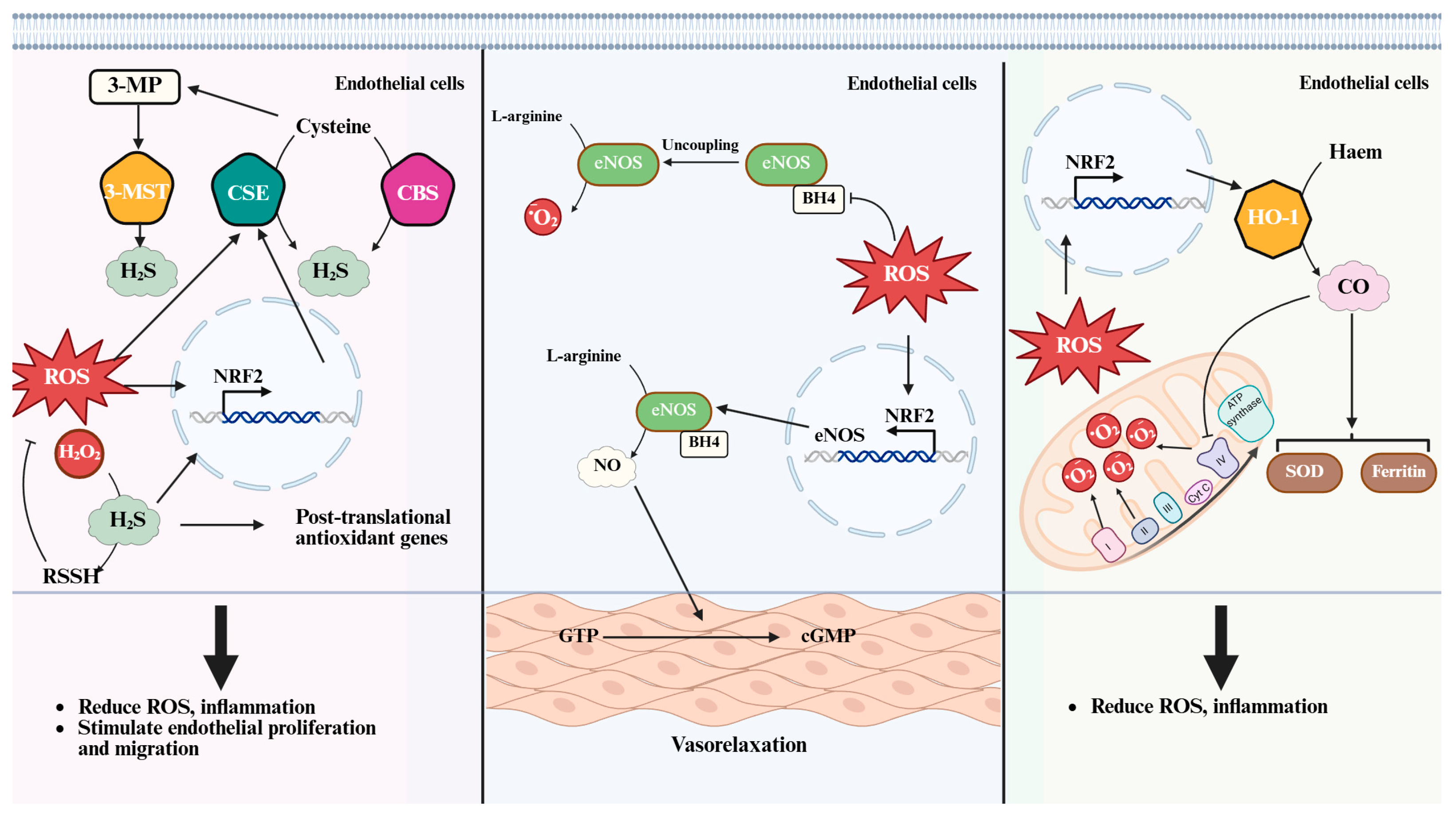
3.3. Enzymatic Biosynthesis and Redox Regulation
4. Hydrogen Sulfide (H2S)
5. Nitric Oxide (NO)
6. Carbon Monoxide (CO)
7. Comparative Analysis of Gasotransmitters
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of Cystathionine γ-Lyase/Hydrogen Sulfide Pathway in Cardiovascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal 2012, 17, 106–118. [Google Scholar] [CrossRef]
- Durán, W.N.; Breslin, J.W.; Sánchez, F.A. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 2010, 87, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, E.; Comita, S.; Pagliaro, P.; Penna, C.; Mancardi, D. Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet? Int. J. Mol. Sci. 2023, 24, 12480. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.Z.; Chen, F.R.; Cheng, Y.Q.; Tang, C.S.; Du, J.B. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003, 21, 1879–1885. [Google Scholar] [CrossRef]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G.; Gojon, G.; et al. H2S Protects Against Pressure Overload-Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef]
- Li, H.G.; Förstermann, U. Prevention of Atherosclerosis by Interference with the Vascular Nitric Oxide System. Curr. Pharm. Des. 2009, 15, 3133–3145. [Google Scholar] [CrossRef]
- Volti, G.L.; Sacerdoti, D.; Sangras, B.; Vanella, A.; Mezentsev, A.; Scapagnini, G.; Falck, J.R.; Abraham, N.G. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid. Redox Signal 2005, 7, 704–710. [Google Scholar] [CrossRef]
- Chung, H.S.; Wang, S.B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine Oxidative Posttranslational Modifications Emerging Regulation in the Cardiovascular System. Circ. Res. 2013, 112, 382–392. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Madhani, M.; Cuello, F.; Charles, R.L.; Brennan, J.P.; Schröder, E.; Browning, D.D.; Eaton, P. Cysteine redox sensor in PKGIα enables oxidant-induced activation. Science 2007, 317, 1393–1397. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wu, S.W.; Yang, J.J.; Chen, W.Y.; Chen, C.J.; Chen, H.H.; Lee, Y.C.; Su, C.H.; Kuan, Y.H. Vascular endothelial dysfunction induced by 3-bromofluoranthene via MAPK-mediated-NFκB pro-inflammatory pathway and intracellular ROS generation. Arch. Toxicol. 2024, 98, 2247–2259. [Google Scholar] [CrossRef]
- Alaaeddine, R.; Elkhatib, M.A.W.; Mroueh, A.; Fouad, H.; Saad, E.; El-Sabban, M.E.; Plane, F.; El-Yazbi, A.F. Impaired Endothelium-Dependent Hyperpolarization Underlies Endothelial Dysfunction during Early Metabolic Challenge: Increased ROS Generation and Possible Interference with NO Function. J. Pharmacol. Exp. Ther. 2019, 371, 567–582. [Google Scholar] [CrossRef]
