Elevated Sirtuin 1 Levels in Patients with Chronic Kidney Disease, Including on Peritoneal Dialysis: Associations with Cardiovascular Risk and Peritoneal Fibrosis
Abstract
1. Introduction
2. Results
2.1. Studied Population
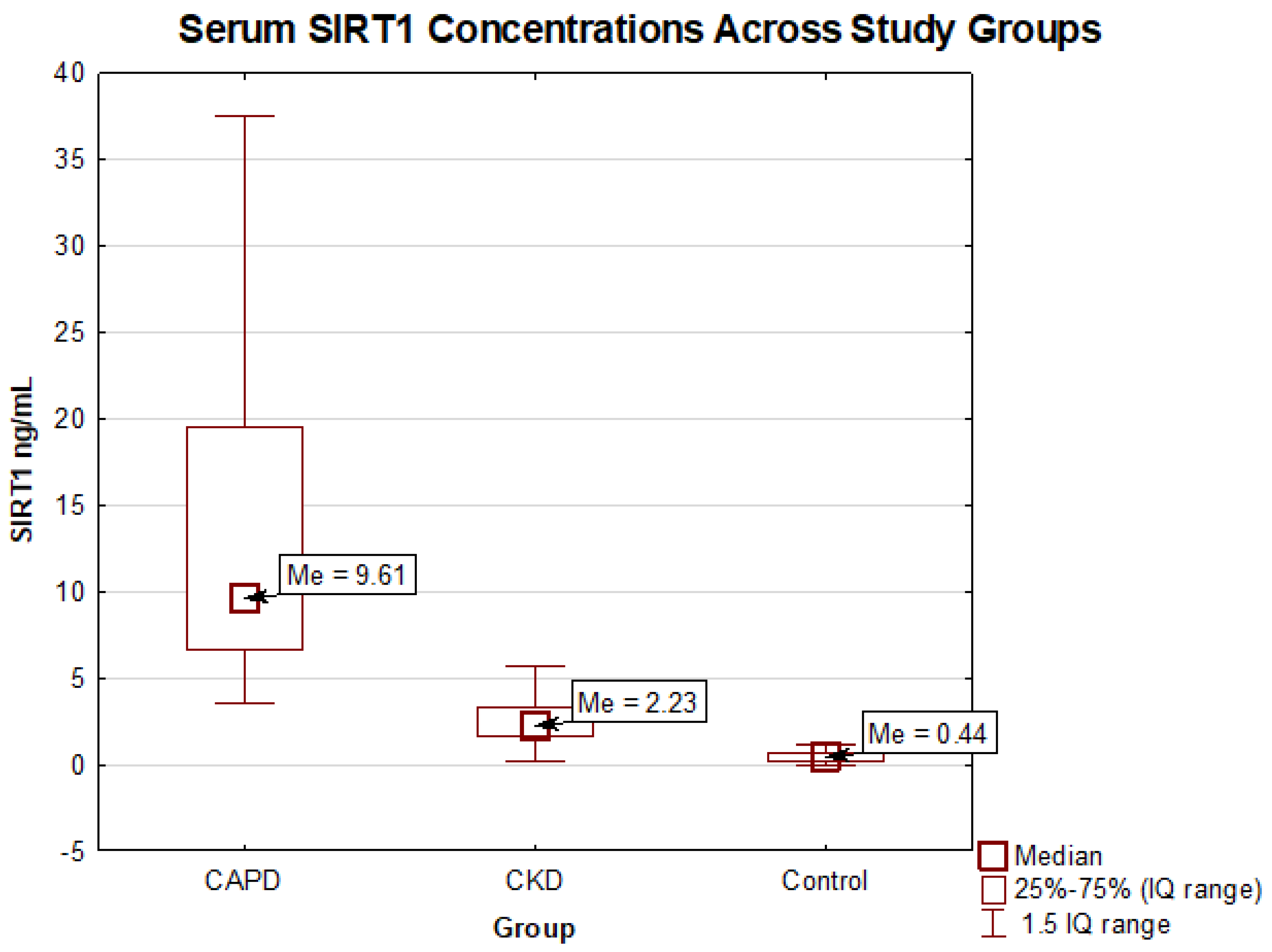
2.2. Comparison of the Sirtuin Level in Chronic Kidney Disease Patients and the Control Group
2.3. Sirtuin Level in CAPD Patients and Its Associations with Selected Variables
2.4. The Relationship Between Sirtuin 1 Level and Selected Variables in the Whole Studied Group
3. Discussion
3.1. The Emerging Role of Sirtuin 1 in Renal Pathophysiology and Clinical Implications in CKD
3.2. Mechanistic Insights into Peritoneal Fibrosis with a Focus on SIRT1
3.2.1. Pathogenesis of Peritoneal Fibrosis
3.2.2. The Anti-Fibrotic Role of SIRT1
3.2.3. Macrophage Polarization, Neovascularization, and SIRT1 in Fibrosis
3.3. SIRT1 as a Potential Biomarker of Peritoneal Filtration Failure in Dialysis Patients
3.4. SIRT1 in Calcium-Phosphate Metabolism
3.5. SIRT1 in Lipid Metabolism
3.6. SIRT1 Concentration in Diabetic Patients
3.7. SIRT1 and Cardiovascular Risk Among At-Risk Patients
3.8. The Impact of Antihypertensive and Lipid-Lowering Therapies on SIRT1 Levels
3.9. SIRT1 in Ventricular Dysfunction
3.10. Hypothesizing a Compensatory Role of SIRT1 in Cardiorenal Dysfunction
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.3. Data Collection
4.4. Statistical Analysis
4.5. Bioethical Considerations and Equipment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| ABCA1/ABCG1 | ATP-binding cassette transporter A1/G1 |
| ACEi | Angiotensin-converting enzyme inhibitor |
| AGEs-RAGE axis | Advanced glycation end products and receptor for advanced glycation end products |
| AMPK/SIRT1 | AMP-activated protein kinase/ sirtuin 1 |
| BMI | Body mass index |
| CKD | Chronic kidney disease |
| CKD-MBD | Chronic kidney disease-mineral bone disease |
| CAPD | Continuous ambulatory peritoneal dialysis |
| CAPE | Caffeic acid phenethyl ester |
| CT | Conservatively treated subgroup of the studied population |
| eGFR | Estimated glomerular filtration rate |
| ELISA | Enzyme-linked immunosorbent assay |
| EMT | Mesothelial to mesenchymal transition |
| ESA | Erythropoiesis-stimulating agent |
| FoxO1 | Forkhead box O1 |
| FXR | Farnesoid X receptor |
| HDL | High-density lipoprotein |
| HGB | Hemoglobin |
| hUCMSCs | Human umbilical cord mesenchymal stem cells |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| iPTH | Intact parathyroid hormone |
| IQR | Interquartile ranges |
| LXR | Liver X receptor |
| MACE | Major adverse cardiovascular events |
| MALE | Major adverse limb events |
| mDBP | Mean diastolic blood pressure |
| mSBP | Mean systolic blood pressure |
| NYHA | New York Heart Association functional classification |
| NAFLD | Non-alcoholic fatty liver disease |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| SIRT1 | Sirtuin 1 |
| SREBP | Sterol regulatory element-binding protein |
| TGFβ | Tumor growth Factor β |
| TNFα | Tumor necrosis factor α |
| TIBC | Total iron-binding capacity |
| RAAS | Renin–angiotensin–aldosterone system |
| VEGF | Vascular endothelial growth factor |
References
- Haigis, M.C.; Guarente, L.P. Mammalian Sirtuins—Emerging Roles in Physiology, Aging, and Calorie Restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef]
- Julien, C.; Tremblay, C.; Émond, V.; Lebbadi, M.; Salem, N.; Bennett, D.A.; Calon, F. Sirtuin 1 Reduction Parallels the Accumulation of Tau in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2009, 68, 48–58. [Google Scholar] [CrossRef]
- Ren, Y.; Du, C.; Shi, Y.; Wei, J.; Wu, H.; Cui, H. The Sirt1 Activator, SRT1720, Attenuates Renal Fibrosis by Inhibiting CTGF and Oxidative Stress. Int. J. Mol. Med. 2017, 39, 1317–1324. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Mehmood, A.; Kanatani, Y.; Kado, T.; Igarashi, Y.; Takikawa, A.; Yamamoto, S.; Okabe, K.; Nakagawa, T.; Yagi, K.; et al. Sirt1 Activator Induces Proangiogenic Genes in Preadipocytes to Rescue Insulin Resistance in Diet-Induced Obese Mice. Sci. Rep. 2018, 8, 11370. [Google Scholar] [CrossRef]
