Hyperuricemia in Chronic Kidney Disease: Emerging Pathophysiology and a Novel Therapeutic Strategy
Abstract
1. Introduction
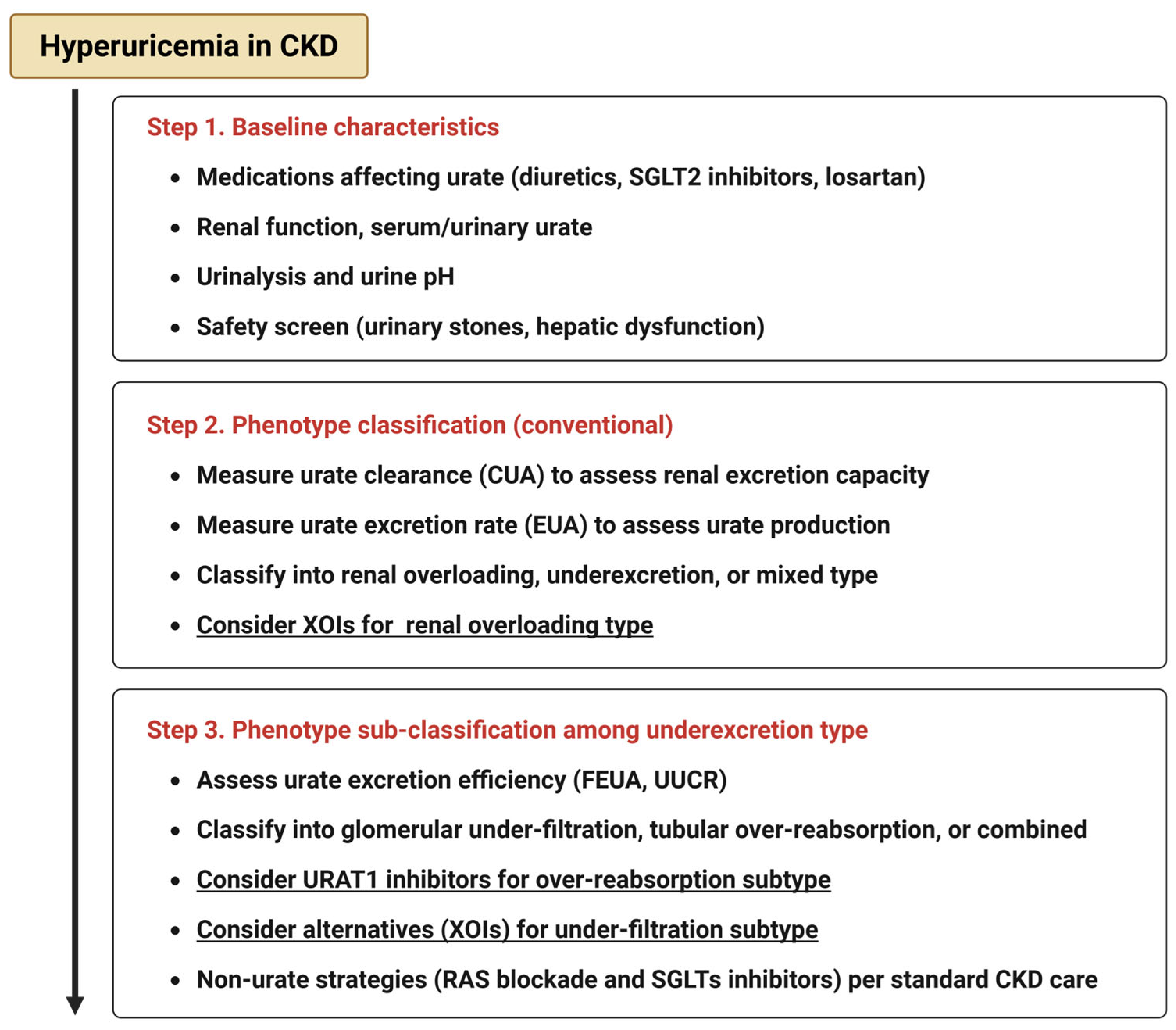
2. Classification of Hyperuricemia in CKD
3. Mechanistic Links Between Hyperuricemia and CKD Progression
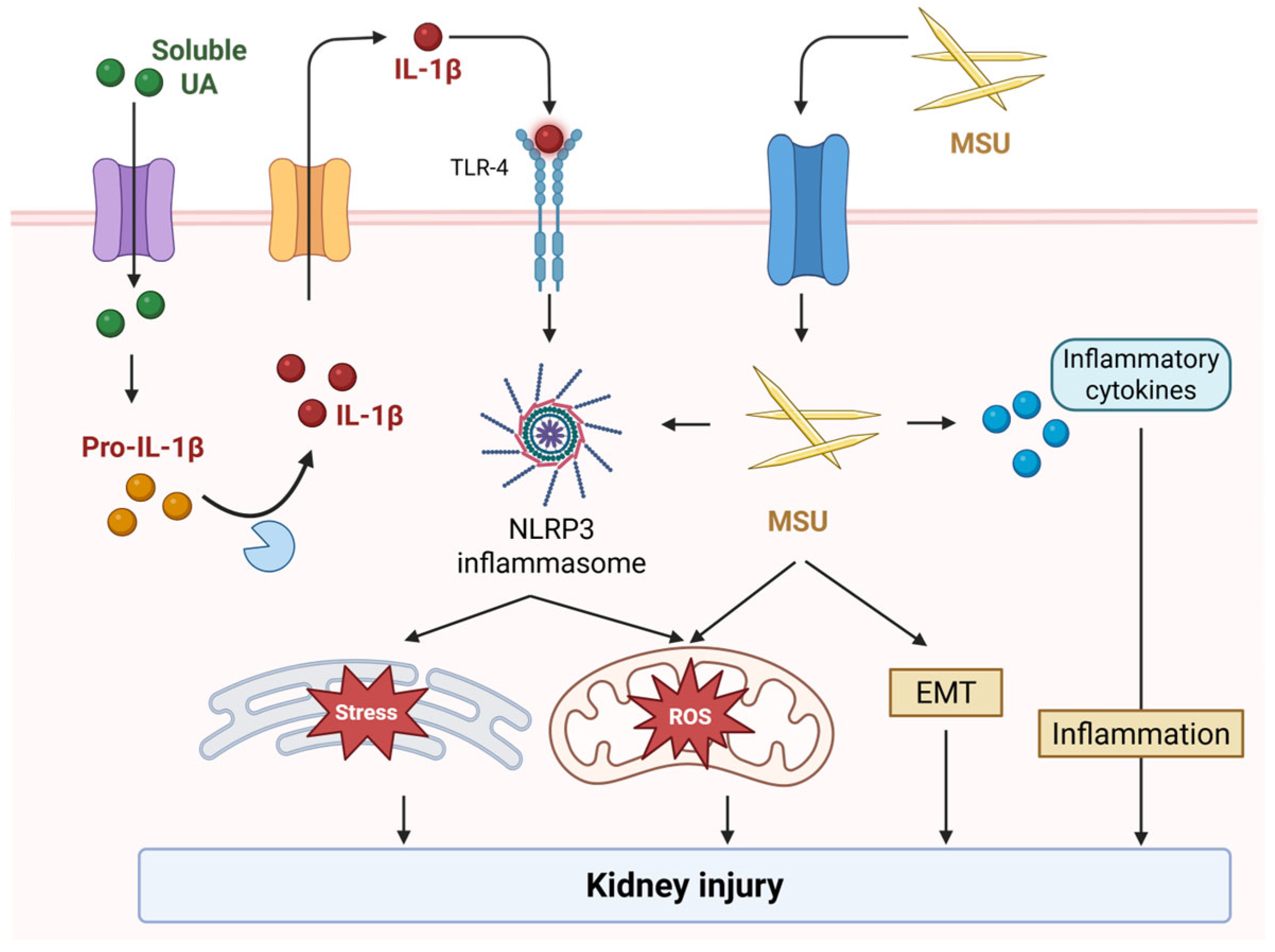
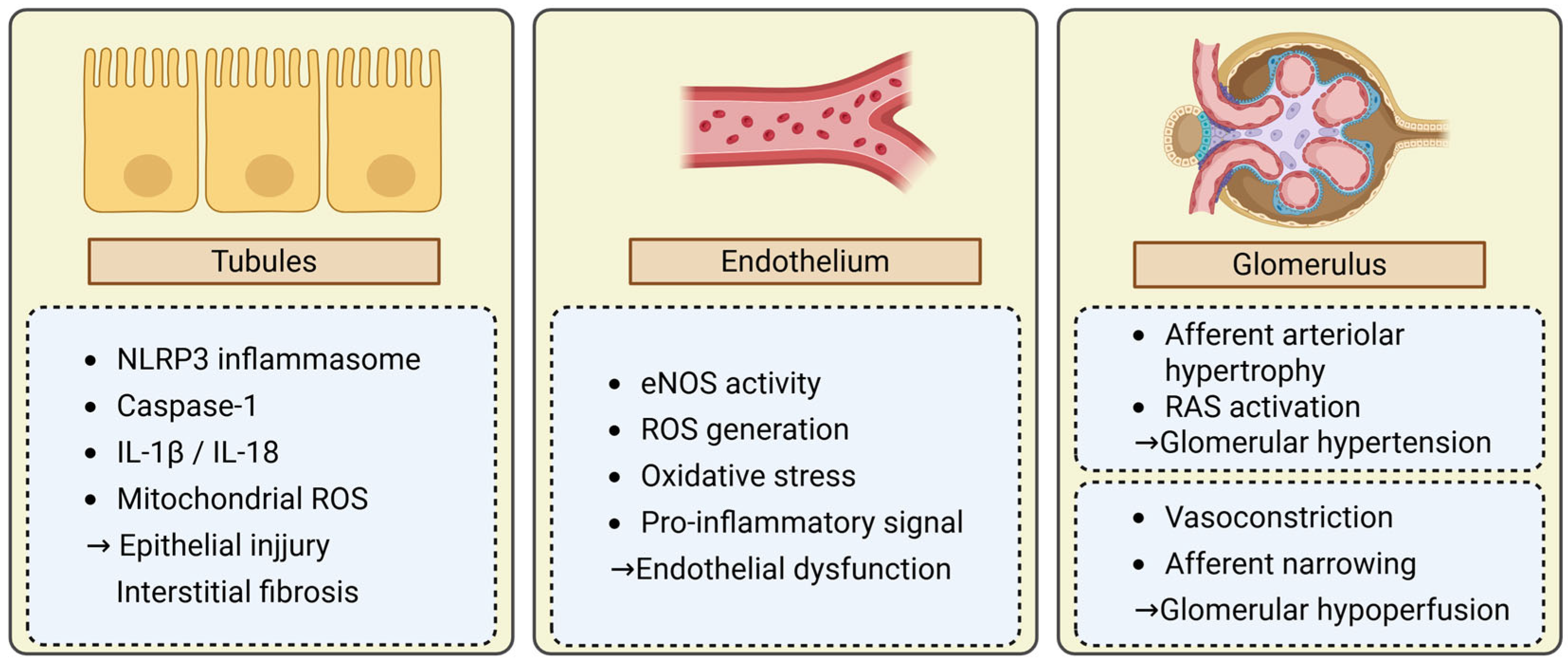
3.1. Tubular Injury
3.2. Endothelial Dysfunction
3.3. Glomerular Hemodynamic Alterations
4. Clinical Evidence of Hyperuricemia and CKD Progression
5. Therapeutic Approaches to Hyperuricemia in CKD
6. Novel and Emerging Therapeutic Strategies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| SURI | Selective urate reabsorption inhibitor |
| URAT1 | Urate transporter 1 |
| GLUT9 | Glucose transporter 9 |
| ABCG2 | ATP-binding cassette transporter G2 |
| GFR | Glomerular filtration rate |
| FEUA | Fractional excretion of uric acid |
| UACR | Urinary uric acid-to-creatinine ratio |
| MSU | Monosodium urate |
| NLRP3 | NOD-, LRR-, and pyrin domain–containing 3 |
| IL | Interleukin |
| eNOS | Endothelial nitric oxide synthase |
| NO | Nitric oxide |
| RO | Reactive oxygen species |
| HIF | Hypoxia-inducible factor |
| RAS | Renin–angiotensin system |
| SUCR | Serum uric acid-to-creatinine ratio |
| ULT | Urate-lowering therapy |
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Horio, M.; Watanabe, T.; Iseki, K.; Yamagata, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Moriyama, T.; Ando, Y.; et al. Prevalence of chronic kidney disease in the Japanese general population. Clin. Exp. Nephrol. 2009, 13, 621–630. [Google Scholar] [CrossRef]
- Marreiros, C.; Viegas, C.; Simes, D. Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 16114. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takata, T.; Hanada, H.; Taniguchi, S.; Hamada, S.; Mae, Y.; Iyama, T.; Kanda, T.; Isomoto, H. Zinc deficiency induces hypertension by paradoxically amplifying salt sensitivity under high salt intake in mice. Clin. Exp. Nephrol. 2024, 28, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Sofue, T.; Nakagawa, N.; Kanda, E.; Nagasu, H.; Matsushita, K.; Nangaku, M.; Maruyama, S.; Wada, T.; Terada, Y.; Yamagata, K.; et al. Prevalences of hyperuricemia and electrolyte abnormalities in patients with chronic kidney disease in Japan: A nationwide, cross-sectional cohort study using data from the Japan Chronic Kidney Disease Database (J-CKD-DB). PLoS ONE 2020, 15, e0240402. [Google Scholar] [CrossRef]
- Piani, F.; Johnson, R.J. Does gouty nephropathy exist, and is it more common than we think? Kidney Int. 2021, 99, 31–33. [Google Scholar] [CrossRef]
- Takata, T.; Isomoto, H. Back to the physiology: Renal tubular handling of uric acid and emerging strategies for managing hyperuricemia. Intern. Med. 2025, in press. [CrossRef]
- Takata, T.; Mae, Y.; Hoi, S.; Hisatome, I.; Isomoto, H. Redefining hyperuricemia in chronic kidney disease: A focus on tubulo-glomerular urate handling. Hypertens. Res. 2025, in press. [CrossRef]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; Takada, Y.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef]
- Hisatome, I.; Ichida, K.M.I. Japanese Society of Gout and Uric & Nucleic Acids 2019 Guidelines for Management of Hyperuricemia and Gout 3rd edition. Gout Uric Nucleic Acids 2020, 44, 1–40. [Google Scholar]
