Potential Benefits of Glycine, Proline and Hydroxyproline on Growth and Flesh Quality of Mirror Carp (Cyprinus carpio var. specularis)
Abstract
1. Introduction
2. Results
2.1. Growth and Growth Hormones
2.2. Flesh Quality and Nutrient Profiles
2.3. Collagen Content
2.4. Targeted Amino Acids Quantification
2.5. Gene Expression
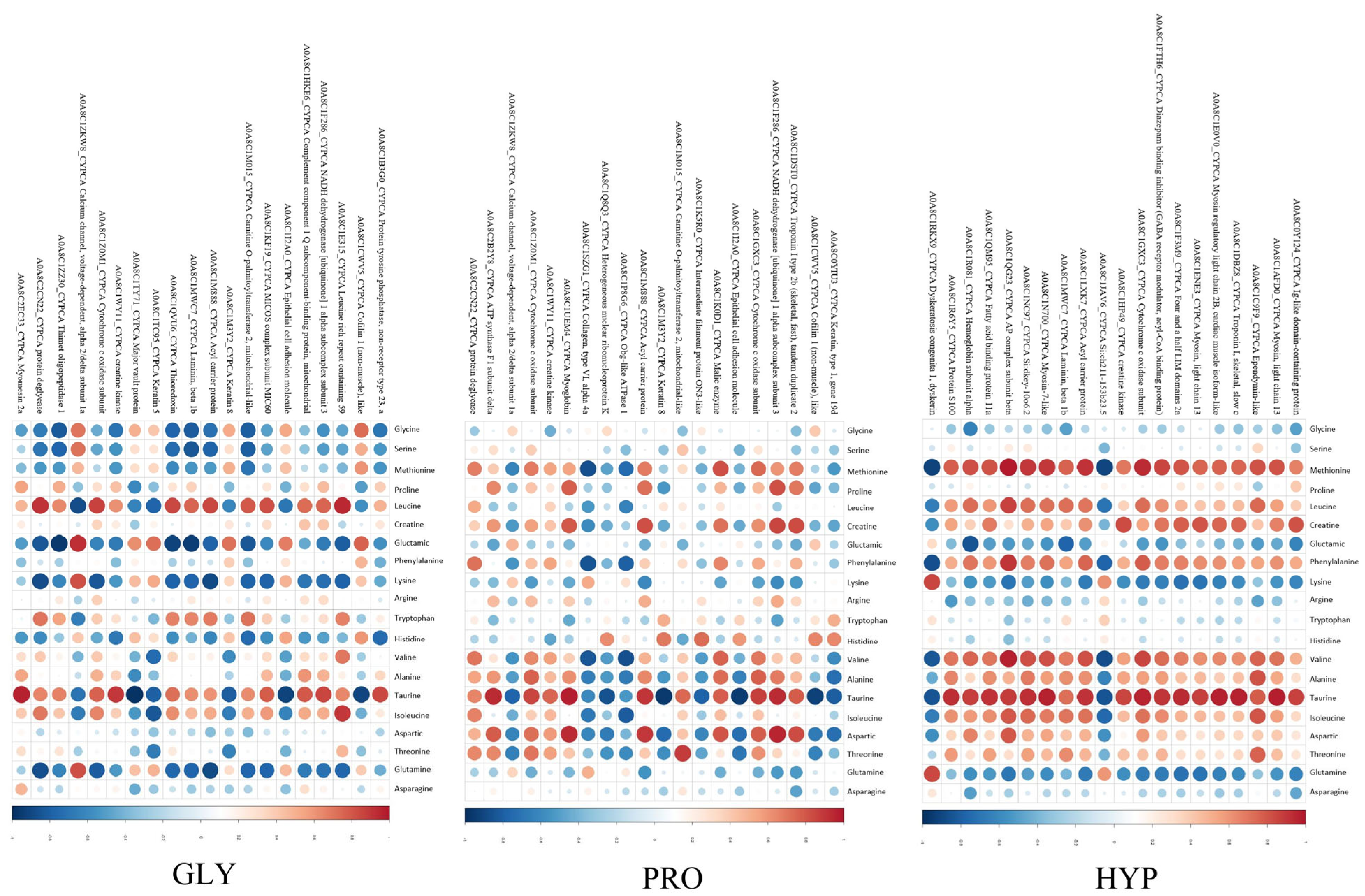
2.6. Proteomics Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Sampling
4.3. Measurements
4.3.1. Growth and Growth Hormones
4.3.2. Flesh Quality, Nutrient Profiles and Collagen Content
4.3.3. Targeted Amino Acid Quantification
4.3.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.3.5. Total Protein Extraction, TMT Labeling, and LC−MS/MS Proteomics Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Qiao, F.; Zhang, W.B.; Parisi, G.; Du, Z.Y.; Zhang, M.L. The flesh texture of teleost fish: Characteristics and interventional strategies. Rev. Aquac. 2024, 16, 508–535. [Google Scholar] [CrossRef]
- Periago, M.J.; Ayala, M.D.; López-Albors, O.; Abdel, I.; Martínez, C.; García-Alcázar, A.; Ros, G.; Gil, F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture 2005, 249, 175–188. Aquaculture 2005, 249, 175–188. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, H.; Peng, Z.; Yu, J.; Yan, L.; Wang, W.; Ren, T.; Han, Y. Comparison of nutritional quality, flesh quality, muscle cellularity, and expression of muscle growth-related genes between wild and recirculating aquaculture system (RAS)-farmed black rockfish (Sebastes schlegelii). Aquac. Int. 2023, 31, 2263–2280. [Google Scholar] [CrossRef]
- Rincón, L.; Castro, P.L.; Álvarez, B.; Hernández, M.D.; Álvarez, A.; Claret, A.; Guerrero, L.; Ginés, R. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture 2016, 451, 195–204. [Google Scholar] [CrossRef]
- Moreno, H.M.; Montero, M.P.; G’omez-Guill’en, M.C.; Fern’andez-Martín, F.; Mørkøre, T.; Borderías, J. Collagen characteristics of farmed Atlantic salmon with firm and soft fillet texture. Food Chem. 2012, 134, 678–685. [Google Scholar] [CrossRef]
