Effects of Photobiomodulation on Osteoarthritis from In Vivo and In Vitro Studies: A Narrative Review
Abstract
1. Introduction
2. Search Strategy and Study Selection
3. Effects of PBMT on OA Studies In Vivo
| Study ID | Author | Year | Irradiation Conditions | Animal OA Model | Results |
|---|---|---|---|---|---|
| [23] | Micheli et al. | 2017 |
| Rat MIA, CFA | PBMT ameliorated the mechanical hyperalgesia induced by MIA and CFA. |
| [24] | Yamada et al. | 2020 |
| Rat MIA | PBMT reduced oxidative stress and suppressed mechanical hyperalgesia in joints, sera, and spinal cord and IL-1β, IL-6, and TNF-α expression. |
| [25] | Yamada et al. | 2022 |
| Rat MIA | PBMT reduced pain sensation and oxidative stress-induced injury, NO levels, and inflammatory cytokines and alleviated oxidative stress in sites distant from the lesion. |
| [26] | Balbinot et al. | 2021 |
| Rat MIA | PBMT ameliorated hyperalgesia and motor deficits, suppressed extracellular matrix disruption in articular cartilage, and reduced the number of glial cells in the spinal dorsal horn. |
| [28] | de Oliveira et al. | 2017 |
| Rat Papain | PBMT ameliorated hyperalgesia and suppressed the expression of TNF-α, CINC-1, and bradykinin receptors B1 and B2. |
| [29] | Alves et al. | 2014 |
| Rat Papain | PBMT reduced collagen type III expression and increased collagen type I expression. LLLT at 50 mW significantly reduced MMP-2 expression at 21 days compared with the injured group. LLLT at 50 mW significantly reduced MMP-9 expression at 21 days compared to LLLT at 100 mW. |
| [30] | Tamazoni et al. | 2017 |
| Rat Papain | PBMT suppressed IL-1β, IL-6, TNF-α, and PGE2 expression. |
| [31] | Tomazoni et al. | 2016 |
| Rat Papain | PBMT and NSAID administration reduced the total number of cells in the inflammatory infiltrate and MMP3 expression. PBMT strongly repressed the expression of myeloperoxidase involved in joint degradation and MMP13 expression. |
| [32] | dos Anjos et al. | 2022 |
| Mouse Zymosan | PBMT suppressed MMP-2, MMP-9, MMP-13, and MMP-14 expression and promoted TIMP-2 expression. |
| [33] | Lemos et al. | 2016 |
| Rat CFA | PBMT prevented joint disc thinning and collagenous fibril and glycosaminoglycan reduction and reduced MMP-2 and MMP-9 activity. |
| [34] | Pallotta et al. | 2012 |
| Rat Kaolin Carrageenan | PBMT decreased white blood cell count, myeloperoxidase activity, IL-1 and IL-6 expression, and prostaglandin E2 levels in joint lavage fluid. |
| [35] | Wang et al. | 2014 |
| Rabbit Anterior cruciate ligament excision | PBMT relieved knee-related pain and reduced synovial inflammation. IL-1β, iNOS, and MMP-3 expression was significantly reduced at 6 weeks. After 8 weeks, PBMT significantly reduced IL-1β, iNOS, MMP-1, MMP-3, and MMP-13 expression. PBMT increased collagen-2, aggrecan, and TIMP-1. |
| [36] | Assis et al. | 2016 |
| Rat Anterior cruciate ligament excision | PBMT improved OARSI scoring of osteoarthritis and suppressed IL-1β, caspase-3, and MMP-13 expression. |
| [37] | Assis et al. | 2018 |
| Rat Anterior cruciate ligament excision | PBMT reduced OA grading of joints and promoted IL-10 and collagen II expression. |
| [38] | Trevisan et al. | 2020 |
| Rat Anterior cruciate ligament excision | PBMT improved joint OARSI scoring and promoted type II collagen and TGF-β expression. |
