Interactions of Hematopoietic and Associated Mesenchymal Stem Cell Populations in the Bone Marrow Microenvironment, In Vivo and In Vitro Model
Abstract
1. Introduction
2. Heterogeneous Populations of Bone Marrow Stem Cells
2.1. Hematopoietic Stem/Progenitor Cells
HSC/HPC Phenotype
2.2. Subsection Bone-Marrow Derived Mesenchymal Stem Cells and Subtypes of MSCs
2.2.1. Endosteal Osteoblasts–Osteolineage Cells
2.2.2. Leptin Receptor+ (LepR) Cells/Leptin+ MSC Osteoprogenitore
2.2.3. CXCL12 Abundant Reticular Cells (CAR)
2.2.4. Nestin and Neuron-Glial Antigen 2 Cells (NG2)
2.2.5. Pericytes
2.2.6. Endothelial Cells (ECs)
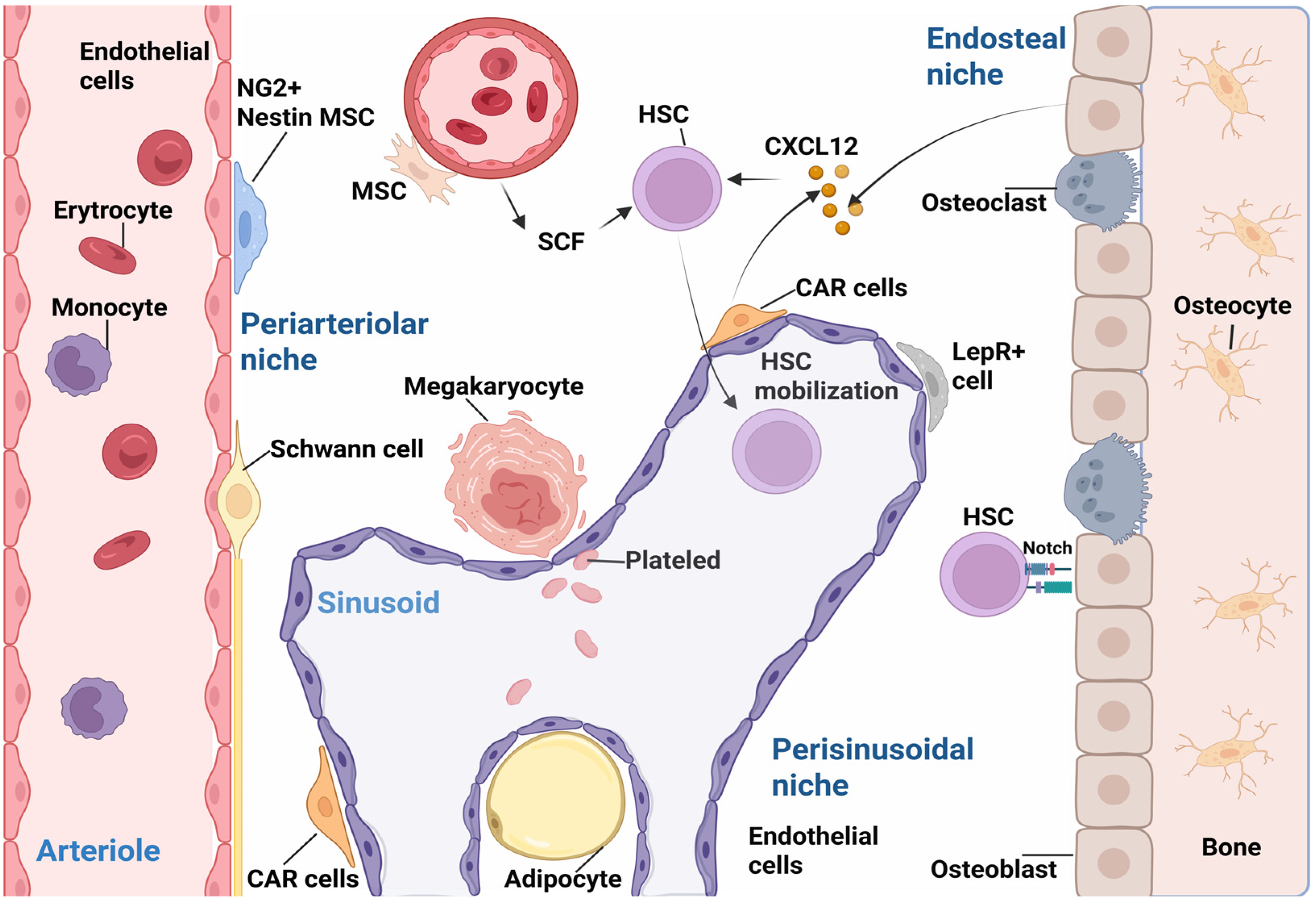
2.3. Mobilization and Homing of Stem Cells
| Type of Niche Bone Marrow | Cell Type | Cell Phenotype | HSC Assignment | References |
|---|---|---|---|---|
| Endosteal niche | Osteoblasts | ALP, COL1, osteopontin | Influence quiescence of HSCs Homing for exogenous HSCs | [1,121] |
| Periarteriolar niche | Nestin+ cells perivascular MSCs | Nestin, PDGFR-α | Circadian oscillations of HSC release, HSC homing | [93] |
| NG2+ cells perivascular MSCs | NG 2 Ang-1, VCAM-1 | HSC maintenance and activation | [87,123] | |
| Sinusoidal Endothelial/Perisinusoidal niche | CAR cells | CXCL12, BMP 4 | HSCs of interaction with CAR cells via CXCL12-CXCR4 | [58,99] |
| LepR+ cells | LepR co-expresion CXCL12 | Influence quiescence of HSCs | [87] | |
| Nestin+ cells | Nestin, PDGFR-alpha | HSC homing | [93] | |
| Endothelial cells | Stro-1, VEGFR2 Notch, CXCL12 | The surrounding vascular microenvironment and acts on HSC self-renewal and retention | [1,114] |
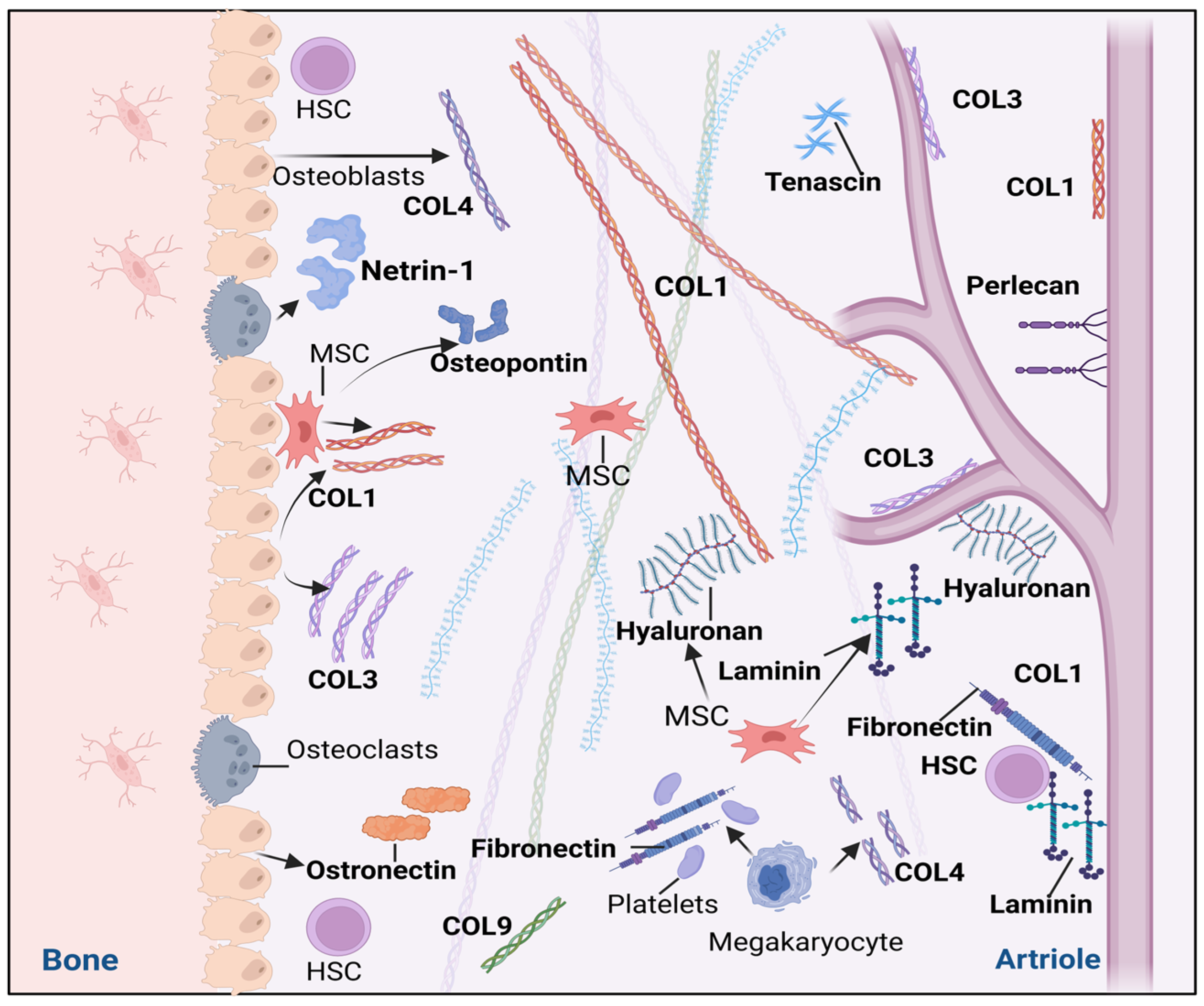
3. The Extracellular Matrix of Hematopoietic Stem Cell Niches
3.1. Glycoproteins
3.2. Collagens (COLs)
3.3. Proteoglycans
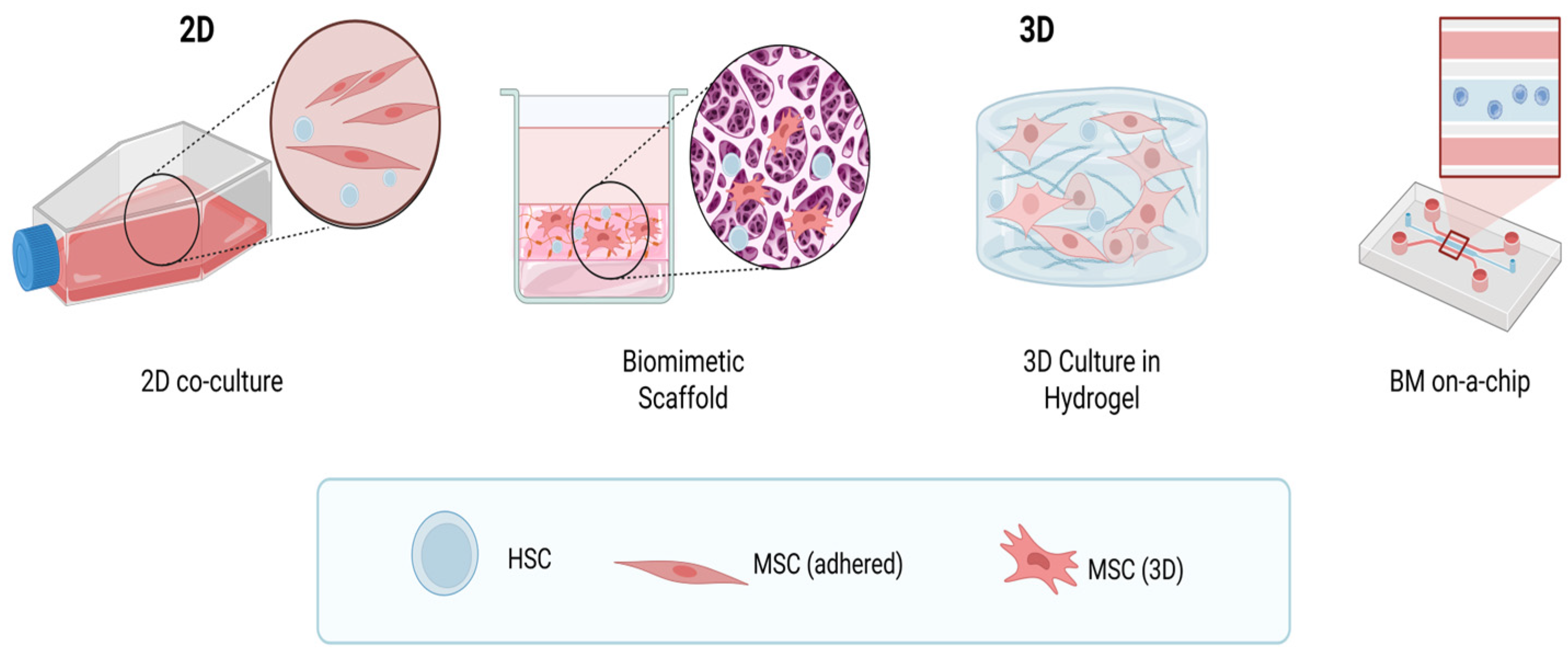
4. Modeling the Hematopoietic Niche
4.1. Two-Dimensional Suspension Cultures of Human Hematopoietic Stem and Progenitor Cells (HSCs/HPCs) and Co-Culture with Mesenchymal Stem Cells
Surfaces with ECM Component Properties
4.2. Three-Dimensional Culture
4.2.1. Biocompatible Synthetic and Natural Scaffolds
4.2.2. Hydrogels
4.2.3. Synthetic Gels with Scaffolds
4.2.4. Bone Marrow-on-a-Chip
4.2.5. Biomimetic 3D Model of Bone Marrow
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ang | Angiopoietin |
| BM | Bone marrow |
| BMM | Bone marrow microenvironment |
| CAMs | Cell adhesion molecules |
| COL | Collagen |
| CXCR4 | C-X-C chemokine receptor type 4 |
| CXCL12; SDF-1 | CXC motif chemokine ligand 12; Stromal-derived factor-1 |
| CAR | CXCL12 abundant reticular |
| ECs | Endothelial cells |
| FN | Fibronectin |
| GAG | Glycosaminoglycan |
| HSPCs | Hematopoietic stem and progenitor cells |
| HPCs | Hematopoetic progenitor cells/multipotent progenitor |
| CD34 | Hematopoietic Progenitor Cell Antigen CD34 |
| HSCs | Hematopoietic stem cells |
| HA | Hyaluronic acid |
| CS | Chondroitin sulfate |
| ITGA6; VLA-6; CD49f | Integrin Subunit Alpha 6 |
| IL | Interleukin |
| LepR | Leptin receptor |
| Lin- | Lineage-negative |
| LT-HSC | Long-term hematopoietic stem cells |
| LFA-1 | Lymphocyte function-associated antigen |
| MSCs | Mesenchymal stem cells |
| MPP | Multipotent progenitor |
| NGF | Nerve growth factor |
| Notch | Neurogenic locus notch homolog protein |
| NG2 | Neuron-glial Antigen 2; Chondroitin sulfate proteoglycan 4 |
| RANKL | Nuclear factor-kappa B ligand |
| Prx-1 | Paired-related homeobox 1 |
| P-glycoprotein | Permeability-glycoprotein |
| AC133; CD133 | Prominin-1 |
| PGE2 | Prostaglandin E2 |
| c-KIT | Proto-oncogene c-KIT |
| ST-HSC | Short-term hematopoietic stem cells |
| SECs | Sinusoidal ECs |
| CFU-S | Spleen colonies formation |
| SCF-1 | Stem cell factor-1 |
| STRO | Stromal cell surface marker |
| Thy-1; CD90 | THYmocyte differentiation antigen 1 |
| VCAM1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VLA-4 | Very late antigen-4; Integrin α4β1 |
References
- Xiao, Y.; McGuinness, C.S.; Doherty-Boyd, W.S. Current insights into the bone marrow niche: From biology in vivo to bioengineering ex vivo. Biomaterials 2022, 286, 121568. [Google Scholar] [CrossRef] [PubMed]
- Lee-Thedieck, C.; Schertl, P.; Klein, G. The extracellular matrix of hematopoietic stem cell niches. Adv. Drug Deliv. Rev. 2022, 181, 114069. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Nyamondo, K.; Wheadon, H. Complexities of modelling the bone marrow microenvironment to facilitate hematopoietic research. Exp. Hematol. 2024, 135, 104233. [Google Scholar] [CrossRef] [PubMed]