- Kuwabara, M. Hyperuricemia, Cardiovascular Disease, and Hypertension. Pulse 2015, 3, 242–252. [Google Scholar] [CrossRef]
- Wiederkehr, M.R.; Moe, O.W. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clin. Rev. Bone Min. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef]
- Kurts, C. A crystal-clear mechanism of chronic kidney disease. Kidney Int. 2013, 84, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Kim, S.M.; Kim, Y.G.; Lee, S.H.; Moon, J.Y. Uric acid and inflammation in kidney disease. Am. J. Physiol. Renal Physiol. 2020, 318, F1327–F1340. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Chen, X.; Ouyang, J.; Lin, D.; Wu, Q.; Fu, X.; Quan, H.; Wang, X.; Wu, S.; et al. The pathogenic mechanism of monosodium urate crystal-induced kidney injury in a rat model. Front. Endocrinol. 2024, 15, 1416996. [Google Scholar] [CrossRef] [PubMed]
- Sellmayr, M.; Hernandez Petzsche, M.R.; Ma, Q.; Krüger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792. [Google Scholar] [CrossRef]
- Yang, S.; Liu, H.; Fang, X.-M.; Yan, F.; Zhang, Y. Signaling pathways in uric acid homeostasis and gout: From pathogenesis to therapeutic interventions. Int. Immunopharmacol. 2024, 132, 111932. [Google Scholar] [CrossRef]
- Crișan, T.O.; Cleophas, M.C.P.; Oosting, M.; Lemmers, H.; Toenhake-Dijkstra, H.; Netea, M.G.; Jansen, T.L.; Joosten, L.A.B. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 2016, 75, 755–762. [Google Scholar] [CrossRef]
- Cabău, G.; Crișan, T.O.; Klück, V.; Popp, R.A.; Joosten, L.A.B. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.L.; Fu, C.; Han, R.; Chen, W.; Lu, Y.; Ye, Z. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int. J. Mol. Med. 2015, 35, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, X.; Yang, X.; Yang, Y. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren. Fail. 2022, 44, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Cao, R.; Wang, G.; Li, S.; Cao, Y.; Zhang, H.; Liu, M.; Liu, G.; Zhang, J.; et al. Soluble uric acid induces myocardial damage through activating the NLRP3 inflammasome. J. Cell. Mol. Med. 2020, 24, 8849–8861. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Nie, Q.; Liu, M.; Zhang, Z.; Zhang, X.; Wang, C.; Song, G. The effects of hyperuricemia on endothelial cells are mediated via GLUT9 and the JAK2/STAT3 pathway. Mol. Biol. Rep. 2021, 48, 8023–8032. [Google Scholar] [CrossRef]
- Mazzali, M.; Hughes, J.; Kim, Y.-G.; Jefferson, J.A.; Kang, D.-H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, W.; Zhou, J.; Zhang, Y.; Huang, F.; Chen, M. Uric acid promotes aortic valve calcification via mediating valve interstitial cell osteogenic differentiation and endothelial dysfunction. FASEB J. 2025, 39, e70437. [Google Scholar] [CrossRef]
- Carlström, M.; Wilcox, C.S.; Arendshorst, W.J. Renal Autoregulation in Health and Disease. Physiol. Rev. 2015, 95, 405–511. [Google Scholar] [CrossRef]
- Griffin, K.A.; Bidani, A.K. Progression of Renal Disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 1054–1065. [Google Scholar] [CrossRef]
