Biphasic Adaptations of Gastric Epithelial Cells in Chronic H. pylori Infection from Stress to Tolerance
Abstract
1. Introduction
2. Results and Discussion
2.1. GES-1 Cells Undergo Morphological Reversion, CagA Fluctuation, and Immune Desensitization During H. pylori Infection
2.2. GES-1 Cell Proliferation Is Impaired in Acute H. pylori Infection and Gradually Adapts upon Sustained Exposure
2.3. Migration and Invasion Capacity of GES-1 Decreased in Acute H. pylori Infection, with Gradual Adaptation Under Sustained Exposure
2.4. Acute H. pylori Infection Disrupts Autophagy and Apoptosis in GES-1 Cells, with Adaptation Restoring Homeostasis in Chronic Stages
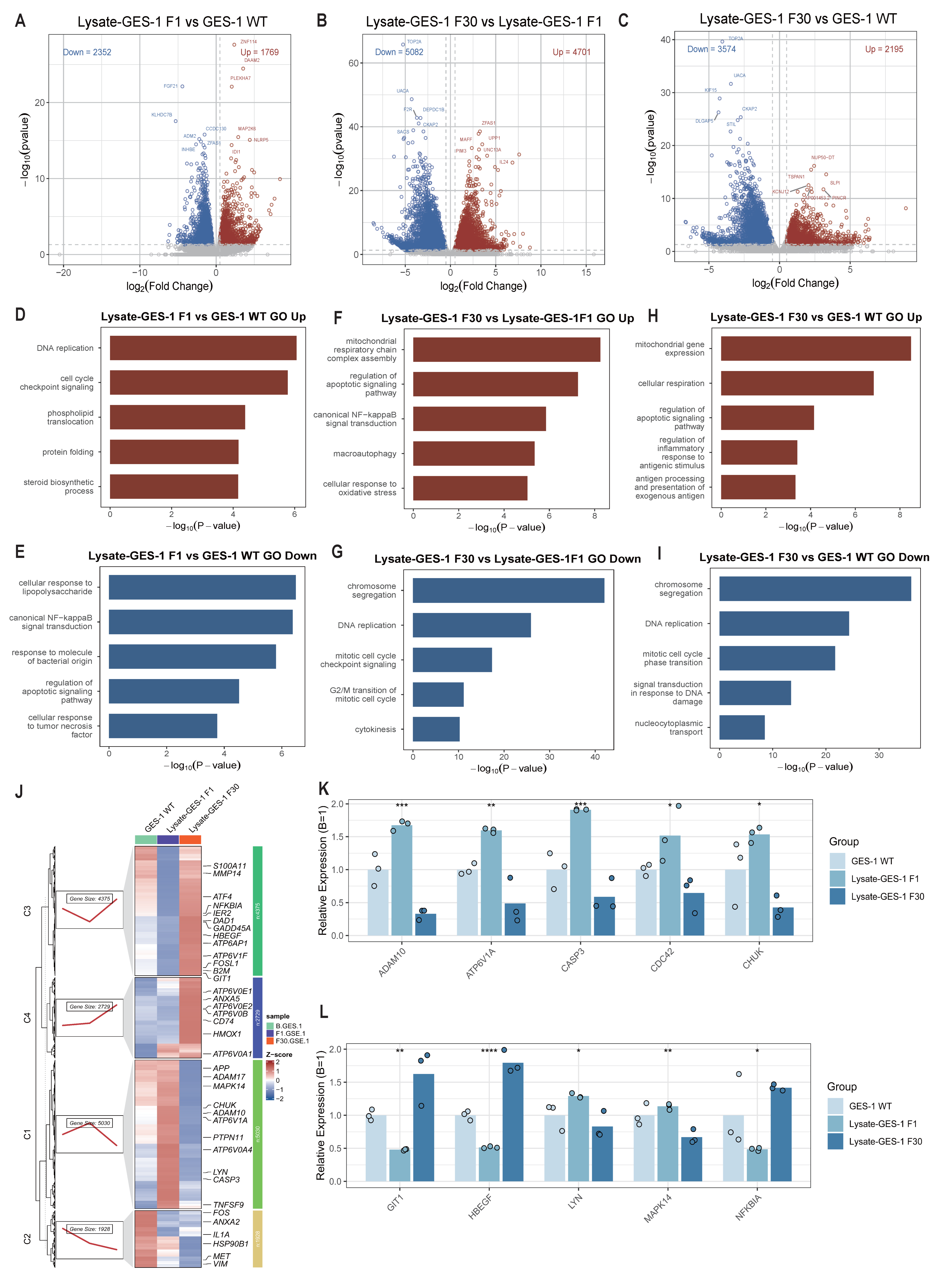
2.5. Bulk RNA-Seq Reveals Inflammation Reprogramming in GES-1 Cells During Sustained H. pylori Lysate Stimulation
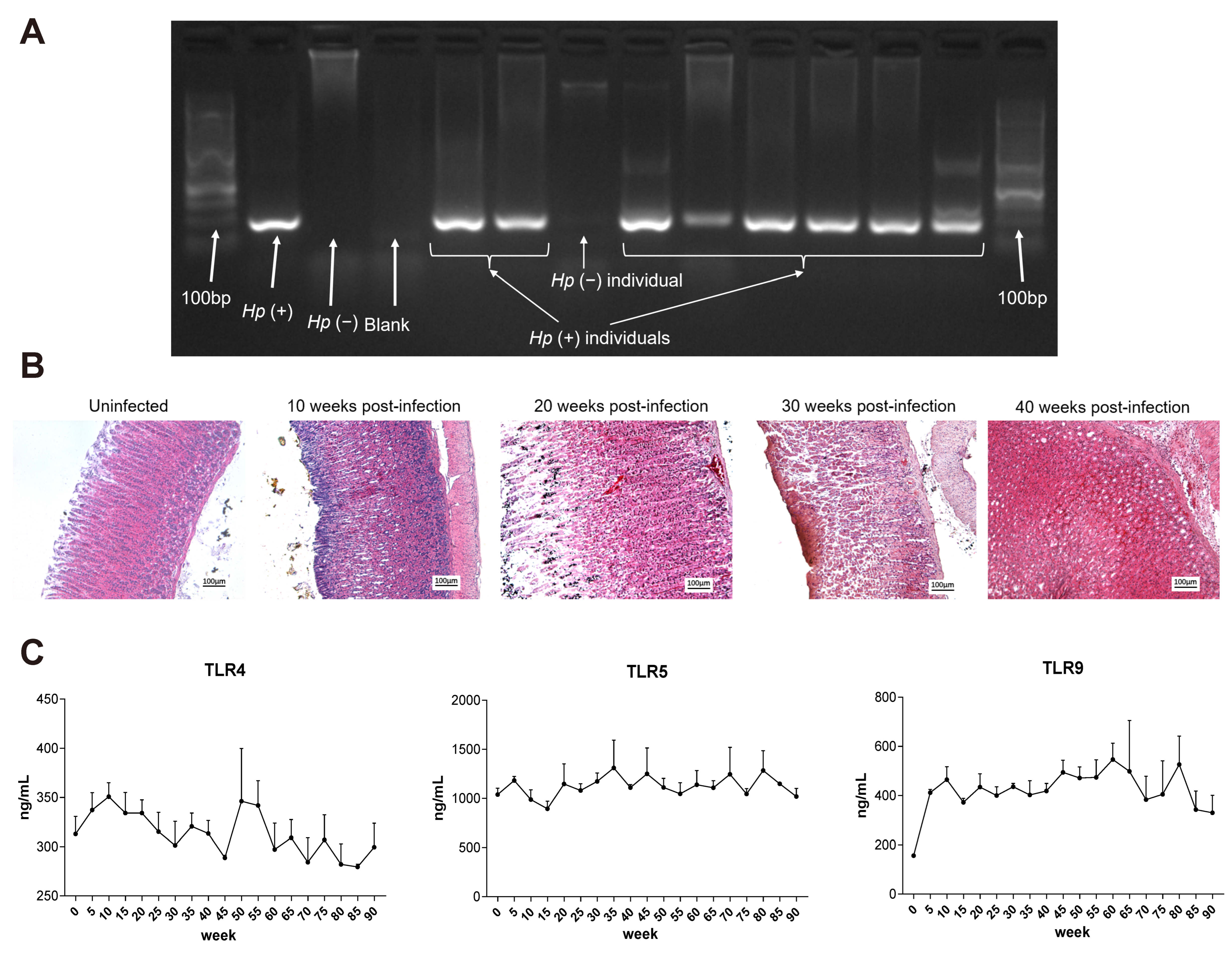
2.6. Chronic H. pylori Infection Induces Progressive Gastric Pathology and Immune Tolerance in Mongolian gerbils
3. Material and Methods
3.1. Bacteria Strain and Bacteria Culture
3.2. Cell Lines and Cell Treatment
3.3. Animal Infection
3.4. Immunofluorescence
3.5. CCK-8 Cell Proliferation Assay
3.6. Wound-Healing Assay
3.7. Colony Formation Assay
3.8. Transwell Migration and Invasion Assay
3.9. mCherry-EGFP-LC3 Lentivirus Transfection
3.10. Apoptosis Assay
