Environmental Insults to Glucose Metabolism: The Role of Pollutants in Insulin Resistance
Abstract
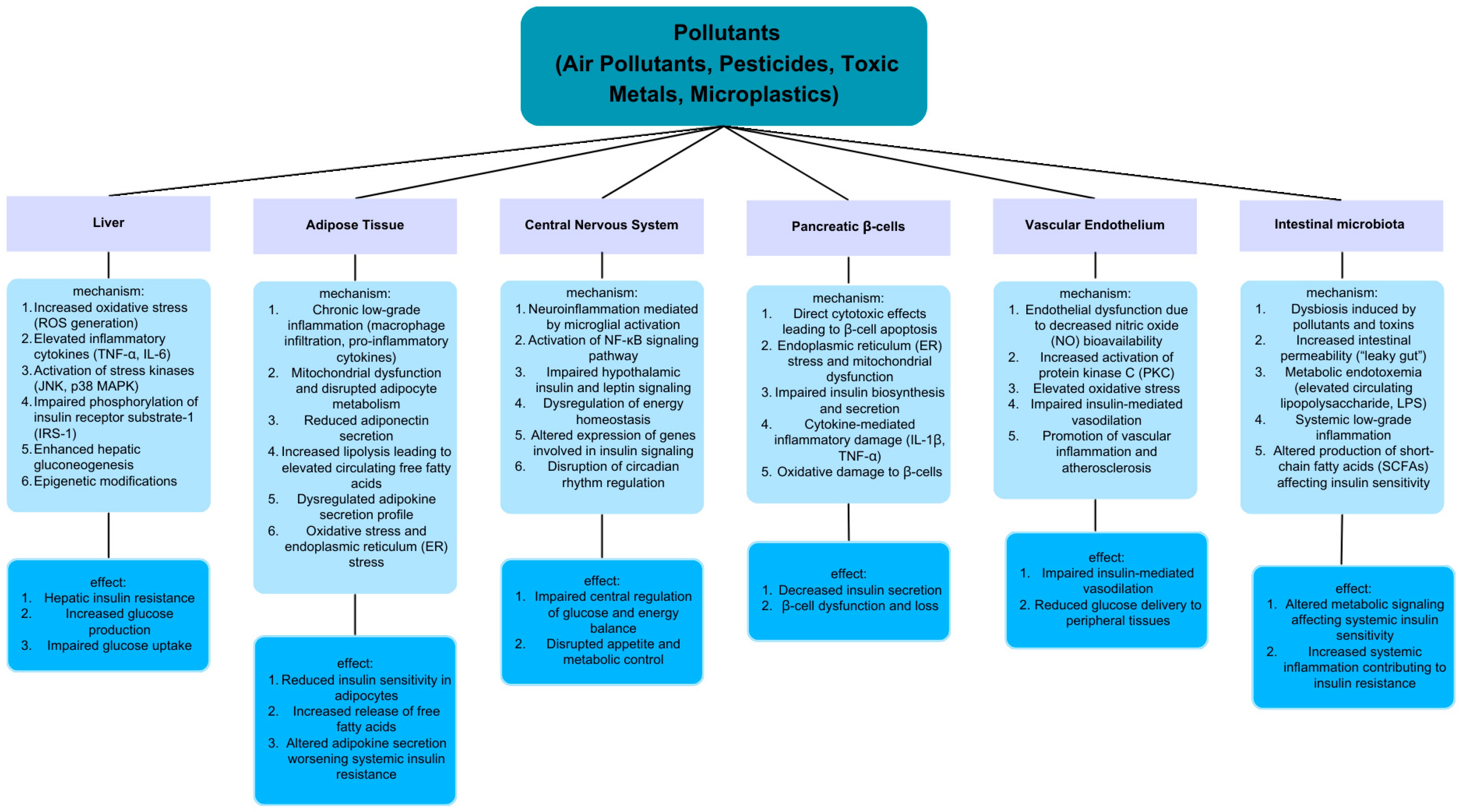
1. Introduction
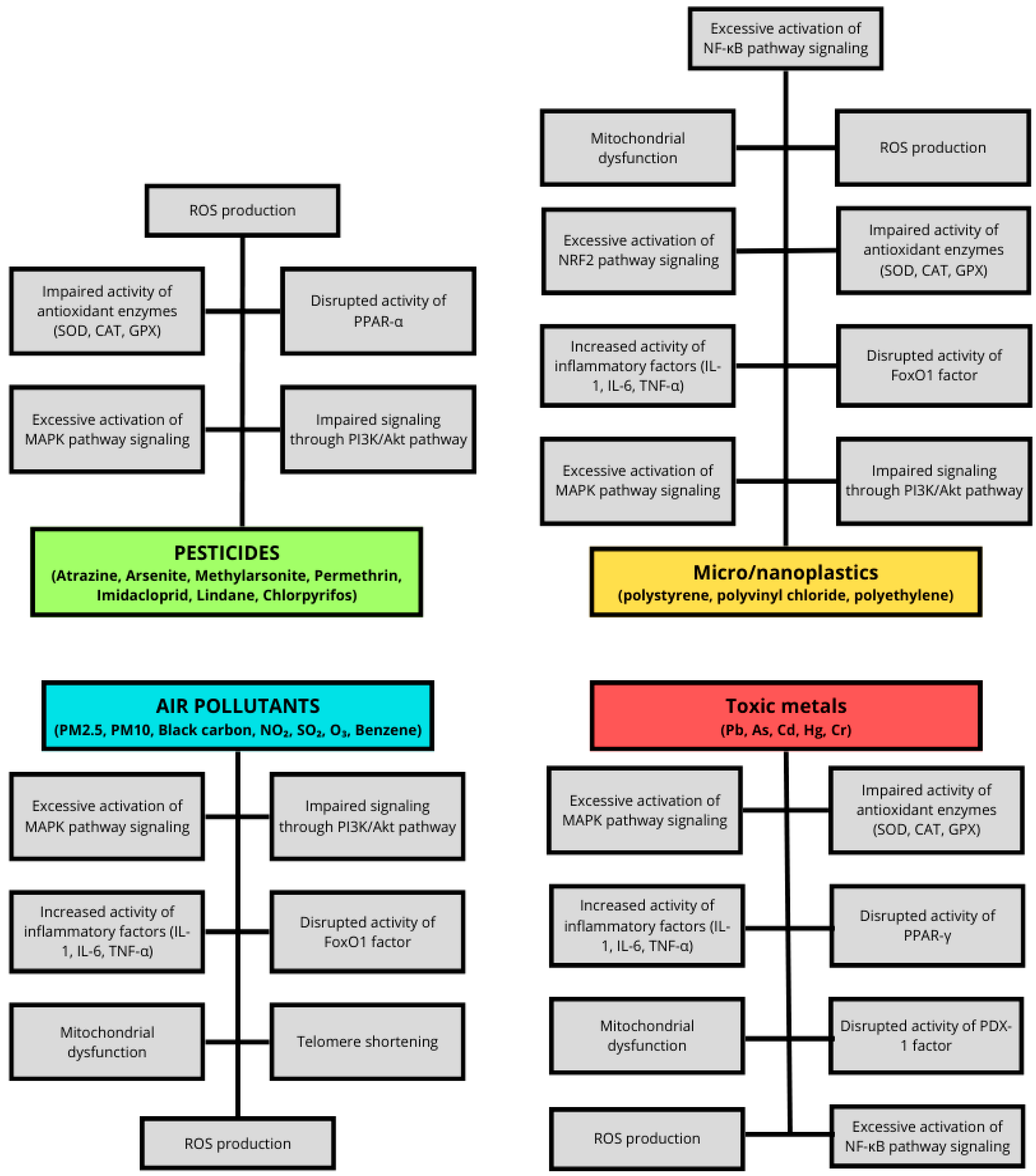
2. The Role of Air Pollutants in Insulin Resistance
2.1. Particulate Matter (PM)
2.2. Black Carbon (BC)
- Personal PM2.5 levels dropped by 48%: from a median of 82 μg/m3 (traditional stove) to 43 μg/m3 (Justa stove).
- Personal BC levels fell by 70%: from 11.8 μg/m3 to 3.5 μg/m3.
- Kitchen BC levels decreased by 86%: from 47.0 μg/m3 to 6.5 μg/m3.
2.3. Nitrogen Dioxide and Sulfur Dioxide
2.4. Ozone
2.5. Benzene
2.6. Comparative Impact of Air Pollutants on Insulin Resistance
| Pollutant | Proposed Mechanisms of Insulin Resistance | Key References |
|---|---|---|
| PM2.5 |
| [20,41,42,44] |
| PM10 |
| [20,36] |
| BC |
| [48] |
| NO2 |
| [50,66] |
| SO2 |
| [54,55] |
| O3 |
| [56,57] |
| Benzene |
| [58,60] |
3. The Role of Pesticides in Insulin Resistance
3.1. Pesticide Bioaccumulation and Markers of Insulin Resistance, Type 2 Diabetes and Metabolic Disorders
3.2. Effects of Selected Pesticides on Glucose Metabolism Disturbances: Evidence from In Vivo and In Vitro Studies
3.2.1. Atrazine
3.2.2. Arsenite and Methylarsonite
3.2.3. Permethrin
3.2.4. Imidacloprid
3.2.5. Lindane
3.2.6. Chlorpyrifos
4. The Role of Toxic Metals in Insulin Resistance
4.1. Lead
4.2. Arsenic
4.3. Cadmium
| Metal | Mechanisms of Action | Epidemiological Evidence | Key References |
|---|---|---|---|
| Pb |
|
| [112,115,116,118,119] |
| As |
|
| [121,122,123,125,126,127] |
| Cd |
|
| [131,132,140,141,144,148] |
| Hg |
|
| [151,152,153,154,155] |
| Cr |
|
| [156,157,158,159,160,161,162] |
4.4. Mercury
4.5. Chromium
5. The Role of Micro- and Nanoplastics in Insulin Resistance
5.1. Micro- and Nanoplastics Characteristics and Sources
5.2. Micro- and Nanoplastics Influence on Glucose Homeostasis and Insulin Resistance
6. Future Directions and Translational Perspectives
6.1. Standardized Biomarkers of Environmental Exposure and Metabolic Effect
6.2. Exposomics for Stratifying Metabolic Risk
6.3. Preventive and Therapeutic Opportunities
6.4. Regulatory and Public Health Implications
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| ADHD | attention deficit hyperactivity disorder |
| As | arsenic |
| As3+ | arsenic trioxide |
| As5+ | arsenates |
| ATR | atrazine |
| BKMR | Bayesian kernel machine regression |
| BMI | body mass index |
| CAT | catalase |
| Cd | cadmium |
| CD36 | cluster of differentiation 36 |
| CHLs | chlordanes |
| COPD | chronic obstructive pulmonary disease |
| COVID-19 | coronavirus disease 2019 |
| COX-2 | cyclooxygenase-2 |
| CR | cortisol receptor |
| Cr | chromium |
| CRH | corticotropin-releasing hormone |
| CRP | c-reactive protein |
| DAG | directed acyclic graph |
| DDTs | dichlorodiphenyltrichloroethanes |
| DMA | dimethylarsinic acid |
| eNOS | endothelial nitric oxide synthase |
| ER-α | estrogen receptor α |
| FoxO1 | forkhead box protein O1 |
| FPG | fasting plasma glucose |
| G6PC | glucose-6-phosphatase |
| GHR | growth hormone receptor |
| GLUT | glucose transporter |
| GP | glycogen phosphorylase |
| GPX | glutathione peroxidase |
| GS | glycogen synthase |
| HBM4EU | European Human Biomonitoring Initiative |
