Ab Initio Study of Formation Mechanisms and Thermochemical Properties of Reactive Oxygen Species (ROS) in Photocatalytic Processes
Abstract
1. Introduction
2. Results
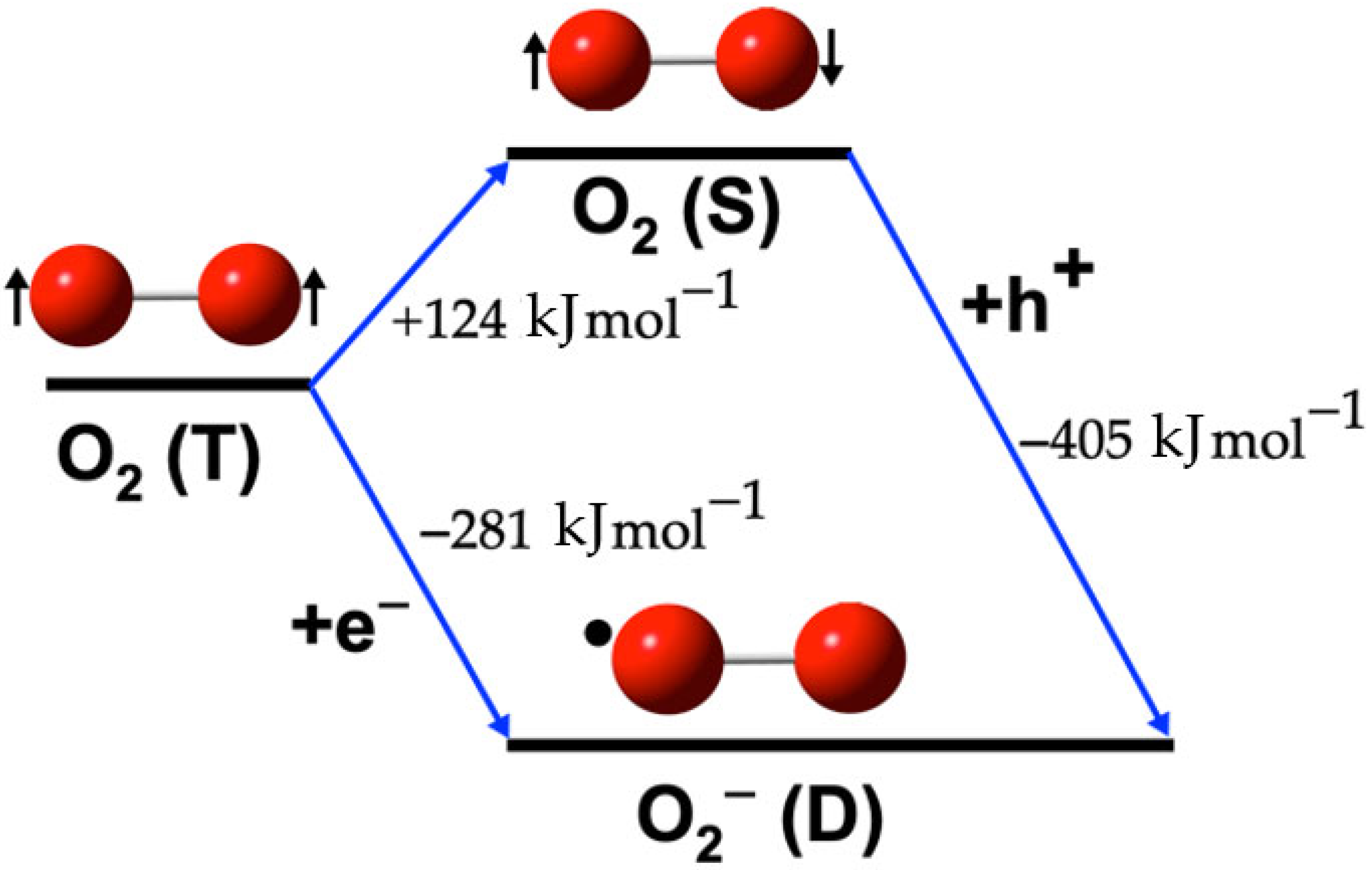
2.1. O2 Species
Formation Reactions of O2 Species
2.2. OH Species
Formation Reactions of OH Species
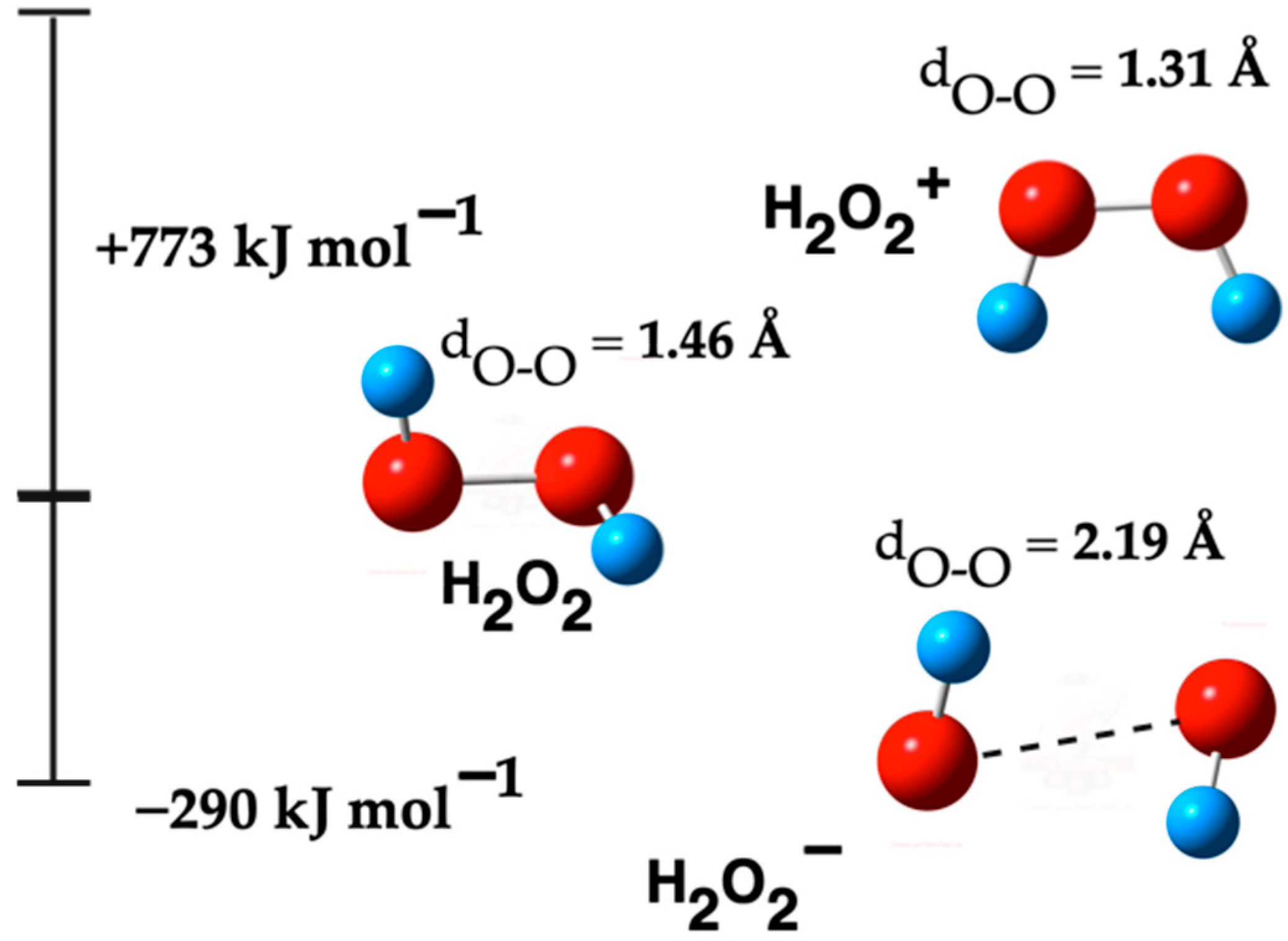
2.3. H2O2 Species
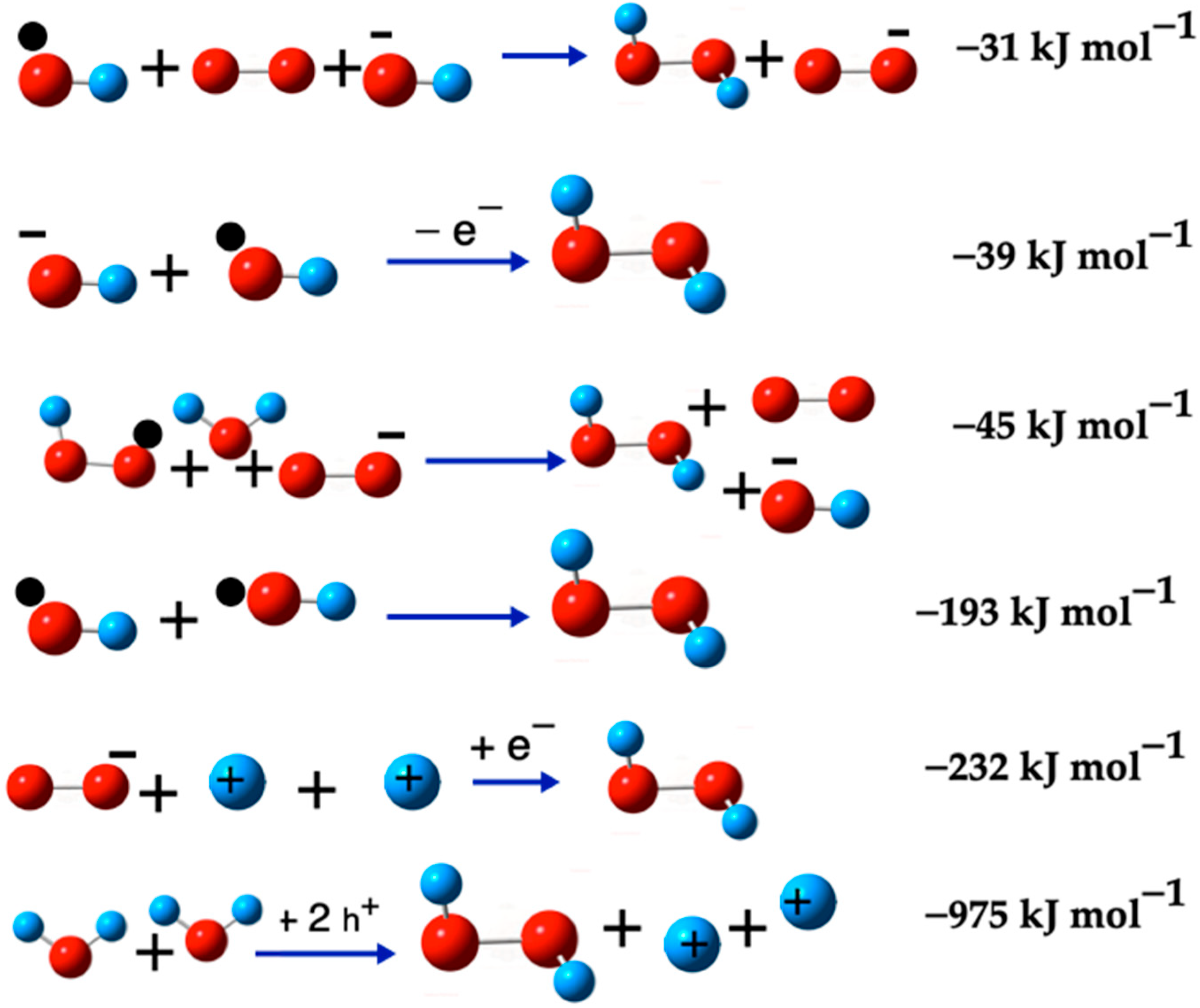
2.3.1. Formation Reactions of H2O2 Species

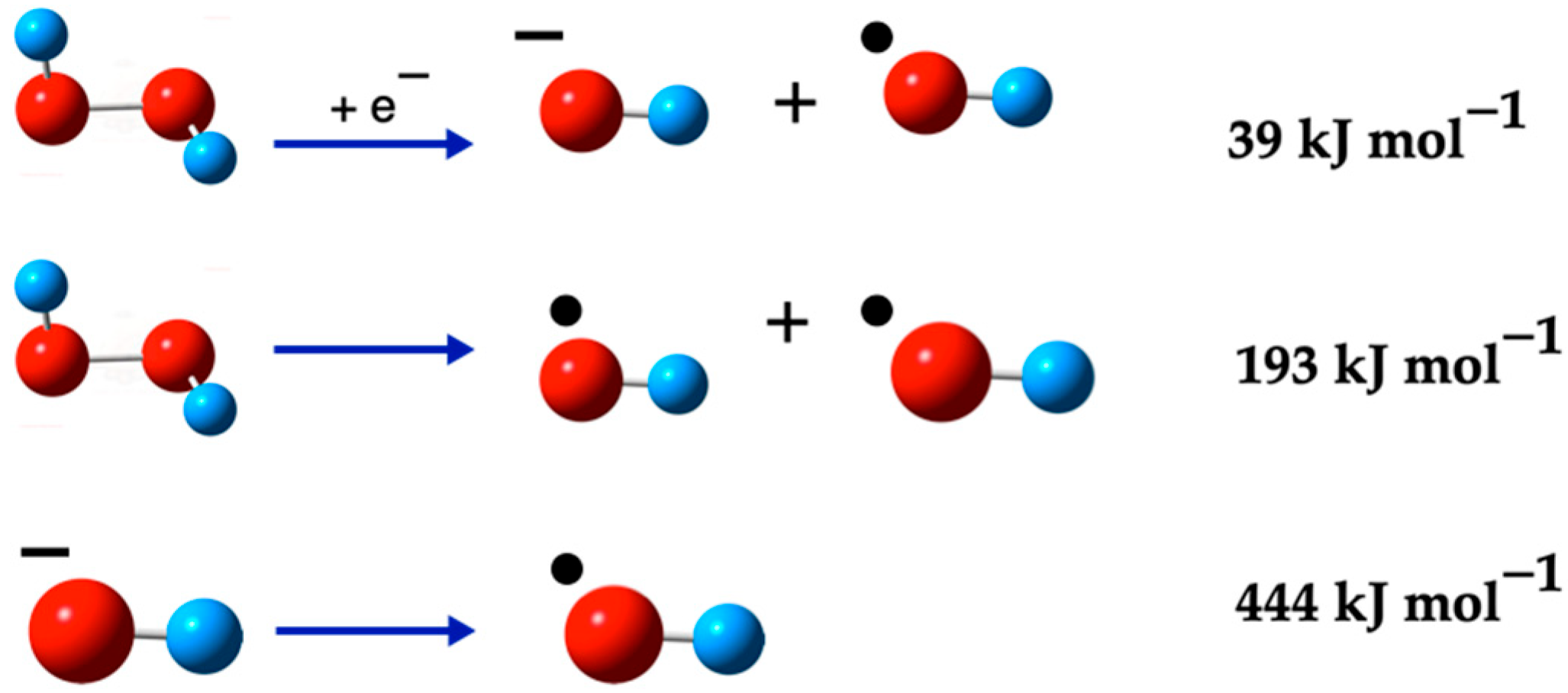
2.3.2. Dissociation Reactions of H2O2 Species
2.4. Other Oxygen Species
3. Discussion
3.1. O2 Species
Formation Reactions of O2 Species
3.2. OH Species
Formation Reactions of OH Species
3.3. H2O2 Species
3.3.1. Formation Reactions of H2O2 Species
3.3.2. Dissociation Reactions of H2O2 Species
4. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| •O2− | Superoxide anion radical |
| H2O2 | Hydrogen peroxide |
| 1O2 | Singlet oxygen |
| •OH | Hydroxyl radical |
| H2O2+ | Protonated hydrogen peroxide |
| H2O2− | Deprotonated hydrogen peroxide |
| OH− | Hydroxide ion |
| H+ | Hydrogen ion or proton |
| H2O | Water |
| HO2− | Perhydroxide ion |
| •O2H | Hydroperoxyl radical |
| e− | Electron |
| h+ | Hole |
| T | Triplet state |
| S | Singlet state |
| D | Doublet state |
| G°, E° | Standard Gibbs free energy and total energy |
| ΔG | Change in Gibbs Free Energy |
| ΔE | Change in Energy |
| ΔG0 | Standard change in Gibbs Free Energy |
| dO-O, dO-H | Bond distances between O atoms and between O and H atoms |
| νO-O, νO-H | Vibrational frequencies of O-O and O-H bonds |
| μ | Dipole moment |
| R2 | Electronic spatial extent |
| ZPE | Zero-Point Energy |
| B3LYP | Becke, 3-parameter, Lee-Yang-Parr |
| SCF | Self-Consistent Field |
| PCM | Polarizable Continuum Model |
| MP2 | Moller-Pleset 2 |
References
- Ong, W.-J.J.W.; Maeda, K. Emerging Nanomaterials for Light-Driven Reactions: Past, Present, and Future; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; Volume 4, p. 2000354. [Google Scholar]
- Ortiz, I.; Rivero, M.J.; Margallo, M. Advanced oxidative and catalytic processes. In Sustainable Water and Wastewater Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–201. ISBN 9780128161708. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; Volume 7, p. 1700841. [Google Scholar]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism; Elsevier: Amsterdam, The Netherlands, 2020; Volume 401, p. 126158. [Google Scholar]
- Jaramillo-Fierro, X.; Cuenca, M.F. Novel Semiconductor Cu(C3H3N3S3)3/ZnTiO3/TiO2 for the Photoinactivation of E. coli and S. aureus under Solar Light. Nanomaterials 2023, 13, 173. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.H. TiO2-Based Photocatalytic Disinfection of Microbes in Aqueous Media: A Review; Academic Press: Cambridge, MA, USA, 2017; Volume 154, pp. 296–303. [Google Scholar]
- Rettig, I.D.; McCormick, T.M. Enrolling Reactive Oxygen Species in Photon-to-Chemical Energy Conversion: Fundamentals, Technological Advances, and Applications; Taylor & Francis: Abingdon, UK, 2021; Volume 6, p. 1950049. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Kiruthika, A.R. Photocatalytic disinfection of micro-organisms: Mechanisms and applications. Environ. Technol. Innov. 2021, 24, 101909. [Google Scholar] [CrossRef]