- Gao, P.; Xu, T.-T.; Lu, J.; Li, L.; Xu, J.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. Overexpression of SIRT1 in Vascular Smooth Muscle Cells Attenuates Angiotensin II-Induced Vascular Remodeling and Hypertension in Mice. J. Mol. Med. 2014, 92, 347–357. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal Tubular Sirt1 Attenuates Diabetic Albuminuria by Epigenetically Suppressing Claudin-1 Overexpression in Podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef]
- Tagawa, A.; Yasuda, M.; Kume, S.; Yamahara, K.; Nakazawa, J.; Chin-Kanasaki, M.; Araki, H.; Araki, S.; Koya, D.; Asanuma, K.; et al. Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes 2016, 65, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 Promotes Endothelium-Dependent Vascular Relaxation by Activating Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Ricardo, S.D.; Bertram, J.F.; Nikolic-Paterson, D.J. Resveratrol Inhibits Renal Fibrosis in the Obstructed Kidney. Am. J. Pathol. 2010, 177, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Zbroch, E.; Bielach-Bazyluk, A.; Malyszko, J.; Koc-Zorawska, E.; Rydzewska-Rosolowska, A.; Kakareko, K.; Hryszko, T. The Serum Concentration of Anti-Aging Proteins, Sirtuin1 and αKlotho in Patients with End-Stage Kidney Disease on Maintenance Hemodialysis. Clin. Interv. Aging 2020, 15, 387–393. [Google Scholar] [CrossRef]
- Ramkaran, P.; Phulukdaree, A.; Khan, S.; Moodley, D.; Chuturgoon, A. Sirtuin 1 Rs1467568 and Rs7895833 in South African Indians with Early-Onset Coronary Artery Disease. Cardiovasc. J. Afr. 2016, 27, 213–217. [Google Scholar] [CrossRef]
- Spoto, B.; Ntounousi, E.; Testa, A.; Liakopoulos, V.; D’Arrigo, G.; Tripepi, G.; Parlongo, R.M.; Sanguedolce, M.C.; Mallamaci, F.; Zoccali, C. The Sirtuin1 Gene Associates with Left Ventricular Myocardial Hypertrophy and Remodeling in Two Chronic Kidney Disease Cohorts: A Longitudinal Study. J. Hypertens. 2018, 36, 1705–1711. [Google Scholar] [CrossRef]
- Zainabadi, K.; Liu, C.J.; Caldwell, A.L.M.; Guarente, L. SIRT1 Is a Positive Regulator of in Vivo Bone Mass and a Therapeutic Target for Osteoporosis. PLoS ONE 2017, 12, e0185236. [Google Scholar] [CrossRef]
- Thomas, B.; Matsushita, K.; Abate, K.H.; Al-Aly, Z.; Ärnlöv, J.; Asayama, K.; Atkins, R.; Badawi, A.; Ballew, S.H.; Banerjee, A.; et al. Global Cardiovascular and Renal Outcomes of Reduced GFR. J. Am. Soc. Nephrol. 2017, 28, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Ramil-Gómez, O.; López-Pardo, M.; Fernández-Rodríguez, J.A.; Rodríguez-Carmona, A.; Pérez-López, T.; Vaamonde-García, C.; Pérez-Fontán, M.; López-Armada, M.J. Involvement of Mitochondrial Dysfunction in the Inflammatory Response in Human Mesothelial Cells from Peritoneal Dialysis Effluent. Antioxidants 2022, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Kariya, T.; Nishimura, H.; Mizuno, M.; Suzuki, Y.; Matsukawa, Y.; Sakata, F.; Maruyama, S.; Takei, Y.; Ito, Y. TGF-Β1-VEGF-A Pathway Induces Neoangiogenesis with Peritoneal Fibrosis in Patients Undergoing Peritoneal Dialysis. Am. J. Physiol.-Ren. Physiol. 2018, 314, F167–F180. [Google Scholar] [CrossRef]
- Ferrantelli, E.; Farhat, K.; Ederveen, A.L.H.; Reiding, K.R.; Beelen, R.H.J.; Van Ittersum, F.J.; Wuhrer, M.; Dotz, V. Effluent and Serum Protein N-Glycosylation Is Associated with Inflammation and Peritoneal Membrane Transport Characteristics in Peritoneal Dialysis Patients. Sci. Rep. 2018, 8, 979. [Google Scholar] [CrossRef]