3.11. RNA Extraction and Real-Time PCR Analysis
3.12. Western Blotting
3.13. H&E Staining
3.14. ELISA Analysis
3.15. Multiplex Assay
3.16. RNA Sequencing
3.17. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Salahi-Niri, A.; Nabavi-Rad, A.; Monaghan, T.M.; Rokkas, T.; Doulberis, M.; Sadeghi, A.; Zali, M.R.; Yamaoka, Y.; Tacconelli, E.; Yadegar, A. Global prevalence of Helicobacter pylori antibiotic resistance among children in the world health organization regions between 2000 and 2023: A systematic review and meta-analysis. BMC Med. 2024, 22, 598. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Georges, D.; Alberts, C.J.; Bray, F.; Clifford, G.; Baussano, I. Global lifetime estimates of expected and preventable gastric cancers across 185 countries. Nat. Med. 2025. [Google Scholar] [CrossRef]
- National Toxicology Program. 15th report on carcinogens. Rep. Carcinog. 2021, 15, roc15. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First american cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori cag type iv secretion system. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef]
- Tran, S.C.; Bryant, K.N.; Cover, T.L. The Helicobacter pylori cag pathogenicity island as a determinant of gastric cancer risk. Gut Microbes 2024, 16, 2314201. [Google Scholar] [CrossRef] [PubMed]