- Kong, L.; Zepp, R.G. Production and consumption of reactive oxygen species by fullerenes. Environ. Toxicol. Chem. 2012, 31, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, M.; Zhao, Y.; Zhang, H.; Shang, J.; Zhong, J.; Sheng, H.; Chen, C.; Zhao, J. The vital role of surface Brönsted acid/base sites for the photocatalytic formation of free ·OH radicals. Appl. Catal. B Environ. 2020, 266, 118634. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.-J.; Hang, Z.-F.; Xu, S.-F.; Liu, Q.-Q.; Tang, H.; Zhao, X.-W. Insights into the Effect of Reactive Oxygen Species Regulation on Photocatalytic Performance via Construction of a Metal-Semiconductor Heterojunction. J. Nanosci. Nanotechnol. 2020, 20, 3478–3485. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S.-Q.; Fan, L.; Cai, J.; Han, W.; Zhang, F. Molecular oxygen activation in photocatalysis: Generation, detection and application. Surf. Interfaces 2024, 46, 104033. [Google Scholar] [CrossRef]
- González, S.; Jaramillo-Fierro, X. Density Functional Theory Study of Methylene Blue Demethylation as a Key Step in Degradation Mediated by Reactive Oxygen Species. Int. J. Mol. Sci. 2025, 26, 1756. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver Nanoparticles: Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Beneficial and Toxicological Effects. Mini Review; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; Volume 39, pp. 16–26. [Google Scholar]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef]
- Lu, X.; Qiu, W.; Ma, J.; Xu, H.; Wang, D.; Cheng, H.; Zhang, W.; He, X. The overestimated role of singlet oxygen for pollutants degradation in some non-photochemical systems. Chem. Eng. J. 2020, 401, 126128. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis; American Chemical Society: New York, NY, USA, 2016; Volume 1, pp. 356–359. [Google Scholar]
- Zhu, S.; Zheng, W.; Duan, W.; Feng, C. Reaction: Harnessing reactive oxygen species (ROS) for water purification. Chem 2023, 9, 1340–1341. [Google Scholar] [CrossRef]
- Bi, Z.; Wang, W.; Zhao, L.; Wang, X.; Xing, D.; Zhou, Y.; Lee, D.-J.; Ren, N.; Chen, C. The generation and transformation mechanisms of reactive oxygen species in the environment and their implications for pollution control processes: A review. Environ. Res. 2024, 260, 119592. [Google Scholar] [CrossRef]
- Edalati, P.; Itagoe, Y.; Ishihara, H.; Ishihara, T.; Emami, H.; Arita, M.; Fuji, M.; Edalati, K. Visible-light photocatalytic oxygen production on a high-entropy oxide by multiple-heterojunction introduction. J. Photochem. Photobiol. A Chem. 2022, 433, 114167. [Google Scholar] [CrossRef]
- Foglietta, F.; Serpe, L.; Canaparo, R. ROS-generating nanoplatforms as selective and tunable therapeutic weapons against cancer. Discov. Nano 2023, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Raja, M.A.; Sayed, M.; Shah, L.A.; Sohail, M. Advanced Oxidation and Reduction Processes. In Advances in Water Purification Techniques: Meeting the Needs of Developed and Developing Countries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–164. ISBN 9780128147900. [Google Scholar]
