UCH-L1 in Alzheimer’s Disease: A Crucial Player in Dementia-Associated Mechanisms
Abstract
1. Mechanistic Insights into UCH-L1 Enzyme Structure and Function
2. Unveiling the Role of UCH-L1 in Governing Crucial AD-Associated Hallmarks
2.1. Amyloid-β Plaque Deposition
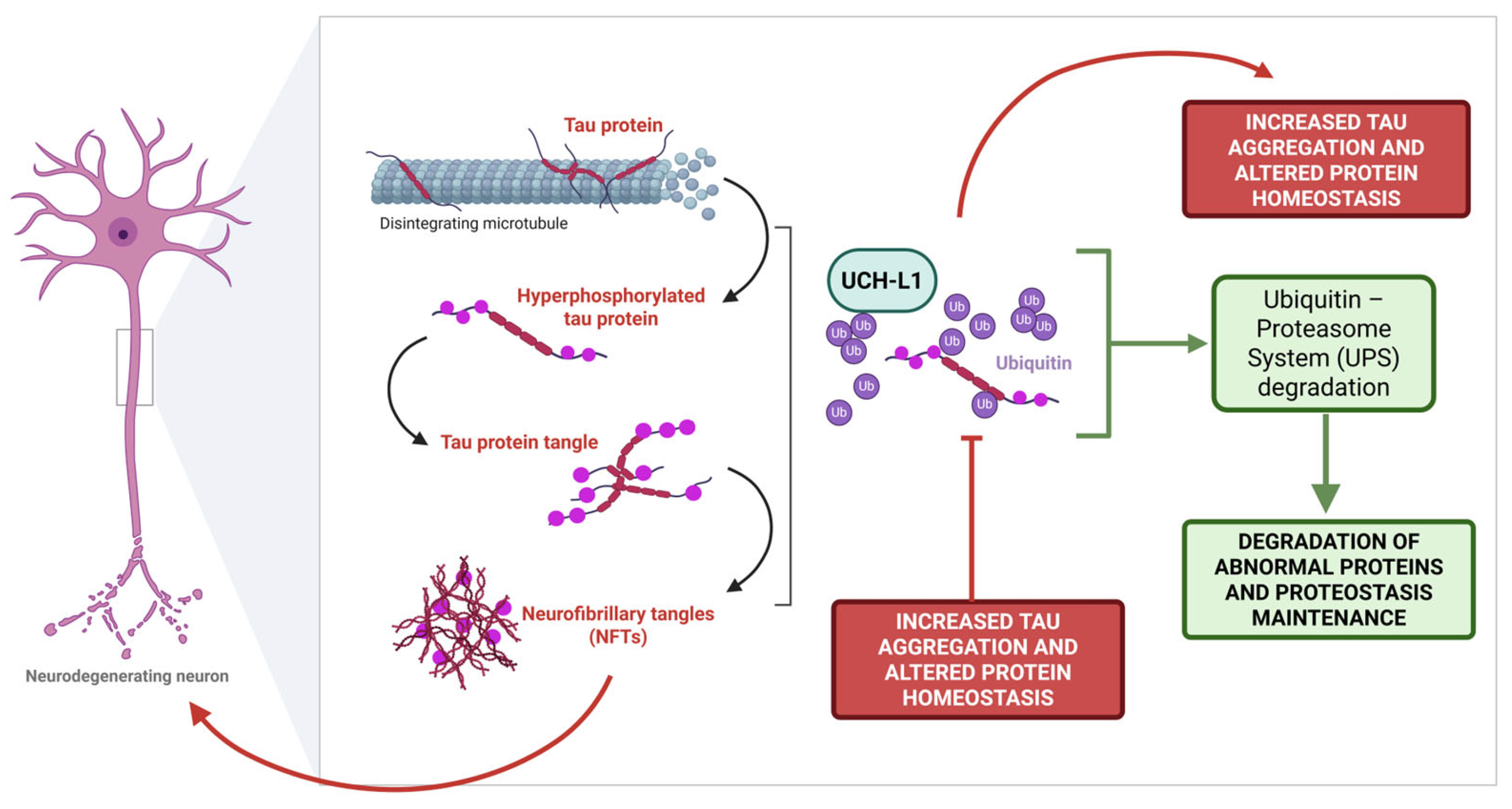
2.2. Tau Protein
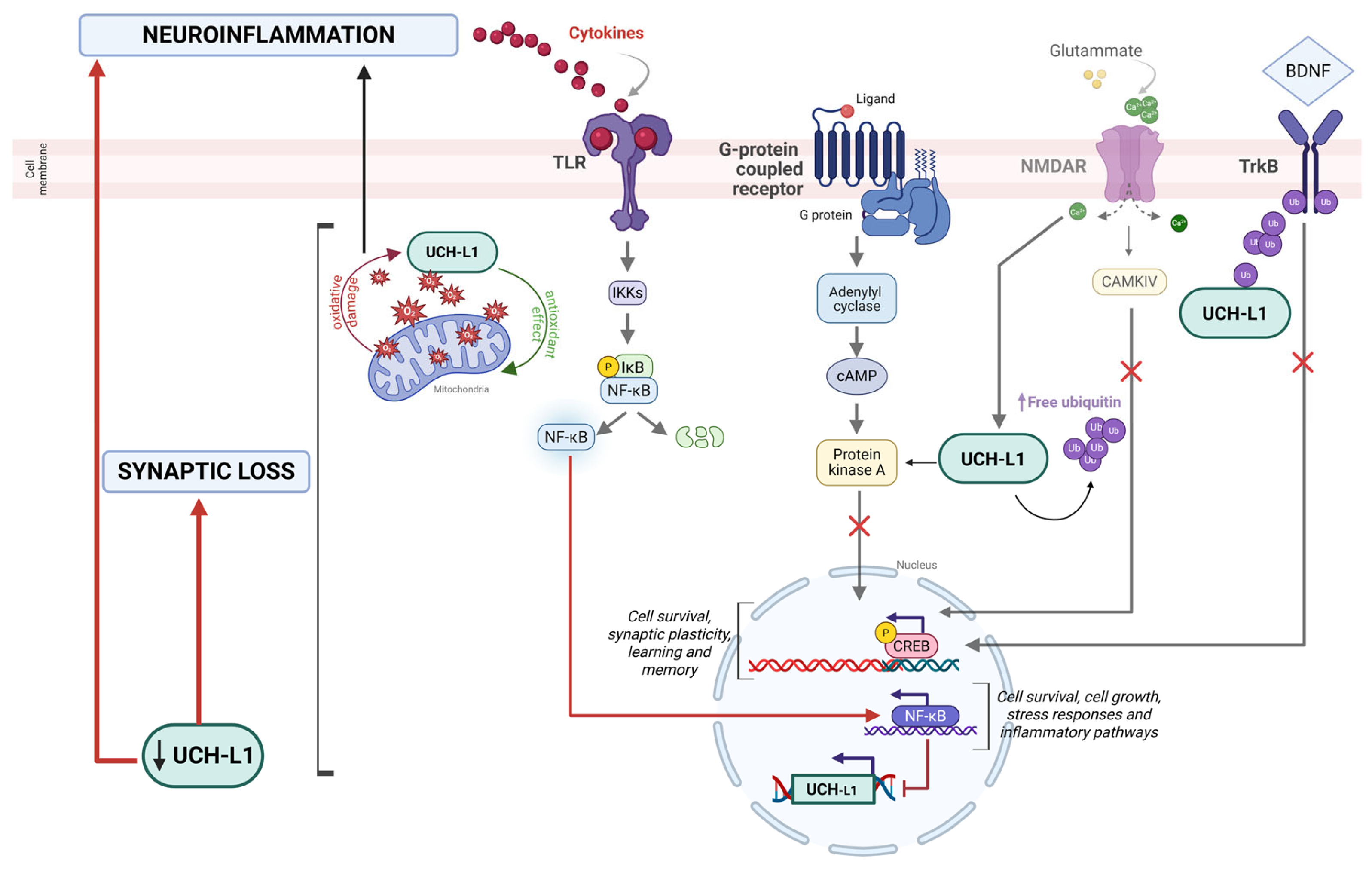
2.3. Synaptic Loss
3. UCH-L1 as a Target and Modulator of Neuroinflammatory Pathways
4. UCH-L1 as a Predictive Cerebrospinal Fluid (CSF) Biomarker for AD
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| Aβ | Amyloid-β |
| BACE1 | β-site amyloid precursor protein cleaving enzyme 1 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| MMSE | Mini Mental State Examination |
| NFTs | Neurofibrillary tangles |
| NF-κB | Nuclear Factor kappa B |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| UCH-L1 | Ubiquitin C-terminal hydrolase |
References
- Kumar, D.; Ambasta, R.K.; Kumar, P. Ubiquitin biology in neurodegenerative disorders: From impairment to therapeutic strategies. Ageing Res. Rev. 2020, 61, 101078. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Shi, X.H.; Chang, X.; Han, Y.; Liu, C.; Jiang, Z.; Yang, X. The mechanism of linear ubiquitination in regulating cell death and correlative diseases. Cell Death Dis. 2023, 14, 659. [Google Scholar] [CrossRef]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–78. [Google Scholar] [CrossRef]
- Ramirez, J.; Prieto, G.; Olazabal-Herrero, A.; Borràs, E.; Fernandez-Vigo, E.; Alduntzin, U.; Osinalde, N.; Beaskoetxea, J.; Lectez, B.; Aloria, K.; et al. A Proteomic Approach for Systematic Mapping of Substrates of Human Deubiquitinating Enzymes. Int. J. Mol. Sci. 2021, 22, 4851. [Google Scholar] [CrossRef]
- Snyder, N.A.; Silva, G.M. Deubiquitinating enzymes (DUBs): Regulation, homeostasis, and oxidative stress response. J. Biol. Chem. 2021, 297, 101077. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.D.; Lee, K.; Deshpande, S.; Duerksen-Hughes, P.; Boss, J.M.; Pohl, J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 1989, 246, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Tylicka, M.; Komarowska, M.D.; Debek, W.A. Hermanowicz, Ubiquitin carboxy-terminal hydrolase L1-physiology and pathology. Cell Biochem. Funct. 2020, 38, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Yang, Z.; Sarkis, G.; Torres, I.; Raghavan, V. Ubiquitin C-terminal hydrolase-L1 (UCH-L1) as a therapeutic and diagnostic target in neurodegeneration, neurotrauma and neuro-injuries. Expert. Opin. Ther. Targets 2017, 21, 627–638. [Google Scholar] [CrossRef]
- Sjölin, K.; Kultima, K.; Larsson, A.; Freyhult, E.; Zjukovskaja, C.; Alkass, K.; Burman, J. Distribution of five clinically important neuroglial proteins in the human brain. Mol. Brain 2022, 15, 52. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Li, M.; Hong, T.; Meng, W.; Ouyang, T. Ubiquitin C-terminal hydrolase-L1: A new cancer marker and therapeutic target with dual effects (Review). Oncol. Lett. 2023, 25, 123. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-Terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Puri, S.; Hsu, S.-T.D. Functional dynamics of human ubiquitin C-terminal hydrolases. Front. Biophys. 2024, 2, 1479898. [Google Scholar] [CrossRef]
- Bishop, P.; Rubin, P.; Thomson, A.R.; Rocca, D.; Henley, J.M. The Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) C Terminus Plays a Key Role in Protein Stability, but Its Farnesylation Is Not Required for Membrane Association in Primary Neurons. J. Biol. Chem. 2014, 289, 36140–36149. [Google Scholar] [CrossRef]