- Shuman, J.; Lin, A.S.; Westland, M.D.; Bryant, K.N.; Fortier, G.E.; Piazuelo, M.B.; Reyzer, M.L.; Judd, A.M.; Tsui, T.; Mcdonald, W.H.; et al. Helicobacter pylori caga and cag type iv secretion system activity have key roles in triggering gastric transcriptional and proteomic alterations. Infect. Immun. 2025, 93, e0059524. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Graham, D.Y. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014, 10, 1487–1500. [Google Scholar] [CrossRef]
- Takahashi Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef]
- Karayiannis, I.; Martinez-Gonzalez, B.; Kontizas, E.; Kokkota, A.V.; Petraki, K.; Mentis, A.; Kollia, P.; Sgouras, D.N. Induction of mmp-3 and mmp-9 expression during Helicobacter pylori infection via mapk signaling pathways. Helicobacter 2023, 28, e12987. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers. 2023, 9, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Y.; Zhang, X.; Fu, B.; Song, Z.; Wang, L.; Fu, R.; Lu, X.; Xing, J.; Lv, J.; et al. Sustained exposure to Helicobacter pylori induces immune tolerance by desensitizing tlr6. Gastric Cancer 2024, 27, 324–342. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Zhang, X.; Fu, B.; Xing, J.; Fu, R.; Lv, J.; Guo, M.; Huo, X.; Liu, X.; et al. Hypoxia exacerbates the malignant transformation of gastric epithelial cells induced by long-term H. pylori infection. Microbiol. Spectr. 2024, 12, e0031124. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef]