- Hagen, Ø.; Solberg, C.; Sirnes, E.; Johnston, I.A. Biochemical and structural factors contributing to seasonal variation in the texture of farmed Atlantic halibut (Hippoglossus hippoglossus L.) flesh. J. Agric. Food Chem. 2007, 55, 5803–5808. [Google Scholar] [CrossRef]
- Hansen, A.C.; Rosenlund, G.; Karlsen, Ø.; Koppe, W.; Hemre, G.I. Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I—Effects on growth and protein retention. Aquaculture 2007, 272, 599–611. [Google Scholar] [CrossRef]
- Li, X.; Bickerdike, R.; Lindsay, E.; Campbell, P.; Nickell, D.; Dingwall, A.; Johnston, I.A. Hydroxylysyl pyridinoline cross-link concentration affects the textural properties of fresh and smoked Atlantic salmon (Salmo salar L.) flesh. J. Agric. Food Chem. 2005, 53, 6844–6850. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Peng, D.; Liang, X.F.; Chai, F.; Tang, S.; Li, J. Effect of dietary hydroxyproline supplementation on Chinese perch (Siniperca chuatsi) fed with fish meal partially replaced by fermented soybean meal. Aquaculture 2022, 547, 737454. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, G. Nutritionally essential amino acids. Adv. Nutr. 2018, 9, 849–851. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Myllyharju, J.; Kivirikko, K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Wu, Z.; Hou, Y.; Dai, Z.; Hu, C.; Wu, G. Metabolism, nutrition, and redox signaling of Hydroxyproline. Antioxid. Redox Signal. 2019, 30, 674–682. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological Actions of Taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 2009, 31, 1–58. [Google Scholar] [CrossRef]
- Tesseraud, S.; Everaert, N.; Boussaid-Om Ezzine, S.; Collin, A.; M’etayer-Coustard, S.; Berri, C. Manipulating tissue metabolism by amino acids. World’s Poult. Sci. J. 2011, 67, 243–252. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Yan, J.J.; Rauan, A.; Ji, H. Impact of iron supplementation on media bed aquaponic system: Focusing on growth performance, fatty acid composition and health status of juvenile mirror carp (Cyprinus carpio var. specularis), production of lettuce (Lactuca sativa var. ramosa Hort), water quality and nitrogen utilization rate. Aquaculture 2024, 582, 740509. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef]