| [39] | Sanches et al. | 2018 |
| Rat Anterior cruciate ligament excision | OARSI score for cartilage degeneration was significantly higher for control than for CS/Gl and CS/Gl + PBMT groups. CS/GS + PBM decreased IL-1β expression and increased IL-10 and Col II immunoexpression. |
| [13] | Xiang et al. | 2020 | Systematic review and meta-analysis | PBMT positively affected cartilage defects in animal knee models under proper irradiance and adequate irradiation time. | |
| [40] | Nambi | 2021 | Systematic review | PBMT was associated with mild to moderate decreases in IL-1β, TNF-α, and MMP-13 expression, which provided inflammatory relief. IL-6 was not reduced. |
4. Effects of PBMT on Articular Cartilage-Related Cells In Vitro
5. Effect of Mesenchymal Stem Cells with PBMT on OA
| Study ID | Author | Year | Irradiation Conditions | Animal OA Model | Results |
|---|---|---|---|---|---|
| [54] | Stancker et al. | 2018 |
| Rat Papain | Combined intra-articular injection of PBMT and ADSCs prevented degenerative modification of COL2-1 and reduced cytokines and MMPs. |
| [55] | Tanideh et al. | 2024 |
| Guinea pig Anterior cruciate ligament excision | ADSCs or PBMT alone results in good radiological and histological indices. ADSC and PBMT combined improved radiological OA scoring more effectively than either method alone. |
| [56] | El-Qashty et al. | 2023 |
| Rat TMJ Complete Freund’s adjuvant injection | ADSCs + LLLT and ADSCs-CM + LLLT restored joint structure with normal cartilage and disc thickness. Inflammation was significantly suppressed based on the significant reduction in TNF-α-positive immunostaining compared to the arthritic group. Cartilage proteoglycan content also significantly increased relative to that in the arthritic group. |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACL | anterior cruciate ligament |
| ADSC | adipose-derived stem cell |
| CINC-1 | chemoattractant-1 |
| CS/Gl | chondroitin sulfate and glucosamine sulfate |
| ECM | extracellular matrix |
| IL | interleukin |
| LED | light-emitting diode |
| MIA | monoidoacetate |
| MMP | matrix metalloproteinase |
| MSC | mesenchymal stem cells |
| NIR | near-infrared |
| NO | nitric oxide |
| NSAID | non-steroidal anti-inflammatory drug |
| OA | osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| PBMT | photobiomodulation therapy |
| PGE2 | prostaglandin E2 |
| ROS | reactive oxygen species |
| TMJ | temporomandibular joint |
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheumatol. 2008, 58, 26–35. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. 2023, 5, E508–E522. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Ziaei Ziabari, E.; Ebrahimi, A.; Ashkani-Esfahani, S.; Razi, M. Stem-cell therapy in human osteoarthritis: A debate. Thrita 2020, 8, e101358. [Google Scholar] [CrossRef]
- Pasternak-Mnich, K.; Ziemba, B.; Szwed, A.; Kopacz, K.; Synder, M.; Bryszewska, M.; Kujawa, J. Effect of photobiomodulation therapy on the increase of viability and proliferation of human mesenchymal stem cells. Lasers Surg. Med. 2019, 51, 824–833. [Google Scholar] [CrossRef]