| HCB | hexachlorobenzene |
| HCHs | hexachlorocyclohexanes |
| Hg | mercury |
| HMGB1 | high-mobility group box 1 |
| HOMA-B | homeostatic assessment for β-cell function |
| HOMA-IR | homeostasic model assessment of insulin resistance |
| HPA | hypothalamic–pituitary–adrenal |
| HRMS | High-resolution mass spectrometry |
| HSP | heat shock protein |
| I3A | indole-3-carbaldehyde |
| IGFBP-1 | insulin-like growth factor binding protein-1 |
| IκB-α | inhibitor of nuclear factor kappa-B kinase subunit α |
| IκK-β | inhibitor of nuclear factor kappa-B kinase subunit β |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| IR | insulin receptor |
| IRS | insulin receptor substrate |
| ITT | insulin tolerance test |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MeHg | methylmercury |
| MetS | metabolic syndrome |
| MMA | monomethylarsinic acid |
| NAFLD | nonalcoholic fatty liver disease |
| NF-kB | nuclear factor kappaB |
| NHANES | National Health and Nutrition Examination Survey |
| NO2 | nitric dioxide |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| OC | organochlorine |
| OGTT | oral glucose tolerance test |
| OP | organophosphorous |
| OR | odds ratio |
| PAHs | polycyclic aromatic hydrocarbons |
| Pb | lead |
| PDK-1 | pyruvate dehydrogenase lipoamide kinase isozyme 1 |
| PDX1 | pancreatic and duodenal homeobox 1 |
| PEPCK | phosphoenolpyruvate carboxykinase |
| PI3K | phosphatidylinositol 3-kinase |
| PKB | protein kinase B |
| PM | particulate matter |
| POCS | polycystic ovarian syndrome |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| PVC-MPs | virgin polyvinyl chloride microplastics |
| RAGE | receptor for advanced glycation end-product |
| ROS | reactive oxygen species |
| SAT | subcutaneous adipose tissue |
| SO2 | sulfur dioxide |
| SOD | superoxide dismutase |
| SPECT | Survey on Prevalence in East China for Metabolic Diseases and Risk Factors |
| SREBP1c | sterol regulatory element-binding protein 1c |
| t,t-MA | trans,trans-muconic acid |
| T2DM | type 2 diabetes mellitus |
| TLR-4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor α |
| UPAS | ultrasonic personal aerosol sampler |
| VAT | visceral adipose tissue |
| WHO | World Health Organization |
| Zn | zinc |
References
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Carr, A.L.J.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 years of insulin: Celebrating the past, present and future of diabetes therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef]
- Gierach, M.; Gierach, J.; Junik, R. Insulinooporność a choroby tarczycy. Endokrynol. Pol. 2014, 65, 70–76. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Lewis, S.T.; Greenway, F.; Tucker, T.R.; Alexander, M.; Jackson, L.K.; Hepford, S.A.; Loveridge, B.; Lakey, J.R.T. A Receptor Story: Insulin Resistance Pathophysiology and Physiologic Insulin Resensitization’s Role as a Treatment Modality. Int. J. Mol. Sci. 2023, 24, 10927. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 101964. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.M.; Goyal, R.K. Liver and insulin resistance: New wine in old bottle!!! Eur. J. Pharmacol. 2019, 862, 172657. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Mukharjee, S.; Bank, S.; Maiti, S. Chronic Tobacco Exposure by Smoking Develops Insulin Resistance. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.O.; Conde, S.V. Impact of Diet Composition on Insulin Resistance. Nutrients 2022, 14, 3716. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Jurkovičová, J.; Hirošová, K.; Vondrová, D.; Samohýl, M.; Štefániková, Z.; Filová, A.; Kachútová, I.; Babjaková, J.; Argalášová, Ľ. The Prevalence of Insulin Resistance and the Associated Risk Factors in a Sample of 14–18-Year-Old Slovak Adolescents. Int. J. Environ. Res. Public Health 2021, 18, 909. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, E.; Fernandez, M.F.; Mustieles, V.; Tolonen, H. Metabolic Syndrome and Endocrine Disrupting Chemicals: An Overview of Exposure and Health Effects. Int. J. Environ. Res. Public Health 2021, 18, 13047. [Google Scholar] [CrossRef]
- Fathallah, N.; Slim, R.; Larif, S.; Hmouda, H.; Ben Salem, C. Drug-Induced Hyperglycaemia and Diabetes. Drug Saf. 2015, 38, 1153–1168. [Google Scholar] [CrossRef]
- Pearlman, M.; Obert, J. Casey The Association Between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef]
- Dang, J.; Yang, M.; Zhang, X.; Ruan, H.; Qin, G.; Fu, J.; Shen, Z.; Tan, A.; Li, R.; Moore, J. Associations of Exposure to Air Pollution with Insulin Resistance: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2593. [Google Scholar] [CrossRef]
- Compendium of WHO and Other UN Guidance on Health and Environment (2024 Update). Available online: https://iris.who.int/bitstream/handle/10665/378095/9789240095380-eng.pdf?sequence=1 (accessed on 28 August 2025).
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef]
- Identification of Risks from Exposure to Endocrine-Disrupting Chemicals at the Country Level. Available online: https://iris.who.int/bitstream/handle/10665/344588/9789289050142-eng.pdf?sequence=1 (accessed on 28 August 2025).
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating Burden and Disease Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Colucci, M.; Deng, L.; Yang, M.; Huang, X.; Zhou, X.; Jin, Y.; Lazzarini, E.; Balbi, C.; et al. The mixed effect of Endocrine-Disrupting chemicals on biological age Acceleration: Unveiling the mechanism and potential intervention target. Environ. Int. 2024, 184, 108447. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009, 304, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.A.; Wheeler, H.B.; Blumberg, B. Obesity and endocrine-disrupting chemicals. Endocr. Connect. 2021, 10, R87–R105. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Fan, Y.; Schlezinger, J.J.; Ehrlich, S.D.; Webster, T.F.; Hyötyläinen, T.; Pedersen, O.; Orešič, M. Exposure to environmental toxicants is associated with gut microbiome dysbiosis, insulin resistance and obesity. Environ. Int. 2024, 186, 108569. [Google Scholar] [CrossRef]
- Prasad, M.; Gatasheh, M.K.; Alshuniaber, M.A.; Krishnamoorthy, R.; Rajagopal, P.; Krishnamoorthy, K.; Periyasamy, V.; Veeraraghavan, V.P.; Jayaraman, S. Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways. Antioxidants 2022, 11, 2436. [Google Scholar] [CrossRef]