- Kasai, K.; Suzuki, H.; Nakamura, T.; Shiina, H.; Shimoda, S.I. Glycine stimulated growth hormone release in man. Eur. J. Endocrinol. 1980, 93, 283–286. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Moutou, K.A.; Conceiçao, L.E.C.; Engrola, S.; Fernandes, J.M.O.; Johnston, I.A. What determines growth potential and juvenile quality of farmed fish species? Rev. Aquac. 2013, 5, S168–S193. [Google Scholar] [CrossRef]
- Xie, S.W.; Tian, L.X.; Jin, Y.; Yang, H.J.; Liang, G.Y.; Liu, Y.J. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture 2014, 418–419, 159–164. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, W.; Tian, L.; Niu, J.; Liu, Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, anti-oxidative and immune ability of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 55, 233–241. [Google Scholar] [CrossRef]
- Xie, S.; Tian, L.; Niu, J.; Liang, G.; Liu, Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, and antioxidative ability of grass carp, Ctenopharyngodon idella. Fish Physiol. Biochem. 2017, 43, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Deng, K.; Zhang, W.; Mai, K. Interactions of dietary vitamin C and proline on growth performance, anti-oxidative capacity and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 2020, 528, 735558. [Google Scholar] [CrossRef]
- Zhang, K.K.; Mai, K.S.; Xu, W.; Zhou, H.H.; Liufu, Z.G.; Zhang, Y.J.; Peng, M.; Ai, Q.H. Proline with or without hydroxyproline influences collagen concentration and regulates prolyl 4-hydroxylase alpha (I) gene expression in juvenile turbo (Scophthalmus maximus L.). J. Ocean Univ. China 2015, 14, 541–548. [Google Scholar] [CrossRef]
- Sunde, J.; Taranger, G.L.; Rungruangsak-Torrissen, K. Digestive protease activitiesand free amino acids in white muscle as indicators for feed conversion efficiencyand growth rate in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2001, 25, 335–345. [Google Scholar] [CrossRef]
- Aksnes, A.; Mundheim, H.; Toppe, J.; Albrektsen, S. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture 2008, 275, 242–249. [Google Scholar] [CrossRef]