- Martinelli, G.; Fumagalli, M.; Piazza, S.; Maranta, N.; Genova, F.; Sperandeo, P.; Sangiovanni, E.; Polissi, A.; Dell’Agli, M.; De Fabiani, E. Investigating the Molecular Mechanisms Underlying Early Response to Inflammation and Helicobacter pylori Infection in Human Gastric Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 15147–15169. [Google Scholar] [CrossRef]
- Amalia, R.; Panenggak, N.; Doohan, D.; Rezkitha, Y.; Waskito, L.A.; Syam, A.F.; Lubis, M.; Yamaoka, Y.; Miftahussurur, M. A comprehensive evaluation of an animal model for Helicobacter pylori-associated stomach cancer: Fact and controversy. Helicobacter 2023, 28, e12943. [Google Scholar] [CrossRef]
- Tohidpour, A.; Gorrell, R.J.; Roujeinikova, A.; Kwok, T. The middle fragment of Helicobacter pylori caga induces actin rearrangement and triggers its own uptake into gastric epithelial cells. Toxins 2017, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Tohidpour, A. Caga-mediated pathogenesis of Helicobacter pylori. Microb. Pathog. 2016, 93, 44–55. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.; Zhang, X.; Lu, X.; Xing, J.; Lv, J.; Guo, M.; Huo, X.; Liu, X.; Lu, J.; et al. Sustained exposure to Helicobacter pylori lysate inhibits apoptosis and autophagy of gastric epithelial cells. Front. Oncol. 2020, 10, 581364. [Google Scholar] [CrossRef]