- Bai, J.; Ma, Y.; Zhao, Y.; Yang, D.; Mubarik, S.; Yu, C. Mixed exposure to phenol, parabens, pesticides, and phthalates and insulin resistance in NHANES: A mixture approach. Sci. Total Environ. 2022, 851, 158218. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari, S.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide: Executive Summary. 2021. Available online: https://www.who.int/publications/i/item/9789240034433 (accessed on 21 July 2025).
- Parasin, N.; Amnuaylojaroen, T.; Saokaew, S.; Sittichai, N.; Tabkhan, N.; Dilokthornsakul, P. Outdoor air pollution exposure and the risk of type 2 diabetes mellitus: A systematic umbrella review and meta-analysis. Environ. Res. 2025, 269, 120885. [Google Scholar] [CrossRef]
- Gong, X.; Wang, S.; Wang, X.; Zhong, S.; Yuan, J.; Zhong, Y.; Jiang, Q. Long-term exposure to air pollution and risk of insulin resistance: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2024, 271, 115909. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Gui, Z.-H.; Zou, Z.-Y.; Yang, B.-Y.; Ma, J.; Jing, J.; Wang, H.-J.; Luo, J.-Y.; Zhang, X.; Luo, C.-Y.; et al. Long-term exposure to ambient air pollution and metabolic syndrome in children and adolescents: A national cross-sectional study in China. Environ. Int. 2021, 148, 106383. [Google Scholar] [CrossRef]
- Gilles, L.; Govarts, E.; Rodriguez Martin, L.; Andersson, A.-M.; Appenzeller, B.M.R.; Barbone, F.; Castaño, A.; Coertjens, D.; Den Hond, E.; Dzhedzheia, V.; et al. Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants. Int. J. Environ. Res. Public Health 2022, 19, 6787. [Google Scholar] [CrossRef]
- Wilson, W.E.; Suh, H.H. Fine Particles and Coarse Particles: Concentration Relationships Relevant to Epidemiologic Studies. J. Air Waste Manag. Assoc. 1997, 47, 1238–1249. [Google Scholar] [CrossRef]
- Kim, J.-A.; Jeong, S.; Lim, S.; Choi, Y.; Kim, H.; Lee, M. Oxidative potential of urban PM2.5 in relation to chemical composition: Importance of fossil driven sources. Environ. Int. 2025, 198, 109424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, J.; Chen, R.; Zhao, Z.; Ying, Z.; Wang, L.; Chen, J.; Hao, K.; Kinney, P.L.; Chen, H.; et al. Particulate Matter Exposure and Stress Hormone Levels. Circulation 2017, 136, 618–627, Erratum in Circulation 2017, 136, e199. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, R.; Cai, J.; Cui, X.; Huang, N.; Kan, H. Short-term exposure to fine particulate air pollution and genome-wide DNA methylation: A randomized, double-blind, crossover trial. Environ. Int. 2018, 120, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Rohatgi, N.; Fukunaga, T.; Wang, Q.-T.; Silva, M.J.; Gardner, M.J.; McDaniel, M.L.; Abumrad, N.A.; Semenkovich, C.F.; Teitelbaum, S.L.; et al. ASXL2 Regulates Glucose, Lipid, and Skeletal Homeostasis. Cell Rep. 2015, 11, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.; Du, Y.; Cui, L.; et al. PM2.5 and Serum Metabolome and Insulin Resistance, Potential Mediation by the Gut Microbiome: A Population-Based Panel Study of Older Adults in China. Environ. Health Perspect. 2022, 130, 027007. [Google Scholar] [CrossRef]
- Heindel, J.J.; Alvarez, J.A.; Atlas, E.; Cave, M.C.; Chatzi, V.L.; Collier, D.; Corkey, B.; Fischer, D.; Goran, M.I.; Howard, S.; et al. Obesogens and Obesity: State-of-the-Science and Future Directions Summary from a Healthy Environment and Endocrine Disruptors Strategies Workshop. Am. J. Clin. Nutr. 2023, 118, 329–337. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Hoek, G.; Simic-Lawson, M.; Fischer, P.; van Bree, L.; ten Brink, H.; Keuken, M.; Atkinson, R.W.; Anderson, H.R.; Brunekreef, B.; et al. Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM 10 and PM 2.5. Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef]