- Trentin, J.J. Hemopoietic Microenvironments: Historical Perspectives, Status, and Projections. In Handbook of the Hemopoietic Microenvironment, 1st ed.; Tavassoli, M., Ed.; Humana Press: Totowa, NJ, USA, 1989; pp. 1–86. [Google Scholar]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Becker, A.J.; McCulloch, E.A.; Till, J.E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963, 197, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Till, J.E.; McCulloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Adams, G.B.; Scadden, D.T. The hematopoietic stem cell in its place. Nat. Immunol. 2006, 7, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Iwashita, T.; Yilmaz, Ö.H. Spatial differences in hematopoiesis but not in stem cells indicate a lack of regional patterning in definitive hematopoietic stem cells. Dev. Biol. 2005, 283, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kopp, H.G.; Avecilla, S.T.; Hooper, A.T.; Rafii, S. The bone marrow vascular niche: Home of HSC differentiation and mobilization. Physiology 2005, 20, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Trumpp, A.; Milsom, M.D. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell 2018, 22, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Abkowitz, J.L.; Linenberger, M.L.; Newton, M.A. Evidence for the maintenance of hematopoiesis in a large animal by the sequential activation of stem-cell clones. Proc. Natl. Acad. Sci. USA 1990, 87, 9062–9066. [Google Scholar] [CrossRef]
- Acar, M.; Kocherlakota, K.S.; Murphy, M.M. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015, 526, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A. Niche regulation of hematopoiesis: The environment is “micro,” but the influence is large. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 691–699. [Google Scholar] [CrossRef]
- Kristensen, H.B.; Andersen, T.L.; Patriarca, A. Human hematopoietic microenvironments. PLoS ONE 2021, 16, e0250081. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, C.; Ye, L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef]
- Cosgrove, J.; Hustin, L.S.; de Boer, R.J. Hematopoiesis in numbers. Trends Immunol. 2021, 42, 1100–1112. [Google Scholar] [CrossRef]
- Notta, F.; Doulatov, S.; Laurenti, E.; Poeppl, A. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 2011, 333, 218–221. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Wang, J. Functions and regulatory mechanisms of resting hematopoietic stem cells: A promising targeted therapeutic strategy. Stem Cell Res. Ther. 2023, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; DeLay, M.; Reynaud, D. Phenotypic analysis of the mouse hematopoietic hierarchy using spectral cytometry: From stem cell subsets to early progenitor compartments. Cytom. Part A 2020, 97, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Tárnok, A.; Ulrich, H.; Bocsi, J. Phenotypes of stem cells from diverse origin. Cytom. Part A 2010, 77, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Morita, Y.; Ooehara, J. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Challen, G.A.; Sirin, O. Hematopoietic stem cell characterization and isolation. Stem Cell Migr. Methods Protoc. 2011, 750, 47–59. [Google Scholar]
- Ogawa, M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993, 81, 844–853. [Google Scholar] [CrossRef]
- Civin, C.I.; Strauss, L.C.; Brovall, C.H.; Fackler, M.J. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J. Immunol. 1984, 133, 157–165. [Google Scholar] [CrossRef]
- Gangenahalli, G.U.; Singh, V.K.; Verma, Y.K. Hematopoietic stem cell antigen CD34: Role in adhesion or homing. Stem Cells Dev. 2006, 15, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ebihara, Y.; Xu, M.J. CD34 expression on long-term repopulating hematopoietic stem cells changes during developmental stages. Blood 2001, 97, 419–425. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Anjos-Afonso, F.; Currie, E.; Palmer, H. CD34− cells at the apex of the human hematopoietic stem cell hierarchy have distinctive cellular and molecular signatures. Cell Stem Cell 2013, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Musto, P.; Cascavilla, N. Thy-1 (CDw90) and c-kit receptor (CD117) expression on CD34+ hematopoietic progenitor cells: A five dimensional flow cytometric study. Haematologica 1998, 83, 587–592. [Google Scholar]
- Hordyjewska, A.; Popiołek, Ł.; Horecka, A. Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology 2015, 67, 387–396. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Colvin, G.A.; Berz, D.; Quesenberry, P.J. Short-term hematopoietic stem cells (ST-HSC) have full long-term capacity with sustained but reduced potential compared with LT-HSC. Blood 2009, 114, 2550. [Google Scholar] [CrossRef]
- Buckley, R.H.; Schiff, S.E.; Schiff, R.I. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N. Engl. J. Med. 1999, 340, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Huntsman, H.D.; Bat, T.; Cheng, H. Human hematopoietic stem cells from mobilized peripheral blood can be purified based on CD49f integrin expression. Blood 2015, 126, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Schippel, N.; Sharma, S. Dynamics of human hematopoietic stem and progenitor cell differentiation to the erythroid lineage. Exp. Hematol. 2023, 123, 1–17. [Google Scholar] [CrossRef]
- Bhatia, M.; Wang, J.C.; Kapp, U. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 5320–5325. [Google Scholar] [CrossRef] [PubMed]
- Mayani, H.; Lansdorp, P.M. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood 1994, 83, 2410–2417. [Google Scholar] [CrossRef][Green Version]