- Luo, Z.M.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Activation of Nuclear Factor Erythroid 2-Related Factor 2 Coordinates Dimethylarginine Dimethylaminohydrolase/PPAR-γ/Endothelial Nitric Oxide Synthase Pathways That Enhance Nitric Oxide Generation in Human Glomerular Endothelial Cells. Hypertension 2015, 65, 896–902. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Agbor, T.A.; Motta, J.P.; Ferraz, J.G.P.; Wang, R.; Buret, A.G.; Wallace, J.L. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. Faseb J. 2015, 29, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal 2001, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.S.; Wang, Q.W.; Zhu, J.H.; Xiao, Q.Z.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, Y.M. Regulation of Endothelial and Vascular Functions by Carbon Monoxide via Crosstalk With Nitric Oxide. Front. Cardiovasc. Med. 2021, 8, 649630. [Google Scholar] [CrossRef]
- Mistry, R.K.; Brewer, A.C. Redox regulation of gasotransmission in the vascular system: A focus on angiogenesis. Free Radic. Biol. Med. 2017, 108, 500–516. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Ralph, S.J. Targeting the redox imbalance in mitochondria: A novel mode for cancer therapy. Mitochondrion 2022, 62, 50–73. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS signaling and redox biology in endothelial cells. Cell. Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal 2007, 9, 731–739. [Google Scholar] [CrossRef]
- Garrido-Urbani, S.; Jemelin, S.; Deffert, C.; Carnesecchi, S.; Basset, O.; Szyndralewiez, C.; Heitz, F.; Page, P.; Montet, X.; Michalik, L.; et al. Targeting Vascular NADPH Oxidase 1 Blocks Tumor Angiogenesis through a PPARα Mediated Mechanism. PLoS ONE 2011, 6, e14665. [Google Scholar] [CrossRef]
- Vogel, J.; Kruse, C.; Zhang, M.; Schröder, K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J. Physiol. 2015, 593, 2145–2154. [Google Scholar] [CrossRef]
- Chen, W.; Xiang, H.; Chen, R.F.; Yang, J.; Yang, X.P.; Zhou, J.D.; Liu, H.D.; Zhao, S.L.; Xiao, J.; Chen, P.; et al. S1PR2 antagonist ameliorate high glucose-induced fission and dysfunction of mitochondria in HRGECs via regulating ROCK1. BMC Nephrol. 2019, 20, 135. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Munteanu, C.; Galaction, A.I.; Onose, G.; Turnea, M.; Rotariu, M. Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells. Pharmaceuticals 2025, 18, 344. [Google Scholar] [CrossRef]
- Li, J.S.; Shi, C.; Wang, X.F.; Liu, C.X.; Ding, X.T.; Ma, P.Y.; Wang, X.; Jia, H.L. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to Copper Oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol. Biochem. 2020, 156, 257–266. [Google Scholar] [CrossRef]
- Peluffo, G.; Calcerrada, P.; Piacenza, L.; Pizzano, N.; Radi, R. Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: Studies in cultured cells and smokers. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H1781–H1792. [Google Scholar] [CrossRef] [PubMed]
- Benchoam, D.; Cuevasanta, E.; Möller, M.N.; Alvarez, B. Hydrogen Sulfide and Persulfides Oxidation by Biologically Relevant Oxidizing Species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Por, E.D.; Kwon, Y.G.; Kim, Y.M. Regulation of ROS Production and Vascular Function by Carbon Monoxide. Oxidative Med. Cell. Longev. 2012, 2012, 794237. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological Roles of Hydrogen Sulfide in Mammalian Cells, Tissues, and Organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Ploska, A.; Wieronska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Cheng, H.T.; Yen, C.J.; Chang, C.C.; Huang, K.T.; Chen, K.H.; Zhang, R.Y.; Lee, P.Y.; Miaw, S.C.; Huang, J.W.; Chiang, C.K.; et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. BBA-Gen. Subj. 2015, 1850, 2506–2517. [Google Scholar] [CrossRef]