- Mazzali, M.; Kim, Y.-G.; Suga, S.; Gordon, K.L.; Kang, D.-H.; Ashley Jefferson, J.; Hughes, J.; Kivlighn, S.D.; Lan, H.Y.; Johnson, R.J. Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation 2001, 71, 900–905. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Gumieniak, O.; Hopkins, P.N.; Murphey, L.J.; Brown, N.J.; Williams, G.H.; Hollenberg, N.K.; Fisher, N.D. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int. 2004, 66, 1465–1470. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Bakir, C.N.; Covic, A.; Ortiz, A.; Tuttle, K.R. Glomerular hyperfiltration as a therapeutic target for CKD. Nephrol. Dial. Transplant. 2024, 39, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Semenikhina, M.; Mathew, R.O.; Barakat, M.; Van Beusecum, J.P.; Ilatovskaya, D.V.; Palygin, O. Blood Pressure Management Strategies and Podocyte Health. Am. J. Hypertens. 2025, 38, 85–96. [Google Scholar] [CrossRef]
- Hill, G.S.; Heudes, D.; Bariéty, J. Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation11See Editorial by Olson, p. 1162. Kidney Int. 2003, 63, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Kohagura, K.; Kochi, M.; Miyagi, T.; Kinjyo, T.; Maehara, Y.; Nagahama, K.; Sakima, A.; Iseki, K.; Ohya, Y. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: A biopsy-based study. Hypertens. Res. 2013, 36, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sofue, T. Hyperuricemia: The third key player for nephrosclerosis with ischemia. Hypertens. Res. 2023, 46, 1707–1709. [Google Scholar] [CrossRef]
- Xia, X.; Luo, Q.; Li, B.; Lin, Z.; Yu, X.; Huang, F. Serum uric acid and mortality in chronic kidney disease: A systematic review and meta-analysis. Metabolism 2016, 65, 1326–1341. [Google Scholar] [CrossRef]
- Rosolowsky, E.T.; Ficociello, L.H.; Maselli, N.J.; Niewczas, M.A.; Binns, A.L.; Roshan, B.; Warram, J.H.; Krolewski, A.S. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 2008, 3, 706–713. [Google Scholar] [CrossRef]
- Ben-Dov, I.Z.; Kark, J.D. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: The Jerusalem Lipid Research Clinic cohort study. Nephrol. Dial. Transplant. 2011, 26, 2558–2566. [Google Scholar] [CrossRef]
- Kawashima, M.; Wada, K.; Ohta, H.; Terawaki, H.; Aizawa, Y. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: A long-term retrospective cohort study. BMC Nephrol. 2011, 12, 31. [Google Scholar] [CrossRef]
- Wu, N.; Xia, J.; Chen, S.; Yu, C.; Xu, Y.; Xu, C.; Yan, T.; Li, N.; Liu, Y.; Pan, X.-F. Serum uric acid and risk of incident chronic kidney disease: A national cohort study and updated meta-analysis. Nutr. Metab. 2021, 18, 94. [Google Scholar] [CrossRef]
- Yen, C.; Chiang, C.; Ho, L.; Hsu, S.H.; Hung, K.; Wu, K.; Tsai, T. Hyperuricemia associated with rapid renal function decline in elderly Taiwanese subjects. J. Formos. Med. Assoc. 2009, 108, 921–928. [Google Scholar] [CrossRef]
- Kuwata, H.; Okamura, S.; Hayashino, Y.; Ishii, H.; Tsujii, S. Serum uric acid levels are associated with a high risk of rapid chronic kidney disease progression among patients with type 2 diabetes: A prospective cohort study [Diabetes Distress and Care Registry at Tenri (DDCRT 12)]. Diabetol. Int. 2016, 7, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Sarnak, M.J.; Wang, X.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Menon, V. Uric acid and long-term outcomes in CKD. Am. J. Kidney Dis. 2009, 53, 796–803. [Google Scholar] [CrossRef]