- Tramutola, A.; Di Domenico, F.; Barone, E.; Perluigi, M.; Butterfield, D.A. It Is All about (U)biquitin: Role of Altered Ubiquitin-Proteasome System and UCHL1 in Alzheimer Disease. Oxidative Med. Cell. Longev. 2016, 2016, 2756068. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Hoang, Q.Q.; Kreinbring, C.A.; Luchansky, S.J.; Meray, R.K.; Ray, S.S.; Lansbury, P.T.; Ringe, D.; Petsko, G.A. Structural basis for conformational plasticity of the Parkinson’s disease-associated ubiquitin hydrolase UCH-L1. Proc. Natl. Acad. Sci. USA 2006, 103, 4675–4680, Erratum in Proc. Natl. Acad. Sci. USA 2006, 103, 6776. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Fu, L. Roles of Ubiquitin Ligases and Deubiquitylases in Alzheimer’s Disease. Mol. Neurobiol. 2025, 62, 7747–7761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meray, R.K.; Grammatopoulos, T.N.; Fredenburg, R.A.; Cookson, M.R.; Liu, Y.; Logan, T.; Lansbury, P.T. Membrane-associated farnesylated UCH-L1 promotes α-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 4635–4640. [Google Scholar] [CrossRef]
- Donovan, L.E.; Higginbotham, L.; Dammer, E.B.; Gearing, M.; Rees, H.D.; Xia, Q.; Duong, D.M.; Seyfried, N.T.; Lah, J.J.; Levey, A.I. Analysis of a membrane-enriched proteome from postmortem human brain tissue in Alzheimer’s disease. Proteom. Clin. Appl. 2012, 6, 201–211. [Google Scholar] [CrossRef]
- Mi, Z.; Graham, S.H. Role of UCHL1 in the pathogenesis of neurodegenerative diseases and brain injury. Ageing Res. Rev. 2023, 86, 101856. [Google Scholar] [CrossRef]
- Castegna, A.; Aksenov, M.; Aksenova, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of oxidatively modified proteins in alzheimer’s disease brain. part I: Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic. Biol. Med. 2002, 33, 562–571. [Google Scholar] [CrossRef]
- Choi, J.; Levey, A.I.; Weintraub, S.T.; Rees, H.D.; Gearing, M.; Chin, L.S.; Li, L. Oxidative Modifications and Down-regulation of Ubiquitin Carboxyl-terminal Hydrolase L1 Associated with Idiopathic Parkinson’s and Alzheimer’s Diseases. J. Biol. Chem. 2004, 279, 13256–13264. [Google Scholar] [CrossRef]
- Sultana, R.; Boyd-Kimball, D.; Poon, H.F.; Cai, J.; Pierce, W.M.; Klein, J.B.; Merchant, M.; Markesbery, W.R.; Butterfield, D.A. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging 2006, 27, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Gnjec, A.; Poon, H.F.; Castegna, A.; Pierce, W.M.; Klein, J.B.; Martins, R.N. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer’s disease: An initial assessment. J. Alzheimer’s Dis. 2006, 10, 391–397. [Google Scholar] [CrossRef]
- Nakamura, T.; Oh, C.K.; Liao, L.; Zhang, X.; Lopez, K.M.; Gibbs, D.; Deal, A.K.; Scott, H.R.; Spencer, B.; Masliah, E.; et al. Noncanonical transnitrosylation network contributes to synapse loss in Alzheimer’s disease. Science 2021, 371, eaaw0843. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.I.; Liu, H.; Di Maio, R.; Kodali, R.B.; Graham, S.H.; Gronenborn, A.M. Cyclopentenone prostaglandin-induced unfolding and aggregation of the Parkinson disease-associated UCH-L1. Proc. Natl. Acad. Sci. USA 2010, 107, 6835–6840. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Abiko, Y.; Katayama, Y.; Kaji, T.; Kumagai, Y. Mercuration of ubiquitin carboxyl-terminal hydrolase L1 through Cys152 by methylmercury causes inhibition of its catalytic activity and reduction of monoubiquitin levels in SH-SY5Y cells. J. Toxicol. Sci. 2015, 40, 887–893. [Google Scholar] [CrossRef][Green Version]