- Doeller, J.E.; Isbell, T.S.; Benavides, G.; Koenitzer, J.; Patel, H.; Patel, R.P.; Lancaster, J.R., Jr.; Darley-Usmar, V.M.; Kraus, D.W. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem. 2005, 341, 40–51. [Google Scholar] [CrossRef]
- Chen, X.L.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.G.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide-Biol. Chem. 2013, 35, 5–20. [Google Scholar] [CrossRef]
- Tanizawa, K. Production of H2S by 3-mercaptopyruvate sulphurtransferase. J. Biochem. 2011, 149, 357–359. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-sulfuration Reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, A.; Kanaumi, T.; Takashima, S.; Enokido, Y.; Nagai, Y.; Kimura, H. Cystathionine β-synthase is enriched in the brains of Down’s patients. Biochem. Biophys. Res. Commun. 2005, 338, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Enokido, Y.; Suzuki, E.; Iwasawa, K.; Namekata, K.; Okazawa, H.; Kimura, H. Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. Faseb J. 2005, 19, 1854–1856. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Li, J.B.; Teng, X.; Jin, S.; Dong, J.H.; Guo, Q.; Tian, D.Y.; Wu, Y.M. Hydrogen sulfide improves endothelial dysfunction by inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in spontaneously hypertensive rats. J. Hypertens. 2019, 37, 1633–1643. [Google Scholar] [CrossRef]
- Jackson-Weaver, O.; Osmond, J.M.; Riddle, M.A.; Naik, J.S.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca-activated K channels and smooth muscle Ca sparks. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1446–H1454. [Google Scholar] [CrossRef]
- Cai, W.J.; Wang, M.J.; Moore, P.K.; Jin, H.M.; Yao, T.; Zhu, Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007, 76, 29–40. [Google Scholar] [CrossRef]
- Naik, J.S.; Osmond, J.M.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H1437–H1444. [Google Scholar] [CrossRef]
- Xie, L.P.; Gu, Y.; Wen, M.L.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.L.; Han, Y.; Wang, Y.H.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Wang, M.X.; Guo, Z.Y.; Wang, S.L. Cystathionine Gamma-Lyase Expression Is Regulated by Exogenous Hydrogen Peroxide in the Mammalian Cells. Gene Expr. 2012, 15, 235–241. [Google Scholar] [CrossRef]
- Mistry, R.K.; Murray, T.V.A.; Prysyazhna, O.; Martin, D.; Burgoyne, J.R.; Santos, C.; Eaton, P.; Shah, A.M.; Brewer, A.C. Transcriptional Regulation of Cystathionine-γ-Lyase in Endothelial Cells by NADPH Oxidase 4-Dependent Signaling. J. Biol. Chem. 2016, 291, 1774–1788. [Google Scholar] [CrossRef] [PubMed]