- Majeti, R.; Park, C.Y.; Weissman, I.L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 2007, 1, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Dick, J.E. Molecular and functional characterization of early human hematopoiesis. Ann. N. Y. Acad. Sci. 2012, 1266, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Takeshita, A.; Zou, P. Multidrug resistance pglycoprotein function of bone marrow hematopoietic cells and the reversal agent effect. J. Tongji Med. Univ. 1999, 19, 260–263. [Google Scholar] [PubMed]
- Nooka, A.K.; Kaufman, J.L. Daratumumab in multiple myeloma. Cancer 2019, 125, 2364–2382. [Google Scholar] [CrossRef] [PubMed]
- Szilvassy, S.J. The biology of hematopoietic stem cells. Arch. Med. Res. 2003, 34, 446–460. [Google Scholar] [CrossRef]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997, 90, 5002–5012. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Mess, J.; Aizarani, N. Hyaluronic acid–GPRC5C signalling promotes dormancy in haematopoietic stem cells. Nat. Cell Biol. 2022, 24, 1038–1048. [Google Scholar] [CrossRef]
- Kollet, O.; Shivtiel, S.; Chen, Y.Q. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Investig. 2003, 112, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Wang, D.A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011, 11, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Avraham, I. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 2006, 124, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Ratajczak, J.; Reca, R. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol. Dis. 2004, 32, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Grabovsky, V.; Habler, L. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Investig. 1999, 104, 1199–1211. [Google Scholar] [CrossRef]
- Kijowski, J.; Baj-Krzyworzeka, M.; Majka, M. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells 2001, 19, 453–466. [Google Scholar] [CrossRef]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Moll, N.M.; Ransohoff, R.M. CXCL12 and CXCR4 in bone marrow physiology. Expert Rev. Hematol. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Cordeiro-Spinetti, E.; Taichman, R.S. The bone marrow endosteal niche: How far from the surface? J. Cell. Biochem. 2015, 116, 6–11. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Dennis, J.E.; Charbord, P. Origin and differentiation of human and murine stroma. Stem Cells 2002, 20, 205–214. [Google Scholar] [CrossRef]
- Blazsek, I.; Chagraoui, J.; Péault, B. Ontogenic emergence of the hematon, a morphogenetic stromal unit that supports multipotential hematopoietic progenitors in mouse bone marrow. Blood 2000, 96, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Stute, N.; Holtz, K.; Bubenheim, M.; Lange, C. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp. Hematol. 2004, 32, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, K.; Quaini, F.; Tasca, G. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2003, 100, 10440–10445. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Zachar, L. Analysis of same selected immunomodulatory properties of chorionic mesenchymal stem cells. Appl. Sci. 2020, 10, 9040. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Keane-Moore, M.; Buyaner, D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003, 10, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Tormin, A.; Li, O.; Brune, J.C.; Walsh, S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 2011, 117, 5067–5077. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yu, W.; Ye, G.; Li, J.; Zheng, G. Single-cell RNA sequencing analysis of human bone-marrow-derived mesenchymal stem cells and functional subpopulation identification. Exp. Mol. Med. 2022, 54, 483–492. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Morochovič, R. Interaction between mesenchymal stem cells and the immune system in rheumatoid arthritis. Pharmaceuticals 2022, 15, 941. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.K. Endosteal marrow: A rich source of hematopoietic stem cells. Science 1978, 199, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kohara, H.; Noda, M. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Taichman, R.S.; Emerson, S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 1994, 179, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Katayama, Y. Regulation of hematopoiesis in endosteal microenvironments. Int. J. Hematol. 2014, 99, 679–684. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Wang, L.; Li, T. Role of Prx1-expressing skeletal cells and Prx1-expression in fracture repair. Bone 2020, 139, 115521. [Google Scholar] [CrossRef]
- Smith, J.N.; Calvi, L.M. Concise review: Current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells 2013, 31, 1044–1050. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Mansour, A.; Abou-Ezzi, G.; Sitnicka, E. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J. Exp. Med. 2012, 209, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Simmons, P.J.; Moore, R.J. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood 1998, 92, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Udagawa, N.; Takami, M. Cells of bone: Osteoclast generation. In Principles of Bone Biology; Academic Press: Cambridge, MA, USA, 2002; Volume 1, pp. 109–126. [Google Scholar]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A. CXC chemokines in angiogenesis. J. Leukocyte Biol. 2000, 68, 1–8. [Google Scholar] [CrossRef]