- Benka-Coker, M.L.; Young, B.N.; Keller, J.P.; Walker, E.S.; Rajkumar, S.; Vockens, J.; Good, N.; Quinn, C.; L’Orange, C.; Weller, Z.D.; et al. Impact of the wood-burning Justa cookstove on fine particulate matter exposure: A stepped-wedge randomized trial in rural Honduras. Sci. Total Environ. 2021, 767, 144369. [Google Scholar] [CrossRef] [PubMed]
- Young, B.N.; Peel, J.L.; Rajkumar, S.; Keller, K.P.; Benka-Coker, M.L.; Good, N.; Walker, E.S.; Brook, R.D.; Nelson, T.L.; Volckens, J.; et al. Impact of the Wood-Burning Justa Cookstove on Glycated Hemoglobin (HbA1c): A Stepped-Wedge Randomized Trial in Rural Honduras. Environ. Health Perspect. 2025, 133, 057021. [Google Scholar] [CrossRef]
- Samoli, E.; Aga, E.; Toulomi, G.; Nisiotis, K.; Forsberg, B.; Lefranc, A.; Pekkanen, J.; Wojtyniak, B.; Schindler, C.; Niciu, E.; et al. Short-term effects of nitrogen dioxide on mortality: An analysis within the APHEA project. Eur. Respir. J. 2006, 27, 1129–1138. [Google Scholar] [CrossRef]
- Vasishta, S.; Adiga, U. Air pollution and its role in the rising burden of type 2 diabetes in India: Urgent call for action. Environ. Sci. Pollut. Res. 2025, 32, 13527–13538. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Wang, Y.; Gong, J.; Wang, G.; Ge, W.; Chen, R.; Meng, X.; Zhao, Y.; Kan, H. Associations of long-term exposure to ambient nitrogen dioxide with indicators of diabetes and dyslipidemia in China: A nationwide analysis. Chemosphere 2021, 269, 128724. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chen, Z.; Zhou, L.; Huang, S. Air pollutants and early origins of respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 75–94. [Google Scholar] [CrossRef]
- Espinoza-Guillen, J.A.; Alderete-Malpartida, M.B.; Cañari-Cancho, J.H.; Pando-Huerta, D.L.; Rosa, D.F.V.-L.; Bernabé-Meza, S.J. Immission levels and identification of sulfur dioxide sources in La Oroya city, Peruvian Andes. Environ. Dev. Sustain. 2023, 25, 12843–12872. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, E.M.; Mohammadi, M.J.; Sulistiyani, S.; Ramírez-Coronel, A.A.; Kiani, F.; Jalil, A.T.; Almulla, A.F.; Asban, P.; Farhadi, M.; Derikondi, M. Effects of sulfur dioxide inhalation on human health: A review. Rev. Environ. Health 2024, 39, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.E.; Kwon, H.; Yun, J.M.; Min, K.; Kim, H.-J.; Park, J.-H. Association between long-term air pollution exposure and insulin resistance independent of abdominal adiposity in Korean adults. Sci. Rep. 2022, 12, 19147. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, G.; Liao, W.; Yuchi, Y.; Yang, X.; Zhang, Z.; Liu, X.; Mao, Z.; Li, L.; Zhao, J.; et al. The role of telomere shortening in ambient ozone exposure-related insulin resistance. J. Hazard. Mater. 2025, 484, 136768. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, K.; Peng, S.; Tan, Y.; Tong, J.; Wang, B.; Cai, H.; Liu, F.; Xiang, H. Climate change and air pollution can amplify vulnerability of glucose metabolism: The mediating effects of biological aging. Environ. Res. 2025, 272, 121183. [Google Scholar] [CrossRef]