- Charlesworth, J.; Fitzpatrick, J.; Perera, N.K.P.; Orchard, J. Osteoarthritis-a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord. 2019, 20, 151. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Bijlsma, J.W.J.; Berenbaum, F.; Lafeber, F.P.J.G. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Lu, K.; Ma, F.; Yi, D.; Yu, H.; Tong, L.; Chen, D. Molecular signaling in temporomandibular joint osteoarthritis. J. Orthop. Transl. 2022, 32, 21–27. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.Y.; Huh, K.H.; Park, J.W. Long-term changes of temporomandibular joint osteoarthritis on computed tomography. Sci. Rep. 2020, 10, 6731. [Google Scholar] [CrossRef]
- Tanne, K. Degenerative changes of articular cartilage in association with mechanical stimuli. Jpn. Dent. Sci. Rev. 2008, 44, 38–47. [Google Scholar] [CrossRef]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Deng, H.; Cheng, K.; Liu, H.; Lin, L.; Qu, X.; Liu, S.; Shen, X. Laser photobiomodulation for cartilage defect in animal models of knee osteoarthritis: A systematic review and meta-analysis. Lasers Med. Sci. 2020, 35, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Barbora, A.; Bohar, O.; Sivan, A.A.; Magory, E.; Nause, A.; Minnes, R. Higher pulse frequency of near-infrared laser irradiation increases penetration depth for novel biomedical applications. PLoS ONE 2021, 16, e0245350. [Google Scholar] [CrossRef]
- Prabhu, V.; Rao, S.B.S.; Chandra, S.; Kumar, P.; Rao, L.; Guddattu, V.; Satyamoorthy, K.; Mahato, K.K. Spectroscopic and histological evaluation of wound healing progression following low level laser therapy (LLLT). J. Biophotonics 2012, 5, 168–184. [Google Scholar] [CrossRef]
- de Almeida, A.L.P.F.; Medeiros, I.L.; Cunha, M.J.; Sbrana, M.C.; de Oliveira, P.G.; Esper, L.A. The effect of low-level laser on bone healing in critical size defects treated with or without autogenous bone graft: An experimental study in rat calvaria. Clin. Oral Implant. Res. 2014, 25, 1131–1136. [Google Scholar] [CrossRef]
- Eslamian, L.; Borzabadi-Farahani, A.; Hassanzadeh-Azhiri, A.; Badiee, M.R.; Fekrazad, R. The effect of 810-nm low-level laser therapy on pain caused by orthodontic elastomeric separators. Lasers Med. Sci. 2014, 29, 559–564. [Google Scholar] [CrossRef]
- Sanz-Moliner, J.D.; Nart, J.; Cohen, R.E.; Ciancio, S.G. The effect of an 810-nm diode laser on postoperative pain and tissue response after modified Widman flap surgery: A pilot study in humans. J. Periodontol. 2013, 84, 152–158. [Google Scholar] [CrossRef]
- Marini, I.; Gatto, M.R.; Bonetti, G.A. Effects of superpulsed low-level laser therapy on temporomandibular joint pain. Clin. J. Pain 2010, 26, 611–616. [Google Scholar] [CrossRef]
- Pitcher, T.; Sousa-Valente, J.; Malcangio, M. The monoiodoacetate model of osteoarthritis pain in the mouse. J. Vis. Exp. 2016, 111, 53746. [Google Scholar] [CrossRef]
- Micheli, L.; Di Cesare Mannelli, L.; Lucarini, E.; Cialdai, F.; Vignali, L.; Ghelardini, C.; Monici, M. Photobiomodulation therapy by NIR laser in persistent pain: An analytical study in the rat. Lasers Med. Sci. 2017, 32, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.F.; Bobinski, F.; Martins, D.F.; Palandi, J.; Folmer, V.; da Silva, M.D. Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J. Biophotonics 2020, 13, e201900204. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.F.; Dos Santos Stein, C.; Moresco, R.N.; Bobinski, F.; Palandi, J.; Fernandes, P.F.; Folmer, V.; da Silva, M.D. Photobiomodulation and Sida tuberculata combination declines the inflammation’s markers in knee-induced osteoarthritis. Lasers Med. Sci. 2022, 37, 193–204. [Google Scholar] [CrossRef]