- Forero, D.A.; Benítez, B.; Arboleda, G.; Yunis, J.J.; Pardo, R.; Arboleda, H. Analysis of functional polymorphisms in three synaptic plasticity-related genes (BDNF, COMT AND UCHL1) in Alzheimer’s disease in Colombia. Neurosci. Res. 2006, 55, 334–341. [Google Scholar] [CrossRef]
- Xue, S.; Jia, J. Genetic association between Ubiquitin Carboxy-terminal Hydrolase-L1 gene S18Y polymorphism and sporadic Alzheimer’s disease in a Chinese Han population. Brain Res. 2006, 1087, 28–32. [Google Scholar] [CrossRef]
- Zetterberg, M.; Sjölander, A.; von Otter, M.; Palmér, M.; Landgren, S.; Minthon, L.; Wallin, A.; Andreasen, N.; Blennow, K.; Zetterberg, H. Ubiquitin carboxy-terminal hydrolase L1 (UCHL1) S18Y polymorphism in Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Fallon, L.; Lashuel, H.A.; Liu, Z.; Lansbury, P.T. The UCH-L1 Gene Encodes Two Opposing Enzymatic Activities that Affect-Synuclein Degradation and Parkinson’s Disease Susceptibility. Cell 2002, 111, 209–218. [Google Scholar] [CrossRef]
- Setsuie, R.; Wada, K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem. Int. 2007, 51, 105–111. [Google Scholar] [CrossRef]
- Bdarneh, A.; Maniv, I.; Glickman, M.H. Ubiquitin C-Terminal Hydrolase L1 (UCHL1), Beyond Hydrolysis. BioEssays 2025, 47, e70028. [Google Scholar] [CrossRef] [PubMed]
- Bilguvar, K.; Tyagi, N.K.; Ozkara, C.; Tuysuz, B.; Bakircioglu, M.; Choi, M.; Delil, S.; Caglayan, A.O.; Baranoski, J.F.; Erturk, O.; et al. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Osaka, H. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum. Mol. Genet. 2003, 12, 1945–1958. [Google Scholar] [CrossRef]
- Larsen, C.N.; Krantz, B.A.; Wilkinson, K.D. Substrate Specificity of Deubiquitinating Enzymes: Ubiquitin C-Terminal Hydrolases. Biochemistry 1998, 37, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, F.W.; de Kleijn, D.P.V.; van den Hurk, H.H.; Neubauer, A.; Sonnemans, M.A.F.; Sluijs, J.A.; Köycü, S.; Ramdjielal, R.D.J.; Salehi, A.; Martens, G.J.M.; et al. Frameshift Mutants of β Amyloid Precursor Protein and Ubiquitin-B in Alzheimer’s and Down Patients. Science 1998, 279, 242–247. [Google Scholar] [CrossRef]
- Maniv, I.; Sarji, M.; Bdarneh, A.; Feldman, A.; Ankawa, R.; Koren, E.; Magid-Gold, I.; Reis, N.; Soteriou, D.; Salomon-Zimri, S.; et al. Altered ubiquitin signaling induces Alzheimer’s disease-like hallmarks in a three-dimensional human neural cell culture model. Nat. Commun. 2023, 14, 5922. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, G.; Yang, J.; Pang, L.; Li, X. Pathological mechanisms and treatment progression of Alzheimer’s disease. Eur. J. Med. Res. 2025, 30, 625. [Google Scholar] [CrossRef]
- Gong, B.; Cao, Z.; Zheng, P.; Vitolo, O.V.; Liu, S.; Staniszewski, A.; Moolman, D.; Zhang, H.; Shelanski, M.; Arancio, O. Ubiquitin Hydrolase Uch-L1 Rescues β-Amyloid-Induced Decreases in Synaptic Function and Contextual Memory. Cell 2006, 126, 775–788. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, F.; Zhang, S.; Zhang, S.; Song, W. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer’s progression in vivo. Sci. Rep. 2014, 4, 7298. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Monteleone, D.; Boido, M.; Piras, A.; Giliberto, L.; Borghi, R.; Vercelli, A.; Fornaro, M.; Tabaton, M.; Tamagno, E. Aβ1-42-mediated down-regulation of Uch-L1 is dependent on NF-κB activation and impaired BACE1 lysosomal degradation. Aging Cell 2012, 11, 834–844. [Google Scholar] [CrossRef]
- Poon, W.W.; Carlos, A.J.; Aguilar, B.L.; Berchtold, N.C.; Kawano, C.K.; Zograbyan, V.; Yaopruke, T.; Shelanski, M.; Cotman, C.W. β-Amyloid (Aβ) Oligomers Impair Brain-derived Neurotrophic Factor Retrograde Trafficking by Down-regulating Ubiquitin C-terminal Hydrolase, UCH-L1. J. Biol. Chem. 2013, 288, 16937–16948. [Google Scholar] [CrossRef]