- Feng, D.; Li, X.; Liu, Y.; Chen, X.; Li, S. Emerging Bismuth-Based Step-Scheme Heterojunction Photocatalysts for Energy and Environmental Applications. Renewables 2023, 1, 485–513. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, G.; Yang, C.; Li, S. Recent progress on S-scheme heterojunction strategy enabling polymer carbon nitrides C3N4 and C3N5 enhanced photocatalysis in energy conversion and environmental remediation. Curr. Opin. Chem. Eng. 2024, 45, 101040. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Cuenca, G.; Ramón, J. Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems. Int. J. Mol. Sci. 2023, 24, 3780. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Li, Q.; Li, F.T. Recent advances in molecular oxygen activation via photocatalysis and its application in oxidation reactions. Chem. Eng. J. 2021, 421, 129915. [Google Scholar] [CrossRef]
- Litter, M.I. Introduction to Oxidative Technologies for Water Treatment; Springer: Cham, Switzerland, 2020; pp. 119–175. [Google Scholar]
- Luo, L.; Zhang, T.; Wang, M.; Yun, R.; Xiang, X. Recent Advances in Heterogeneous Photo-Driven Oxidation of Organic Molecules by Reactive Oxygen Species. ChemSusChem 2020, 13, 5173–5184. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Dong, K.; Chen, X.; Li, S. Floatable S-scheme Bi2WO6/C3N4/carbon fiber cloth composite photocatalyst for efficient water decontamination. Chin. J. Catal. 2023, 52, 239–251. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yang, Z.; Ying, G.-G.; Shih, K.; Feng, Y. Hydrogen peroxide as a key intermediate for hydroxyl radical generation during catalytic ozonation of biochar: Mechanistic insights into the evolution and contribution of radicals. Sep. Purif. Technol. 2023, 324, 124525. [Google Scholar] [CrossRef]
- Liu, J.; Dong, G.; Jing, J.; Zhang, S.; Huang, Y.; Ho, W. Photocatalytic reactive oxygen species generation activity of TiO2 improved by the modification of persistent free radicals. Environ. Sci. Nano 2021, 8, 3846–3854. [Google Scholar] [CrossRef]
- Vuckovic, S.; Fabiano, E.; Gori-Giorgi, P.; Burke, K. MAP: An MP2 Accuracy Predictor for Weak Interactions from Adiabatic Connection Theory. J. Chem. Theory Comput. 2020, 16, 4141–4149. [Google Scholar] [CrossRef]
- Shang, H.; Yang, J. Implementation of Laplace Transformed MP2 for Periodic Systems with Numerical Atomic Orbitals. Front. Chem. 2020, 8, 589992. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Peng, Y.; Bian, Z.; Wang, F.; Li, S.; Xu, S.; Wang, H. Electrocatalytic degradation of p-nitrophenol on metal-free cathode: Superoxide radical (O2•−) production via molecular oxygen activation. J. Hazard. Mater. 2024, 462, 132797. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 production Systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.b.; Huang, X.; Hung, S.F.; Cai, W.; Jia, C.; Miao, S.; Chen, H.M.; Yang, X.; Huang, Y.; et al. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2020, 6, 658–674. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef] [PubMed]
- Mahne, N.; Schafzahl, B.; Leypold, C.; Leypold, M.; Grumm, S.; Leitgeb, A.; Strohmeier, G.A.; Wilkening, M.; Fontaine, O.; Kramer, D.; et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium-oxygen batteries. Nat. Energy 2017, 2, 17036. [Google Scholar] [CrossRef]
- Dalrymple, R.M.; Carfagno, A.K.; Sharpless, C.M. Correlations between dissolved organic matter optical properties and quantum yields of singlet oxygen and hydrogen peroxide. Environ. Sci. Technol. 2010, 44, 5824–5829. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Alnashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Khan, A.U.; Kasha, M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 12365–12367. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.T.S.; Ogrodnik, A.; Gasteiger, H.A. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries. Mater. Today 2018, 21, 825–833. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhu, J. BODIPY parent compound: Excited triplet state and singlet oxygen formation exhibit strong molecular oxygen enhancing effect. J. Lumin. 2019, 212, 286–292. [Google Scholar] [CrossRef]