- Sturm, G.; Kollerits, B.; Neyer, U.; Ritz, E.; Kronenberg, F. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp. Gerontol. 2008, 43, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Sakaguchi, Y.; Oka, T.; Hattori, K.; Kawaoka, T.; Doi, Y.; Yamamoto, R.; Matsui, I.; Mizui, M.; Kaimori, J.-Y.; et al. Association between urinary uric acid excretion and kidney outcome in patients with CKD. Sci. Rep. 2024, 14, 5119. [Google Scholar] [CrossRef] [PubMed]
- López Iglesias, A.; Blanco Pardo, M.; Rodríguez Magariños, C.; Pértega, S.; Sierra Castro, D.; García Falcón, T.; Rodríguez-Carmona, A.; Fontán, M.P.; Mukhopadhyay, P. Association of urinary excretion rates of uric acid with biomarkers of kidney injury in patients with advanced chronic kidney disease. PLoS ONE 2024, 19, e0304105. [Google Scholar] [CrossRef]
- Kawamoto, R.; Asuka, K.; Ninomiya, D.; Kumagi, T.; Abe, M. High serum uric acid/creatinine ratio is a useful predictor of hypertension among Japanese community-dwelling persons. Clin. Hypertens. 2025, 31, e9. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, H.; Ding, Y.; Yuan, C.; Shao, Y. Association between serum uric acid to serum creatinine ratio with cardiovascular and all-cause mortality in adults with hypertension. Sci. Rep. 2024, 14, 18008. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Masulli, M.; Cirillo, P.; Virdis, A.; Casiglia, E.; Tikhonoff, V.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cappelli, F.; et al. Serum Uric Acid/Serum Creatinine Ratio and Cardiovascular Mortality in Diabetic Individuals—The Uric Acid Right for Heart Health (URRAH) Project. Metabolites 2024, 14, 164. [Google Scholar] [CrossRef]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Grassi, G.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid / serum creatinine ratio as a predictor of cardiovascular events. Detection of prognostic cardiovascular cut-off values. J. Hypertens. 2023, 41, 180–186. [Google Scholar] [CrossRef]
- Sircar, D.; Chatterjee, S.; Waikhom, R.; Golay, V.; Raychaudhury, A.; Chatterjee, S.; Pandey, R. Efficacy of Febuxostat for Slowing the GFR Decline in Patients With CKD and Asymptomatic Hyperuricemia: A 6-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Kidney Dis. 2015, 66, 945–950. [Google Scholar] [CrossRef]
- Siu, Y.P.; Leung, K.T.; Tong, M.K.H.; Kwan, T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hosoya, T.; Uchida, S.; Inaba, M.; Makino, H.; Maruyama, S.; Yamamoto, T.; Tomino, Y.; Ohno, I.; Shibagaki, Y.; et al. Febuxostat Therapy for Patients With Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am. J. Kidney Dis. 2018, 72, 798–810. [Google Scholar] [CrossRef]
- Doria, A.; Galecki, A.T.; Spino, C.; Pop-Busui, R.; Cherney, D.Z.; Lingvay, I.; Parsa, A.; Rossing, P.; Sigal, R.J.; Afkarian, M.; et al. Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N. Engl. J. Med. 2020, 382, 2493–2503. [Google Scholar] [CrossRef]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; Dalbeth, N.; Day, R.O.; de Zoysa, J.R.; et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef]
- Wang, Y.; Dalbeth, N.; Terkeltaub, R.; Zhang, Y.; Li, X.; Zeng, C.; Lei, G.; Wei, J. Target Serum Urate Achievement and Chronic Kidney Disease Progression in Patients With Gout and Kidney Disease. JAMA Intern. Med. 2025, 185, 74–82. [Google Scholar] [CrossRef]