- Grassi, F.; Piacentini, A.; Cristino, S. Human osteoclasts express different CXC chemokines depending on cell culture substrate: Molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem. Cell Biol. 2003, 120, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Rosenberg, O.; Soudry, M. Osteoblasts in bone physiology—Mini review. Rambam Maimonides Med. J. 2012, 3, e0013. [Google Scholar] [CrossRef]
- Pinho, S.; Lacombe, J.; Hanoun, M. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 2013, 210, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Cancelas, J.A. From marrow to bone and fat: Exploring the multifaceted roles of leptin receptor positive bone marrow mesenchymal stromal cells. Cells 2024, 13, 910. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Michienzi, S. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef]
- Hosokawa, K.; Arai, F.; Yoshihara, H. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood 2010, 116, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.A.; Hemperly, J.J. Human N-cadherin: Nucleotide and deduced amino acid sequence. Nucleic Acids Res. 1990, 18, 5896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yin, T.; Wiegraebe, W. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 2009, 457, 97–101, Erratum in Nature 2010, 466, 1134. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Moore, R.; Negishi, M. Nuclear receptor CAR (NR1I3) is essential for DDC-induced liver injury and oval cell proliferation in mouse liver. Lab. Investig. 2011, 91, 1624–1633. [Google Scholar] [CrossRef]
- Nilsson, S.K.; Johnston, H.M.; Whitty, G.A. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005, 106, 1232–1239. [Google Scholar] [CrossRef]
- Eltoukhy, H.S.; Sinha, G.; Moore, C. CXCL12-abundant reeticular (CAR) cells: A review of the literature with relevance to cancer stem cell survival. J. Cancer Stem Cell Res. 2016, 4, e1004. [Google Scholar] [CrossRef]
- Nagasawa, T. Cxcl12/sdf-1 and cxcr4. Front. Immunol. 2015, 6, 301. [Google Scholar] [CrossRef] [PubMed]
- Mosteo, L.; Storer, J.; Batta, K. The dynamic interface between the bone marrow vascular niche and hematopoietic stem cells in myeloid malignancy. Front. Cell Dev. Biol. 2021, 9, 635189. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Hsu, Y.M.S.; Day, R.B. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235, Erratum in Nature 2014, 514, 262. [Google Scholar] [CrossRef]
- Li, H.; Bräunig, S.; Dhapolar, P. Identification of phenotypically, functionally, and anatomically distinct stromal niche populations in human bone marrow based on single-cell RNA sequencing. eLife 2023, 12, e81656. [Google Scholar] [CrossRef] [PubMed]
- Nyamondo, K.; Wheadon, H. Micro-environment alterations through time leading to myeloid malignancies. Br. J. Pharmacol. 2024, 181, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.W.C.; Scadden, D.T. Hematopoietic stem cell and its bone marrow niche. Curr. Top. Dev. Biol. 2016, 118, 21–44. [Google Scholar] [PubMed]
- Asada, N.; Kunisaki, Y.; Pierce, H. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017, 19, 214–223. [Google Scholar] [CrossRef]
- Mach, D.B.; Rogers, S.D.; Sabino, M.C. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience 2002, 113, 155–166. [Google Scholar] [CrossRef]
- Maryanovich, M.; Takeishi, S.; Frenette, P.S. Neural regulation of bone and bone marrow. Cold Spring Harb. Perspect. Med. 2018, 8, a031344. [Google Scholar] [CrossRef]
- Hirschi, K.K.; D’Amore, P.A. Pericytes in the microvasculature. Cardiovasc. Res. 1996, 32, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lamagna, C.; Bergers, G. The bone marrow constitutes a reservoir of pericyte progenitors. J. Leukocyte Biol. 2006, 80, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Allt, G.; Lawrenson, J.G. Pericytes: Cell biology and pathology. Cells Tissues Organs 2001, 169, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Cleary, K. Morphology and properties of pericytes. Blood-Brain Other Neural Barriers Rev. Protoc. 2010, 686, 49–68. [Google Scholar]
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Himburg, H.A.; Harris, J.R.; Ito, T. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012, 2, 964–975. [Google Scholar] [CrossRef]
- Kobayashi, H.; Butler, J.M.; O’donnell, R. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 2010, 12, 1046–1056. [Google Scholar] [CrossRef]
- Xu, C.; Gao, X.; Wei, Q. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat. Commun. 2018, 9, 2449. [Google Scholar] [CrossRef]