- Yanai, N.; Kimizuka, N. New Triplet Sensitization Routes for Photon Upconversion: Thermally Activated Delayed Fluorescence Molecules, Inorganic Nanocrystals, and Singlet-to-Triplet Absorption. Acc. Chem. Res. 2017, 50, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Prasad, A.; Rác, M. Mechanism of the formation of electronically excited species by oxidative metabolic processes: Role of reactive oxygen species. Biomolecules 2019, 9, 258. [Google Scholar] [CrossRef]
- Petit, Y.K.; Mourad, E.; Prehal, C.; Leypold, C.; Windischbacher, A.; Mijailovic, D.; Slugovc, C.; Borisov, S.M.; Zojer, E.; Brutti, S.; et al. Mechanism of mediated alkali peroxide oxidation and triplet versus singlet oxygen formation. Nat. Chem. 2021, 13, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kőrösi, L.; Bognár, B.; Bouderias, S.; Castelli, A.; Scarpellini, A.; Pasquale, L.; Prato, M. Highly-efficient photocatalytic generation of superoxide radicals by phase-pure rutile TiO2 nanoparticles for azo dye removal. Appl. Surf. Sci. 2019, 493, 719–728. [Google Scholar] [CrossRef]
- Yu, W.; Chen, F.; Wang, Y.; Zhao, L. Rapid evaluation of oxygen vacancies-enhanced photogeneration of the superoxide radical in nano-TiO2suspensions. RSC Adv. 2020, 10, 29082–29089. [Google Scholar] [CrossRef]
- Li, X.; Lyu, S.; Lang, X. Superoxide generated by blue light photocatalysis of g-C3N4/TiO2 for selective conversion of amines. Environ. Res. 2021, 195, 110851. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Su, H.; Zhu, Y.; Vafaei Molamahmood, H.; Long, M. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Res. 2018, 145, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Ji, J.; Shen, B.; Dong, C.; Liu, J.; Zhang, J.; Xing, M. Singlet Oxygen Triggered by Superoxide Radicals in a Molybdenum Cocatalytic Fenton Reaction with Enhanced REDOX Activity in the Environment. Environ. Sci. Technol. 2019, 53, 9725–9733. [Google Scholar] [CrossRef]
- Zaichenko, A.; Schröder, D.; Janek, J.; Mollenhauer, D. Pathways to Triplet or Singlet Oxygen during the Dissociation of Alkali Metal Superoxides: Insights by Multireference Calculations of Molecular Model Systems. Chem. A Eur. J. 2020, 26, 2395–2404. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Cao, Q.; Liao, H.; Gao, J.; Zhang, L.; Wei, W.; Li, H.; Lu, J. Structural Regulation of Photocatalyst to Optimize Hydroxyl Radical Production Pathways for Highly Efficient Photocatalytic Oxidation. Adv. Mater. 2024, 36, 2306758. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Litorja, M.; Ruscic, B. A photoionization study of the hydroperoxyl radical, HO2, and hydrogen peroxide, H2O2. J. Electron Spectros. Relat. Phenom. 1998, 97, 131–146. [Google Scholar] [CrossRef]
- Thürmer, S.; Unger, I.; Slavíček, P.; Winter, B. Relaxation of Electronically Excited Hydrogen Peroxide in Liquid Water: Insights from Auger-Electron Emission. J. Phys. Chem. C 2013, 117, 22268–22275. [Google Scholar] [CrossRef]
- Hrušák, J.; Iwata, S. The vibrational spectrum of H2O2+ radical cation: An illustration of symmetry breaking. J. Chem. Phys. 1997, 106, 4877–4888. [Google Scholar] [CrossRef]