- Kataoka, H.; Mochizuki, T.; Ohara, M.; Tsuruta, Y.; Iwasa, N.; Yoshida, R.; Tsuchiya, K.; Nitta, K.; Kimura, K.; Hosoya, T. Urate-lowering therapy for CKD patients with asymptomatic hyperuricemia without proteinuria elucidated by attribute-based research in the FEATHER Study. Sci. Rep. 2022, 12, 3784. [Google Scholar] [CrossRef] [PubMed]
- Ghang, B.; Park, J.; Lee, J.S.; Lim, J.S.; Kim, H.; Liew, D.F.L.; Kim, J.; Kang, D.-H.; Yoo, B. Post-hoc analysis of the CARES trial suggests delayed progression of chronic kidney disease in patients with gout during urate-lowering therapy. Kidney Int. 2025, 107, 521–529. [Google Scholar] [CrossRef]
- Chou, H.W.; Chiu, H.T.; Tsai, C.W.; Ting, I.W.; Yeh, H.C.; Huang, H.C.; Kuo, C.-C.; CMUH Kidney Research Group. Comparative effectiveness of allopurinol, febuxostat and benzbromarone on renal function in chronic kidney disease patients with hyperuricemia: A 13-year inception cohort study. Nephrol. Dial. Transplant. 2017, 33, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Kohagura, K.; Satoh, A.; Kochi, M.; Nakamura, T.; Zamami, R.; Tana, T.; Kinjyo, K.; Funakoshi, R.; Yamazato, M.; Ishida, A.; et al. Urate-lowering drugs for chronic kidney disease with asymptomatic hyperuricemia and hypertension: A randomized trial. J. Hypertens. 2023, 41, 1420–1428. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Sundy, J.S.; Miner, J.A.; Cravets, M.; Storgard, C. Lesinurad in combination with allopurinol: Results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol. Ann. Rheum. Dis. 2016, 75, 1074–1080. [Google Scholar] [CrossRef]
- Bardin, T.; Keenan, R.T.; Khanna, P.P.; Kopicko, J.; Fung, M.; Bhakta, N.; Adler, S.; Storgard, C.; Baumgartner, S.; So, A. Lesinurad in combination with allopurinol: A randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann. Rheum. Dis. 2017, 76, 811–820. [Google Scholar] [CrossRef]
- Saag, K.G.; Fitz-Patrick, D.; Kopicko, J.; Fung, M.; Bhakta, N.; Adler, S.; Storgard, C.; Baumgartner, S.; Becker, M.A. Lesinurad Combined With Allopurinol: A Randomized, Double-Blind, Placebo-Controlled Study in Gout Patients With an Inadequate Response to Standard-of-Care Allopurinol (a US-Based Study). Arthritis Rheumatol. 2017, 69, 203–212. [Google Scholar] [CrossRef]
- Dalbeth, N.; Jones, G.; Terkeltaub, R.; Khanna, D.; Kopicko, J.; Bhakta, N.; Adler, S.; Fung, M.; Storgard, C.; Baumgartner, S.; et al. Lesinurad, a Selective Uric Acid Reabsorption Inhibitor, in Combination With Febuxostat in Patients With Tophaceous Gout: Findings of a Phase III Clinical Trial. Arthritis Rheumatol. 2017, 69, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Tausche, A.K.; Alten, R.; Dalbeth, N.; Kopicko, J.; Fung, M.; Adler, S.; Bhakta, N.; Storgard, C.; Baumgartner, S.; Saag, K. Lesinurad monotherapy in gout patients intolerant to a xanthine oxidase inhibitor: A 6 month phase 3 clinical trial and extension study. Rheumatology 2017, 56, 2170–2178. [Google Scholar] [CrossRef]
- Shiramoto, M.; Liu, S.; Shen, Z.; Yan, X.; Yamamoto, A.; Gillen, M.; Ito, Y.; Hall, J. Verinurad combined with febuxostat in Japanese adults with gout or asymptomatic hyperuricaemia: A phase 2a, open-label study. Rheumatology 2018, 57, 1602–1610. [Google Scholar] [CrossRef]