- Xie, S.; Xu, F.; Lu, Y.; Zhang, Y.; Li, X.; Yu, M.; Cui, W. Elabela Attenuates the TGF-Β1-Induced Epithelial-Mesenchymal Transition of Peritoneal Mesothelial Cells in Patients Receiving Peritoneal Dialysis. Front. Pharmacol. 2022, 13, 890881. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chow, K.M.; Cho, Y.; Fan, S.; Figueiredo, A.E.; Harris, T.; Kanjanabuch, T.; Kim, Y.-L.; Madero, M.; Malyszko, J.; et al. ISPD Peritonitis Guideline Recommendations: 2022 Update on Prevention and Treatment. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2022, 42, 110–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, L.; Zhang, W.; Zeng, Y.; Hu, J.; Song, K. Caffeic Acid Phenethyl Ester Restores Mitochondrial Homeostasis against Peritoneal Fibrosis Induced by Peritoneal Dialysis through the AMPK/SIRT1 Pathway. Ren. Fail. 2024, 46, 2350235. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Gou, R.; Wang, Y.; Shi, X.; Pang, X.; Tang, L. SIRT1-Modified Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Experimental Peritoneal Fibrosis by Inhibiting the TGF-β/Smad3 Pathway. Stem Cell Res. Ther. 2020, 11, 362. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Gou, R.; Wang, Y.; Shi, X.; Zhang, Y.; Pang, X.; Tang, L. Ameliorative Role of SIRT1 in Peritoneal Fibrosis: An in Vivo and in Vitro Study. Cell Biosci. 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xian, S.; Cheng, X.; Hou, Y.; Jia, W.; Ma, Y. Efficacy of Oroxylin A in Ameliorating Renal Fibrosis with Emphasis on Sirt1 Activation and TGF-β/Smad3 Pathway Modulation. Front. Pharmacol. 2024, 15, 1499012. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Zhang, M.-Z.; You, L.; Davis, L.S.; Fan, H.; Yang, H.-C.; Fogo, A.B.; Zent, R.; Harris, R.C.; et al. Sirt1 Activation Protects the Mouse Renal Medulla from Oxidative Injury. J. Clin. Investig. 2010, 120, 1056–1068. [Google Scholar] [CrossRef]
- Huang, K.-P.; Chen, C.; Hao, J.; Huang, J.-Y.; Liu, P.-Q.; Huang, H.-Q. AGEs-RAGE System Down-Regulates Sirt1 Through the Ubiquitin-Proteasome Pathway to Promote FN and TGF-Β1 Expression in Male Rat Glomerular Mesangial Cells. Endocrinology 2015, 156, 268–279. [Google Scholar] [CrossRef]
- Bielach-Bazyluk, A.; Zbroch, E.; Czajkowska, K.; Koc-Zorawska, E.; Kakareko, K.; Rydzewska-Rosolowska, A.; Hryszko, T. Serum Sirtuin 1 Is Independently Associated with Intact PTH among Patients with Chronic Kidney Disease. Clin. Interv. Aging 2021, 16, 525–536. [Google Scholar] [CrossRef]
- Batko, K.; Sączek, A.; Banaszkiewicz, M.; Małyszko, J.; Koc-Żórawska, E.; Żórawski, M.; Niezabitowska, K.; Siek, K.; Bętkowska-Prokop, A.; Małyszko, J.A.; et al. Comprehensive Assessment of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: Novel Tools for Assessment of Cardiovascular Risk and Kidney Outcomes in Long-Term Kidney Transplant Patients. Pol. Heart J. 2024, 82, 760–770. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Y.; Fu, P.; Ma, L. Peritoneal Fibrosis: From Pathophysiological Mechanism to Medicine. Front. Physiol. 2024, 15, 1438952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hu, H.; Xia, F. Correlation between Serum SIRT1 and EZH2 Expressions and Peritoneal Function in Patients with Diabetic Nephropathy Undergoing Peritoneal Dialysis. Pak. J. Med. Sci. 2022, 38, 2318–2323. [Google Scholar] [CrossRef]
- Isaza-Restrepo, A.; Martin-Saavedra, J.S.; Velez-Leal, J.L.; Vargas-Barato, F.; Riveros-Dueñas, R. The Peritoneum: Beyond the Tissue—A Review. Front. Physiol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, W.; Yao, K.; Xie, Y.; Liao, C.; Lin, Y.; Zhou, T. Efficacy of Mesenchymal Stem Cells in the Treatment of Peritoneal Fibrosis in Animal Models: A Systematic Review and Meta-Analysis. Ren. Fail. 2024, 46, 2438863. [Google Scholar] [CrossRef]
- Lu, H.; Shen, J.; Sun, J.; Sun, J. Prognostic Value of Elevated Plasma Galectin-3 for Renal Adverse Events in Dialysis Patients: A Systematic Review and Meta-Analysis. Altern. Ther. Health Med. 2023, 29, 8. [Google Scholar]
- Li, Y.-C.; Sung, P.-H.; Yang, Y.-H.; Chiang, J.Y.; Yip, H.-K.; Yang, C. Dipeptidyl Peptidase 4 Promotes Peritoneal Fibrosis and Its Inhibitions Prevent Failure of Peritoneal Dialysis. Commun. Biol. 2021, 4, 144. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and Low–Glucose Degradation Product Dialysis Fluids Induce Major Early Alterations of the Peritoneal Membrane in Children on Peritoneal Dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Ma, M.; Zhuang, X.; Lu, Y.; Miao, L.; Lu, X.; Cui, Y.; Cui, W. Mechanisms Underlying the Involvement of Peritoneal Macrophages in the Pathogenesis and Novel Therapeutic Strategies for Dialysis-Induced Peritoneal Fibrosis. Front. Immunol. 2024, 15, 1507265. [Google Scholar] [CrossRef]