- Himburg, H.A.; Termini, C.M.; Schlussel, L. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell 2018, 23, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Chen, L.S.; Singh, R.P. Secreted protein Del-1 regulates myelopoesis in the hematopoietic stem cell niche. J. Clin. Investig. 2017, 127, 3624–3639. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem cell homing: From physiology to therapeutics. Stem Cells 2020, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Abkowitz, J.L.; Robinson, A.E.; Kale, S. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 2003, 102, 1249–1253. [Google Scholar] [CrossRef]
- Mazo, I.B.; von Andrian, U.H. Adhesion and homing of blood-borne cells in bone marrow microvessels. J. Leukocyte Biol. 1999, 66, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, J.P.; Takamatsu, Y.; Nilsson, S.K. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood 2001, 98, 1289–1297. [Google Scholar] [CrossRef]
- Li, D.; Xue, W.; Li, M. VCAM-1+ macrophages guide the homing of HSPCs to a vascular niche. Nature 2018, 564, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, V.; Mikkola, H.K. The genesis of human hematopoietic stem cells. Blood 2023, 142, 519–532. [Google Scholar] [CrossRef]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Balasubramani, M.; Schreiber, E.M. Biomechanical properties of native basement membranes. FEBS J. 2007, 274, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57, 1–11. [Google Scholar] [CrossRef]
- Siler, U.; Seiffert, M.; Puch, S.; Richards, A. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood 2000, 96, 4194–4203. [Google Scholar] [CrossRef]
- Gu, Y.C.; Kortesmaa, J.; Tryggvason, K. Laminin isoform–specific promotion of adhesion and migration of human bone marrow progenitor cells. Blood 2003, 101, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, V.; Yebra, M. Netrins: Beyond the brain. Nat. Rev. Mol. Cell Biol. 2007, 8, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Renders, S.; Svendsen, A.F.; Panten, J. Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1. Nat. Commun. 2021, 12, 608. [Google Scholar] [CrossRef]
- Mediero, A.; Ramkhelawon, B.; Perez-Aso, M. Netrin-1 is a critical autocrine/paracrine factor for osteoclast differentiation. J. Bone Miner. Res. 2015, 30, 837–854. [Google Scholar] [CrossRef]
- Enoki, Y.; Sato, T.; Tanaka, S. Netrin-4 derived from murine vascular endothelial cells inhibits osteoclast differentiation in vitro and prevents bone loss in vivo. FEBS Lett. 2014, 588, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde-Zimmermann, D.; Verdaasdonk, M.A.M.; Rademakers, L.H.P.M. Fibronectin distribution in human bone marrow stroma: Matrix assembly and tumor cell adhesion via α5β1 integrin. Exp. Cell Res. 1997, 230, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- Hautanen, A.; Gailit, J.; Mann, D.M. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J. Biol. Chem. 2009, 264, 1437–1442. [Google Scholar] [CrossRef]
- Petrie, T.A.; Capadona, J.R.; Reyes, C.D. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials 2006, 27, 5459–5470. [Google Scholar] [CrossRef] [PubMed]
- Vuillet-Gaugler, M.H.; Breton-Gorius, J.; Vainchenker, W. Loss of attachment to fibronectin with terminal human erythroid differentiation. Blood 1990, 75, 865–873. [Google Scholar] [CrossRef]
- Bourgine, P.E.; Klein, T.; Paczulla, A.M. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl. Acad. Sci. USA 2018, 115, 5688–5695. [Google Scholar] [CrossRef]
- Klein, G.; Beck, S.; Müller, C.A. Tenascin is a cytoadhesive extracellular matrix component of the human hematopoietic microenvironment. J. Cell Biol. 1993, 123, 1027–1035. [Google Scholar] [CrossRef]
- Seki, M.; Kameoka, J.; Takahashi, S.; Harigae, H. Identification of tenascin-C as a key molecule determining stromal cell-dependent erythropoiesis. Exp. Hematol. 2006, 34, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Grassinger, J.; Haylock, D.N.; Storan, M.J. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with α9β1 and α4β1 integrins. Blood 2009, 114, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, A.; Boch, T.; Medyouf, H. Loss of SPARC protects hematopoietic stem cells from chemotherapy toxicity by accelerating their return to quiescence. Blood 2014, 123, 4054–4063. [Google Scholar] [CrossRef]
- Siewe, B.T.; Kalis, S.L.; Le, P.T.; Witte, P.L. In vitro requirement for periostin in B lymphopoiesis. Blood 2011, 117, 3770–3779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. The extracellular matrix of the hematopoietic microenvironment. Experientia 1995, 51, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Simmons, P.J.; Graves, S.E. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone 2001, 28, 174–181. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Malara, A.; Currao, M.; Gruppi, C. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef]
- Semeniak, D.; Kulawig, R.; Stegner, D. Proplatelet formation is selectively inhibited by collagen type I through Syk-independent GPVI signaling. J. Cell Sci. 2016, 129, 3473–3484. [Google Scholar] [CrossRef]