- Toyama, Y.; Nirasawa, T.; Morishima, M.; Saito, Y.; Irie, K.; Murayama, S.; Ikegawa, M. Integrated Spatial Multi-Omics Study of Postmortem Brains of Alzheimer’s Disease. Acta Histochem. Cytochem. 2024, 57, 119–130. [Google Scholar] [CrossRef]
- Ichihara, N.; Wu, J.; Chui, D.H.; Yamazaki, K.; Wakabayashi, T.; Kikuchi, T. Axonal degeneration promotes abnormal accumulation of amyloid β-protein in ascending gracile tract of gracile axonal dystrophy (GAD) mouse. Brain Res. 1995, 695, 173–178. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease Beta-secretase enzyme. BACE1 Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chan, C.H.; Ma, Q.; Xu, X.; Xiao, Z.; Tan, E.-K. The roles of amyloid precursor protein (APP) in neurogenesis. Cell Adhes. Migr. 2011, 5, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Qing, H.; Zhou, W.; Christensen, M.A.; Sun, X.; Tong, Y.; Song, W. Degradation of BACE by the ubiquitin-proteasome pathway. FASEB J. 2004, 18, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, Y.; Luo, Y.; Zhang, S.; Zou, H.; Cai, F.; Wada, K.; Song, W. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J. Neurochem. 2012, 120, 1129–1138. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Monteleone, D.; Vasciaveo, V.; Repetto, I.E.; Manassero, G.; Tabaton, M.; Tamagno, E. The decrease of Uch-L1 activity is a common mechanism responsible for Aβ 42 accumulation in Alzheimer’s and vascular disease. Front. Aging Neurosci. 2017, 9, 320. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Zhou, W.; Ly, P.T.T.; Cai, F.; Song, W. NF-κB signaling inhibits ubiquitin carboxyl-terminal hydrolase L1 gene expression. J. Neurochem. 2011, 116, 1160–1170. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernandez, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xie, G.; Li, X.; Xiong, J. Cytoskeletal Proteins and Alzheimer’s Disease Pathogenesis: Focusing on the Interplay with Tau Pathology. Biomolecules 2025, 15, 831. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflamm. 2023, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Poppek, D.; Keck, S.; Ermak, G.; Jung, T.; Stolzing, A.; Ullrich, O.; Davies, K.J.A.; Grune, T. Phosphorylation inhibits turnover of the tau protein by the proteasome: Influence of RCAN1 and oxidative stress. Biochem. J. 2006, 400, 511–520. [Google Scholar] [CrossRef]
- Xie, M.; Han, Y.; Yu, Q.; Wang, X.; Wang, S.; Liao, X. UCH-L1 Inhibition Decreases the Microtubule-Binding Function of Tau Protein. J. Alzheimer’s Dis. 2015, 49, 353–363. [Google Scholar] [CrossRef]
- Minjarez, B.; Calderón-González, K.G.; Rustarazo, M.L.V.; Herrera-Aguirre, M.E.; Labra-Barrios, M.L.; Rincon-Limas, D.E.; del Pino, M.M.S.; Mena, R.; Luna-Arias, J.P. Identification of proteins that are differentially expressed in brains with Alzheimer’s disease using iTRAQ labeling and tandem mass spectrometry. J. Proteom. 2016, 139, 103–121. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, H.; Li, Y.; Liu, C.; Wang, S.; Liao, X. UCH-L1 Inhibition Suppresses tau Aggresome Formation during Proteasomal Impairment. Mol. Neurobiol. 2017, 55, 3812–3821. [Google Scholar] [CrossRef]
- Zhao, Z.-B.; Wu, L.; Xiong, R.; Wang, L.-L.; Zhang, B.; Wang, C.; Li, H.; Liang, L.; Chen, S.-D. MicroRNA-922 promotes tau phosphorylation by downregulating ubiquitin carboxy-terminal hydrolase L1 (UCHL1) expression in the pathogenesis of Alzheimer’s disease. Neuroscience 2014, 275, 232–237. [Google Scholar] [CrossRef]