- Tawada, M.; Hamada, C.; Suzuki, Y.; Sakata, F.; Sun, T.; Kinashi, H.; Katsuno, T.; Takei, Y.; Maruyama, S.; Honda, K.; et al. Effects of Long-Term Treatment with Low-GDP, pH-Neutral Solutions on Peritoneal Membranes in Peritoneal Dialysis Patients. Clin. Exp. Nephrol. 2019, 23, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Gensberger-Reigl, S.; Weigel, I.; Stützer, J.; Auditore, A.; Nikolaus, T.; Pischetsrieder, M. Degradation and de Novo Formation of Nine Major Glucose Degradation Products during Storage of Peritoneal Dialysis Fluids. Sci. Rep. 2022, 12, 4268. [Google Scholar] [CrossRef]
- Lui, S.L.; Yung, S.; Yim, A.; Wong, K.M.; Tong, K.L.; Wong, K.S.; Li, C.S.; Au, T.C.; Lo, W.K.; Ho, Y.W.; et al. A Combination of Biocompatible Peritoneal Dialysis Solutions and Residual Renal Function, Peritoneal Transport, and Inflammation Markers: A Randomized Clinical Trial. Am. J. Kidney Dis. 2012, 60, 966–975. [Google Scholar] [CrossRef]
- Cheng, Y.; Takeuchi, H.; Sonobe, Y.; Jin, S.; Wang, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Suzumura, A. Sirtuin 1 Attenuates Oxidative Stress via Upregulation of Superoxide Dismutase 2 and Catalase in Astrocytes. J. Neuroimmunol. 2014, 269, 38–43. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The Sirtuin Family in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Qin, X.; Chen, K.; Wang, R.; Yuan, L.; Chen, X.; Hao, C.; Huang, X. SIRT1 Attenuates Renal Fibrosis by Repressing HIF-2α. Cell Death Discov. 2021, 7, 59. [Google Scholar] [CrossRef]
- Wu, H.; Qiu, Z.; Wang, L.; Li, W. Renal Fibrosis: SIRT1 Still of Value. Biomedicines 2024, 12, 1942. [Google Scholar] [CrossRef]
- Huang, X.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C. Sirt1 Activation Ameliorates Renal Fibrosis by Inhibiting the TGF-β/Smad3 Pathway. J. Cell. Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef]
- Zerr, P.; Palumbo-Zerr, K.; Huang, J.; Tomcik, M.; Sumova, B.; Distler, O.; Schett, G.; Distler, J.H.W. Sirt1 Regulates Canonical TGF-β Signalling to Control Fibroblast Activation and Tissue Fibrosis. Ann. Rheum. Dis. 2016, 75, 226–233. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Lee, K.; He, J.C. The Role of SIRT1 in Diabetic Kidney Disease. Front. Endocrinol. 2014, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, J.; He, J.C.; Zhong, Y. Sirtuin 1 in Chronic Kidney Disease and Therapeutic Potential of Targeting Sirtuin 1. Front. Endocrinol. 2022, 13, 917773. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, M.; Ji, W.; Yu, M.; Tang, C.; Tian, K.; Gao, Z.; Su, L.; Tang, J.; Zhao, X. The Role and Regulation of SIRT1 in Pulmonary Fibrosis. Mol. Biol. Rep. 2024, 51, 338. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Zhang, Y.; Wang, X.; Fan, X.-F.; Zhang, Y.; Li, X.; Gong, Y.-S.; Han, L.-P. SIRT1 Activation Attenuates Cardiac Fibrosis by Endothelial-to-Mesenchymal Transition. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 118, 109227. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Jiang, S.; Liu, Q.; Ma, Y.; Zhu, X.; Liang, M.; Shi, X.; Ding, W.; Zhou, X.; Zou, H.; et al. Sirtuin1 Protects against Systemic Sclerosis-Related Pulmonary Fibrosis by Decreasing Proinflammatory and Profibrotic Processes. Am. J. Respir. Cell Mol. Biol. 2018, 58, 28–39. [Google Scholar] [CrossRef]