- Balbinot, G.; Schuch, C.P.; Nascimento, P.S.D.; Lanferdini, F.J.; Casanova, M.; Baroni, B.M.; Vaz, M.A. Photobiomodulation therapy partially restores cartilage integrity and reduces chronic pain behavior in a rat model of osteoarthritis: Involvement of spinal glial modulation. Cartilage 2021, 13, 1309S–1321S. [Google Scholar] [CrossRef]
- Meng, X.; Grad, S.; Wen, C.; Lai, Y.; Alini, M.; Qin, L.; Wang, X. An impaired healing model of osteochondral defect in papain-induced arthritis. J. Orthop. Transl. 2021, 26, 101–110. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.L.C.; Silva, J.A., Jr.; Serra, A.J.; Pallotta, R.C.; da Silva, E.A.; de Farias Marques, A.C.; Feliciano, R.D.; Marcos, R.L.; Leal-Junior, E.C.; de Carvalho, P.T. Photobiomodulation therapy in the modulation of inflammatory mediators and bradykinin receptors in an experimental model of acute osteoarthritis. Lasers Med. Sci. 2017, 32, 87–94. [Google Scholar] [CrossRef]
- Alves, A.C.A.; Albertini, R.; dos Santos, S.A.; Leal-Junior, E.C.; Santana, E.; Serra, A.J.; Silva, J.A., Jr.; de Carvalho, T. Effect of low-level laser therapy on metalloproteinase MMP-2 and MMP-9 production and percentage of collagen types I and III in a papain cartilage injury model. Lasers Med. Sci. 2014, 29, 911–919. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.P.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Lopes-Martins, R.Á. Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med. Sci. 2017, 32, 101–108. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.P.; Frigo, L.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Bjordal, J.M.; Lopes-Martins, R.Á. Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J. Biomed. Opt. 2016, 21, 108001. [Google Scholar] [CrossRef]
- dos Anjos, L.M.J.; Quirino-Teixeira, A.C.; Hottz, E.D.; da Fonseca, A.S.; Gameiro, J.; de Paoli, F. Photobiomodulation effects in metalloproteinases expression in zymosan-induced arthritis. Lasers Med. Sci. 2022, 37, 3661–3670. [Google Scholar] [CrossRef]
- Lemos, G.A.; Rissi, R.; de Souza Pires, I.L.; de Oliveira, L.P.; de Aro, A.A.; Pimentel, E.R.; Palomari, E.T. Low-level laser therapy stimulates tissue repair and reduces the extracellular matrix degradation in rats with induced arthritis in the temporomandibular joint. Lasers Med. Sci. 2016, 31, 1051–1059. [Google Scholar] [CrossRef]
- Pallotta, R.C.; Bjordal, J.M.; Frigo, L.; Leal Junior, E.C.; Teixeira, S.; Marcos, R.L.; Ramos, L.; Messias Fde, M.; Lopes-Martins, R.A. Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med. Sci. 2012, 27, 71–78. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Yang, X.; Zhou, Y.; Wei, X.; Ji, Q.; Yang, L.; He, C. Effects of low-level laser therapy on joint pain, synovitis, anabolic, and catabolic factors in a progressive osteoarthritis rabbit model. Lasers Med. Sci. 2014, 29, 1875–1885. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Assis, L.; Tim, C.; Magri, A.; Fernandes, K.R.; Vassão, P.G.; Renno, A.C.M. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med. Sci. 2018, 33, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, E.S.; Martignago, C.C.S.; Assis, L.; Tarocco, J.C.; Salman, S.; Dos Santos, L.; Liebano, R.; Tim, C.R. Effectiveness of led photobiomodulation therapy on treatment with knee osteoarthritis: A rat study. Am. J. Phys. Med. Rehabil. 