- Dalbeth, N.; Jones, G.; Terkeltaub, R.; Khanna, D.; Fung, M.; Baumgartner, S.; Perez-Ruiz, F. Efficacy and safety during extended treatment of lesinurad in combination with febuxostat in patients with tophaceous gout: CRYSTAL extension study. Arthritis Res. Ther. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Patrick, D.; Roberson, K.; Niwa, K.; Fujimura, T.; Mori, K.; Hall, J.; Yan, X.; Shen, Z.; Liu, S.; Ito, Y.; et al. Safety and efficacy of verinurad, a selective URAT1 inhibitor, for the treatment of patients with gout and/or asymptomatic hyperuricemia in the United States and Japan: Findings from two phase II trials. Mod. Rheumatol. 2019, 29, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Furuno, K.; Kanda, S. A non-inferiority study of the novel selective urate reabsorption inhibitor dotinurad versus febuxostat in hyperuricemic patients with or without gout. Clin Exp Nephrol. 2020, 24 (Suppl. S1), 71–79. [Google Scholar] [CrossRef]
- Hosoya, T.; Sano, T.; Sasaki, T.; Fushimi, M.; Ohashi, T. Clinical efficacy and safety of dotinurad, a novel selective urate reabsorption inhibitor, in Japanese hyperuricemic patients with or without gout: An exploratory, randomized, multicenter, double-blind, placebo-controlled, parallel-group early phase 2 study. Clin. Exp. Nephrol. 2020, 24 (Suppl. S1), 44–52. [Google Scholar] [CrossRef]
- Hosoya, T.; Sano, T.; Sasaki, T.; Fushimi, M.; Ohashi, T. Clinical efficacy and safety of dotinurad, a novel selective urate reabsorption inhibitor, in Japanese hyperuricemic patients with or without gout: Randomized, multicenter, double-blind, placebo-controlled, parallel-group, confirmatory phase 2 study. Clin. Exp. Nephrol. 2020, 24 (Suppl. S1), 53–61. [Google Scholar] [CrossRef]
- Hosoya, T.; Sano, T.; Sasaki, T.; Fushimi, M.; Ohashi, T. Dotinurad versus benzbromarone in Japanese hyperuricemic patient with or without gout: A randomized, double-blind, parallel-group, phase 3 study. Clin. Exp. Nephrol. 2020, 24 (Suppl. S1), 62–70. [Google Scholar] [CrossRef]
- Stack, A.G.; Dronamraju, N.; Parkinson, J.; Johansson, S.; Johnsson, E.; Erlandsson, F.; Terkeltaub, R. Effect of Intensive Urate Lowering With Combined Verinurad and Febuxostat on Albuminuria in Patients With Type 2 Diabetes: A Randomized Trial. Am. J. Kidney Dis. 2021, 77, 481–489. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stack, A.G.; Terkeltaub, R.; Jongs, N.; Inker, L.A.; Bjursell, M.; Maklad, N.; Perl, S.; Eklund, O.; Rikte, T.; et al. Combination Treatment with Verinurad and Allopurinol in CKD: A Randomized Placebo and Active Controlled Trial. J. Am. Soc. Nephrol. 2024, 35, 594–606. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Voors, A.A.; Mentz, R.J.; Lewis, G.D.; Perl, S.; Myte, R.; Kaguthi, G.; Sjöström, C.D.; Källgren, C.; Shah, S.J. Verinurad Plus Allopurinol for Heart Failure With Preserved Ejection Fraction: The AMETHYST Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Nunoue, T.; Itabashi, N.; Katayama, A.; Nakamura, A.; Ohbayashi, H.; Onishi, Y.; Watanabe, K.; Maruyama, K.; Hosoya, T.; et al. Dotinurad Treatment for Patients With Hyperuricemia Complicating CKD. Kidney Int. Reports. 2025, 10, 1711–1720. [Google Scholar] [CrossRef]