- Thompson, W.E.; Lugez, C.L.; Jacox, M.E. The infrared spectrum of HOOH+ trapped in solid neon. J. Chem. Phys. 2012, 137, 144305. [Google Scholar] [CrossRef]
- Messer, B.M.; Stielstra, D.E.; Cappa, C.D.; Scholtens, K.W.; Elrod, M.J. Computational and experimental studies of chemical ionization mass spectrometric detection techniques for atmospherically relevant peroxides. Int. J. Mass Spectrom. 2000, 197, 219–235. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Ye, Z.; He, J.; Zheng, Z.; Gong, X.; Zhang, J.; Lo, I.M.C. Superoxide radicals dominated visible light driven peroxymonosulfate activation using molybdenum selenide (MoSe2) for boosting catalytic degradation of pharmaceuticals and personal care products. Appl. Catal. B Environ. 2021, 296, 120223. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, D.; Niu, S.; Liu, S.; Liu, Y.; Zhao, B.; Zhang, Y.; Liu, H.; Zhao, Z.; Li, M.; et al. Modulating electron density enable efficient cascade conversion from peroxymonosulfate to superoxide radical driven by electron-rich/poor dual sites. J. Hazard. Mater. 2024, 468, 133749. [Google Scholar] [CrossRef]
- Ren, Q.; Liu, J.; Yang, Z.; Yang, Q. Boosting transformation of dissolved oxygen to superoxide radical: Function of P25. Water Environ. Res. 2023, 95, e10898. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Wan, Q.; Zeng, H.; Wang, H.; Yu, H.; Pang, H.; Zhang, W.; Yuan, X.; Huang, J. Built-in electric field boosted exciton dissociation in sulfur doped BiOCl with abundant oxygen vacancies for transforming the pathway of molecular oxygen activation. Appl. Catal. B Environ. 2024, 343, 123557. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, B.; Zhang, Y.; Zhao, Y.; Wang, S. Directional regulation of reactive oxygen species in titanium dioxide boosting the photocatalytic degradation performance of azo dyes. J. Colloid Interface Sci. 2024, 673, 275–283. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Q.; Fan, J.; Yu, T.; Zhang, C.; Zhou, X. Efficient decontamination and detoxification of phenols by photocatalytic CQDs@Ag3PO4: Re-insights into the targeted reduction and coupling processes of ·O2− and 1O2. Sep. Purif. Technol. 2025, 353, 128306. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Chen, T.; Yang, N.; Wang, S.; Ding, X.; Chen, H. Deep insight into ROS mediated direct and hydroxylated dichlorination process for efficient photocatalytic sodium pentachlorophenate mineralization. Appl. Catal. B Environ. 2021, 296, 120352. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Qin, H.; Huang, J.; Yu, Y. Identification of Reactive Oxygen Species and Mechanism on Visible Light-Induced Photosensitized Degradation of Oxytetracycline. Int. J. Environ. Res. Public Health 2022, 19, 15550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lei, J.; Wang, F.; Wang, L.; Hoffmann, M.R.; Liu, Y.; In, S.-I.; Zhang, J. Carbon nitride nanotubes with in situ grafted hydroxyl groups for highly efficient spontaneous H2O2 production. Appl. Catal. B Environ. 2021, 288, 119993. [Google Scholar] [CrossRef]