- Zhu, X.; Chu, H.; Jiang, S.; Liu, Q.; Liu, L.; Xue, Y.; Zheng, S.; Wan, W.; Qiu, J.; Wang, J.; et al. Sirt1 Ameliorates Systemic Sclerosis by Targeting the mTOR Pathway. J. Dermatol. Sci. 2017, 87, 149–158. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, F.; Yu, W.; Zhang, X.; Yin, G.; Liang, P.; Feng, Y.; Chen, S.; Liu, H. Biological Role and Related Natural Products of SIRT1 in Nonalcoholic Fatty Liver. Diabetes Metab. Syndr. Obes. 2023, 16, 4043–4064. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Li, L.; Gao, P.; Chen, H.-Z.; Zhang, R.; Wei, Y.-S.; Liu, D.-P.; Liang, C.-C. Involvement of the P65/RelA Subunit of NF-kappaB in TNF-Alpha-Induced SIRT1 Expression in Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2010, 397, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Kim, S.; Lee, H.J.; Park, I.; Kim, H.; Shin, G.-T. Tumor Necrosis Factor α Is a Risk Factor for Infection in Peritoneal Dialysis Patients. Korean J. Intern. Med. 2016, 31, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Hylander, B.; Wretlind, B. Interleukin-6 and Interleukin-8 in Dialysate and Serum from Patients on Continuous Ambulatory Peritoneal Dialysis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1993, 22, 430–435. [Google Scholar] [CrossRef]
- Song, Q.; Yang, X.; Shi, Y.; Yan, H.; Yu, Z.; Li, Z.; Yuan, J.; Ni, Z.; Gu, L.; Fang, W. High Intraperitoneal Interleukin-6 Levels Predict Ultrafiltration (UF) Insufficiency in Peritoneal Dialysis Patients: A Prospective Cohort Study. Front. Med. 2022, 9, 836861. [Google Scholar] [CrossRef]
- Zhang, K.-W.; Wang, D.; Cai, H.; Cao, M.-Q.; Zhang, Y.-Y.; Zhuang, P.-Y.; Shen, J. IL-6 Plays a Crucial Role in Epithelial-mesenchymal Transition and Pro-metastasis Induced by Sorafenib in Liver Cancer. Oncol. Rep. 2021, 45, 1105–1117. [Google Scholar] [CrossRef]
- Shrestha, R.; Bridle, K.R.; Crawford, D.H.G.; Jayachandran, A. TNF-α-mediated Epithelial-to-mesenchymal Transition Regulates Expression of Immune Checkpoint Molecules in Hepatocellular Carcinoma. Mol. Med. Rep. 2020, 21, 1849–1860. [Google Scholar] [CrossRef]
- Guo, K.; Feng, Y.; Zheng, X.; Sun, L.; Wasan, H.S.; Ruan, S.; Shen, M. Resveratrol and Its Analogs: Potent Agents to Reverse Epithelial-to-Mesenchymal Transition in Tumors. Front. Oncol. 2021, 11, 644134. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Zhao, X.; Li, M.; Lu, Y.; Wang, M.; Xiao, J.; Xie, Q.; He, X.; Shuai, S. Sirt1 Inhibits Macrophage Polarization and Inflammation in Gouty Arthritis by Inhibiting the MAPK/NF-κB/AP-1 Pathway and Activating the Nrf2/HO-1 Pathway. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2024, 73, 1173–1184. [Google Scholar] [CrossRef]
- He, T.; Bai, X.; Li, Y.; Zhang, D.; Xu, Z.; Yang, X.; Hu, D.; Han, J. Insufficient SIRT1 in Macrophages Promotes Oxidative Stress and Inflammation during Scarring. J. Mol. Med. Berl. Ger. 2023, 101, 1397–1407. [Google Scholar] [CrossRef]
- Hassan, H.A.; Nageeb, M.M.; Mohammed, H.O.; Samy, W.; Fawzy, A.; Afifi, R.; Abbas, N.A.T. Dapagliflozin Dampens Liver Fibrosis Induced by Common Bile Duct Ligation in Rats Associated with the Augmentation of the Hepatic Sirt1/AMPK/PGC1α/FoxO1 Axis. Toxicol. Appl. Pharmacol. 2024, 489, 116991. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 (PGC-1) Family in Physiological and Pathophysiological Process and Diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.W.; Lee, S.Y.; Hong, K.W.; Bae, S.S.; Kim, K.; Kim, C.D. SIRT1/Adenosine Monophosphate-Activated Protein Kinase α Signaling Enhances Macrophage Polarization to an Anti-Inflammatory Phenotype in Rheumatoid Arthritis. Front. Immunol. 2017, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kong, Q.; Hao, W.; Hu, W. High Glucose Contributes to the Polarization of Peritoneal Macrophages to the M2 Phenotype in Vivo and in Vitro. Mol. Med. Rep. 2020, 22, 127–134. [Google Scholar] [CrossRef]
- Laemmle, A.; Lechleiter, A.; Roh, V.; Schwarz, C.; Portmann, S.; Furer, C.; Keogh, A.; Tschan, M.P.; Candinas, D.; Vorburger, S.A.; et al. Inhibition of SIRT1 Impairs the Accumulation and Transcriptional Activity of HIF-1α Protein under Hypoxic Conditions. PLoS ONE 2012, 7, e33433. [Google Scholar] [CrossRef]