- Pachathundikandi, S.K.; Tegtmeyer, N.; Backert, S. Masking of typical TLR4 and TLR5 ligands modulates inflammation and resolution by Helicobacter pylori. Trends Microbiol. 2023, 3, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Dooyema, S.D.R.; Noto, J.M.; Wroblewski, L.E.; Piazuelo, M.B.; Krishna, U.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Peek, R.M. Helicobacter pylori actively suppresses innate immune nucleic acid receptors. Gut Microbes 2022, 14, 2105102. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.; Chang, H.; Lee, H.S.; Park, S.M.; Park, J.H.; Shin, C.M.; Kim, J.M.; Kim, J.S.; Lee, D.H.; et al. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis 2015, 36, 553–563. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, H.S.; Lee, Y.S.; Kim, S.; Cha, S.Y.; Ota, I.; Kim, N.H.; Cha, Y.H.; Yang, D.H.; Lee, Y.; et al. Helicobacter pylori CagA promotes Snail-mediated epithelial-mesenchymal transition by reducing GSK-3 activity. Nat. Commun. 2014, 5, 4423. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, T.; Zhang, J.; Ning, J.; Fu, W.; Wang, Y.; Shi, Y.; Wei, G.; Zhang, J.; Chen, X.; et al. AUF1-mediated inhibition of autophagic lysosomal degradation contributes to CagA stability and Helicobacter pylori-induced inflammation. Gut Microbes 2024, 1, 2382766. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Douraghi, M.; Azadmanesh, K.; Shokrgozar, M.A.; Zeraati, H.; Hosseini, M.E.; Mohagheghi, M.A.; Parsaeian, M.; Mohammadi, M. A potential association between Helicobacter pylori caga epiya and multimerization motifs with cytokeratin 18 cleavage rate during early apoptosis. Helicobacter 2012, 17, 350–357. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Gao, W.; An, Y.; Dong, G.; Jia, J.; Yang, Q. Helicobacter pylori inhibits autophagic flux and promotes its intracellular survival and colonization by down-regulating sirt1. J. Cell. Mol. Med. 2021, 25, 3348–3360. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, L.; Liu, X.; Wang, Q.; Gui, S.; Bao, L.; Wang, Z.; He, X.; Zhao, Y.; Zhou, J.; et al. Helicobacter pylori CagA mediated mitophagy to attenuate the NLRP3 inflammasome activation and enhance the survival of infected cells. Sci. Rep. 2024, 1, 21648. [Google Scholar] [CrossRef]
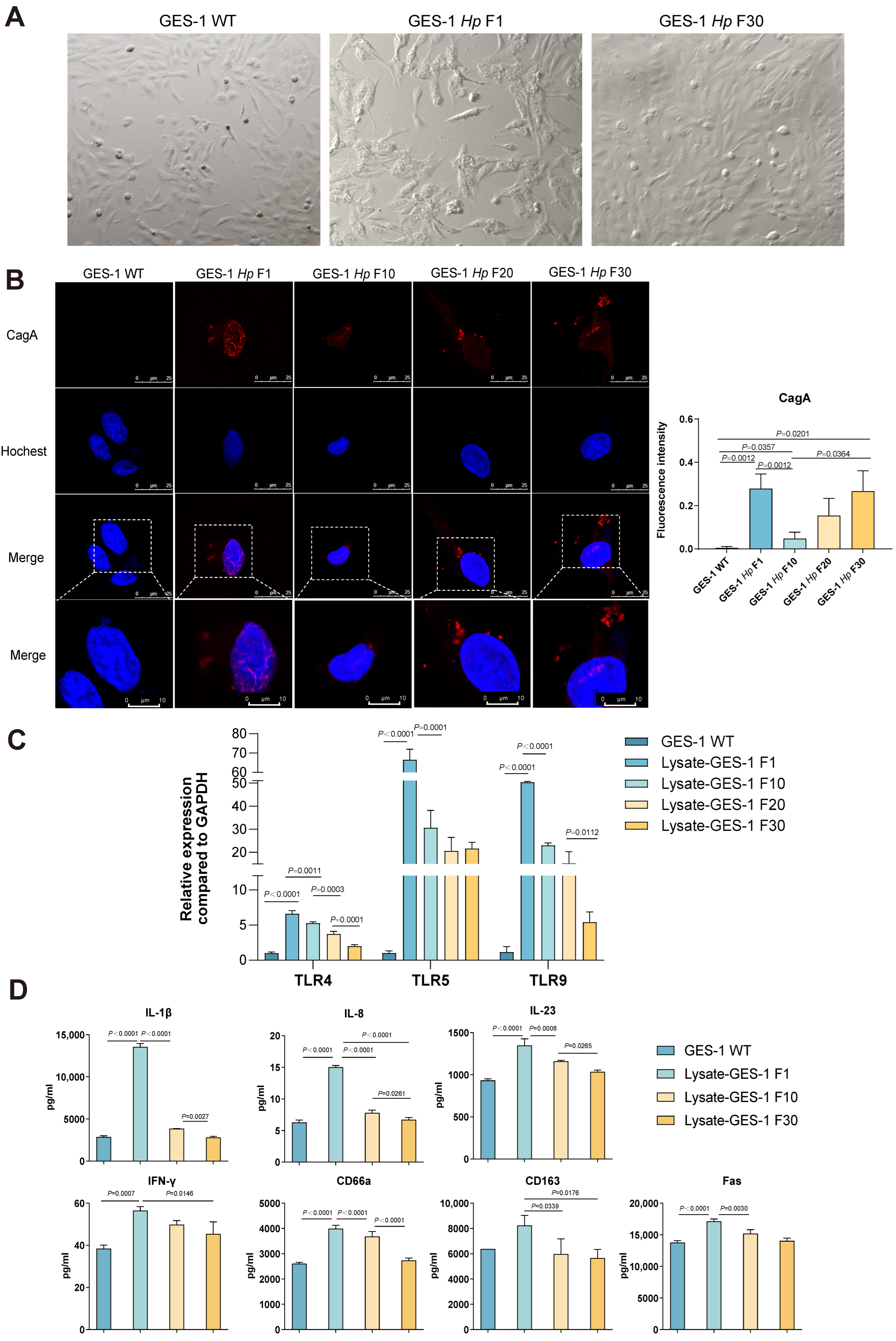
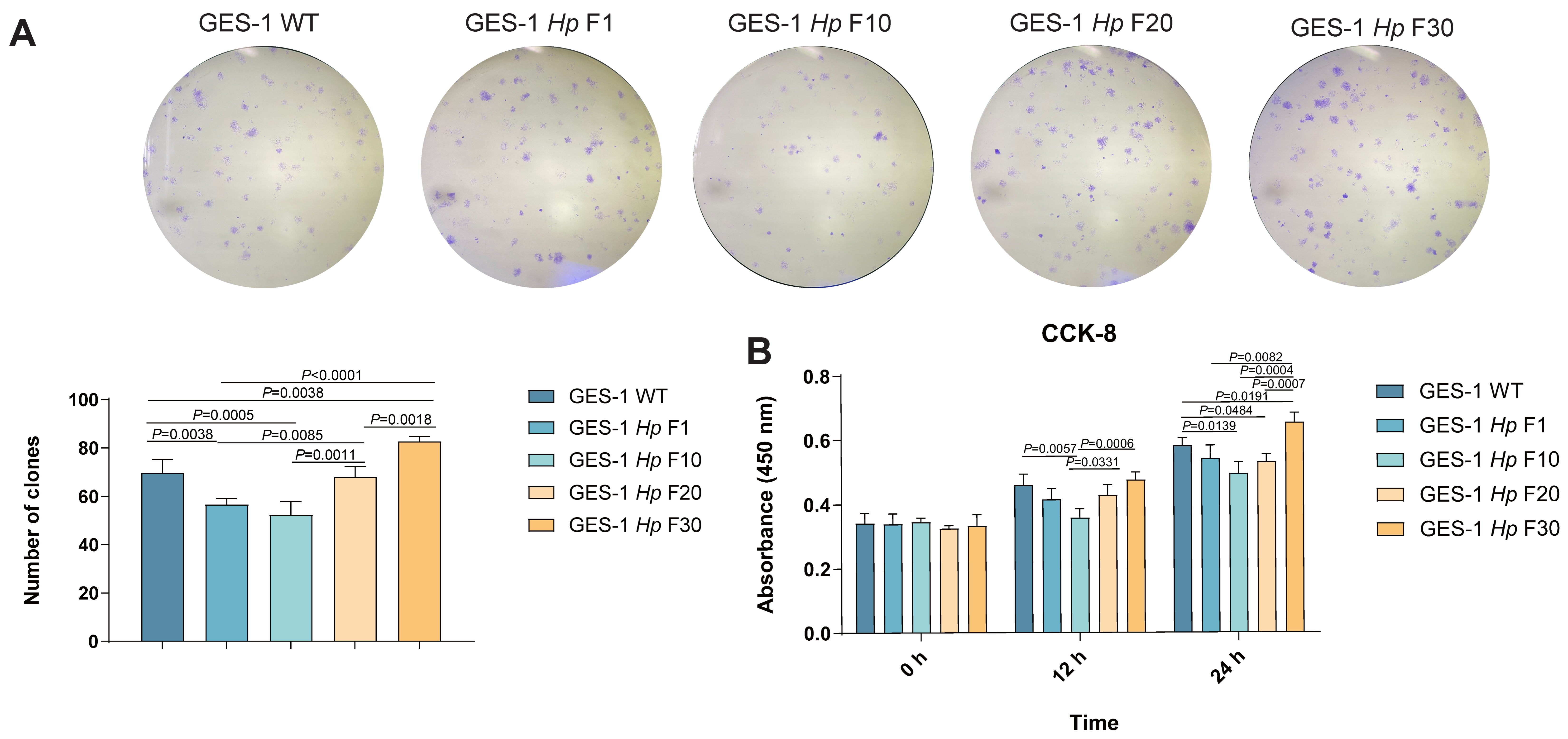
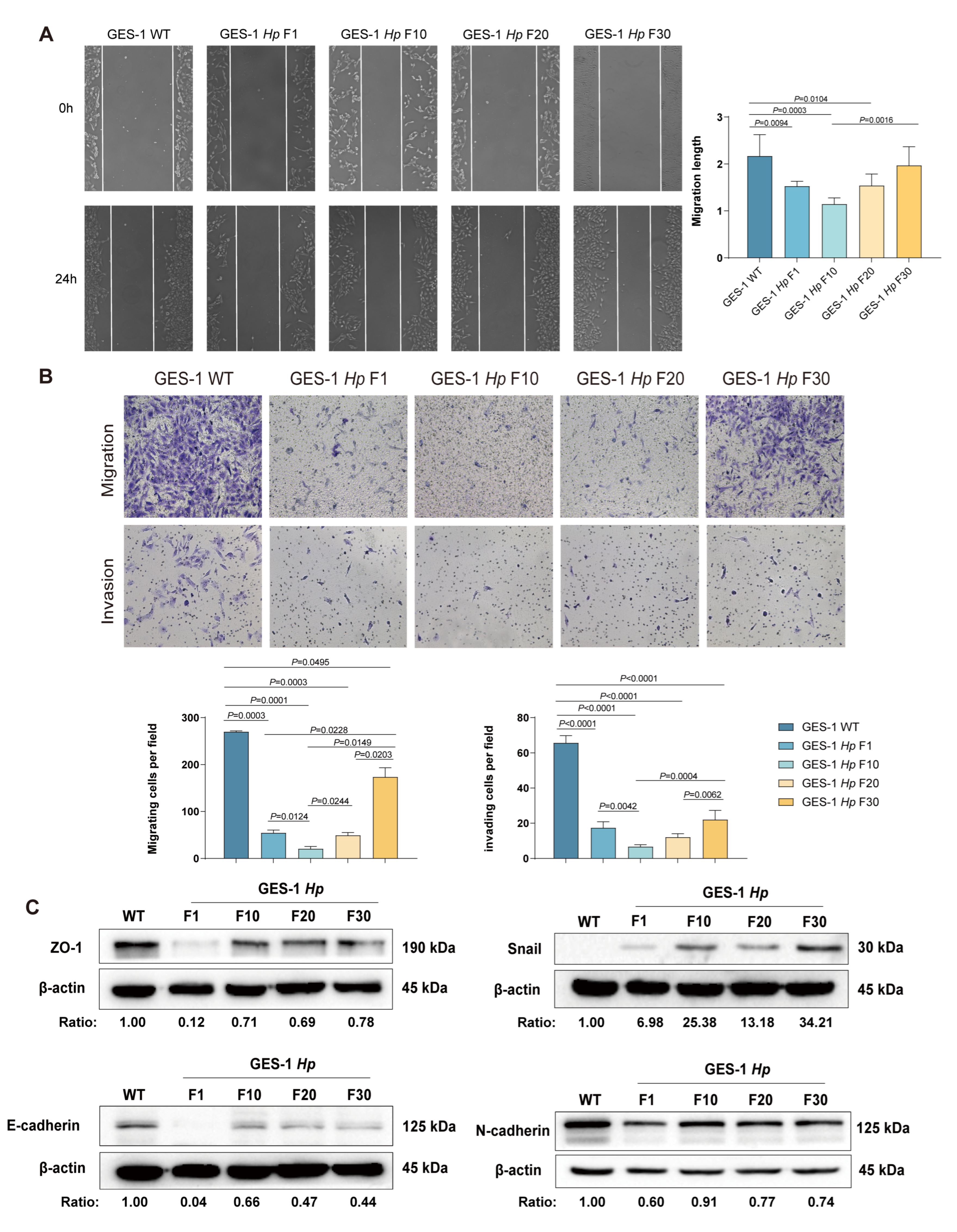
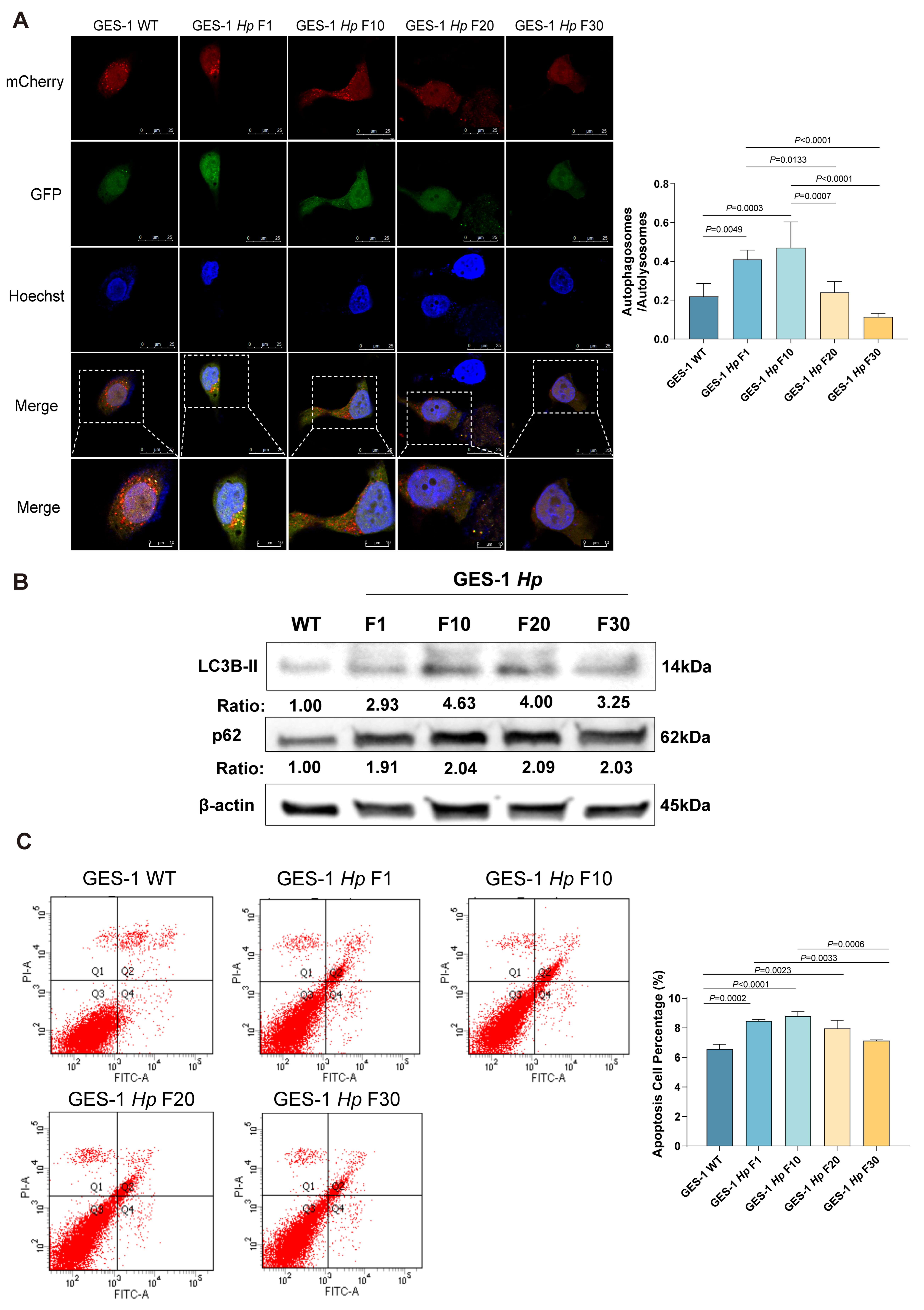
| Category | Acute Phase | Chronic Phase | Outcome of Chronic H. pylori Infection |