2020, 99, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.; Assis, L.; Criniti, C.; Fernandes, D.; Tim, C.; Renno, A.C.M. Chondroitin sulfate and glucosamine sulfate associated to photobiomodulation prevents degenerative morphological changes in an experimental model of osteoarthritis in rats. Lasers Med. Sci. 2018, 33, 549–557. [Google Scholar] [CrossRef]
- Nambi, G. Does low level laser therapy has effects on inflammatory biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in osteoarthritis of rat models-a systemic review and meta-analysis. Lasers Med. Sci. 2021, 36, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Su, C.Y.; Fang, C.H.; Fang, H.W. Preventative treatment of red light-emitting diode protected osteoarthritis-like chondrocytes from oxidative stress-induced inflammation and promoted matrix gene expression. J. Taiwan Inst. Chem. Eng. 2021, 127, 23–31. [Google Scholar] [CrossRef]
- Yang, X.; Liu, T.C.; Liu, S.; Zhu, W.; Li, H.; Liang, P.; Ye, S.; Cui, S. Promoted viability and differentiated phenotype of cultured chondrocytes with low level laser irradiation potentiate efficacious cells for therapeutics. Front. Bioeng. Biotechnol. 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Hang, N.L.T.; Chuang, A.E.; Chang, C.J.; Yen, Y.; Wong, C.C.; Yang, T.S. Photobiomodulation associated with alginate-based engineered tissue on promoting chondrocytes-derived biological responses for cartilage regeneration. Int. J. Biol. Macromol. 2024, 280, 135982. [Google Scholar] [CrossRef]
- Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Hiraki, T.; Gunji, H.; Hirose, N.; Yanoshita, M.; Putranti, N.A.R.; Tanimoto, K. High-frequency near-infrared diode laser irradiation attenuates IL-1beta-induced expression of inflammatory cytokines and matrix metalloproteinases in human primary chondrocytes. J. Clin. Med. 2020, 9, 881. [Google Scholar] [CrossRef]
- Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Hiraki, T.; Gunji, H.; Hirose, N.; Yanoshita, M.; Putranti, N.A.R.; Tanimoto, K. High-frequency near-infrared diode laser irradiation suppresses IL-1β-induced inflammatory cytokine expression and NF-κB signaling pathways in human primary chondrocytes. Lasers Med. Sci. 2022, 37, 1193–1201. [Google Scholar] [CrossRef]
- Tim, C.R.; Martignago, C.C.S.; Assis, L.; Neves, L.M.; Andrade, A.L.; Silva, N.C.; Parizotto, N.; Pinto, K.Z.; Rennó, A.C. Effects of photobiomodulation therapy in chondrocyte response by in vitro experiments and experimental model of osteoarthritis in the knee of rats. Lasers Med. Sci. 2022, 37, 1677–1686. [Google Scholar] [CrossRef]
- Torricelli, P.; Giavaresi, G.; Fini, M.; Guzzardella, G.A.; Morrone, G.; Carpi, A.; Giardino, R. Laser biostimulation of cartilage: In vitro evaluation. Biomed. Pharmacother. 2001, 55, 117–120. [Google Scholar] [CrossRef]