- Greiner, R.; Pálinkás, Z.; Bäsell, K.; Becher, D.; Antelmann, H.; Nagy, P.; Dick, T.P. Polysulfides Link H2S to Protein Thiol Oxidation. Antioxid. Redox Signal 2013, 19, 1749–1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Q.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.F.; et al. Response to Letter Regarding Article, “Dysregulation of Hydrogen Sulfide (H2S) Producing Enzyme Cystathionine γ-lyase (CSE) Contributes to Maternal Hypertension and Placental Abnormalities in Preeclampsia”. Circulation 2014, 129, E517–E518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szijártó, I.A.; Markó, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine γ-Lyase Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.J.; Li, Z.; Sharp, T.E.; Polhemus, D.J.; Carnal, J.; Moles, K.H.; Tao, Y.X.; Elrod, J.; Pfeilschifter, J.; Beck, K.F.; et al. Endothelial Cell Cystathionine γ-Lyase Expression Level Modulates Exercise Capacity, Vascular Function, and Myocardial Ischemia Reperfusion Injury. J. Am. Heart Assoc. 2020, 9, e017544. [Google Scholar] [CrossRef]
- Peleli, M.; Lyngso, K.S.; Poulsen, F.R.; Hansen, P.B.L.; Papapetropoulos, A.; Stubbe, J. Inhibition of cystathionine-gamma lyase dampens vasoconstriction in mouse and human intracerebral arterioles. Acta Physiol. 2023, 239, e14021. [Google Scholar] [CrossRef]
- Diwakar, L.; Ravindranath, V. Inhibition of cystathionine-γ-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem. Int. 2007, 50, 418–426. [Google Scholar] [CrossRef]
- Sun, L.L.; Jin, H.F.; Sun, L.J.; Chen, S.Y.; Huang, Y.Q.; Liu, J.; Li, Z.Z.; Zhao, M.M.; Sun, Y.; Tang, C.S.; et al. Hydrogen Sulfide Alleviates Myocardial Collagen Remodeling in Association with Inhibition of TGF-β/Smad Signaling Pathway in Spontaneously Hypertensive Rats. Mol. Med. 2014, 20, 503–515. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.K.; Zhang, C.Y.; Zeng, X.J.; Yan, H.; Jin, H.F.; Tang, C.S.; Du, J.B. Regulatory Effect of Hydrogen Sulfide on Vascular Collagen Content in Spontaneously Hypertensive Rats. Hypertens. Res. 2008, 31, 1619–1630. [Google Scholar] [CrossRef]
- Ciccone, V.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Matteo, R.; Pecchioni, N.; Montanaro, R.; Mannelli, L.D.; et al. Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. Int. J. Mol. Sci. 2022, 23, 15593. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim. Biophys. Acta-Bioenerg. 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Mannick, J.B. Regulation of apoptosis by protein S-nitrosylation. Amino Acids 2007, 32, 523–526. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Milovanova, T.N.; Yang, M.; Bogush, M.; Buerk, D.G. Nitric-oxide Synthase-2 Linkage to Focal Adhesion Kinase in Neutrophils Influences Enzyme Activity and β2 Integrin Function. J. Biol. Chem. 2013, 288, 4810–4818. [Google Scholar] [CrossRef]
- Matrullo, G.; Filomeni, G.; Rizza, S. Redox regulation of focal adhesions. Redox Biol. 2025, 80, 103514. [Google Scholar] [CrossRef]
- Oh, C.K.; Nakamura, T.; Zhang, X.; Lipton, S.A. Redox regulation, protein S-nitrosylation, and synapse loss in Alzheimer’s and related dementias. Neuron 2024, 112, 3823–3850. [Google Scholar] [CrossRef]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef] [PubMed]