- Çelebi, B.; Pineault, N.; Mantovani, D. The role of collagen type I on hematopoietic and mesenchymal stem cells expansion and differentiation. Adv. Mater. Res. 2012, 409, 111–116. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Shah, S.R.; Cohen, A.J. Type III collagen regulates osteoblastogenesis and the quantity of trabecular bone. Calcif. Tissue Int. 2014, 94, 621–631. [Google Scholar] [CrossRef]
- Balduini, A.; Pallotta, I.; Malara, A. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J. Thromb. Haemost. 2008, 6, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Schofield, K.P.; Gallagher, J.T. Expression of proteoglycan core proteins in human bone marrow stroma. Biochem. J. 1999, 343, 663–668. [Google Scholar] [CrossRef]
- Haylock, D.N.; Nilsson, S.K. The role of hyaluronic acid in hemopoietic stem cell biology. Regen. Med. 2006, 1, 437–445. [Google Scholar] [CrossRef]
- Pilarski, L.M.; Pruski, E.; Wizniak, J. Potential role for hyaluronan and the hyaluronan receptor RHAMM in mobilization and trafficking of hematopoietic progenitor cells. Blood 1999, 93, 2918–2927. [Google Scholar] [CrossRef]
- Ingavle, G.; Vaidya, A.; Kale, V. Constructing three-dimensional microenvironments using engineered biomaterials for hematopoietic stem cell expansion. Tissue Eng. Part B 2019, 25, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Zaldívar, H.; Santos, L.; De León Rodríguez, A. Expansion of human hematopoietic stem cells for transplantation: Trends and perspectives. Cytotechnology 2008, 56, 151–160. [Google Scholar] [CrossRef]
- Jaroscak, J.; Goltry, K.; Smith, A. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo–expanded UCB cells: Results of a phase 1 trial using the AastromReplicell System. Blood 2003, 101, 5061–5067. [Google Scholar] [CrossRef]
- Leisten, I.; Kramann, R.; Ferreira, M.S.V. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Ibrahim, A.M.; El-Masry, M.W. Ex vivo expansion of stem cells: Defining optimum conditions using various cytokines. Lab. Hematol. 2006, 12, 86–93. [Google Scholar] [CrossRef]
- Ueda, T.; Tsuji, K.; Yoshino, H. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J. Clin. Investig. 2000, 105, 1013–1021. [Google Scholar] [CrossRef]
- Wohrer, S.; Knapp, D.J.; Copley, M.R. Distinct stromal cell factor combinations can separately control hematopoietic stem cell survival, proliferation, and self-renewal. Cell Rep. 2014, 7, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.E.; Chao, N.J.; Rizzieri, D.A. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J. Clin. Investig. 2014, 124, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Goessling, W.; Walkley, C.R. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 2007, 447, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- McNiece, I.K.; Robinson, S.N.; Shpall, E.J. MSC for Ex Vivo Expansion of Umbilical Cord Blood Cells. In Mesenchymal Stromal Cells: Biology and Clinical Applications; Humana Press: New York, NY, USA, 2013; pp. 485–501. [Google Scholar]
- Delorme, B.; Charbord, P. Culture and characterization of human bone marrow mesenchymal stem cells. In Tissue Engineering; Humana Press: Totowa, NJ, USA, 2007; pp. 67–81. [Google Scholar]
- McNiece, I.; Harrington, J.; Turney, J. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy 2004, 6, 311–317. [Google Scholar] [CrossRef]
- Purdy, M.H.; Hogan, C.J.; Hami, L. Large volume ex vivo expansion of CD34-positive hematopoietic progenitor cells for transplantation. J. Hematother. 1995, 4, 515–525. [Google Scholar] [CrossRef]
- De Lima, M.; McNiece, I.; Robinson, S.N. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N. Engl. J. Med. 2012, 367, 2305–2315. [Google Scholar] [CrossRef]
- Celebi, B.; Mantovani, D.; Pineault, N. Effects of extracellular matrix proteins on the growth of haematopoietic progenitor cells. Biomed. Mater. 2011, 6, 055011. [Google Scholar] [CrossRef] [PubMed]
- Schmal, O.; Seifert, J.; Schäffer, T.E. Hematopoietic stem and progenitor cell expansion in contact with mesenchymal stromal cells in a hanging drop model uncovers disadvantages of 3D culture. Stem Cells Int. 2016, 2016, 4148093. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.E.L.; Wheadon, H.; Lewis, N. A quiescent, regeneration-responsive tissue engineered mesenchymal stem cell bone marrow niche model via magnetic levitation. ACS Nano 2016, 10, 8346–8354. [Google Scholar] [CrossRef]
- Travlos, G.S. Normal structure, function, and histology of the bone marrow. Toxicol. Pathol. 2006, 34, 548–565. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.N.; Simmons, P.J.; Yang, H. Mesenchymal stem cells in ex vivo cord blood expansion. Best Pract. Res. Clin. Haematol. 2011, 24, 83–92. [Google Scholar] [CrossRef]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007, 25, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Futrega, K.; Atkinson, K.; Lott, W.B. Spheroid coculture of hematopoietic stem/progenitor cells and monolayer expanded mesenchymal stem/stromal cells in polydimethylsiloxane microwells modestly improves in vitro hematopoietic stem/progenitor cell expansion. Tissue Eng. Part C 2017, 23, 200–218. [Google Scholar] [CrossRef]