- Anbari, F.; Khalighi, H.; Baharvand, M.; Khosousi Sani, S.; Sharaki, M.; Yadegari, Z.; Mojahedi Nasab, S.M.; Khosousi Sani, M. Effect of low-level laser irradiation on the proliferation of human chondrocytes: An in vitro study. J. Lasers Med. Sci. 2024, 15, e55. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Gunji, H.; Tsuka, Y.; Yoshimi, Y.; Awada, T.; Sumi, K.; Nakajima, K.; Kimura, A.; Hiraki, T.; Abe, T.; et al. Effects of high-frequency near-infrared diode laser irradiation on the proliferation and migration of mouse calvarial osteoblasts. Lasers Med. Sci. 2018, 33, 959–966. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.N.; Zhu, S.Y.; He, H.C.; Yu, X.; Xu, Y.; He, C.Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 2022, 13, 14. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H. Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regen Ther. 2022, 21, 73–80. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal stem cells in osteoarthritis therapy: A review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar]
- Stancker, T.G.; Vieira, S.S.; Serra, A.J.; do Nascimento Lima, R.; Dos Santos Feliciano, R.; Silva, J.A., Jr.; Dos Santos, S.A.; Dos Santos Vieira, M.A.; Simões, M.C.B.; Leal-Junior, E.C.; et al. Can photobiomodulation associated with implantation of mesenchymal adipose-derived stem cells attenuate the expression of MMPs and decrease degradation of type II collagen in an experimental model of osteoarthritis? Lasers Med. Sci. 2018, 33, 1073–1084. [Google Scholar] [CrossRef]
- Tanideh, N.; Ali Behnam, M.; Mohit Ghiri, S.; Koohi-Hosseinabadi, O.; Khajeh-Zadeh, H.; Zare, S.; Azarpira, N.; Akbarzadeh, A.; Ashkani-Esfahani, S.; Ebrahimi, A.; et al. The effects of combined and independent low-level laser and mesenchymal stem cell therapy on induced knee osteoarthritis: An animal study. Knee 2024, 47, 208–218. [Google Scholar] [CrossRef]
- El-Qashty, R.; Elkashty, O.A.; Hany, E. Photobiostimulation conjugated with stem cells or their secretome for temporomandibular joint arthritis in a rat model. BMC Oral Health 2023, 23, 720. [Google Scholar] [CrossRef]
- de Souza Merli, L.A.; de Medeiros, V.P.; Toma, L.; Reginato, R.D.; Katchburian, E.; Nader, H.B.; Faloppa, F. The low level laser therapy effect on the remodeling of bone extracellular matrix. Photochem. Photobiol. 2012, 88, 1293–1301. [Google Scholar] [CrossRef]
- Lucke, L.D.; Bortolazzo, F.O.; Theodoro, V.; Fujii, L.; Bombeiro, A.L.; Felonato, M.; Dalia, R.A.; Carneiro, G.D.; Cartarozzi, L.P.; Vicente, C.P.; et al. Low-level laser and adipose-derived stem cells altered remodelling genes expression and improved collagen reorganization during tendon repair. Cell Prolif. 2019, 52, e12580. [Google Scholar] [CrossRef] [PubMed]
- de Matos Brunelli Braghin, R.; Libardi, E.C.; Junqueira, C.; Rodrigues, N.C.; Nogueira-Barbosa, M.H.; Renno, A.C.M.; Carvalho de Abreu, D.C. The effect of low-level laser therapy and physical exercise on pain, stiffness, function, and spatiotemporal gait variables in subjects with bilateral knee osteoarthritis: A blind randomized clinical trial. Disabil. Rehabil. 2019, 41, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, C.; Bertolutti, G.; Martinello, T.; Van Bruaene, N.; Broeckx, S.Y.; Patruno, M.; Spaas, J.H. Tenogenic induction of equine mesenchymal stem cells by means of growth factors and low-level laser technology. Vet. Res. Commun. 2016, 40, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.H.; Zheng, Y.P.; Lu, M.H.; Chi, Z.R. Development of a portable 3D ultrasound imaging system for musculoskeletal tissues. Ultrasonics 2005, 43, 153–163. [Google Scholar] [CrossRef]