- Debarba, L.K.; Mulka, A.; Lima, J.B.M.; Didyuk, O.; Fakhoury, P.; Koshko, L.; Awada, A.A.; Zhang, K.; Klueh, U.; Sadagurski, M. Acarbose protects from central and peripheral metabolic imbalance induced by benzene exposure. Brain Behav. Immun. 2020, 89, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, E.Y.; Batzer, A.; Li, N.; Lee, C.-H.; Lowenstein, E.; Mohammadi, M.; Margolis, B.; Schlessinger, J. The Function of GRB2 in Linking the Insulin Receptor to Ras Signaling Pathways. Science 1993, 260, 1953–1955. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, J.H.; Lee, B.-E.; Hong, Y.-C. Urinary benzene metabolite and insulin resistance in elderly adults. Sci. Total Environ. 2014, 482–483, 260–268. [Google Scholar] [CrossRef]
- Park, H.-S.; Seo, J.-C.; Kim, J.-H.; Bae, S.-H.; Lim, Y.-H.; Cho, S.-H.; Hong, Y.-C. Relationship Between Urinary t, t-muconic Acid and Insulin Resistance in the Elderly. Korean J. Occup. Environ. Med. 2011, 23, 387. [Google Scholar] [CrossRef]
- Williams, A.D.; Grantz, K.L.; Zhang, C.; Nobles, C.; Sherman, S.; Mendola, P. Ambient Volatile Organic Compounds and Racial/Ethnic Disparities in Gestational Diabetes Mellitus: Are Asian/Pacific Islander Women at Greater Risk? Am. J. Epidemiol. 2019, 188, 389–397. [Google Scholar] [CrossRef]
- Alderete, T.L.; Habre, R.; Toledo-Corral, C.M.; Berhane, K.; Chen, Z.; Lurmann, F.W.; Weigensberg, M.J.; Goran, M.I.; Gil-liland, F.D. Longitudinal Associations Between Ambient Air Pollution With Insulin Sensitivity, β-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes 2017, 66, 1789–1796. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender Differences in Insulin Resistance: New Knowledge and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, G.; Montella, A.; Puci, M.V.; Sotgiu, G.; Muresu, N.; Cherchi, S.; Palermo, M.; Seghieri, G.; Franconi, F.; Campesi, I. Surrogate Indexes of Insulin Resistance Are Affected by Sex and Gender and by the Combination of Smoking and Oral Contraceptives. Diabetology 2024, 5, 677–689. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Luo, M. Relationship between air pollution exposure and insulin resistance in Chinese middle-aged and older populations: Evidence from Chinese cohort. Front. Public Health 2025, 13, 1551851. [Google Scholar] [CrossRef]
- Lamat, H.; Sauvant-Rochat, M.-P.; Tauveron, I.; Bagheri, R.; Ugbolue, U.C.; Maqdasi, S.; Navel, V.; Dutheil, F. Metabolic syndrome and pesticides: A systematic review and meta-analysis. Environ. Pollut. 2022, 305, 119288. [Google Scholar] [CrossRef]
- Czajka, M.; Matysiak-Kucharek, M.; Jodłowska-Jędrych, B.; Sawicki, K.; Fal, B.; Drop, B.; Kruszewski, M.; Kap-ka-Skrzypczak, L. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ. Res. 2019, 178, 108685. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Mrema, E.J.; Rubino, F.M.; Brambilla, G.; Moretto, A.; Tsatsakis, A.M.; Colosio, C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013, 307, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- WHO: Exposure to Highly Hazardous Pesticides: A Major Public Health Concern. Available online: https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19.4.6 (accessed on 19 August 2025).