- Liu, Y.Z.; He, G.; Wang, Q.C.; Mai, K.S.; Xu, W.; Zhou, H.H. Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 2014, 433, 476–480. [Google Scholar] [CrossRef]
- Wei, Z.; Ma, J.; Pan, X.; Mu, H.; Li, J.; Shentu, J.; Zhang, W.; Mai, K. Dietary hydroxyproline improves the growth and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 2016, 464, 497–504. [Google Scholar] [CrossRef]
- Zhang, K.; Ai, Q.; Mai, K.; Xu, W.; Liufu, Z.; Zhang, Y.; Peng, M. Effects of dietaryhydroxyproline on growth performance, body composition, hydroxyproline andcollagen concentrations in tissues in relation to prolyl 4-hydroxylase α (I) geneexpression of juvenile turbot, Scophthalmus maximus L. fed high plant protein diets. Aquaculture 2013, 404, 77–84. [Google Scholar] [CrossRef]
- Song, D.; Yun, Y.; He, Z.; Mi, J.; Wang, L.; Jin, M.; Zhou, Q.; Nie, G. Fillet texture, physicochemical indexes, muscle cellularity and molecular expression in muscle of Yellow River carp (Cyprinus carpio haematopterus) in response to dietary hydroxyproline supplementation. Aquaculture 2022, 549, 737783. [Google Scholar] [CrossRef]
- Aksnes, A.; Hope, B.; Jönsson, E.; Björnsson, B.T.; Albrektsen, S. Size-fractionated fishhydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed highplant protein diets. I: Growth, growth regulation and feed utilization. Aquaculture 2006, 261, 305–317. [Google Scholar] [CrossRef]
- Rezaei, R.; Wang, W.; Wu, Z.; Dai, Z.; Wang, J.; Wu, G. Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J. Anim. Sci. Biotechnol. 2013, 4, 7. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.; Kuang, S.; Jiang, J.; Li, S.; Tang, L.; Zhou, X. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef]
- Johnsen, C.A.; Hagen, Ø.; Adler, M.; Jönsson, E.; Kling, P.; Bickerdike, R.; Solberg, C.; Björnsson, B.T.; Bendiksen, E.Å. Effects of feed, feeding regime and growth rate on flesh quality, connective tissue and plasma hormones in farmed Atlantic salmon (Salmo salar L.). Aquaculture 2011, 318, 343–354. [Google Scholar] [CrossRef]