- Riobó, N.A.; Clementi, E.; Melani, M.; Boveris, A.; Cadenas, E.; Moncada, S.; Poderoso, J.J. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem. J. 2001, 359, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cassina, A.; Radi, R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch. Biochem. Biophys. 1996, 328, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Aguilar, G.; Koning, T.; Ehrenfeld, P.; Sanchez, F.A. Role of NO and S-nitrosylation in the Expression of Endothelial Adhesion Proteins That Regulate Leukocyte and Tumor Cell Adhesion. Front. Physiol. 2020, 11, 595526. [Google Scholar] [CrossRef]
- Wedgwood, S.; Black, S.M. Molecular mechanisms of nitric oxide-induced growth arrest and apoptosis in fetal pulmonary arterial smooth muscle cells. Nitric Oxide 2003, 9, 201–210. [Google Scholar] [CrossRef]
- Dimmeler, S.; Lottspeich, F.; Brune, B. Nitric-Oxide Causes Adp-Ribosylation and Inhibition of Glyceraldehyde-3-Phosphate Dehydrogenase. J. Biol. Chem. 1992, 267, 16771–16774. [Google Scholar] [CrossRef]
- Drapier, J.C.; Hibbs, J.B., Jr. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. 1988, 140, 2829–2838. [Google Scholar]
- Brunelli, L.; Yermilov, V.; Beckman, J. Modulation of catalase peroxidatic and catalatic activity by nitric oxide. Free Radic. Biol. Med. 2001, 30, 709–714. [Google Scholar] [CrossRef]
- Singh, R.R.; McArdle, Z.M.; Booth, L.C.; May, C.N.; Head, G.A.; Moritz, K.M.; Schlaich, M.P.; Denton, K.M. Increase in Bioavailability of Nitric Oxide After Renal Denervation Improves Kidney Function in Sheep With Hypertensive Kidney Disease. Hypertension 2021, 77, 1299–1310. [Google Scholar] [CrossRef]
- Tang, L.; Yi, X.L.; Tan, W.T.; Yang, H.R.; Song, S.S.; Xiong, J.H.; Liu, C.J.; Zhang, Y.F.; Wang, M.L.; Zhu, M.Z.; et al. ELABELA Ameliorates Atherosclerosis Through Restoring the M1/M2 Macrophage Balance in ApoE−/− Mice. J. Am. Heart Assoc. 2025, 14, e041261. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, X.M.; Tarnawski, L.; Peleli, M.; Zhuge, Z.B.; Terrando, N.; Harris, R.A.; Olofsson, P.S.; Larsson, E.; Persson, A.E.G.; et al. Dietary nitrate attenuates renal ischemia-reperfusion injuries by modulation of immune responses and reduction of oxidative stress. Redox Biol. 2017, 13, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Oyovwi, M.O.; Atere, A.D. Exploring the medicinal significance of L-Arginine mediated nitric oxide in preventing health disorders. Eur. J. Med. Chem. Rep. 2024, 12, 100175. [Google Scholar] [CrossRef]
- Li, H.G.; Förstermann, U.; Xia, N.; Kuntic, M.; Münzel, T.; Daiber, A. Pharmacological targeting of endothelial nitric oxide synthase dysfunction and nitric oxide replacement therapy. Free Radic. Biol. Med. 2025, 237, 455–472. [Google Scholar] [CrossRef]
- Wu, L.Y.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From metabolism to molecular therapy. Am. J. Respir. Cell Mol. Biol. 2009, 41, 251–260. [Google Scholar] [CrossRef]
- Leffler, C.W.; Parfenova, H.; Jaggar, J.H. Carbon monoxide as an endogenous vascular modulator. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1–H11. [Google Scholar] [CrossRef]