- Güil-Oumrait, N.; Stratakis, N.; Maitre, L.; Anguita-Ruiz, A.; Urquiza, J.; Fabbri, L.; Basagaña, X.; Heude, B.; Haug, L.S.; Sakhi, A.K.; et al. Prenatal Exposure to Chemical Mixtures and Metabolic Syndrome Risk in Children. JAMA Netw. Open 2024, 7, e2412040. [Google Scholar] [CrossRef]
- Smink, A.; Ribas-Fito, N.; Garcia, R.; Torrent, M.; Mendez, M.A.; Grimalt, J.O.; Sunyer, J. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr. 2008, 97, 1465–1469. [Google Scholar] [CrossRef]
- Warner, M.; Wesselink, A.; Harley, K.G.; Bradman, A.; Kogut, K.; Eskenazi, B. Prenatal Exposure to Dichlorodiphenyltrichloroethane and Obesity at 9 Years of Age in the CHAMACOS Study Cohort. Am. J. Epidemiol. 2014, 179, 1312–1322. [Google Scholar] [CrossRef]
- Song, Y.; Chou, E.L.; Baecker, A.; You, N.Y.; Song, Y.; Sun, Q.; Liu, S. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J. Diabetes 2016, 8, 516–532. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, I.-K.; Jin, S.-H.; Steffes, M.; Jacobs, D.R. Association Between Serum Concentrations of Persistent Organic Pollutants and Insulin Resistance Among Nondiabetic Adults. Diabetes Care 2007, 30, 622–628. [Google Scholar] [CrossRef]
- Kim, K.-S.; Lee, Y.-M.; Kim, S.G.; Lee, I.-K.; Lee, H.-J.; Kim, J.-H.; Kim, J.; Moon, H.-B.; Jacobs, D.R.; Lee, D.-H. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere 2014, 94, 151–157. [Google Scholar] [CrossRef]
- Kim, K.-S.; Hong, N.-S.; Jacobs, D.R.; Lee, D.-H. Interaction Between Persistent Organic Pollutants and C-reactive Protein in Estimating Insulin Resistance Among Non-diabetic Adults. J. Prev. Med. Public Health 2012, 45, 62–69. [Google Scholar] [CrossRef]
- Jansen, A.; Lyche, J.L.; Polder, A.; Aaseth, J.; Skaug, M.A. Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss—A review. J. Toxicol. Environ. Health Part B 2017, 20, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Otaru, S.; Jones, L.E.; Carpenter, D.O. Associations between urine glyphosate levels and metabolic health risks: Insights from a large cross-sectional population-based study. Environ. Health 2024, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, J.R.; Lee, D.-H.; Porta, M.; Steffes, M.W.; Jacobs, D.R. Persistent organic pollutants in young adults and changes in glucose related metabolism over a 23-year follow-up. Environ. Res. 2015, 137, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Hyun, N.; Zeng, D.; Uppal, K.; Tran, V.T.; Yu, T.; Jones, D.; He, J.; Lee, E.T.; et al. Novel Metabolic Markers for the Risk of Diabetes Development in American Indians. Diabetes Care 2015, 38, 220–227. [Google Scholar] [CrossRef]
- Seesen, M.; Pratchayasakul, W.; Pintana, H.; Chattipakorn, N.; Chattipakorn, S.C. Exposure to organophosphates in association with the development of insulin resistance: Evidence from in vitro, in vivo, and clinical studies. Food Chem. Toxicol. 2022, 168, 113389. [Google Scholar] [CrossRef]
- Gupta, H.P.; Fatima, M.; Pandey, R.; Ravi Ram, K. Adult exposure of atrazine alone or in combination with carbohydrate diet hastens the onset/progression of type 2 diabetes in Drosophila. Life Sci. 2023, 316, 121370. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ahn, S.Y.; Song, I.C.; Chung, M.H.; Jang, H.C.; Park, K.S.; Lee, K.-U.; Pak, Y.K.; Lee, H.K. Chronic Exposure to the Herbicide, Atrazine, Causes Mitochondrial Dysfunction and Insulin Resistance. PLoS ONE 2009, 4, e5186. [Google Scholar] [CrossRef]
- Zhang, C.; Fennel, E.M.J.; Douillet, C.; Stýblo, M. Exposures to arsenite and methylarsonite produce insulin resistance and impair insulin-dependent glycogen metabolism in hepatocytes. Arch. Toxicol. 2017, 91, 3811–3821. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, Q.; Kim, Y.; Yang, S.-H.; Qi, W.; Kim, D.; Yoon, K.S.; Clark, J.M.; Park, Y. Exposure to permethrin promotes high fat diet-induced weight gain and insulin resistance in male C57BL/6J mice. Food Chem. Toxicol. 2018, 111, 405–416. [Google Scholar] [CrossRef]
- Xiao, X.; Kim, Y.; Kim, D.; Yoon, K.S.; Clark, J.M.; Park, Y. Permethrin alters glucose metabolism in conjunction with high fat diet by potentiating insulin resistance and decreases voluntary activities in female C57BL/6J mice. Food Chem. Toxicol. 2017, 108, 161–170. [Google Scholar] [CrossRef]
- Sun, Q.; Xiao, X.; Kim, Y.; Kim, D.; Yoon, K.S.; Clark, J.M.; Park, Y. Imidacloprid Promotes High Fat Diet-Induced Adiposity and Insulin Resistance in Male C57BL/6J Mice. J. Agric. Food Chem. 2016, 64, 9293–9306. [Google Scholar] [CrossRef]