- Immonen, K.; Ruusunen, M.; Hissa, K.; Puolanne, E. Bovine muscle glycogen concentration in relation to finishing diet, slaughter and ultimate pH. Meat Sci. 2000, 55, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bjørnevik, M.; Hansen, H.; Roth, B.; Foss, A.; Imsland, A. Effects of starvation, subsequent feeding and photoperiod on flesh quality in farmed cod (Gadus morhua). Aquac. Nutr. 2017, 23, 285–292. [Google Scholar] [CrossRef]
- Lerfall, J.; Roth, B.; Skare, E.F.; Henriksen, A.; Betten, T.; Dziatkowiak-Stefaniak, M.A.; Rotabakk, B.T. Pre-mortem stress and the subsequent effect on flesh quality of pre-rigor filleted Atlantic salmon (Salmo salar L.) during ice storage. Food Chem. 2015, 175, 157–165. [Google Scholar] [CrossRef]
- Suárez, M.; Abad, M.; Ruiz-Cara, T.; Estrada, J.; García-Gallego, M. Changes in muscle collagen content during post mortem storage of farmed sea bream (Sparus aurata): Influence on textural properties. Aquac. Int. 2005, 13, 315–325. [Google Scholar] [CrossRef]
- Purslow, P.P. New developments on the role of intramuscular connective tissue in meat toughness. Annu. Rev. Food Sci. Technol. 2014, 5, 133–153. [Google Scholar] [CrossRef]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef]
- Ospina-Rojas, I.C.; Murakami, A.E.; Eyng, C.; Nunes, R.V.; Duarte, C.R.A.; Vargas, M.D. Commercially available amino acid supplementation of low-protein diets for broiler chickens with different ratios of digestible glycine+serine:lysine. Poult. Sci. 2012, 91, 3148–3155. [Google Scholar] [CrossRef]
- Corzo, A.; Kidd, M.T.; Burnham, D.J.; Kerr, B.J. Dietary glycine needs of broiler chicks. Poult. Sci. 2004, 83, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Rong, H.; Lin, J.L.; Yuan, Y.Y.; Yu, J.; Yu, C.Q.; You, C.H.; Wang, S.Q.; Sun, Z.J.; Wen, X.B. Enhancement of collagen deposition in swim bladder of Chu’s croaker (Nibea coibor) by proline: View from in-vitro and in-vivo study. Aquaculture 2020, 523, 735175. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef]
- Garbutt, J.T.; Lack, L.; Tyor, M.P. The enterohepatic circulation of bile salts in gastrointestinal disorders. Am. J. Med. 1971, 51, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Pasantes, M.H.; Quesada, O.; Moran, J. Taurine: An osmolyte in mammalian tissues. In Taurine 3; Springer: Berlin/Heidelberg, Germany, 1998; Volume 442, pp. 209–217. [Google Scholar] [CrossRef]