- Gende, O.A. Carbon monoxide inhibits capacitative calcium entry in human platelets. Thromb. Res. 2004, 114, 113–119. [Google Scholar] [CrossRef]
- Chi, P.L.; Chuang, Y.C.; Chen, Y.W.; Lin, C.C.; Hsiao, L.D.; Yang, C.M. The CO donor CORM-2 inhibits LPS-induced vascular cell adhesion molecule-1 expression and leukocyte adhesion in human rheumatoid synovial fibroblasts. Brit J. Pharmacol. 2014, 171, 2993–3009. [Google Scholar] [CrossRef]
- Le, L.N.V.; Joyce, J.P.; Oyala, P.H.; DeBeer, S.; Agapie, T. Highly Activated Terminal Carbon Monoxide Ligand in an Iron-Sulfur Cluster Model of FeMco with Intermediate Local Spin State at Fe. J. Am. Chem. Soc. 2024, 146, 5045–5050. [Google Scholar] [CrossRef]
- Atamna, H.; Walter, P.B.; Ames, B.N. The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch. Biochem. Biophys. 2002, 397, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Smyrnias, I.; Zhang, X.H.; Zhang, M.; Murray, T.V.A.; Brandes, R.P.; Schröder, K.; Brewer, A.C.; Shah, A.M. Nicotinamide Adenine Dinucleotide Phosphate Oxidase-4-Dependent Upregulation of Nuclear Factor Erythroid-Derived 2-Like 2 Protects the Heart During Chronic Pressure Overload. Hypertension 2015, 65, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M.K. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef]
- Beuneu, C.; Auger, R.; Löffler, M.; Guissani, A.; Lemaire, G.; Lepoivre, M. Indirect inhibition of mitochondrial dihydroorotate dehydrogenase activity by nitric oxide. Free Radic. Biol. Med. 2000, 28, 1206–1213. [Google Scholar] [CrossRef]
- Piantadosi, C.A. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 2008, 45, 562–569. [Google Scholar] [CrossRef]
- Abid, S.; Houssaïni, A.; Mouraret, N.; Marcos, E.; Amsellem, V.; Wan, F.; Dubois-Randé, J.L.; Derumeaux, G.; Boczkowski, J.; Motterlini, R.; et al. p21-Dependent Protective Effects of a Carbon Monoxide-Releasing Molecule-3 in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 304–312. [Google Scholar] [CrossRef]
- Fujimoto, H.; Ohno, M.; Ayabe, S.; Kobayashi, H.; Ishizaka, N.; Kimura, H.; Yoshida, K.; Nagai, R. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arter. Throm Vas. 2004, 24, 1848–1853. [Google Scholar] [CrossRef]
- Lancel, S.; Montaigne, D.; Marechal, X.; Marciniak, C.; Hassoun, S.M.; Decoster, B.; Ballot, C.; Blazejewski, C.; Corseaux, D.; Lescure, B.; et al. Carbon Monoxide Improves Cardiac Function and Mitochondrial Population Quality in a Mouse Model of Metabolic Syndrome. PLoS ONE 2012, 7, e41836. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses–Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Chan, K.H.; Ng, M.K.; Stocker, R. Haem oxygenase-1 and cardiovascular disease: Mechanisms and therapeutic potential. Clin. Sci. 2011, 120, 493–504. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Turnea, M.A.; Rotariu, M. Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis—A Comprehensive One-Year Review. Antioxidants 2023, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Cottin, Y.; Zeller, M.; Vergely, C. Carbon monoxide: Mechanisms of action and potential clinical implications. Pharmacol. Ther. 2013, 137, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Nilius, B.; Han, J. Gaseous Signaling Molecules in Cardiovascular Function: From Mechanisms to Clinical Translation. Rev. Physiol. Biochem. Pharmacol. 2018, 174, 81–156. [Google Scholar] [CrossRef]