- Chen, J.; Huang, R.Y.C.; Turko, I.V. Mass spectrometry assessment of ubiquitin carboxyl-terminal hydrolase L1 partitioning between soluble and particulate brain homogenate fractions. Anal. Chem. 2013, 85, 6011–6017. [Google Scholar] [CrossRef]
- Crimins, J.L.; Pooler, A.; Polydoro, M.; Luebke, J.I.; Spires-Jones, T.L. The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 757–763. [Google Scholar] [CrossRef]
- Hegde, A.N.; Inokuchi, K.; Pei, W.; Casadio, A.; Ghirardi, M.; Chain, D.G.; Martin, K.C.; Kandel, E.R.; Schwartz, J.H. Ubiquitin C-Terminal Hydrolase Is an Immediate-Early Gene Essential for Long-Term Facilitation in Aplysia. Cell 1997, 89, 115–126. [Google Scholar] [CrossRef][Green Version]
- Cookson, M.R.; Hardy, J. The Persistence of Memory. N. Engl. J. Med. 2006, 355, 2697–2698. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Sekiguchi, M.; Zushida, K.; Yamada, K.; Nagamine, S.; Kabuta, T.; Wada, K. Reduction in memory in passive avoidance learning, exploratory behaviour and synaptic plasticity in mice with a spontaneous deletion in the ubiquitin C-terminal hydrolase L1 gene. Eur. J. Neurosci. 2008, 27, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.E.; Djakovic, S.N.; Salehi, A.; Wilson, S.M.; Masliah, E.; Patrick, G.N. Regulation of Synaptic Structure by Ubiquitin C-Terminal Hydrolase L1. J. Neurosci. 2009, 29, 7857–7868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, J.; Xu, B.; Yang, J.; Wu, Z.; Cheng, L. TrkB/BDNF signaling pathway and its small molecular agonists in CNS injury. Life Sci. 2024, 336, 122282. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Lu, Y.; Zheng, Y.; Chen, X.-R.; Dong, J.-L.; Yuan, R.-R.; Huang, S.-H.; Yu, H.; Wang, Y.; Chen, Z.-Y.; et al. Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) Promotes Hippocampus-Dependent Memory via Its Deubiquitinating Effect on TrkB. J. Neurosci. 2017, 37, 5978–5995. [Google Scholar] [CrossRef]
- Chen, F.; Sugiura, Y.; Myers, K.G.; Liu, Y.; Lin, W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2010, 107, 1636–1641. [Google Scholar] [CrossRef]
- Konsman, J.P. Cytokines in the Brain and Neuroinflammation: We Didn’t Starve the Fire! Pharmaceuticals 2022, 15, 140. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood–brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef]
- Pasko, V.I.; Churkina, A.S.; Shakhov, A.S.; Kotlobay, A.A.; Alieva, I.B. Modeling of Neurodegenerative Diseases: ‘Step by Step’ and ‘Network’ Organization of the Complexes of Model Systems. Int. J. Mol. Sci. 2023, 24, 604. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood–brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Thibaudeau, T.A.; Anderson, R.T.; Smith, D.M. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat. Commun. 2018, 9, 1097. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The Ubiquitin system for protein degradation. Annu. Rev. Biochem. 1992, 61, 761–807. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zheng, W.; Wang, X.; Chen, Z. Proteostasis of α-Synuclein and Its Role in the Pathogenesis of Parkinson’s Disease. Front. Cell. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef]
- Thapa, R.; Bhat, A.A.; Shahwan, M.; Ali, H.; PadmaPriya, G.; Bansal, P.; Rajotiya, S.; Barwal, A.; Prasad, G.V.S.; Pramanik, A.; et al. Proteostasis disruption and senescence in Alzheimer’s disease pathways to neurodegeneration. Brain Res. 2024, 1845, 149202. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Olanow, C.W. Schapira, Jenner, Isacson, Hunot, Tatton, Beal, Proteolytic stress: A unifying concept for the etiopathogenesis of Parkinson’s disease. Ann. Neurol. 2003, 53, S73–S86. [Google Scholar] [CrossRef]