- Ferreira, M.S.V.; Jahnen-Dechent, W.; Labude, N. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 2012, 33, 6987–6997, Erratum in Biomaterials 2012, 33, 9165. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chai, C.; Jiang, X.S. Expansion of engrafting human hematopoietic stem/progenitor cells in three-dimensional scaffolds with surface-immobilized fibronectin. J. Biomed. Mater. Res. Part A 2006, 78, 781–791. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Abroun, S.; Soleimani, M. 3-Dimensional nano-fibre scaffold for ex vivo expansion of cord blood haematopoietic stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 740–748. [Google Scholar] [CrossRef]
- Bianco, J.E.R.; Rosa, R.G.; Congrains-Castillo, A. Characterization of a novel decellularized bone marrow scaffold as an inductive environment for hematopoietic stem cells. Biomater. Sci. 2019, 7, 1516–1528. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Vallmajo-Martin, Q.; Broguiere, N.; Millan, C. PEG/HA hybrid hydrogels for biologically and mechanically tailorable bone marrow organoids. Adv. Funct. Mater. 2020, 30, 1910282. [Google Scholar] [CrossRef]
- Mahadik, B.P.; Wheeler, T.D.; Skertich, L.J. Microfluidic generation of gradient hydrogels to modulate hematopoietic stem cell culture environment. Adv. Healthc. Mater. 2014, 3, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Braham, M.V.; Li Yim, A.S.; Garcia Mateos, J. A human hematopoietic niche model supporting hematopoietic stem and progenitor cells in vitro. Adv. Healthc. Mater. 2019, 8, 1801444. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, A.E.; Lee, S.; Hu, Y. Soluble signals and remodeling in a synthetic gelatin-based hematopoietic stem cell niche. Adv. Healthc. Mater. 2019, 8, 1900751. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2005; pp. 378–386. [Google Scholar]
- Baghaban, E.M.; Bagheri, F.; Zomorodian, E. Matrigel enhances in vitro bone differentiation of human marrow-derived mesenchymal stem cells. Iran. J. Basic Med. Sci. 2010, 13, 187–194. [Google Scholar]
- Trebuňová, M.; Petroušková, P.; Balogová, A.F. Evaluation of biocompatibility of PLA/PHB/TPS polymer scaffolds with different additives of ATBC and OLA plasticizers. J. Funct. Biomater. 2023, 14, 412. [Google Scholar] [CrossRef]
- Čajková, J.; Trebuňová, M.; Modrák, M. Influence of Oligomeric Lactic Acid and Structural Design on Biodegradation and Absorption of PLA-PHB Blends for Tissue Engineering. Polymers 2024, 16, 2969. [Google Scholar] [CrossRef]
- Bello, A.B.; Park, H.; Lee, S.H. Current approaches in biomaterial-based hematopoietic stem cell niches. Acta Biomater. 2018, 72, 1–15. [Google Scholar] [CrossRef]
- Shakesheff, K.M.; Cannizzaro, S.M.; Langer, R. Creating biomimetic micro-environments with synthetic polymer-peptide hybrid molecules. J. Biomater. Sci. Polym. Ed. 1998, 9, 507–518. [Google Scholar] [CrossRef]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef] [PubMed]
- Klass, C.M.; Couchman, J.R.; Woods, A. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J. Cell Sci. 2000, 113, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wu, H.; Huang, Y. Structurally and mechanically tuned macroporous hydrogels for scalable mesenchymal stem cell–extracellular matrix spheroid production. Proc. Natl. Acad. Sci. USA 2024, 121, e2404210121. [Google Scholar] [CrossRef]
- Kandarakov, O.; Belyavsky, A.; Semenova, E. Bone marrow niches of hematopoietic stem and progenitor cells. Int. J. Mol. Sci. 2022, 23, 4462. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, A.; Meinert, C.; Bas, O. Engineering a 3D bone marrow adipose composite tissue loading model suitable for studying mechanobiological questions. Mater. Sci. Eng. C 2021, 128, 112313. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Wirth, L.; Cavak, N. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 2018, 12, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Evans, R.; Mezan, R. Three-dimensional microfluidic tri-culture model of the bone marrow microenvironment for study of acute lymphoblastic leukemia. PLoS ONE 2015, 10, e0140506, Erratum in PLoS ONE 2015, 10, e0146203. [Google Scholar] [CrossRef]
- Kefallinou, D.; Grigoriou, M.; Boumpas, D.T. Mesenchymal Stem Cell and Hematopoietic Stem and Progenitor Cell Co-Culture in a Bone-Marrow-on-a-Chip Device toward the Generation and Maintenance of the Hematopoietic Niche. Bioengineering 2024, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Fortea, M.; Marciano, D.; Gautrot, J.E. Biomimetic Artificial Bone Marrow Niches for the Scale Up of Hematopoietic Stem and Progenitor Cells. Biomaterials 2025, 325, 123612. [Google Scholar] [CrossRef] [PubMed]
- Baccin, C.; Al-Sabah, J.; Velten, L. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 2020, 22, 38–48. [Google Scholar] [CrossRef] [PubMed]
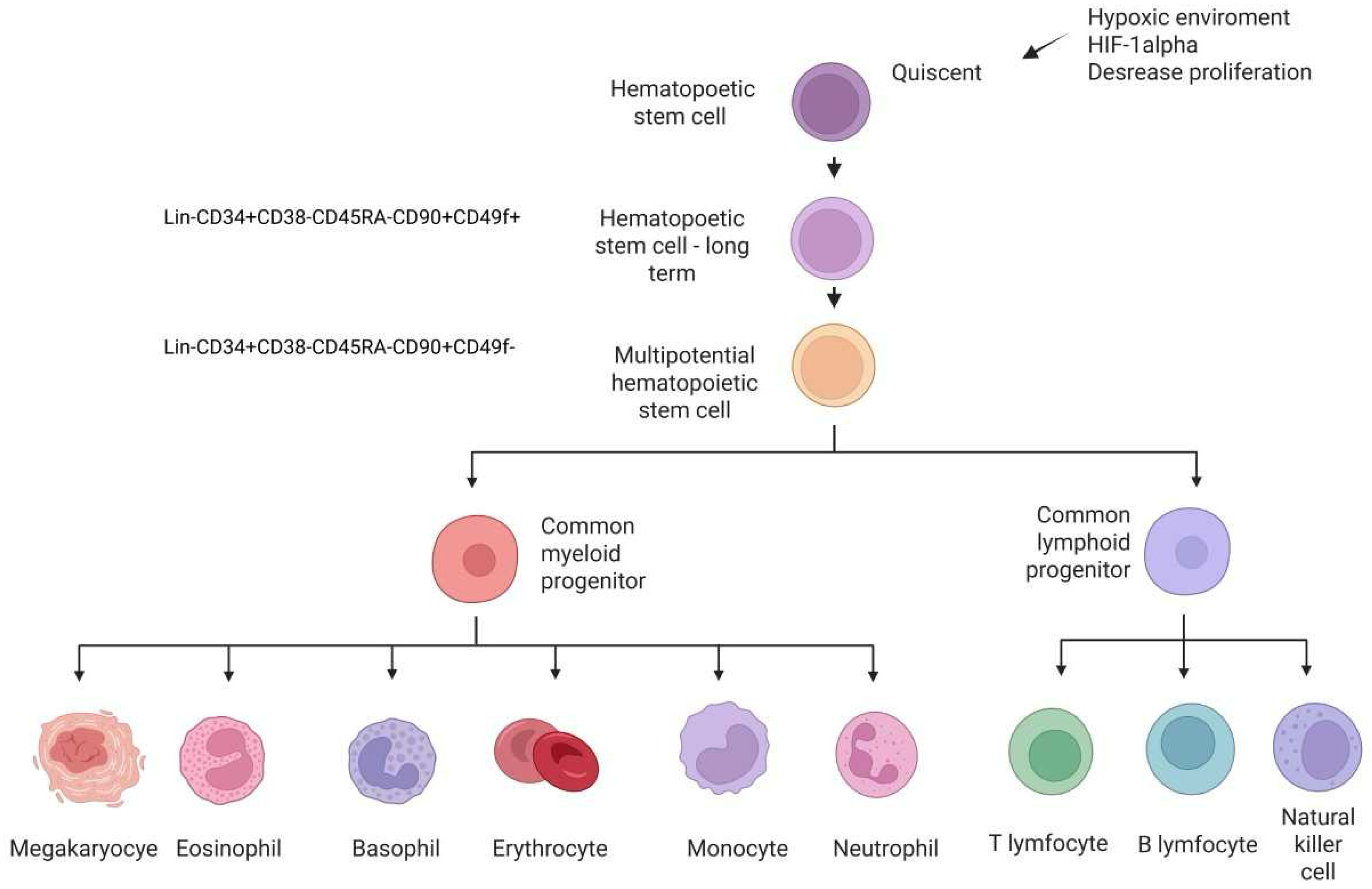
| Panel of Surface Marker Human Hematopoietic and Progenitor Stem Cells | Cell-Type Specificity | Localization | References |
|---|---|---|---|