- Li, Y.F.; Selloni, A. Theoretical study of interfacial electron transfer from reduced anatase TiO2(101) to adsorbed O2. J. Am. Chem. Soc. 2013, 135, 9195–9199. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.H.; Gul, N.S.; Sabahat, S.; Sun, J.; Tahir, K.; Shah, N.S.; Muhammad, N.; Rahim, A.; Imran, M.; Iqbal, J.; et al. Removal of organic pollutants through hydroxyl radical-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2023, 267, 115564. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Kjellsson, L.; Nanda, K.D.; Rubensson, J.E.; Doumy, G.; Southworth, S.H.; Ho, P.J.; March, A.M.; Al Haddad, A.; Kumagai, Y.; Tu, M.F.; et al. Resonant Inelastic X-Ray Scattering Reveals Hidden Local Transitions of the Aqueous OH Radical. Phys. Rev. Lett. 2020, 124, 236001. [Google Scholar] [CrossRef]
- Berbille, A.; Li, X.F.; Su, Y.; Li, S.; Zhao, X.; Zhu, L.; Wang, Z.L. Mechanism for Generating H2O2 at Water-Solid Interface by Contact-Electrification. Adv. Mater. 2023, 35, 2304387. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Chapter 1. Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Royal Society of Chemistry: London, UK, 2016; pp. 1–21. [Google Scholar]
- Huang, S.; Xu, Y.; Liu, Q.; Zhou, T.; Zhao, Y.; Jing, L.; Xu, H.; Li, H. Enhancing reactive oxygen species generation and photocatalytic performance via adding oxygen reduction reaction catalysts into the photocatalysts. Appl. Catal. B Environ. 2017, 218, 174–185. [Google Scholar] [CrossRef]
- Liu, C.; Ji, Y.; Shao, Q.; Feng, X.; Lu, X. Thermodynamic analysis for synthesis of advanced materials. Struct. Bond. 2009, 131, 193–270. [Google Scholar] [CrossRef]
- Cheng, B.; Ceriotti, M. Computing the absolute Gibbs free energy in atomistic simulations: Applications to defects in solids. Phys. Rev. B 2018, 97, 054102. [Google Scholar] [CrossRef]
- Tuchagues, J.-P.; Bousseksou, A.; Molnár, G.; McGarvey, J.J.; Varret, F. The Role of Molecular Vibrations in the Spin Crossover Phenomenon. In Spin Crossover in Transition Metal Compounds III; Springer: Berlin/Heidelberg, Germany, 2006; pp. 84–103. ISBN 978-3-540-44984-3. [Google Scholar]
- Hanh, T.T.T.; Van Hoa, N. Zero-Point Vibration of the Adsorbed Hydrogen on the Pt(110) Surface. Divers. Quasipart. Prop. Emerg. Mater. 2022, 303–315. [Google Scholar] [CrossRef]
- Bajpai, A.; Mehta, P.; Frey, K.; Lehmer, A.M.; Schneider, W.F. Benchmark First-Principles Calculations of Adsorbate Free Energies. ACS Catal. 2018, 8, 1945–1954. [Google Scholar] [CrossRef]
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 photocatalysis of oxygenated and deoxygenated aqueous systems: A probe for photocatalytically produced hydroxyl radicals. J. Phys. Chem. C 2014, 118, 10083–10087. [Google Scholar] [CrossRef]
- Friesner, R.A. Ab initio quantum chemistry: Methodology and applications. Proc. Natl. Acad. Sci. USA 2005, 102, 6648–6653. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D. Møller–Plesset perturbation theory: From small molecule methods to methods for thousands of atoms. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 509–530. [Google Scholar] [CrossRef]
- Ayala, P.Y.; Scuseria, G.E. Linear scaling second-order Moller–Plesset theory in the atomic orbital basis for large molecular systems. J. Chem. Phys. 1999, 110, 3660–3671. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Riley, K.E.; Hobza, P. Assessment of the MP2 method, along with several basis sets, for the computation of interaction energies of biologically relevant hydrogen bonded and dispersion bound complexes. J. Phys. Chem. A 2007, 111, 8257–8263. [Google Scholar] [CrossRef]