- Miyazaki, T.; Honda, A.; Ikegami, T.; Matsuzaki, Y. The role of taurine on skeletal muscle cell differentiation. Adv. Exp. Med. Biol. 2013, 776, 321–328. [Google Scholar] [CrossRef]
- An, W.; Huang, Z.; Mao, Z.; Jia, G.; Zhao, H.; Liu, G.; Chen, X. Taurine promotes muscle fiber type transformation through CaN/NFATc1 signaling in porcine myoblasts. J. Cell. Physiol. 2023, 238, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 2009, 6, 26. [Google Scholar] [CrossRef]
- Zhang, S.R.; Chen, X.L.; Huang, Z.Q.; Chen, D.W.; Yu, B.; Chen, H.; He, J.; Luo, J.Q.; Zheng, P.; Yu, J.; et al. Leucine promotes porcine myofibre type transformation from fast-twitch to slow-twitch through the protein kinase B(Akt)/forkhead box 1 signalling pathway and microRNA-27a. Br. J. Nutr. 2019, 121, 1–8. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, Y.J.; Choi, Y.M.; Kim, B.C.; Yoo, B.H.; Hong, K.C. Possible muscle fiber characteristics in the selection for improvement in porcine lean meat production and quality. Asian-Australas. J. Anim. Sci. 2008, 21, 1529–1534. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar] [CrossRef]
- Ismaeel, A.; Kim, J.S.; Kirk, J.S.; Smith, R.S.; Bohannon, W.T.; Koutakis, P. Role of transforming growth factor-β in skeletal muscle fibrosis: A review. Int. J. Mol. Sci. 2019, 20, 2446. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.M.; Ma, L.L.; Ji, H.; Li, Z.F.; Wang, G.J.; Xie, J.; Yu, D.G.; Kaneko, G.; Tian, J.J.; Zhang, K.; et al. Smad4-dependent regulation of type I collagen expression in the muscle of grass carp fed with faba bean. Gene 2019, 685, 32–41. [Google Scholar] [CrossRef]
- Long, D.L.; Loeser, R.F. p38 gamma mitogen-activated protein kinase suppresses chondrocyte production of MMP-13 in response to catabolic stimulation. Osteoarthr. Cartil. 2010, 18, 1203–1210. [Google Scholar] [CrossRef]