- Sonninen, T.M.; Goldsteins, G.; Laham-Karam, N.; Koistinaho, J.; Lehtonen, Š. Proteostasis Disturbances and Inflammation in Neurodegenerative Diseases. Cells 2020, 9, 2183. [Google Scholar] [CrossRef]
- Choi, J.E.; Lee, J.J.; Kang, W.; Kim, H.J.; Cho, J.H.; Han, P.L.; Lee, K.J. Proteomic Analysis of Hippocampus in a Mouse Model of Depression Reveals Neuroprotective Function of Ubiquitin C-terminal Hydrolase L1 (UCH-L1) via Stress-induced Cysteine Oxidative Modifications. Mol. Cell. Proteom. 2018, 17, 1803. [Google Scholar] [CrossRef]
- Puri, S.; Hsu, S.T.D. Cross-over Loop Cysteine C152 Acts as an Antioxidant to Maintain the Folding Stability and Deubiquitinase Activity of UCH-L1 Under Oxidative Stress. J. Mol. Biol. 2021, 433, 166879. [Google Scholar] [CrossRef]
- Gabbita, S.P.; Aksenov, M.Y.; Lovell, M.A.; Markesbery, W.R. Decrease in Peptide Methionine Sulfoxide Reductase in Alzheimer’s Disease Brain. J. Neurochem. 1999, 73, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Head, E.; Butterfield, D.A.; Perluigi, M. Oxidative Stress and Proteostasis Network: Culprit and Casualty of Alzheimer’s-Like Neurodegeneration. Adv. Geriatr. 2014, 2014, 527518. [Google Scholar] [CrossRef]
- Qin, Z.H.; Tao, L.Y.; Chen, X. Dual roles of NF-κB in cell survival and implications of NF-κB inhibitors in neuroprotective therapy. Acta Pharmacol. Sin. 2007, 28, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, N.; Chen, X.; Zhang, F.; Kong, T.; Tang, X.; Yang, Q.; Chen, W.; Xiong, X.; Chen, X. UCHL1 regulates inflammation via MAPK and NF-κB pathways in LPS-activated macrophages. Cell Biol. Int. 2021, 45, 2107–2117. [Google Scholar] [CrossRef]
- Liang, Z.; Damianou, A.; Vendrell, I.; Jenkins, E.; Lassen, F.H.; Washer, S.J.; Grigoriou, A.; Liu, G.; Yi, G.; Lou, H.; et al. Proximity proteomics reveals UCH-L1 as an essential regulator of NLRP3-mediated IL-1β production in human macrophages and microglia. Cell Rep. 2024, 43, 114152. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Repetto, I.E.; Monteleone, D.; Vasciaveo, V.; Franchino, C.; Rinaldi, S.; Tabaton, M.; Tamagno, E. Stroke and Amyloid-β Downregulate TREM-2 and Uch-L1 Expression that Synergistically Promote the Inflammatory Response. J. Alzheimer’s Dis. 2019, 71, 907–920. [Google Scholar] [CrossRef]
- Clayton, K.; Delpech, J.C.; Herron, S.; Iwahara, N.; Ericsson, M.; Saito, T.; Saido, T.C.; Ikezu, S.; Ikezu, T. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Mol. Neurodegener. 2021, 16, 18, Erratum in Mol. Neurodegener. 2021, 16, 24. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Duan, J.; Lv, A.; Guo, Z.; Liu, Q.; Tian, C.; Yang, Y.; Bi, J.; Yu, X.; Peng, G.; Luo, B.; et al. CX3CR1+/UCHL1+ microglial extracellular vesicles in blood: A potential biomarker for multiple sclerosis. J. Neuroinflamm. 2024, 21, 254. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chu, W.; Xia, Y.; Shi, M.; Li, T.; Zhou, F.Q.; Deng, D.Y.B. UCHL1 facilitates protein aggregates clearance to enhance neural stem cell activation in spinal cord injury. Cell Death Dis. 2023, 14, 479. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, C.; Sun, Y.; Li, Y. Serum ubiquitin C-terminal hydrolase L1 as a biomarker for traumatic brain injury: A systematic review and meta-analysis. Am. J. Emerg. Med. 2015, 33, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Evaluation of Automatic Class III Designation for Banyan Brain Trauma Indicator_Decision-Memorandum. 2018. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN170045.pdf (accessed on 14 July 2025).