|---|---|---|---|
| Morphology | Hummingbird phenotype | Morphology reverts to normal | Cellular adaptation and immune tolerance |
| CagA Delivery | High cytoplasmic enrichment | CagA signal decreases then rebounds | Impaired toxin clearance |
| TLR Expression | TLR4/5/9 upregulated | Downregulated | Immune desensitization is established |
| Cytokine Secretion | IL-1β, IL-8, IL-23, IFN-γ peak | Declines | Attenuated inflammatory response |
| Cell Proliferation | Decreased | Recovers | Overcoming of stress-induced growth arrest |
| Migration and Invasion | Reduced | Recovers and then enhanced | Acquisition of motile, invasive phenotype |
| EMT Markers Expression | Unaffected | E-cadherin/ZO-1 down; Snail up | EMT initiation promotes malignancy |
| Autophagic Flux | Blocked | Restored | Shift to pro-survival autophagy |
| Apoptosis Level | Elevated | Declines | Accumulation of damaged cells |
| Transcriptomic Profile | DNA repair and apoptosis pathways active | NF-κB and pro-migration genes active | Reprogramming to pro-carcinogenic signaling |
| In Vivo Pathology | No significant changes | Progressive atrophy to dysplasia | Recapitulation of Correa’s cascade |
| In Vivo TLR Expression | TLR4/9 increased | TLR4/9 decline | Systemic immune tolerance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; He, Y.; Zhang, X.; Liang, Z.; Wang, W.; Da, Z.; Lv, J.; Guo, M.; Huo, X.; Liu, X.; et al. Biphasic Adaptations of Gastric Epithelial Cells in Chronic H. pylori Infection from Stress to Tolerance. Int. J. Mol. Sci. 2025, 26, 9016. https://doi.org/10.3390/ijms26189016
Zhang X, He Y, Zhang X, Liang Z, Wang W, Da Z, Lv J, Guo M, Huo X, Liu X, et al. Biphasic Adaptations of Gastric Epithelial Cells in Chronic H. pylori Infection from Stress to Tolerance. International Journal of Molecular Sciences. 2025; 26(18):9016. https://doi.org/10.3390/ijms26189016
Chicago/Turabian StyleZhang, Xiulin, Yang He, Xiaolu Zhang, Ziyi Liang, Wendong Wang, Zhenyu Da, Jianyi Lv, Meng Guo, Xueyun Huo, Xin Liu, and et al. 2025. "Biphasic Adaptations of Gastric Epithelial Cells in Chronic H. pylori Infection from Stress to Tolerance" International Journal of Molecular Sciences 26, no. 18: 9016. https://doi.org/10.3390/ijms26189016
APA StyleZhang, X., He, Y., Zhang, X., Liang, Z., Wang, W., Da, Z., Lv, J., Guo, M., Huo, X., Liu, X., Lu, J., Cao, L., Du, X., Ge, Z., Chen, Z., Lu, X., Zhang, J., & Li, C. (2025). Biphasic Adaptations of Gastric Epithelial Cells in Chronic H. pylori Infection from Stress to Tolerance. International Journal of Molecular Sciences, 26(18), 9016. https://doi.org/10.3390/ijms26189016