- da Silva, G.M.; da Silva, M.C.; Nascimento, D.V.G.; Silva, E.M.L.; Gouvêa, F.F.F.; Lopes, L.G.D.; Araújo, A.V.; Pereira, K.N.F.; de Queiroz, T.M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Gori, T. More answers to the still unresolved question of nitrate tolerance. Eur. Heart J. 2013, 34, 2666–2673. [Google Scholar] [CrossRef]
- Vukotic, R.; Di Donato, R.; Roncarati, G.; Simoni, P.; Renzulli, M.; Gitto, S.; Schepis, F.; Villa, E.; Berzigotti, A.; Bosch, J.; et al. 5-MTHF enhances the portal pressure reduction achieved with propranolol in patients with cirrhosis: A randomized placebo-controlled trial. J. Hepatol. 2023, 79, 977–988. [Google Scholar] [CrossRef]
- Sedding, D.; Schmidt, T.M.; Bähre, H.; Bavendiek, U.; Casas, A.I.; Chen, S.Z.; Dao, V.T.V.; Elbatreek, M.H.; Gutzki, F.; Hahn, A.; et al. Nutritional L-Citrulline and Tetrahydrobiopterin in Peripheral Artery Disease A Phase II Randomized Trial (CIPER Study). JACC Adv. 2025, 4, 101590. [Google Scholar] [CrossRef]
- Bibli, S.I.; Hu, J.; Sigala, F.; Wittig, I.; Heidler, J.; Zukunft, S.; Tsilimigras, D.I.; Randriamboavonjy, V.; Wittig, J.; Kojonazarov, B.; et al. Cystathionine γ Lyase Sulfhydrates the RNA Binding Protein Human Antigen R to Preserve Endothelial Cell Function and Delay Atherogenesis. Circulation 2019, 139, 101–114. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon, G., Sr.; Gojon, G., Jr.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Y.; Li, L.; Lu, H.; Meng, G.L.; Li, X.Z.; Shirhan, M.; Peh, M.T.; Xie, L.P.; Zhou, S.M.; et al. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice. Brit J. Pharmacol. 2013, 169, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Luo, W.; Gui, D.D.; Ren, Z.; Wei, D.H.; Liu, L.S.; Li, G.H.; Tang, Z.H.; Xiong, W.H.; Hu, H.J.; et al. Hydrogen sulfide attenuates atherosclerosis induced by low shear stress by sulfhydrylating endothelium NFIL3 to restrain MEST mediated endothelial mesenchymal transformation. Nitric Oxide-Biol. Chem. 2024, 142, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Mishra, J.S.; Hurt, M.W.; Chen, D.B.; Kumar, S. H2S donor GYY4137 mitigates sFlt-1-induced hypertension and vascular dysfunction in pregnant rats. Biol. Reprod. 2024, 111, 879–889. [Google Scholar] [CrossRef]
- Zheng, X.; Li, H.Y.; Gao, S.W.; Müllen, K.; Zhang, J.; Ji, C.D.; Yin, M.Z. “One-Stone-Three-Birds” H2S-Photothermal Therapy for Enhanced Thrombolysis and Vascular Healing. Small 2024, 20, 2403284. [Google Scholar] [CrossRef]
- Bojakowski, K.; Gaciong, Z.; Grochowiecki, T.; Szmidt, J. Carbon monoxide may reduce ischemia Reperfusion injury: A case report of complicated kidney transplantation from a carbon monoxide poisoned donor. Transplant. Proc. 2007, 39, 2928–2929. [Google Scholar] [CrossRef]