- Redahan, L.; Davenport, A. Peritoneal Dialysate Effluent and Serum CA125 Concentrations in Stable Peritoneal Dialysis Patients. J. Nephrol. 2016, 29, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, T.; Tu, Z.; Zhang, Y.; Wang, X.; Zang, D.; Xu, D.; Feng, Y.; He, F.; Ni, M.; et al. Both High Glucose and Phosphate Overload Promote Senescence-Associated Calcification of Vascular Muscle Cells. Int. Urol. Nephrol. 2022, 54, 2719–2731. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, T.; Jiang, P.; Wang, X.; Yao, J.; Shen, H.; Zhang, Z.; Zheng, B.; Wang, T.; Ren, Y.; et al. Decreased Sirtuin 1 in Type 2 Diabetes Patients with Abnormal BMD. Front. Endocrinol. 2025, 15, 1480847. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Blander, G.; Tse, J.G.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Shen, M.; Kuang, L.; Yang, K.; Wu, S.; Liu, X.; Wang, Y.; Wang, Y. SIRT1/SREBPs-mediated regulation of lipid metabolism. Pharmacol. Res. 2024, 199, 107037. [Google Scholar] [CrossRef]
- Kemper, J.K.; Xiao, Z.; Ponugoti, B.; Miao, J.; Fang, S.; Kanamaluru, D.; Tsang, S.; Wu, S.Y.; Chiang, C.M.; Veenstra, T.D. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009, 10, 392–404. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Zeng, H.T.; Fu, Y.C.; Yu, W.; Lin, J.M.; Zhou, L.; Liu, L.; Wang, W. SIRT1 prevents atherosclerosis via liver X receptor and NF-κB signaling in a U937 cell model. Mol. Med. Rep. 2013, 8, 23–28. [Google Scholar] [CrossRef]
- Purushotham, A.; Xu, Q.; Lu, J.; Foley, J.F.; Yan, X.; Kim, D.H.; Kemper, J.K.; Li, X. Hepatic deletion of SIRT1 decreases hepatocyte nuclear factor 1α/farnesoid X receptor signaling and induces formation of cholesterol gallstones in mice. Mol. Cell. Biol. 2012, 32, 1226–1236. [Google Scholar] [CrossRef]
- Han, J.; Li, S.; Wang, W.; Jiang, X.; Liu, C.; Lei, L.; Li, Y.; Sheng, R.; Zhang, Y.; Wu, Y.; et al. SIRT1 Activator E1231 Alleviates Nonalcoholic Fatty Liver Disease by Regulating Lipid Metabolism. Curr. Issues Mol. Biol. 2023, 45, 5052–5070. [Google Scholar] [CrossRef]
- Kolesnikova, O.; Zaprovalna, O.; Radchenko, A.; Bondar, T. Association of SIRT1 with metabolic parameters and aging rate. Biomed. Res. Ther. 2023, 10, 5575–5583. [Google Scholar] [CrossRef]
- Mariani, S.; Di Giorgio, M.R.; Martini, P.; Persichetti, A.; Barbaro, G.; Basciani, S.; Contini, S.; Poggiogalle, E.; Sarncola, A.; Genco, A.; et al. Inverse Association of Circulating SIRT1 and Adiposity: A Study on Underweight, Normal Weight, and Obese Patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Taghizadeh, M.; Khalese-Ranjbar, B.; Hamedi-Shahraki, S.; Asghari, S. The clinical value of serumsirtuin-1 concentration in the diagnosis of metabolic dysfunction-associated steatotic liver disease. BMC Gastroenterol. 2025, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yang, S.J. Aging-Related Correlation between Serum Sirtuin 1 Activities and Basal Metabolic Rate in Women, but not in Men. Clin. Nutr. Res. 2017, 6, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Baksi, A.; Kraydashenko, O.; Zalevkaya, A.; Stets, R.; Elliott, P.; Haddad, J.; Hoffmann, E.; Vlasuk, G.P.; Jacobson, E.W. A Phase II, Randomized, Placebo-Controlled, Double-Blind, Multi-Dose Study of SRT2104, a SIRT1 Activator, in Subjects with Type 2 Diabetes. Br. J. Clin. Pharmacol. 2014, 78, 69–77. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Ma, X.; Yang, M.; Liu, Y.; Wang, Q. Expression Levels of Serum Vasohibin-1 and Other Biomarkers in Type 2 Diabetes Mellitus Patients with Different Urinary Albumin to Creatinine Ratios. J. Diabetes Complicat. 2019, 33, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Ghanbari, S.; Khazaei, M.; Niroman, E. Comparison of Sirtuin 1 Level and Related Blood Factors in Diabetic and Healthy Subjects. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 17–21. [Google Scholar] [CrossRef]
- Herwana, E.; Yenny; Alvina; Kurniasari; Febinia, C.A.; Pusparini. Sirtuin, Irisin, and Vitamin D as Predictors of Diabetes Mellitus with Uncontrolled Glycemia in Indonesian Patients. Endocr. Metab. Sci. 2025, 17, 100214. [Google Scholar] [CrossRef]
- Pai, D.; Adiga, S.; Suresh, G.; Adiga, U.; Kumari, S.; Chaitra, D.; Desy, T.M. Serum SIRT1 Levels and Genetic Variants in Diabetic Nephropathy: Insights from a Cross-Sectional Study. Res. J. Pharm. Technol. 2024, 2829–2834. [Google Scholar] [CrossRef]