- Stausholm, M.B.; Naterstad, I.F.; Joensen, J.; Lopes-Martins, R.Á.B.; Sæbø, H.; Lund, H.; Fersum, K.V.; Bjordal, J.M. Efficacy of low-level laser therapy on pain and disability in knee osteoarthritis: Systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open 2019, 9, e031142. [Google Scholar] [CrossRef]
- Kheiri, A.; Amid, R.; Kheiri, L.; Namdari, M.; Mojahedi, M.; Kadkhodazadeh, M. Effect of low-level laser therapy on bone regeneration of critical-size bone defects: A systematic review of in vivo studies and meta-analysis. Arch. Oral Biol. 2020, 117, 104782. [Google Scholar] [CrossRef]
- Karic, V.; Chandran, R.; Abrahamse, H. Laser-induced differentiation of human adipose-derived stem cells to temporomandibular joint disc cells. Lasers Surg. Med. 2021, 53, 567–577. [Google Scholar] [CrossRef]
- Shin, M.J.; Park, J.Y.; Park, J.Y.; Lim, S.H.; Lim, H.; Choi, J.K.; Park, C.K.; Kang, Y.J.; Khang, D. Inflammation-targeting mesenchymal stem cells combined with photothermal treatment attenuate severe joint inflammation. Adv. Mater. 2024, 36, e2304333. [Google Scholar] [CrossRef]
- Aleksic, V.; Aoki, A.; Iwasaki, K.; Takasaki, A.A.; Wang, C.Y.; Abiko, Y.; Ishikawa, I.; Izumi, Y. Low-level Er:YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med. Sci. 2010, 25, 559–569. [Google Scholar] [CrossRef]
- Hu, W.P.; Wang, J.J.; Yu, C.L.; Lan, C.C.; Chen, G.S.; Yu, H.S. Helium-neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J. Investig. Dermatol. 2007, 127, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Shingyochi, Y.; Kanazawa, S.; Tajima, S.; Tanaka, R.; Mizuno, H.; Tobita, M. A low-level carbon dioxide laser promotes fibroblast proliferation and migration through activation of Akt, ERK, and JNK. PLoS ONE 2017, 12, e0168937. [Google Scholar] [CrossRef] [PubMed]
- de Paula Gomes, C.A.F.; Leal-Junior, E.C.P.; Dibai-Filho, A.V.; de Oliveira, A.R.; Bley, A.S.; Biasotto-Gonzalez, D.A.; de Tarso Camillo de Carvalho, P. Incorporation of photobiomodulation therapy into a therapeutic exercise program for knee osteoarthritis: A placebo-controlled, randomized, clinical trial. Lasers Surg. Med. 2018, 50, 819–828. [Google Scholar] [CrossRef]
- Fukuda, V.O.; Fukuda, T.Y.; Guimarães, M.; Shiwa, S.; de Lima, C.; Martins, R.Á.; Casarotto, R.A.; Alfredo, P.P.; Bjordal, J.M.; Fucs, P.M. Short-term efficacy of low-level laser therapy in patients with knee osteoarthritis: A randomized placebo controlled, double-blind clinical trial. Rev. Bras. De Ortop. 2011, 46, 526–533. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Couppé, C.; Chow, R.T.; Tunér, J.; Ljunggren, E.A. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003, 49, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.L.M.; Bonjardim, L.R.; Quintans, S.; Ribeiro, M.A.; Maia, L.G.; Conti, P.C. Effect of low-level laser therapy on pain levels in patients with temporomandibular disorders: A systematic review. J. Appl. Oral Sci. 2012, 20, 594–602. [Google Scholar] [CrossRef]
- Bülow, P.M.; Jensen, H.; Danneskiold-Samsøe, B. Low power Ga-Al-As laser treatment of painful osteoarthritis of the knee. a double-blind placebo-controlled study. J. Rehabil. Med. 1994, 26, 155–159. [Google Scholar] [CrossRef]
- Tascioglu, F.; Armagan, O.; Tabak, Y.; Corapci, I.; Oner, C. Low power laser treatment in patients with knee osteoarthritis. Swiss Med. Wkly. 2004, 134, 254–258. [Google Scholar] [CrossRef]