- Yanai, K.; Hirai, K.; Kaneko, S.; Mutsuyoshi, Y.; Kitano, T.; Miyazawa, H.; Ito, K.; Ueda, Y.; Ookawara, S.; Morishita, Y. The Efficacy and Safety of Dotinurad on Uric Acid and Renal Function in Patients with Hyperuricemia and Advanced Chronic Kidney Disease: A Single Center, Retrospective Analysis. Drug Des. Dev. Ther. 2023, 17, 3233–3248. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Katsuyama, H.; Hakoshima, M.; Adachi, H. Urate Transporter 1 Can Be a Therapeutic Target Molecule for Chronic Kidney Disease and Diabetic Kidney Disease: A Retrospective Longitudinal Study. Biomedicines 2023, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, O.; Yamada, T.; Kato, K.; Miyauchi, Y. Efficacy of dotinurad in patients with severe renal dysfunction. Clin. Exp. Nephrol. 2024, 28, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Kobayashi, S.; Terawaki, H. Dotinurad restores exacerbated kidney dysfunction in hyperuricemic patients with chronic kidney disease. BMC Nephrol. 2024, 25, 97. [Google Scholar] [CrossRef]
- Takata, T.; Taniguchi, S.; Mae, Y.; Kageyama, K.; Fujino, Y.; Iyama, T.; Hikita, K.; Sugihara, T.; Isomoto, H. Comparative assessment of the effects of dotinurad and febuxostat on the renal function in chronic kidney disease patients with hyperuricemia. Sci. Rep. 2025, 15, 8990. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Design | Population | Exposure | Kidney Outcome | Main Finding |
|---|---|---|---|---|---|
| [40] | Cross-sectional | T1D | SUA | eGFR decline | Higher SUA independently associated with lower eGFR |
| [42] | Retrospective | General | SUA | Incident CKD | Higher SUA is associated with new-onset CKD |
| [43] | Meta-analysis | General/CKD | SUA | Incident CKD | ~15% higher CKD risk per 1 mg/dL higher SUA |
| [44] | Prospective | General/CKD | SUA | eGFR decline | ~20% higher risk of eGFR decline per 1 mg/dL higher SUA |
| [45] | Prospective | T2D | SUA | Rapid eGFR decline | Higher SUA predicted faster decline in eGFR |
| [46] | Prospective | CKD G3-4 | SUA | Renal failure | Neutral association |
| [47] | Prospective | CKD | SUA | Doubling of Cr/renal failure | Neutral association |
| [48] | Prospective | CKD | FEUA/UUCR | eGFR decline | Lower urinary urate excretion predicted eGFR decline |
| [49] | Cross-sectional | CKD | FEUA/UUCR | Kidney injury biomarkers | Urinary excretion of UA associated with injury markers |
| Ref. | Design | Population | Intervention | Kidney Outcome | Main Finding |
|---|---|---|---|---|---|
| [54] | RCT | CKD G3-4 | Febuxostat | eGFR decline | Febuxostat slowed eGFR decline |
| [55] | RCT | CKD | Allopurinol | 40% increase in Cr/dialysis | Allopurinol prevented composite renal endpoint |
| [56] | RCT | CKD G3 | Febuxostat | eGFR slope | Neutral |
| [57] | RCT | T1D | Allopurinol | eGFR slope | Neutral |
| [58] | RCT | CKD G3-4 | Allopurinol | eGFR slope | Neutral |
| [59] | Cohort | CKD G3 | Mostly with allopurinol | Progression to advanced CKD | Target achievement associated with lower risk |
| [60] | Post hoc within RCT | CKD G3 | Febuxostat | eGFR slope | Febuxostat slowed eGFR decline without proteinuria |
| [61] | Post hoc within RCT | Gout with CV risk | Febuxostat/allopurinol | eGFR trajectory | Lower SUA linked to less eGFR decline |