- Priya, S.H.; Kedari, G.S.R.; Naidu, M.P. Higher Serum Sirtuin 1 Levels and GA Heterozygote of SIRT1 Gene Polymorphism Rs10823108 Serve as Independent Risk Factor for Diabetic Nephropathy in Women. Hum. Gene 2022, 34, 201084. [Google Scholar] [CrossRef]
- Gok, O.; Karaali, Z.; Ergen, A.; Ekmekci, S.; Abaci, N. Serum Sirtuin 1 Protein as a Potential Biomarker for Type 2 Diabetes: Increased Expression of Sirtuin 1 and the Correlation with microRNAs. J. Res. Med. Sci. 2019, 24, 56. [Google Scholar] [CrossRef]
- Łukawska-Tatarczuk, M.; Franek, E.; Czupryniak, L.; Joniec-Maciejak, I.; Pawlak, A.; Wojnar, E.; Zieliński, J.; Mirowska-Guzel, D.; Mrozikiewicz-Rakowska, B. Sirtuin 1, Visfatin and IL-27 Serum Levels of Type 1 Diabetic Females in Relation to Cardiovascular Parameters and Autoimmune Thyroid Disease. Biomolecules 2021, 11, 1110. [Google Scholar] [CrossRef]
- Pai, D.; Chaitra, D.; Raghavendra, H.; Kamath, D. Serum Biomarker for Cardiovascular Disease Risk Assessment in Patients With Diabetic Nephropathy. Clin. Ter. 2024, 175, 455–459. [Google Scholar] [CrossRef]
- Biscetti, F.; Rando, M.M.; Nicolazzi, M.A.; Rossini, E.; Santoro, M.; Angelini, F.; Iezzi, R.; Eraso, L.H.; Dimuzio, P.J.; Pitocco, D.; et al. Evaluation of Sirtuin 1 as a Predictor of Cardiovascular Outcomes in Diabetic Patients with Limb-Threatening Ischemia. Sci. Rep. 2024, 14, 26940. [Google Scholar] [CrossRef]
- He, X.; Zheng, J.; Liu, C. Low Serum Level of Sirtuin 1 Predicts Coronary Atherosclerosis Plaques during Computed Tomography Angiography among an Asymptomatic Cohort. Coron. Artery Dis. 2019, 30, 621–625. [Google Scholar] [CrossRef]
- Kilic, U.; Gok, O.; Elibol-Can, B.; Uysal, O.; Bacaksiz, A. Efficacy of Statins on Sirtuin 1 and Endothelial Nitric Oxide Synthase Expression: The Role of Sirtuin 1 Gene Variants in Human Coronary Atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2015, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Yamaç, A.H.; Kılıç, Ü. Effect of Statins on Sirtuin 1 and Endothelial Nitric Oxide Synthase Expression in Young Patients with a History of Premature Myocardial Infarction. Turk. Kardiyol. Dernegi Arsivi Turk. Kardiyol. Derneginin Yayin. Organidir 2018, 46, 205–215. [Google Scholar] [CrossRef]
- Leal, D.P.; Gonçalinho, G.H.F.; Tavoni, T.M.; Kuwabara, K.L.; Paccanaro, A.P.; Freitas, F.R.; Strunz, C.M.C.; César, L.A.M.; Maranhão, R.C.; Mansur, A.D.P. The Interplay of Sirtuin-1, LDL-Cholesterol, and HDL Function: A Randomized Controlled Trial Comparing the Effects of Energy Restriction and Atorvastatin on Women with Premature Coronary Artery Disease. Antioxidants 2022, 11, 2363. [Google Scholar] [CrossRef] [PubMed]
- Van Le, T.N.; Zoungrana, L.I.; Wang, H.; Fatmi, M.K.; Ren, D.; Krause-Hauch, M.; Li, J. Sirtuin 1 Aggravates Hypertrophic Heart Failure Caused by Pressure Overload via Shifting Energy Metabolism. Biochem. Biophys. Res. Commun. 2022, 637, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Zaffran, S.; Kelly, R.G.; Meilhac, S.M.; Buckingham, M.E.; Brown, N.A. Right Ventricular Myocardium Derives from the Anterior Heart Field. Circ. Res. 2004, 95, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.E.; Heijman, J.; Dobrev, D. Differences in Left Versus Right Ventricular Electrophysiological Properties in Cardiac Dysfunction and Arrhythmogenesis. Arrhythmia Electrophysiol. Rev. 2016, 5, 14–19. [Google Scholar] [CrossRef]
- Vázquez-Garza, E.; Bernal-Ramírez, J.; Jerjes-Sánchez, C.; Lozano, O.; Acuña-Morín, E.; Vanoye-Tamez, M.; Ramos-González, M.R.; Chapoy-Villanueva, H.; Pérez-Plata, L.; Sánchez-Trujillo, L.; et al. Resveratrol Prevents Right Ventricle Remodeling and Dysfunction in Monocrotaline-Induced Pulmonary Arterial Hypertension with a Limited Improvement in the Lung Vasculature. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. Editorial Board. Kidney Int. 2024, 105, A1. [Google Scholar] [CrossRef]