- Wang, J.; Ji, C.; Ye, W.; Rong, Y.; Ge, X.; Wang, Z.; Tang, P.; Zhou, Z.; Luo, Y.; Cai, W. Deubiquitinase UCHL1 promotes angiogenesis and blood–spinal cord barrier function recovery after spinal cord injury by stabilizing Sox17. Cell. Mol. Life Sci. 2024, 81, 137. [Google Scholar] [CrossRef]
- Corada, M.; Orsenigo, F.; Bhat, G.P.; Conze, L.L.; Breviario, F.; Cunha, S.I.; Claesson-Welsh, L.; Beznoussenko, G.V.; Mironov, A.A.; Bacigaluppi, M.; et al. Fine-Tuning of Sox17 and Canonical Wnt Coordinates the Permeability Properties of the Blood-Brain Barrier. Circ. Res. 2019, 124, 511–525. [Google Scholar] [CrossRef]
- Leuzy, A.; Bollack, A.; Pellegrino, D.; Teunissen, C.E.; La Joie, R.; Rabinovici, G.D.; Franzmeier, N.; Johnson, K.; Barkhof, F.; Shaw, L.M.; et al. Considerations in the clinical use of amyloid PET and CSF biomarkers for Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e14528. [Google Scholar] [CrossRef]
- Öhrfelt, A.; Johansson, P.; Wallin, A.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Svensson, J. Increased Cerebrospinal Fluid Levels of Ubiquitin Carboxyl-Terminal Hydrolase L1 in Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Dis. Extra 2016, 6, 283–294. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N.; Gera, R.G.; Kremer, T.; Cedarbaum, J.; Barbour, R.; Zago, W.; Schade, S.; Otte, B.; Bartl, M.; et al. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Haque, R.U.; Dammer, E.B.; Duong, D.M.; Ping, L.; Johnson, E.C.B.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Targeted mass spectrometry to quantify brain-derived cerebrospinal fluid biomarkers in Alzheimer’s disease. Clin. Proteom. 2020, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Álvarez-Sánchez, L.; Balaguer, Á.; Raga, L.; García-Vallés, L.; Baquero, M.; Cháfer-Pericás, C. Defining Alzheimer’s Disease through Proteomic CSF Profiling. J. Proteome Res. 2024, 23, 5096–5106. [Google Scholar] [CrossRef]
- Chatziefstathiou, A.; Canaslan, S.; Kanata, E.; Vekrellis, K.; Constantinides, V.C.; Paraskevas, G.P.; Kapaki, E.; Schmitz, M.; Zerr, I.; Xanthopoulos, K.; et al. SIMOA Diagnostics on Alzheimer’s Disease and Frontotemporal Dementia. Biomedicines 2024, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, S.; Wojdała, A.L.; Bellomo, G.; Toja, A.; Chipi, E.; Piersma, S.R.; Pham, T.V.; Gaetani, L.; Jimenez, C.R.; Parnetti, L.; et al. Potential diagnostic value of CSF metabolism-related proteins across the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2023, 15, 124. [Google Scholar] [CrossRef]
- Wojdała, A.L.; Bellomo, G.; Gaetani, L.; Toja, A.; Chipi, E.; Shan, D.; Chiasserini, D.; Parnetti, L. Trajectories of CSF and plasma biomarkers across Alzheimer’s disease continuum: Disease staging by NF-L, p-tau181, and GFAP. Neurobiol. Dis. 2023, 189, 106356. [Google Scholar] [CrossRef]
- Sarto, J.; Ruiz-García, R.; Guillén, N.; Ramos-Campoy, Ó.; Falgàs, N.; Esteller, D.; Contador, J.; Fernández, G.; González, Y.; Tort-Merino, A.; et al. Diagnostic Performance and Clinical Applicability of Blood-Based Biomarkers in a Prospective Memory Clinic Cohort. Neurology 2023, 100, E860–E873. [Google Scholar] [CrossRef]
- Lobue, C.; Stopschinski, B.E.; Calveras, N.S.; Douglas, P.M.; Huebinger, R.; Cullum, C.M.; Hart, J.; Gonzales, M.M. Blood Markers in Relation to a History of Traumatic Brain Injury Across Stages of Cognitive Impairment in a Diverse Cohort. J. Alzheimer’s Dis. 2024, 97, 345–358. [Google Scholar] [CrossRef]