- Feyereisen, M.; Fitzgerald, G.; Komornicki, A. Use of approximate integrals in ab initio theory. An application in MP2 energy calculations. Chem. Phys. Lett. 1993, 208, 359–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Shi, X.; Back, S.; Gill, T.M.; Siahrostami, S.; Zheng, X. Electrochemical Synthesis of H2O2 by Two-Electron Water Oxidation Reaction. Chem 2021, 7, 38–63. [Google Scholar] [CrossRef]
- Keller, E.; Tsatsoulis, T.; Reuter, K.; Margraf, J.T. Regularized second-order correlation methods for extended systems. J. Chem. Phys. 2022, 156, 24106. [Google Scholar] [CrossRef]
- Piris, M. Dynamic electron-correlation energy in the natural-orbital-functional second-order-Møller-Plesset method from the orbital-invariant perturbation theory. Phys. Rev. A 2018, 98, 022504. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Cao, D.; Mao, R.; Zhao, X. Synergetic activation of H2O2 by photo-generated electrons and cathodic Fenton reaction for enhanced self-driven photoelectrocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2018, 235, 1–8. [Google Scholar] [CrossRef]
- Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Niu, J. Selective electrochemical H2O2 generation and activation on a bifunctional catalyst for heterogeneous electro-Fenton catalysis. J. Hazard. Mater. 2020, 382, 121102. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, G.; Ji, Q.; Liu, H.; Hua, X.; Xia, H.; Sillanpää, M.; Qu, J. PH-Independent Production of Hydroxyl Radical from Atomic H*-Mediated Electrocatalytic H2O2 Reduction: A Green Fenton Process without Byproducts. Environ. Sci. Technol. 2020, 54, 14725–14731. [Google Scholar] [CrossRef] [PubMed]
- MacManus-Spencer, L.A.; McNeill, K. Quantification of singlet oxygen production in the reaction of superoxide with hydrogen peroxide using a selective chemiluminescent probe. J. Am. Chem. Soc. 2005, 127, 8954–8955. [Google Scholar] [CrossRef]
- Schürmann, A.; Luerßen, B.; Mollenhauer, D.; Janek, J.; Schröder, D. Singlet Oxygen in Electrochemical Cells: A Critical Review of Literature and Theory. Chem. Rev. 2021, 121, 12445–12464. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6; Semichem Inc.: Shawnee, KS, USA, 2019. [Google Scholar]

| Species | E° (Hartree) | G° (Hartree) | dO-O (Å) | νO-O (cm−1) | μ (Debye) | R2 (Å2) |
|---|---|---|---|---|---|---|
| O2(T) | −150.04 | −150.06 | 1.23 | 1423 | 0 | 12.43 |
| 1O2(S) | −149.99 | −150.01 | 1.26 | 1216 | 0 | 12.75 |
| •O2−(D) | −150.15 | −150.17 | 1.37 | 1064 | 0 | 16.02 |
| Reaction | ΔE (kJ mol−1) * | ΔG (kJ mol−1) | |
|---|---|---|---|
| O2(T) → 1O2(S) | (1) | 124 | 126 |
| 1O2(S) → •O2−(D) + e− | (2) | −405 | −407 |
| •O2−(D) + h+ → 1O2(S) | (3) | 405 | 407 |
| O2(T) + e− → •O2−(D) | (4) | −281 | −281 |
| Reaction | ΔE (kJ mol−1) | ΔG (kJ mol−1) | |
|---|---|---|---|
| •O2− + •OH → O2(S) + OH− | (5) | −38 | −34 |
| •O2− + H+ → •O2H | (6) | 130 | 152 |
| Species | E° (Hartree) | ΔE° (kJ mol−1) | G° (Hartree) | ΔG° (kJ mol−1) | dO-H (Å) | νO-H (cm−1) | μ (Debye) | R2 (Å2) |
|---|---|---|---|---|---|---|---|---|
| OH−(S) | −75.75 | −444 | −75.77 | −442 | 0.96 | 3794 | 6.23 | |
| •OH(D) | −75.58 | −75.60 | 0.97 | 3826 | 2.11 |
| Species | E° (Hartree) | G° (Hartree) | ΔG° (kJ mol−1) | dO-O (Å) | dO-H (Å) | νO-H (cm−1) | νO-O (cm−1) | μ (Debye) | R2 (Å2) |
|---|---|---|---|---|---|---|---|---|---|
| H2O2 | −151.23 | −151.26 | 1.46 | 0.97 | 3799 3803 | 931 | 2.48 | 47.1 | |
| H2O2− | −151.34 | −151.37 | −295 | 2.19 | 0.97 | 3854 3857 | 367 | 1.55 | 32.4 |
| H2O2+ | −151.94 | −151.96 | 773 | 1.31 | 1.00 | 3541 | 896 | 3.49 | 15.8 |