- Tan, J.; Tong, B.D.; Wu, Y.J.; Xiong, W. MicroRNA-29 mediates TGFβ1-induced extracellular matrix synthesis by targeting wnt/β-catenin pathway in human orbital fibroblasts. Int. J. Clin. Exp. Pathol. 2014, 7, 7571–7577. [Google Scholar]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Shegogue, D.; Trojanowska, M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J. Biol. Chem. 2004, 279, 23166–23175. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Babare, I.H.; Reynolds, J.; Mawson, R.F. Freezing and thawing rate effects on drip loss from samples of pork. Meat Sci. 1999, 53, 149–158. [Google Scholar] [CrossRef]
- Wen, M.L.; Wu, P.; Jiang, W.D.; Liu, Y.; Wu, C.M.; Zhong, C.B.; Li, S.W.; Tang, L.; Feng, L.; Zhou, X.Q. Dietary threonine improves muscle nutritional value and muscle hardness associated with collagen synthesis in grass carp (Ctenopharyngodon idella). Food Chem. 2023, 422, 136223. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.; Liu, L.; Li, H.; Zhu, H. Aloe gel polysaccharides and ascorbic acid promote collagen synthesis in mirror carp (Cyprinus carpio var. specularis). Aquaculture 2025, 602, 742291. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Zhang, Z.; Payne, S.H.; Zhang, B.; McDermott, J.E.; Zhou, J.Y.; Petyuk, V.A.; Chen, L.; Ray, D.; et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 2016, 166, 755. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING V9.1: Protein-Protein Interaction Networks, with Increased Coverage and Integration. Nucleic Acids Res. 2012, 41, D808–D815. [Google Scholar] [CrossRef] [PubMed]
- Fazelan, Z.; Hoseini, S.M.; Yousefi, M.; Khalili, M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary eucalyptol administration on antioxidant and inflammatory genes in common carp (Cyprinus carpio) exposed to ambient copper. Aquaculture 2020, 520, 734988. [Google Scholar] [CrossRef]
- Khorshidi, Z.; Paknejad, H.; Sodagar, M.; Hajimoradloo, A.; Shekarabi, S.P.H. Effect of a commercial multi-effect additive (Biotronic® Top3) on growth performance, digestive enzymes, and intestinal barrier gene expression in common carp (Cyprinus carpio). Aquaculture 2022, 560, 738588. [Google Scholar] [CrossRef]
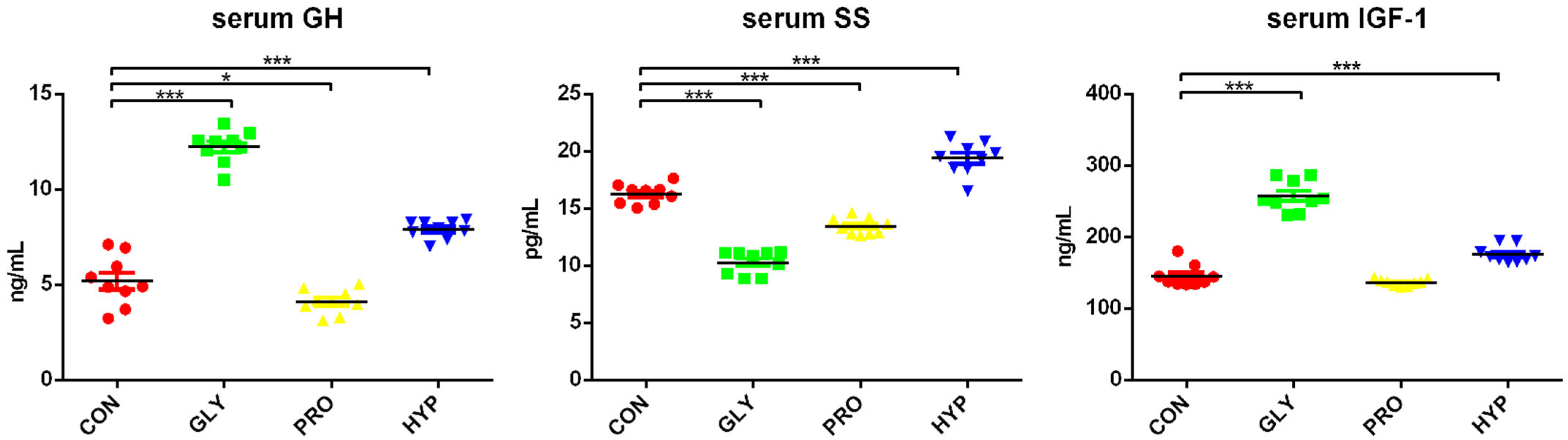

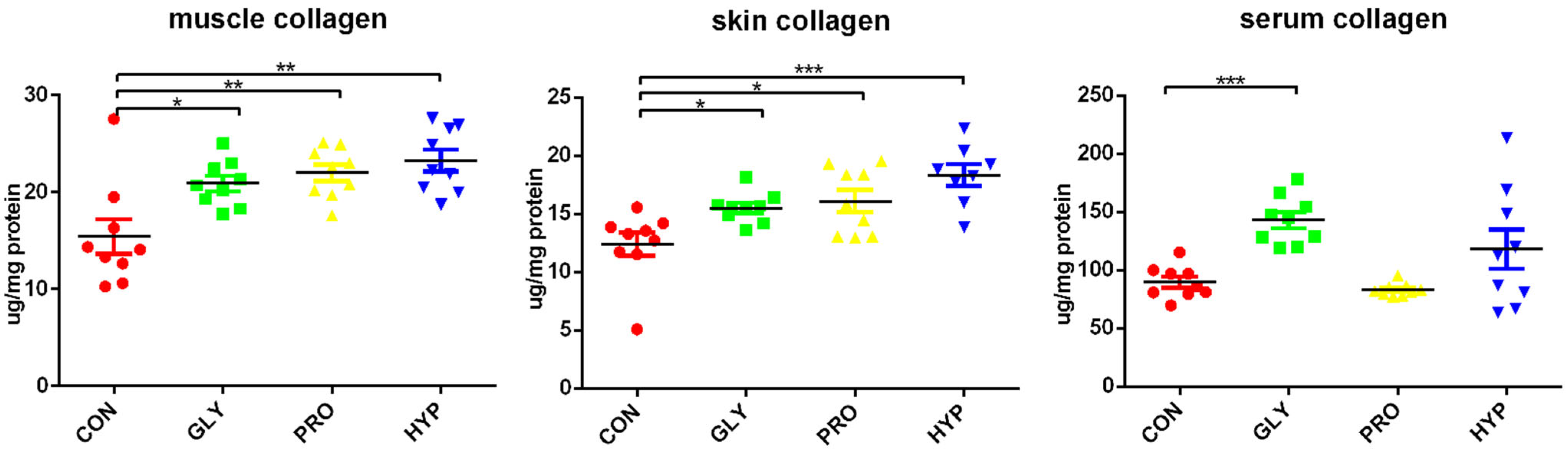