- Sakihama, H.; Lee, G.R.; Chin, B.Y.; Csizmadia, E.; Gallo, D.; Qi, Y.L.; Gagliani, N.; Wang, H.J.; Bach, F.H.; Otterbein, L.E. Carbon Monoxide Suppresses Neointima Formation in Transplant Arteriosclerosis by Inhibiting Vascular Progenitor Cell Differentiation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1915–1927. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; El-Sayed, S.A.; Abdel-Hakim, S.M.; Hassan, M.K.A.; Aziz, N.M. The effect of induction of endogenous CO by heme-oxygenase inducer, hemin versus heme-oxygenase blocker, zinc mesoporphyrin on gastric secretion and ulceration under different conditions in adult male albino rats. Bratisl. Med. J. 2014, 115, 319–329. [Google Scholar] [CrossRef]
- Mansour, A.M.; Khaled, R.M.; Ferraro, G.; Shehab, O.R.; Merlino, A. Metal-based carbon monoxide releasing molecules with promising cytotoxic properties. Dalton Trans. 2024, 53, 9612–9656. [Google Scholar] [CrossRef]
- Niu, Q.S.; Du, F.; Yang, X.J.; Yang, X.J.; Wang, X.H. Carbon monoxide-releasing molecule 2 inhibits inflammation associated with intestinal ischemia-reperfusion injury in a rat model of hemorrhagic shock. Int. Immunopharmacol. 2022, 113, 109441. [Google Scholar] [CrossRef]
- Obara, T.; Yamamoto, H.; Aokage, T.; Igawa, T.; Nojima, T.; Hirayama, T.; Seya, M.; Ishikawa-Aoyama, M.; Nakao, A.; Motterlini, R.; et al. Luminal Administration of a Water-soluble Carbon Monoxide-releasing Molecule (CORM-3) Mitigates Ischemia/Reperfusion Injury in Rats Following Intestinal Transplantation. Transplantation 2022, 106, 1365–1375. [Google Scholar] [CrossRef]
| Feature | Hydrogen Sulfide | Nitric Oxide | Carbon Monoxide |
|---|---|---|---|
| Biosynthetic enzyme | CSE, CBS, 3-MST | data eNOS, nNOS, iNOS | HO-1, HO-2 |
| Primary action | K+ channel opening, thiol persulfidation | data sGC activation, S-nitrosylation | sGC activation, mitochondrial regulation |
| Redox interaction | data Reacts with H2O2 →polysulfides | Reacts with O2− →ONOO− | Inhibits mitochondrial ROS |
| Antioxidant effect | data Upregulates antioxidant proteins | Scavenges ROS, but forms RNS | Induces SOD, ferritin |
| Vascular role | data Vasodilation, angiogenesis | Vasodilation, anti-inflammation | Vasodilation, anti-apoptosis |
| Clinical challenges | Donor stability, specificity | Dual role, oxidative vulnerability | Delivery method, toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, G.-H.; Kim, C.-S. Redox Regulation of Endogenous Gasotransmitters in Vascular Health and Disease. Int. J. Mol. Sci. 2025, 26, 9037. https://doi.org/10.3390/ijms26189037
Vu G-H, Kim C-S. Redox Regulation of Endogenous Gasotransmitters in Vascular Health and Disease. International Journal of Molecular Sciences. 2025; 26(18):9037. https://doi.org/10.3390/ijms26189037
Chicago/Turabian StyleVu, Giang-Huong, and Cuk-Seong Kim. 2025. "Redox Regulation of Endogenous Gasotransmitters in Vascular Health and Disease" International Journal of Molecular Sciences 26, no. 18: 9037. https://doi.org/10.3390/ijms26189037
APA StyleVu, G.-H., & Kim, C.-S. (2025). Redox Regulation of Endogenous Gasotransmitters in Vascular Health and Disease. International Journal of Molecular Sciences, 26(18), 9037. https://doi.org/10.3390/ijms26189037