| Reaction | ΔE (kJ mol−1) | ΔG (kJ mol−1) | |
|---|---|---|---|
| •O2− + 2H+ → H2O2 + h+ | (7) | 542 | 590 |
| HO2− + H2O → H2O2 + OH− | (8) | 492 | 494 |
| •O2H + H+ → H2O2 + h+ | (9) | 412 | 438 |
| •O2− + •O2H + H+ → O2(S) + H2O2 | (10) | 43 | 73 |
| ½ O2 + H2O → H2O2 | (11) | 127 | 142 |
| •O2− + •O2H + H2O → H2O2 + O2 + OH− | (12) | −45 | −40 |
| •OH + O2 + OH− → H2O2 + O2− | (13) | −31 | −2 |
| •OH + OH− → H2O2 + e− | (14) | −39 | −16 |
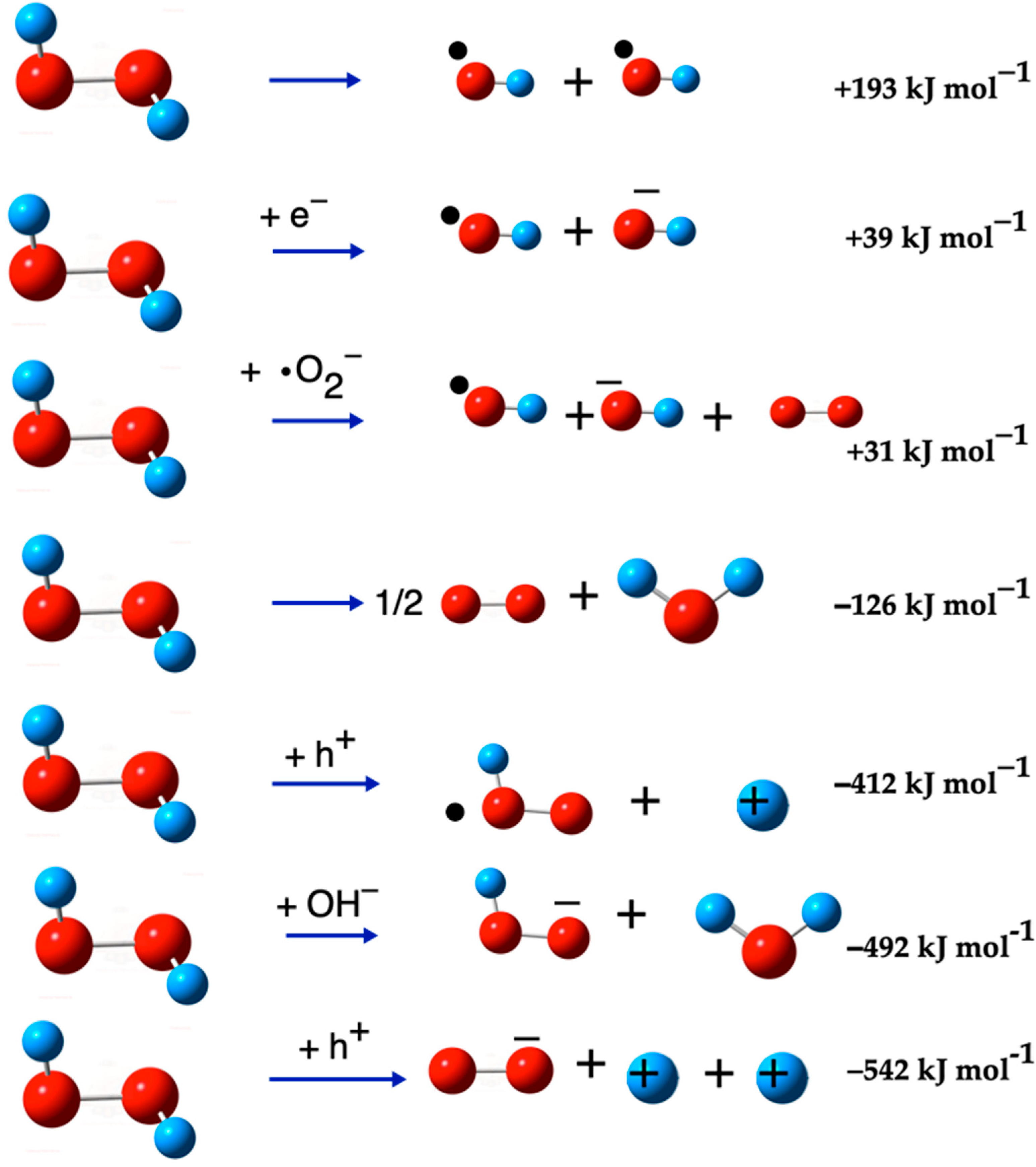
| •OH + •OH → H2O2 | (15) | −193 | −163 |
| •O2− + 2H+ + e− → H2O2 | (16) | −232 | −182 |
| 2H2O + 2h+ → H2O2 + 2H+ | (17) | −975 | −990 |
| Reaction | ΔE (kJ mol−1) | ΔG (kJ mol−1) | |
|---|---|---|---|
| H2O2 → •OH + •OH | (18) | 193 | 163 |
| H2O2 + e− → •OH + OH− | (19) | 39 | 16 |
| H2O2 + •O2− → •OH + O2 + OH− | (20) | 31 | 2 |
| H2O2 → ½ O2 + H2O | (21) | −127 | −142 |
| H2O2 + h+ → •O2H + H+ | (22) | −412 | −438 |
| H2O2 + OH− → HO2− + H2O | (23) | −492 | −494 |
| H2O2 + h+ → •O2− + 2H+ | (24) | −542 | −590 |
| Species | E° (Hartree) | G° (Hartree) | dO-O (Å) | dO-H (Å) | νO-H (cm−1) | νO-O (cm−1) | μ (Debye) | R2 (Å2) |
|---|---|---|---|---|---|---|---|---|
| H+ | −0.50 | −0.51 | 0 | 3.1 | ||||
| O−(S) | −75.08 | −75.09 | 4.8 | |||||
| H2O(S) | −76.26 | −76.28 | 0.96 | 3844 3952 | 1626 | 2.50 | 29.3 | |
| H2O+(D) | −75.93 | −75.95 | 1.00 | 3493 3543 | 1483 | 6.28 | 25.6 | |
| •O2H(D) | −150.60 | −150.62 | 1.33 | 0.98 | 3679 | 1226 1441 | 2.40 | 43.9 |
| O2H−(S) | −150.74 | −150.76 | 1.51 | 0.96 | 3839 | 1177 891 | 9.28 | 50.6 |
| Target ROS | Reaction | ΔE (kJ mol−1) | ΔG (kJ mol−1) | |
|---|---|---|---|---|
| •O2H | 2H2O + 2h+ → H2O2 + 2H+ H2O2 + h+ → •O2− + 2H+ •O2− + H+ → •O2H | (17) (24) (6) | −975 −542 +130 ΔE = −1387 | −990 −590 +152 ΔG = −1428 |
| H2O2 | 2H2O + 2h+ → H2O2 + 2H+ | (17) | −975 | −990 |
| •O2− | 2H2O + 2h+ → H2O2 + 2H+ H2O2 + h+ → •O2− + 2H+ | (17) (24) | −975 −542 ΔE = −1517 | −990 −590 ΔG = −1580 |
| •OH | 2H2O + 2h+ → H2O2 + 2H+ H2O2 + h+ → •O2− + 2H+ H2O2 + •O2− → •OH + O2 + OH− | (17) (24) (20) | −975 −542 +31 ΔE = −1486 | −990 −590 +2 ΔG = −1578 |
| •OH | 2H2O + 2h+ → H2O2 + 2H+ H2O2 + e− → •OH + OH− | (17) (19) | −975 +39 ΔE = −936 | −990 +16 ΔG = −974 |
| 1O2 | O2 → 1O2 | (1) | +124 | +126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, S.; Jaramillo-Fierro, X. Ab Initio Study of Formation Mechanisms and Thermochemical Properties of Reactive Oxygen Species (ROS) in Photocatalytic Processes. Int. J. Mol. Sci. 2025, 26, 8989. https://doi.org/10.3390/ijms26188989
González S, Jaramillo-Fierro X. Ab Initio Study of Formation Mechanisms and Thermochemical Properties of Reactive Oxygen Species (ROS) in Photocatalytic Processes. International Journal of Molecular Sciences. 2025; 26(18):8989. https://doi.org/10.3390/ijms26188989
Chicago/Turabian StyleGonzález, Silvia, and Ximena Jaramillo-Fierro. 2025. "Ab Initio Study of Formation Mechanisms and Thermochemical Properties of Reactive Oxygen Species (ROS) in Photocatalytic Processes" International Journal of Molecular Sciences 26, no. 18: 8989. https://doi.org/10.3390/ijms26188989
APA StyleGonzález, S., & Jaramillo-Fierro, X. (2025). Ab Initio Study of Formation Mechanisms and Thermochemical Properties of Reactive Oxygen Species (ROS) in Photocatalytic Processes. International Journal of Molecular Sciences, 26(18), 8989. https://doi.org/10.3390/ijms26188989