| CD34+ cells | Heterogeneous stem cells include committed progenitors | BM and peripheral blood | [31,32] |
| CD34+ CD38− | LTC-IC, CFCs | BM | [39] |
| Lin− CD34+ CD38+ | In HSCs, expression of CD38 is correlated with increased differentiation | BM and peripheral blood | [42] |
| Lin− CD34+ CD38− CD45RA− CD90− CD49f− | HPC | BM, peripheral blood, cord blood | [40] |
| Lin− CD90+ CD45RA− CD71− | HSC/HPC | peripheral blood mobilisation | [43,44] |
| Lin− CD34+ CD38− CD45RA− CD90+ CD49f+ | Long-term repopulating hematopoietic stem cells | BM and cord blood | [22] |
| Type Culture/Cell Types | Conditions Cell Cultures | Advantages/Disadvantages | References |
|---|---|---|---|
| Suspension culture Human umbilical cord blood (UCB) CD34+ | UCB CD34+ cells: Iscove’s modified Dulbecco’s medium (IMDM), fetal calf serum (FSC) stem cell factor (SCF) thrombopoietin, FMS-like tyrosine kinase 3 ligand (Flt3L) angiopoietin-like proteins (ANGPTLs), IL-6 | Advantages: expansion of UCB CD34+ cells easy-to-use protocol high reproducibility Disadvantages: loss stemness | [164,167] |
| Suspension hematopoietic cells and monolayer mesenchymal stem cells (MSC) co-culture Human UCB HSC/HPC CD34+ and MSC | UCB CD34+ cells: IMDM, FCS, SCF, Granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, Thrombopoietin (TPO), IL-6 MSC: alpha MEM, FCS | Advantages: co-culture ability isolation and maintaining viability during cultivation large number of cells in subculture increase in CD34+ Disadvantages: loss stemness | [163,166,171,180] |
| Surfaces for culture with coating with COL1, fibronectin, laminin human UCB HSC/HPC CD34+ | UCB CD34+ cells: IMDM serum-free medium substitute albumin/insulin/transferrin | Advantages: ex vivo expansion of UCB CD34+ cells unlimited cell growth large numbers of cells by subculturing Disadvantages: absence of BM stromal cells | [176] |
| 3D culture spheroid techniques human UCB CD34+ HSC/HPC bone marrow (BM) MSC | UCB CD34+ cells: Serum-free medium for hematopoietic cells (SFEM) | Advantages: BM stromal cell spheroids improve cell–cell interactions and promote ECM production mimicked the endosteal and perivascular niches Disadvantages: demanding cultivation system | [177,178,179] |
| BM MSC: DMEM low glucose, human thrombocyte lysate, L-glutamine, HEPES sodium salt | |||
| Scaffolds PCL, PLGA, fibrin a collagen UCB HSC/HPC CD34+ UCB MSC | UCB CD34+ cells: SCF, thrombopoetin, fibroblast growth factor-1, angiopoietin like-5, insulin-like growth factor binding-protein 2 UCB MSC: alpha MEM medium, FCS, insulin–transferrin–selenic acid, linoleic acid | Advantages: culture CD34+ cells expanded on 3D fibrin scaffolds with UC MSC 3D scaffold PLGA meshes 3D fibrin scaffolds with stromal support for expansion of CB CD34+ cells in the presence of cytokine supplementation with UCB MSC HSC adhesion to fibrin scaffold, as in the HSC niche Disadvantages: PLGA meshes did not support HSC expansion | [183] |
| Bone marrow-on-a-chip PET membrane in the form of a chip with cells in UB MSC and HSC/HPC CD34+ co-culture | UCB CD34+ cells: serum-free medium for expansion of hematopoietic cells with SCF, Flt3-Ligand UB MSC: alpha MEM, FCS | Advantages: microfluidic system with passive perfusion with contact HSC/HPC and MSC culture cells and produce ECM, increase the population of HSCs/HPCs Disadvantages: technically demanding culture system | [203,205] |
| Biomimetic 3D model of bone marrow co-culture of UCB CD34+ and UCB MSC | UCB CD34+ HSPC and UCB MSC: culture growth medium, SCF | Advantages: Biomimic perfusion system with architecture like BM De novo ECM formation with COL 1, COL 4, fibronectin, osteocalcin Suitable for studying the bone marrow niche Disadvantages: technically demanding culture system | [206,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bačenková, D.; Trebuňová, M.; Dosedla, E.; Čajková, J.; Živčák, J. Interactions of Hematopoietic and Associated Mesenchymal Stem Cell Populations in the Bone Marrow Microenvironment, In Vivo and In Vitro Model. Int. J. Mol. Sci. 2025, 26, 9036. https://doi.org/10.3390/ijms26189036
Bačenková D, Trebuňová M, Dosedla E, Čajková J, Živčák J. Interactions of Hematopoietic and Associated Mesenchymal Stem Cell Populations in the Bone Marrow Microenvironment, In Vivo and In Vitro Model. International Journal of Molecular Sciences. 2025; 26(18):9036. https://doi.org/10.3390/ijms26189036
Chicago/Turabian StyleBačenková, Darina, Marianna Trebuňová, Erik Dosedla, Jana Čajková, and Jozef Živčák. 2025. "Interactions of Hematopoietic and Associated Mesenchymal Stem Cell Populations in the Bone Marrow Microenvironment, In Vivo and In Vitro Model" International Journal of Molecular Sciences 26, no. 18: 9036. https://doi.org/10.3390/ijms26189036
APA StyleBačenková, D., Trebuňová, M., Dosedla, E., Čajková, J., & Živčák, J. (2025). Interactions of Hematopoietic and Associated Mesenchymal Stem Cell Populations in the Bone Marrow Microenvironment, In Vivo and In Vitro Model. International Journal of Molecular Sciences, 26(18), 9036. https://doi.org/10.3390/ijms26189036