| Ref. | Intervention | Comparator | Duration | Renal Outcome/Safety |
|---|---|---|---|---|
| [64] | Lesinurad + allopurinol | Placebo + allopurinol | 4 weeks | Renal parameters neutral |
| [65] | Lesinurad + allopurinol | Placebo + allopurinol | 12 months | Renal AEs and sCr ≥ 1.5× more frequent with 400 mg |
| [66] | Lesinurad + allopurinol | Placebo + allopurinol | 12 months | Renal AEs and sCr ≥ 1.5× higher with 400 mg |
| [67] | Lesinurad + febuxostat | Febuxostat + placebo | 12 months | Renal AEs increased with 400 mg |
| [68] | Lesinurad | Placebo | 6 months | Renal AEs more frequent with 400 mg |
| [69] | Verinurad + febuxostat | Febuxostat, verinurad, benzbromarone | 42 days | No concerning renal signal reported |
| [70] | Lesinurad + febuxostat | No placebo | 12 months | No new renal safety signals |
| [71] | Verinurad | Placebo | 24 weeks | sCr elevations more frequent with verinurad |
| [72] | Dotinurad | Fabuxostat | 14 weeks | AE rates comparable |
| [73] | Dotinurad | Placebo | 8 weeks | Overall renal function stable |
| [74] | Dotinurad | Placebo | 12 weeks | Consistent renal safety |
| [75] | Dotinurad | Benzbromarone | 14 weeks | AE rates comparable |
| [76] | Verinurad + febuxostat | Placebo | 24 weeks | Verinurad reduced UACR, overall safety similar |
| [77] | Verinurad + allopurinol | Alopurinol/placebo | 34 weeks | No improvement in UACR or eGFR decline |
| [78] | Verinurad + allopurinol | Alopurinol/placebo | 32 weeks | No between-group differences in eGFR and UACR |
| Ref. | Design | Population | Intervention | Kidney Outcome | Main Finding |
|---|---|---|---|---|---|
| [79] | Prospective | CKD G1-4 | Dotinurad | eGFR change | eGFR tended to improve |
| [80] | Retrospective | CKD G3-5 | Dotinurad | eGFR slope | eGFR slope improved |
| [81] | Retrospective | CKD | Dotinurad | Albuminuria, Cr | Increase in urate excretion correlated with less Cr rise |
| [82] | Retrospective | CKD | Dotinurad | eGFR change | eGFR improved in eGFR < 30 subgroup |
| [83] | Retrospective | CKD | Dotinurad | eGFR change | eGFR improved |
| [84] | Retrospective | CKD | Dotinurad vs. febuxostat | eGFR change | Only dotinurad group showed eGFR improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, T.; Mae, Y.; Hoi, S.; Iyama, T.; Isomoto, H. Hyperuricemia in Chronic Kidney Disease: Emerging Pathophysiology and a Novel Therapeutic Strategy. Int. J. Mol. Sci. 2025, 26, 9000. https://doi.org/10.3390/ijms26189000
Takata T, Mae Y, Hoi S, Iyama T, Isomoto H. Hyperuricemia in Chronic Kidney Disease: Emerging Pathophysiology and a Novel Therapeutic Strategy. International Journal of Molecular Sciences. 2025; 26(18):9000. https://doi.org/10.3390/ijms26189000
Chicago/Turabian StyleTakata, Tomoaki, Yukari Mae, Shotaro Hoi, Takuji Iyama, and Hajime Isomoto. 2025. "Hyperuricemia in Chronic Kidney Disease: Emerging Pathophysiology and a Novel Therapeutic Strategy" International Journal of Molecular Sciences 26, no. 18: 9000. https://doi.org/10.3390/ijms26189000
APA StyleTakata, T., Mae, Y., Hoi, S., Iyama, T., & Isomoto, H. (2025). Hyperuricemia in Chronic Kidney Disease: Emerging Pathophysiology and a Novel Therapeutic Strategy. International Journal of Molecular Sciences, 26(18), 9000. https://doi.org/10.3390/ijms26189000