- Emshoff, R.; Bösch, R.; Pümpel, E.; Schöning, H.; Strobl, H. Low-level laser therapy for treatment of temporomandibular joint pain: A double-blind and placebo-controlled trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, A.; Sgolastra, F.; Gatto, R.; Mattei, A.; Monaco, A. Effectiveness of low-level laser therapy in temporomandibular disorders: A systematic review and meta-analysis. J. Orofac. Pain 2011, 25, 298–307. [Google Scholar] [PubMed]
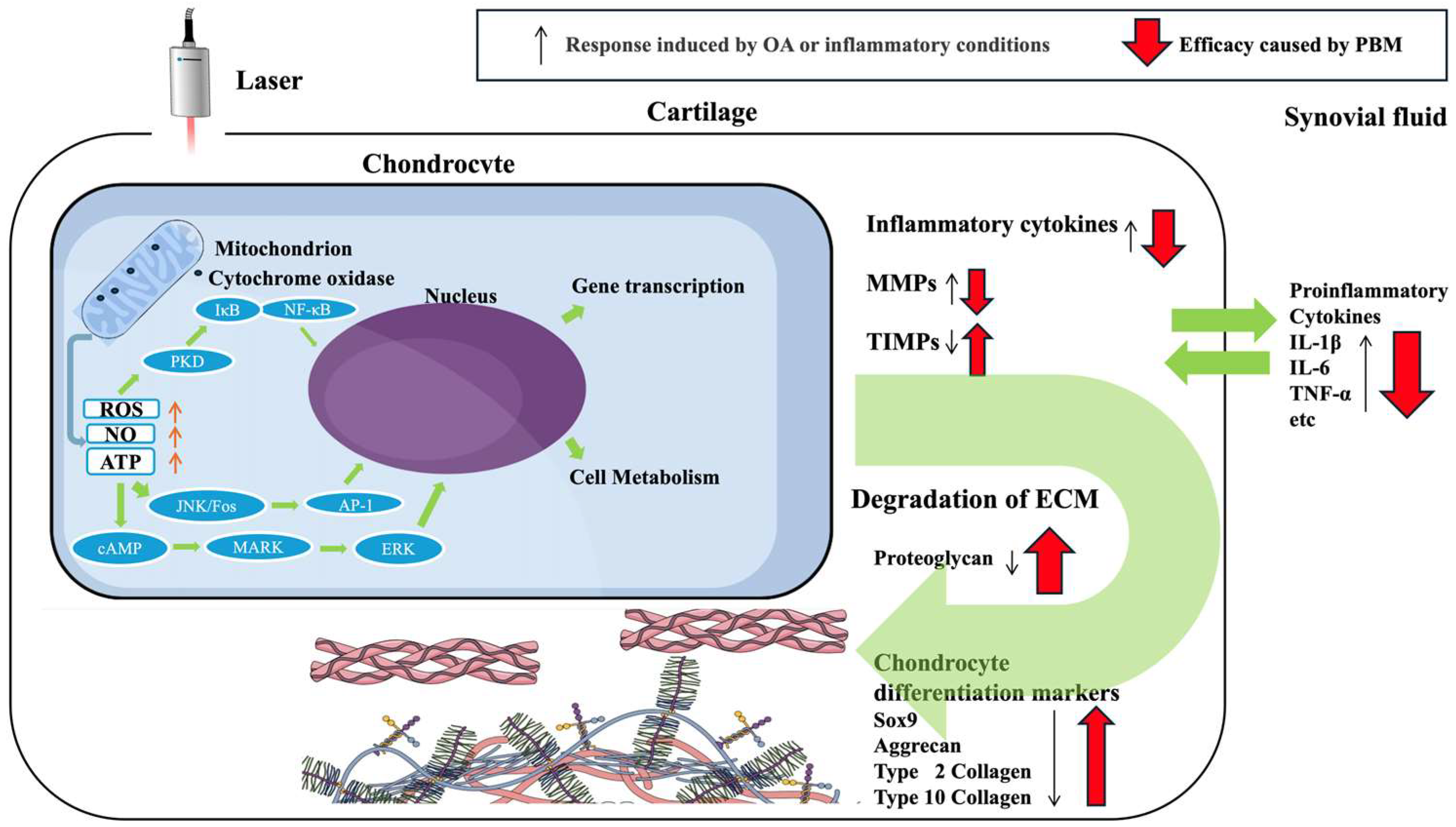
| Study ID | Author | Year | Irradiation Conditions | Cell Inflammation Model | Results |
|---|---|---|---|---|---|
| [41] | Chen et al. | 2021 |
| Porcine chondrocytes H2O2 | PBMT decreased free radical generation and IL-1β and TNF-α expression and promoted type II collagen expression. |
| [42] | Yang et al. | 2020 |
| Rabbit chondrocytes IL-1β | PBMT promoted cellular growth and cartilaginous matrix production and suppressed type I collagen and IL-1β expression and promoted type II collagen, aggrecan, CTNNB1, and SOX9 expression. On IL-1β stimulation, ADAMTS5, caspase-3, FADD, MMP-13, TNF-α, TNFR1, and TRADD expression was suppressed. |
| [43] | Hang et al. | 2024 |
| Rabbit and human chondrocytes None | PBM increased relative intensity of collagen type II immunostaining and collagen type II expression in 2D culture. PBM and alginate-based scaffolds show promise for accelerating and optimizing cartilage regeneration, with potential application in tissue engineering. |
| [44] | Sakata et al. | 2020 |
| Human chondrocytes IL-1β | PBMT suppressed IL-1β, IL-6, TNF-α, MMP-1, and MMP-3 expression. |
| [45] | Sakata et al. | 2022 |
| Human chondrocytes IL-1β | PBMT suppressed NF-κB signaling, which was enhanced by IL-1β stimulation. |
| [46] | Tim et al. | 2022 |
| Rat chondrocytes None | PBMT increased DNA content of chondrocytes. |
| [47] | Torricelli et al. | 2001 |
| Rabbit and human chondrocytes None | PBMT promoted cellular growth. |
| [48] | Anbari et al. | 2024 |
| Human chondrocytes None | 808 nm laser irradiation at energetic doses below 5 J/cm2 does not significantly increase chondrocyte proliferation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunimatsu, R.; Nakatani, A.; Sakata, S.; Tanimoto, K. Effects of Photobiomodulation on Osteoarthritis from In Vivo and In Vitro Studies: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 8997. https://doi.org/10.3390/ijms26188997
Kunimatsu R, Nakatani A, Sakata S, Tanimoto K. Effects of Photobiomodulation on Osteoarthritis from In Vivo and In Vitro Studies: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(18):8997. https://doi.org/10.3390/ijms26188997
Chicago/Turabian StyleKunimatsu, Ryo, Ayaka Nakatani, Shuzo Sakata, and Kotaro Tanimoto. 2025. "Effects of Photobiomodulation on Osteoarthritis from In Vivo and In Vitro Studies: A Narrative Review" International Journal of Molecular Sciences 26, no. 18: 8997. https://doi.org/10.3390/ijms26188997
APA StyleKunimatsu, R., Nakatani, A., Sakata, S., & Tanimoto, K. (2025). Effects of Photobiomodulation on Osteoarthritis from In Vivo and In Vitro Studies: A Narrative Review. International Journal of Molecular Sciences, 26(18), 8997. https://doi.org/10.3390/ijms26188997