- Prejbisz, A.; Dobrowolski, P.; Doroszko, A.; Olszanecka, A.; Tycińska, A.; Tykarski, A.; Adamczak, M.; Begier-Krasińska, B.; Chrostowska, M.; Dzida, G.; et al. Guidelines for the Management of Hypertension in Poland 2024—The Position of the Polish Society of Hypertension/Polish Cardiac Society Experts. Arter. Hypertens. 2024, 28, 91–146. [Google Scholar] [CrossRef]
| CAPD | CT | p Value | |
|---|---|---|---|
| Sirtuin 1 (ng/mL) | 9.61 (6.65–19.45) | 2.23 (1.57–3.24) | <0.001 |
| Age | 54 (46–67) | 74 (62.5–80) | <0.001 |
| Gender (vs. male) | 62.5% female N = 25 | 51% N = 51 | 0.29 |
| BMI (kg/m2) | 24 (22.2–28.1) | 27.7 (24.8–32.9) | 0.002 |
| HGB (mg/dL) | 11.9 (11.2–12.8) | 10.8 (9.9–12.1) | 0.004 |
| Calcium (mmol/L) | 2.17 (2.01–2.25) | 2.23 (2.1–2.31) | 0.11 |
| Phosphate (mmol/L) | 5.84 (4.38–6.76) | 1.22 (1.03–1.5) | <0.001 |
| iPTH (pg/mL) | 331.9 (171–626) | 146.5 (88.1–243.6) | <0.001 |
| Variable | Value |
|---|---|
| Age (yrs) | 57 (46–67) |
| Sex (M/F) | 15/25 |
| Dialysis vintage (months) | 32 (18–54) |
| Diabetes mellitus n (%) | 14 (35) |
| HGB (g/dL) | 11.85 (11.20–12.75) |
| ESA treatment n (%) | 32 (80) |
| Albumin (g/dL) | 3.54 (3.23–3.85) |
| kt/V | 2.00 (1.70–2.29) |
| Residual renal function (ml) | 350 (100–900) |
| CAPD + CT | ||
|---|---|---|
| R | p Value | |
| Age [ln] | −0.37 | <0.001 |
| Gender (vs. male) | - | 0.71 |
| Body mass index (kg/m2) [ln] | −0.29 | <0.001 |
| HGB (mg/dL) | 0.17 | 0.05 |
| Calcium (mmol/L) | −0.07 | 0.43 |
| Pi (mmol/L) [ln] | 0.7 | <0.001 |
| iPTH (pg/mL) | 0.4 | <0.001 |
| Glucose (mg/dL) | −0.02 | 0.79 |
| Total cholesterol | 0.26 | 0.003 |
| Triglycerides (mg/dL) | 0.09 | 0.34 |
| mSBP (mmHg) | 0.04 | 0.64 |
| mDBP (mmHg) | 0.31 | <0.001 |
| Iron (µg/dL) | 0.07 | 0.46 |
| Ferritin (ng/mL) | 0.3 | 0.002 |
| TIBC (µg/dL) | −0.09 | 0.51 |
| Regression Summary R = 0.74, R2 = 0.55; F = 3.8, p < 0.0 | ||||||
|---|---|---|---|---|---|---|
| Variable | Β * | SE of β * | β | SE of β | t | p |
| Ln(Age) | −0.241008 | 0.175360 | −1.24207 | 0.903745 | −1.37436 | 0.181525 |
| Ln(BMI) | 0.056816 | 0.152665 | 0.53827 | 1.446358 | 0.37216 | 0.712911 |
| mDBP | 0.082300 | 0.163090 | 0.01016 | 0.020142 | 0.50463 | 0.618236 |
| Ln(Pi) | 0.521498 | 0.145978 | 1.18510 | 0.331733 | 3.57245 | 0.001472 |
| iPTH | 0.040628 | 0.145586 | 0.14879 | 0.533197 | 0.27906 | 0.782493 |
| Cholesterol | 0.425150 | 0.157242 | 0.01376 | 0.005088 | 2.70379 | 0.012151 |
| Ferritin | 0.043267 | 0.158618 | 0.00067 | 0.002448 | 0.27277 | 0.787268 |
| Right Ventricle Size | 0.132423 | 0.146562 | 0.04121 | 0.045615 | 0.90353 | 0.374871 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielach-Bazyluk, A.; Czajkowska, K.; Koc-Zorawska, E.; Hryszko, T.; Zbroch, E. Elevated Sirtuin 1 Levels in Patients with Chronic Kidney Disease, Including on Peritoneal Dialysis: Associations with Cardiovascular Risk and Peritoneal Fibrosis. Int. J. Mol. Sci. 2025, 26, 9033. https://doi.org/10.3390/ijms26189033
Bielach-Bazyluk A, Czajkowska K, Koc-Zorawska E, Hryszko T, Zbroch E. Elevated Sirtuin 1 Levels in Patients with Chronic Kidney Disease, Including on Peritoneal Dialysis: Associations with Cardiovascular Risk and Peritoneal Fibrosis. International Journal of Molecular Sciences. 2025; 26(18):9033. https://doi.org/10.3390/ijms26189033
Chicago/Turabian StyleBielach-Bazyluk, Angelika, Katarzyna Czajkowska, Ewa Koc-Zorawska, Tomasz Hryszko, and Edyta Zbroch. 2025. "Elevated Sirtuin 1 Levels in Patients with Chronic Kidney Disease, Including on Peritoneal Dialysis: Associations with Cardiovascular Risk and Peritoneal Fibrosis" International Journal of Molecular Sciences 26, no. 18: 9033. https://doi.org/10.3390/ijms26189033
APA StyleBielach-Bazyluk, A., Czajkowska, K., Koc-Zorawska, E., Hryszko, T., & Zbroch, E. (2025). Elevated Sirtuin 1 Levels in Patients with Chronic Kidney Disease, Including on Peritoneal Dialysis: Associations with Cardiovascular Risk and Peritoneal Fibrosis. International Journal of Molecular Sciences, 26(18), 9033. https://doi.org/10.3390/ijms26189033