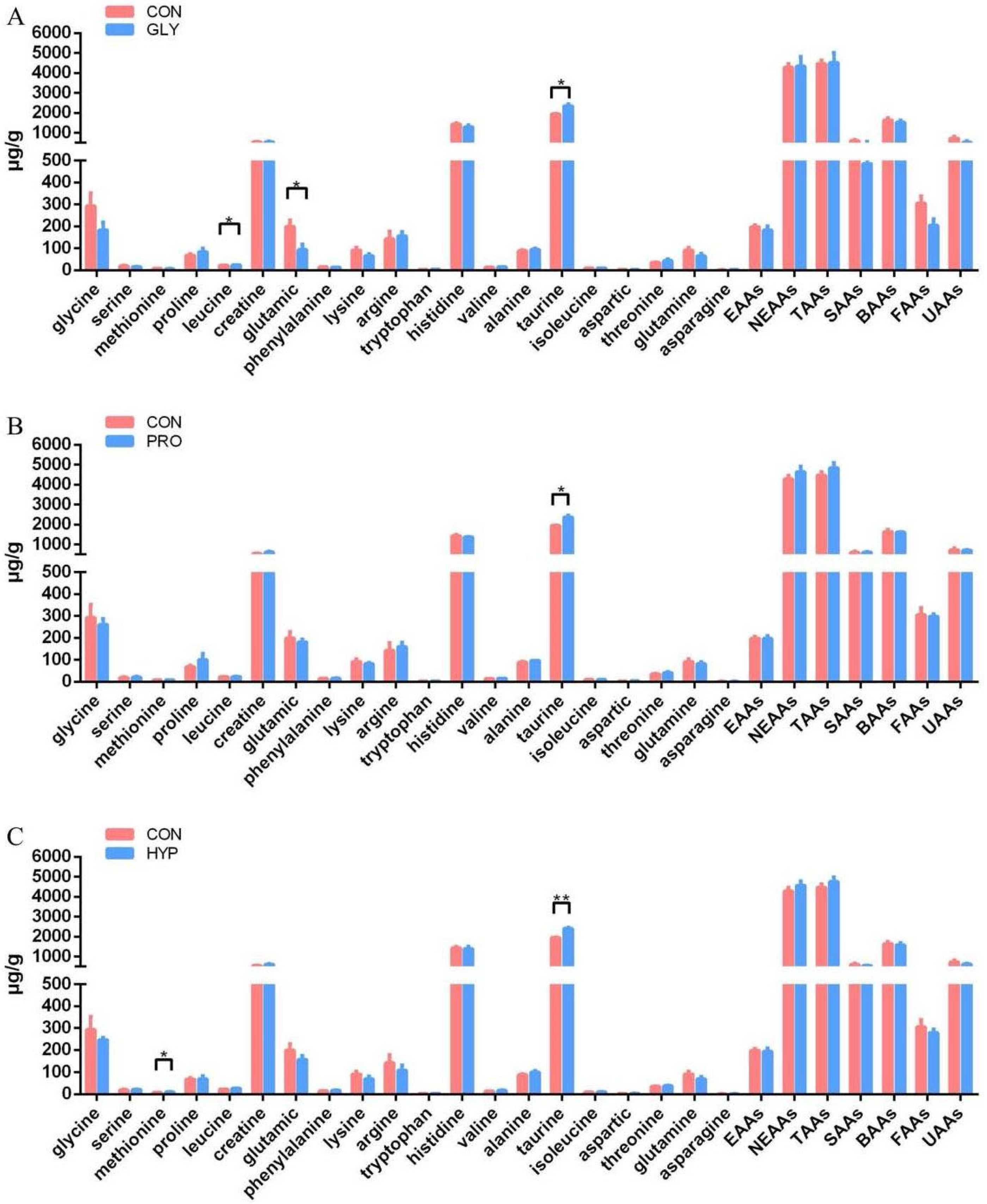
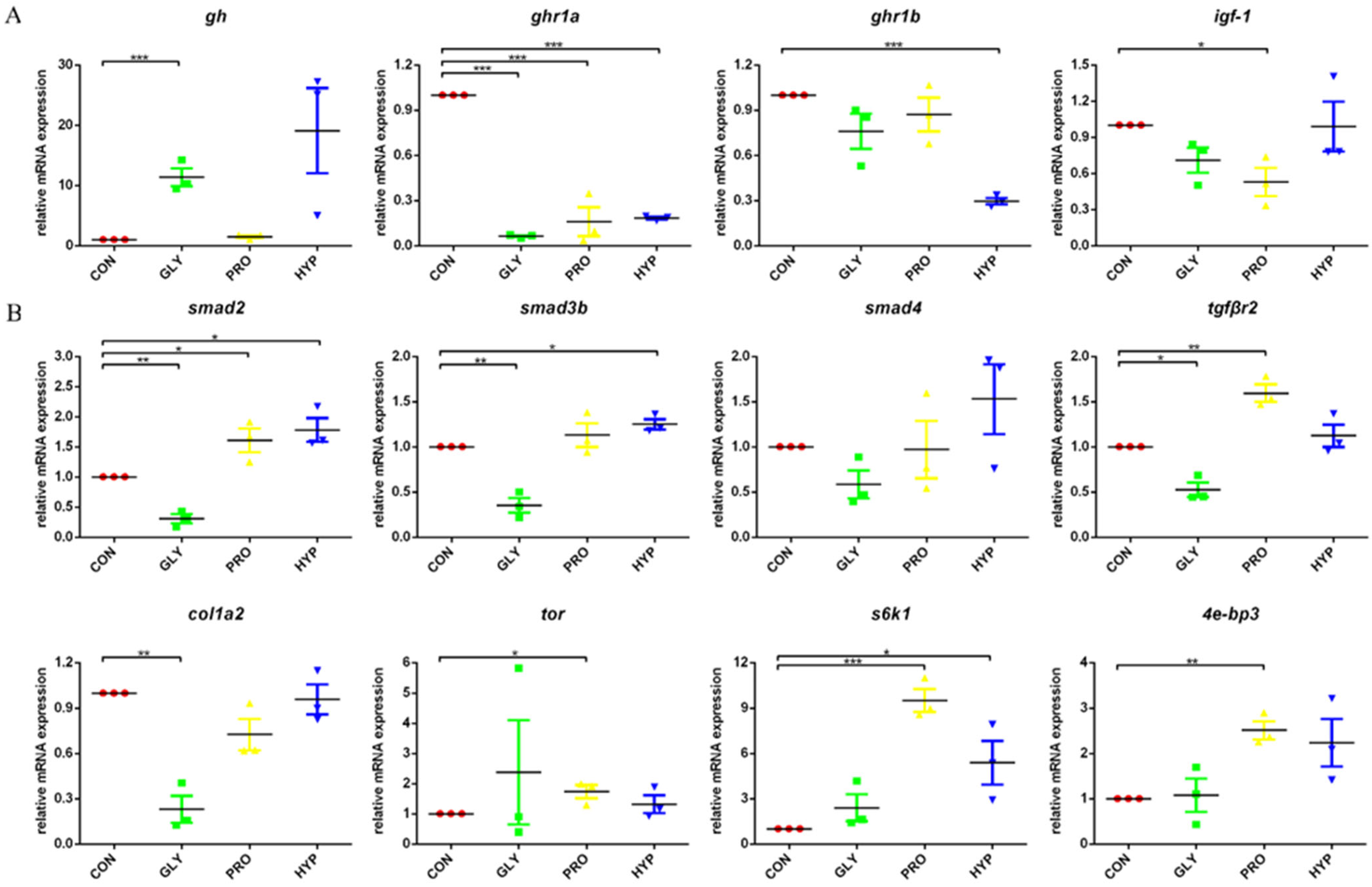

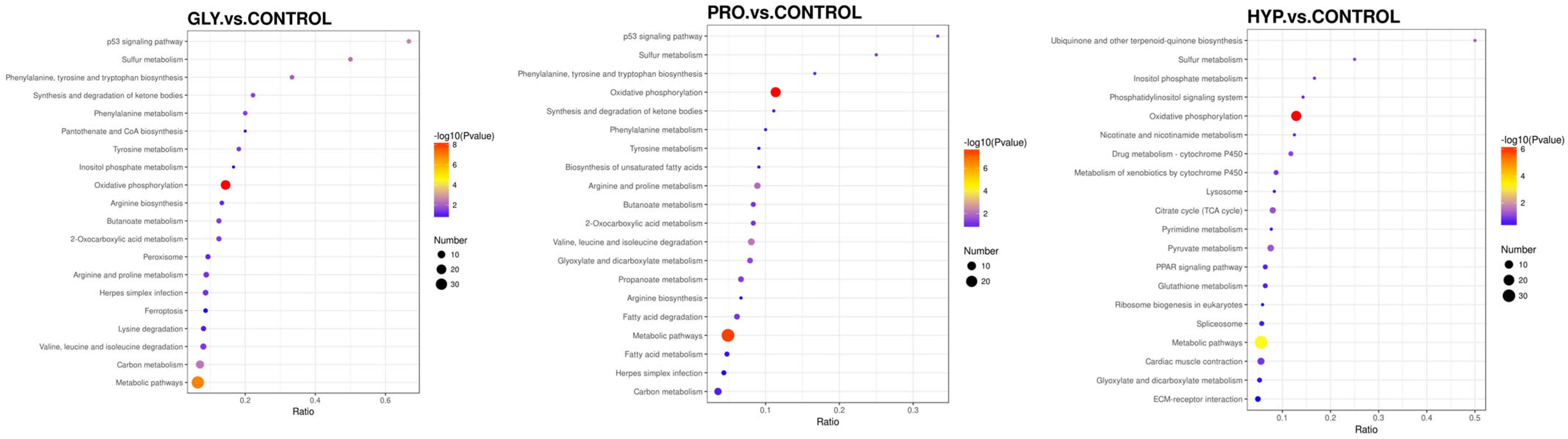
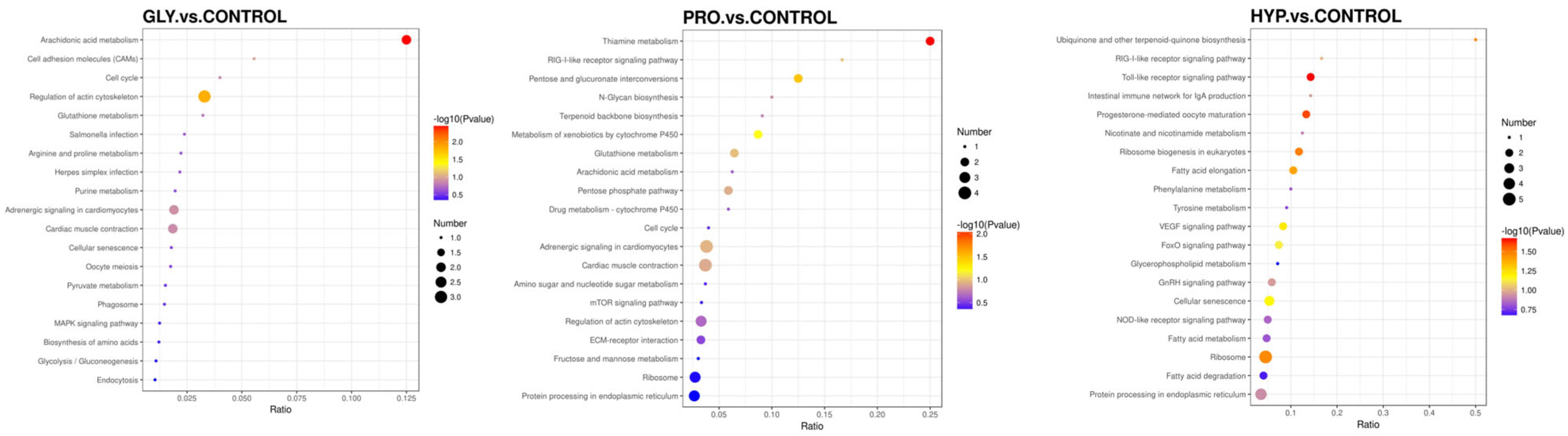
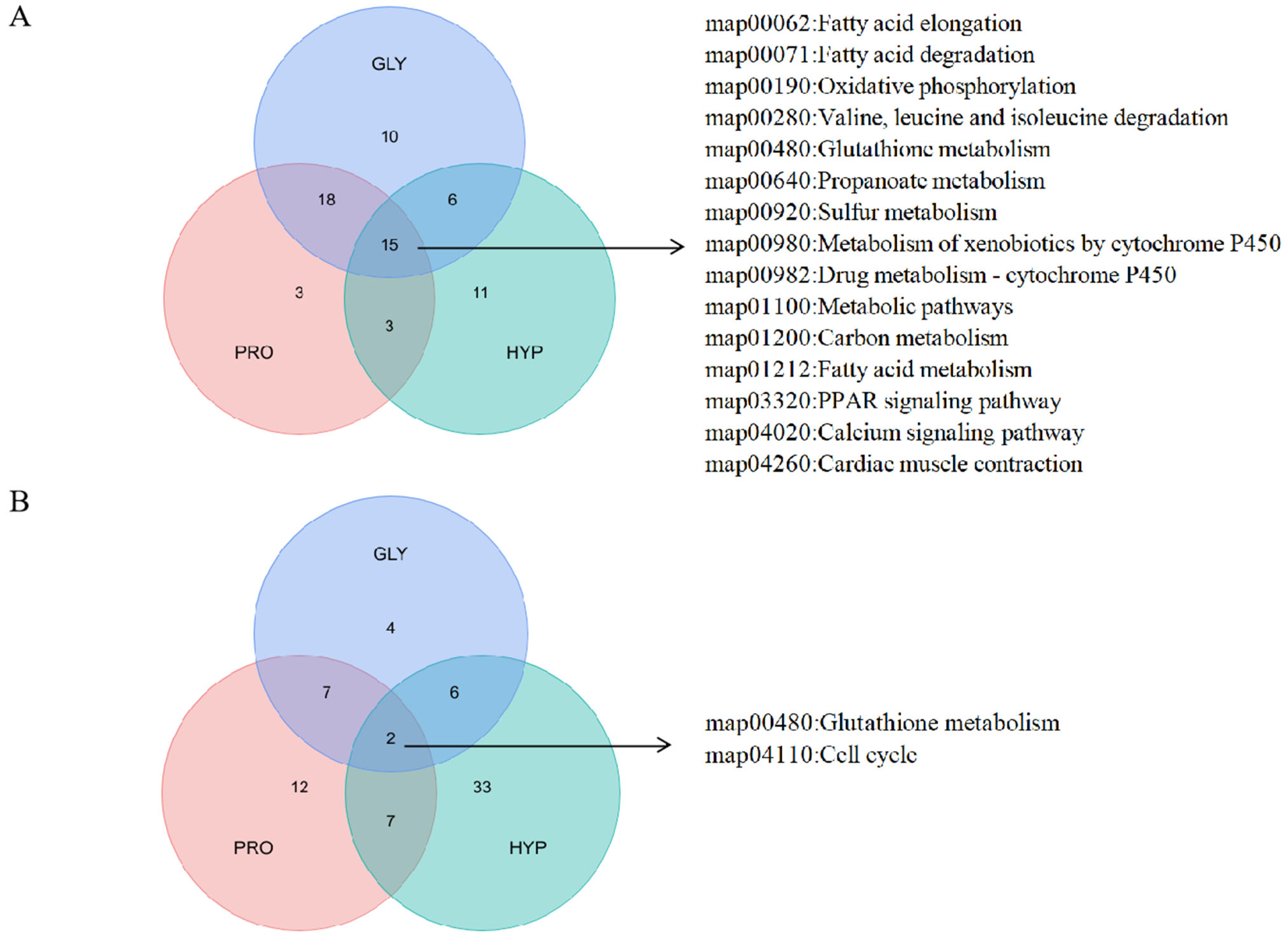
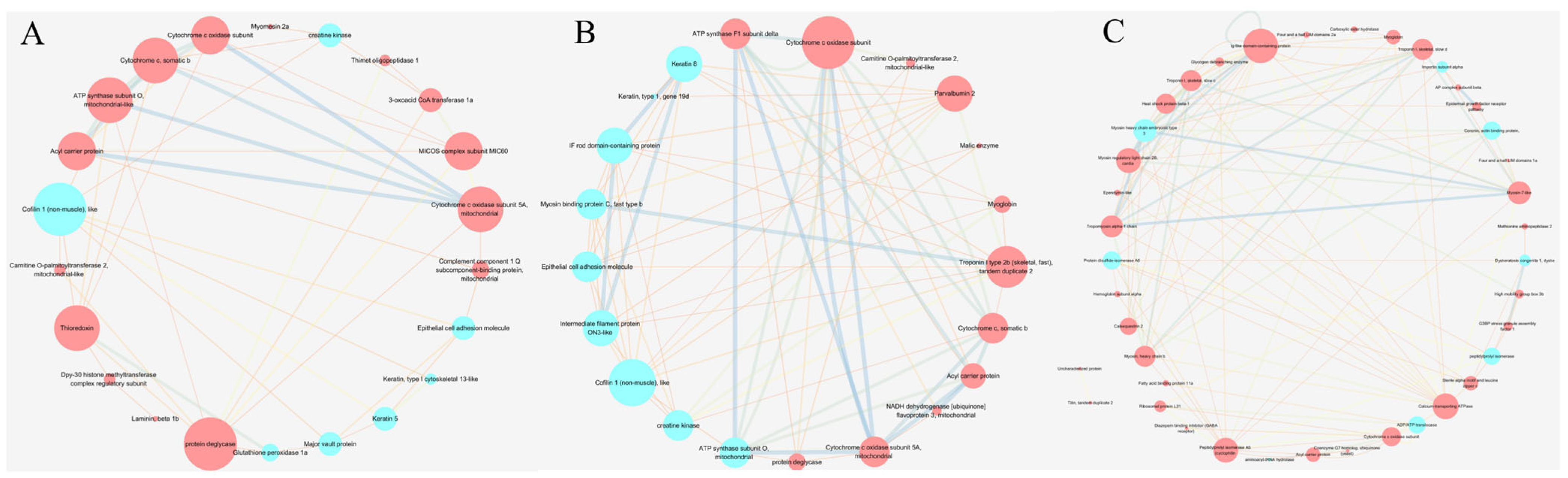
| Items | CON | GLY | PRO | HYP | p-Value |
|---|---|---|---|---|---|
| Survival rate (%) | 100 | 100 | 100 | 100 | - |
| Initial weight (g) | 175.00 ± 2.50 | 177.50 ± 11.46 | 167.50 ± 2.50 | 177.50 ± 11.45 | 0.450 |
| Final weight (g) | 309.83 ± 17.85 | 315.52 ± 20.27 | 299.07 ± 5.54 | 295.23 ± 17.65 | 0.446 |
| Weight gain (g) | 134.83 ± 15.37 | 138.02 ± 16.09 | 131.58 ± 5.47 | 117.73 ± 14.99 | 0.346 |
| Weight gain rate (%) | 76.97 ± 7.67 | 77.97 ± 10.27 | 78.57 ± 3.66 | 66.54 ± 9.77 | 0.307 |
| Specific growth rate (%) | 1.32 ± 0.10 | 1.34 ± 0.14 | 1.35 ± 0.05 | 1.18 ± 0.14 | 0.296 |
| Feed conversion rate | 0.88 ± 0.10 | 0.87 ± 0.11 | 0.90 ± 0.04 | 1.01 ± 0.12 | 0.295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Li, H.; Wang, X.; Liu, L.; Zhu, H. Potential Benefits of Glycine, Proline and Hydroxyproline on Growth and Flesh Quality of Mirror Carp (Cyprinus carpio var. specularis). Int. J. Mol. Sci. 2025, 26, 9011. https://doi.org/10.3390/ijms26189011
Zhang R, Li H, Wang X, Liu L, Zhu H. Potential Benefits of Glycine, Proline and Hydroxyproline on Growth and Flesh Quality of Mirror Carp (Cyprinus carpio var. specularis). International Journal of Molecular Sciences. 2025; 26(18):9011. https://doi.org/10.3390/ijms26189011
Chicago/Turabian StyleZhang, Rong, Huijuan Li, Xiaowen Wang, Lili Liu, and Hua Zhu. 2025. "Potential Benefits of Glycine, Proline and Hydroxyproline on Growth and Flesh Quality of Mirror Carp (Cyprinus carpio var. specularis)" International Journal of Molecular Sciences 26, no. 18: 9011. https://doi.org/10.3390/ijms26189011
APA StyleZhang, R., Li, H., Wang, X., Liu, L., & Zhu, H. (2025). Potential Benefits of Glycine, Proline and Hydroxyproline on Growth and Flesh Quality of Mirror Carp (Cyprinus carpio var. specularis). International Journal of Molecular Sciences, 26(18), 9011. https://doi.org/10.3390/ijms26189011