- Anaya-Cubero, E.; Fernández-Irigoyen, J.; Santamaría, E. Application of Single Molecule Array (SIMOA) in Cerebrospinal Fluid. Methods Mol. Biol. 2025, 2914, 25–39. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Moscoso, A.; Karikari, T.K.; Grothe, M.J.; Ashton, N.J.; Lantero-Rodriguez, J.; Snellman, A.; Zetterberg, H.; Blennow, K.; Schöll, M. CSF biomarkers and plasma p-tau181 as predictors of longitudinal tau accumulation: Implications for clinical trial design. Alzheimer’s Dement. 2022, 18, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Bateman, R.J.; Hirtz, C.; Marin, P.; Becher, F.; Sato, C.; Gabelle, A.; Lehmann, S. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimer’s Res. Ther. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Sekimori, T.; Oizumi, H.; Takeda, A.; Fukunaga, K. Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique. Int. J. Mol. Sci. 2023, 24, 13267. [Google Scholar] [CrossRef]
- Barrachina, M.; Castaño, E.; Dalfó, E.; Maes, T.; Buesa, C.; Ferrer, I. Reduced ubiquitin C-terminal hydrolase-1 expression levels in dementia with Lewy bodies. Neurobiol. Dis. 2006, 22, 265–273. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Xie, W.; Liu, J.; Wang, C. UCHL1 from serum and CSF is a candidate biomarker for amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 1420–1428. [Google Scholar] [CrossRef]
- Mondello, S.; Palmio, J.; Streeter, J.; Hayes, R.L.; Peltola, J.; Jeromin, A. Ubiquitin Carboxy-Terminal Hydrolase L1 (UCH-L1) is increased in cerebrospinal fluid and plasma of patients after epileptic seizure. BMC Neurol. 2012, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.S.L.; Tan, Y.J.; Lu, Z.; Ng, E.Y.; Ng, S.Y.E.; Chia, N.S.Y.; Setiawan, F.; Xu, Z.; Keong, N.C.H.; Tay, K.Y.; et al. Plasma ubiquitin C-terminal hydrolase L1 levels reflect disease stage and motor severity in Parkinson’s disease. Aging 2020, 12, 1488–1495. [Google Scholar] [CrossRef]

| Model/System | Experimental Approach | Findings |
|---|---|---|
| Human AD brain (post mortem) | Proteomic and ICC studies [9,18,21,41,42,43,58,61] |
|
| Knockout mice (gad) | UCH-L1-null mutation [44,65] |
|
| APP/PS1, APP23/PS45 mice | Overexpression or peptide restoration of UCH-L1 [39,40,49] |
|
| Cell lines (HEK293, SH-SY5Y, N2a) | siRNA knockdown/overexpression [40,48,57,59,60] |
|
| Aplysia and hippocampal slices | UCH-L1 modulation [39,63,64] |
|
| AD Hallmark/ Mechanism | Main Findings | Relevance to AD |
|---|---|---|
| Amyloid-β plaques |
|
|
| Tau protein and NFTs |
|
|
| Synaptic dysfunction |
|
|
| Neuroinflammation/ Oxidative stress |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porchietto, E.; Morello, G.; Cicilese, G.; Rainero, I.; Rubino, E.; Tamagno, E.; Boschi, S.; Guglielmotto, M. UCH-L1 in Alzheimer’s Disease: A Crucial Player in Dementia-Associated Mechanisms. Int. J. Mol. Sci. 2025, 26, 9012. https://doi.org/10.3390/ijms26189012
Porchietto E, Morello G, Cicilese G, Rainero I, Rubino E, Tamagno E, Boschi S, Guglielmotto M. UCH-L1 in Alzheimer’s Disease: A Crucial Player in Dementia-Associated Mechanisms. International Journal of Molecular Sciences. 2025; 26(18):9012. https://doi.org/10.3390/ijms26189012
Chicago/Turabian StylePorchietto, Elisa, Giulia Morello, Giulia Cicilese, Innocenzo Rainero, Elisa Rubino, Elena Tamagno, Silvia Boschi, and Michela Guglielmotto. 2025. "UCH-L1 in Alzheimer’s Disease: A Crucial Player in Dementia-Associated Mechanisms" International Journal of Molecular Sciences 26, no. 18: 9012. https://doi.org/10.3390/ijms26189012
APA StylePorchietto, E., Morello, G., Cicilese, G., Rainero, I., Rubino, E., Tamagno, E., Boschi, S., & Guglielmotto, M. (2025). UCH-L1 in Alzheimer’s Disease: A Crucial Player in Dementia-Associated Mechanisms. International Journal of Molecular Sciences, 26(18), 9012. https://doi.org/10.3390/ijms26189012