- Singh, V.K.; Sarkar, S.K.; Saxena, A.; Koner, B.C. Sub-toxic exposure to lindane activates redox sensitive kinases and impairs insulin signaling in muscle cell culture: The possible mechanism of lindane-induced insulin resistance. Toxicol. Vitr. 2019, 54, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Singh, V.K.; Sarkar, S.K.; Shanmugasundaram, B.; Jeevaratnam, K.; Koner, B.C. Effect of sub-toxic chlorpyrifos on redox sensitive kinases and insulin signaling in rat L6 myotubes. J. Diabetes Metab. Disord. 2018, 17, 325–332. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J.; Chojenta, C.; Byles, J.E. Exposure to heavy metals from point pollution sources and risk of incident type 2 diabetes among women: A prospective cohort analysis. Int. J. Environ. Health Res. 2021, 31, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, H.; Zhu, X.; Xiang, R.; Miao, Y.; Zhang, Y.; Song, Y.; Chen, J.; Zhang, L. Effects of multi-metal exposure on the risk of diabetes mellitus among people aged 40–75 years in rural areas in southwest China. J. Diabetes Investig. 2022, 13, 1412–1425. [Google Scholar] [CrossRef]
- Wang, X.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Mukherjee, B.; Harlow, S.D.; Park, S.K. Urinary metals and incident diabetes in midlife women: Study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res. Care 2020, 8, e001233. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Bæcklund, M.; Pedersen, N.L.; Björkman, L.; Vahter, M. Variation in Blood Concentrations of Cadmium and Lead in the Elderly. Environ. Res. 1999, 80, 222–230. [Google Scholar] [CrossRef]
- Gulson, B.L.; Mahaffey, K.R.; Jameson, C.W.; Mizon, K.J.; Korsch, M.J.; Cameron, M.A.; Eisman, J.A. Mobilization of lead from the skeleton during the postnatal period is larger than during pregnancy. J. Lab. Clin. Med. 1998, 131, 324–329. [Google Scholar] [CrossRef]
- Björkman, L.; Vahter, M.; Pedersen, N.L. Both the environment and genes are important for concentrations of cadmium and lead in blood. Environ. Health Perspect. 2000, 108, 719–722. [Google Scholar] [CrossRef][Green Version]
- Bunn, T.L.; Marsh, J.A.; Dietert, R.R. Gender differences in developmental immunotoxicity to lead in the chicken: Analysis following a single early low-level exposure in ovo. J. Toxicol. Environ. Health A 2000, 61, 677–693. [Google Scholar] [CrossRef]
- Bunn, T.L.; Parsons, P.J.; Kao, E.; Dietert, R.R. Gender-based profiles of developmental immunotoxicity to lead in the rat: Assessment in juveniles and adults. J. Toxicol. Environ. Health A 2001, 64, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-M.; Ko, Y.-F.; Huang, Y.-K.; Chen, H.-W.; Chiou, H.-Y.; Huang, Y.-L.; Yang, M.-H.; Chen, C.-J. Determinants of inorganic arsenic methylation capability among residents of the Lanyang Basin, Taiwan: Arsenic and selenium exposure and alcohol consumption. Toxicol Lett. 2003, 137, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtle, T. Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis 2003, 24, 967–974. [Google Scholar] [CrossRef]
- Waalkes, M.P. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: Promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis 2003, 25, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P.; Ward, J.M.; Liu, J.; Diwan, B.A. Transplacental carcinogenicity of inorganic arsenic in the drinking water: Induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol. Appl. Pharmacol. 2003, 186, 7–17. [Google Scholar] [CrossRef]
- Jana, K.; Jana, S.; Samanta, P.K. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: Possible an estrogenic mode of action. Reprod. Biol. Endocrinol. 2006, 4, 9. [Google Scholar] [CrossRef]
- Åkesson, A.; Berglund, M.; Schütz, A.; Bjellerup, P.; Bremme, K.; Vahter, M. Cadmium Exposure in Pregnancy and Lactation in Relation to Iron Status. Am. J. Public Health 2002, 92, 284–287. [Google Scholar] [CrossRef]
- Vahter, M.; Berglund, M.; Åkesson, A.; Lidén, C. Metals and Women’s Health. Environ. Res. 2002, 88, 145–155. [Google Scholar] [CrossRef]
- Pillet, S.; D’Elia, M.; Bernier, J.; Bouquegneau, J.-M.; Fournier, M.; Cyr, D.G. Immunomodulatory Effects of Estradiol and Cadmium in Adult Female Rats. Toxicol. Sci. 2006, 92, 423–432. [Google Scholar] [CrossRef][Green Version]
- Barregård, L.; Svalander, C.; Schütz, A.; Westberg, G.; Sällsten, G.; Blohmé, I.; Mölne, J.; Attman, P.O.; Haglind, P. Cadmium, mercury, and lead in kidney cortex of the general Swedish population: A study of biopsies from living kidney donors. Environ. Health Perspect. 1999, 107, 867–871. [Google Scholar] [CrossRef][Green Version]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Casagrande, C.; Nicolas, G.; Horvath, Z.; Frenoy, P.; Weiderpass, E.; Katzke, V.; Kaaks, R.; Rodri-guez-Barranco, M.; Panico, S.; et al. Dietary intakes of dioxins and polychlorobiphenyls (PCBs) and breast cancer risk in 9 European countries. Environ. Int. 2022, 163, 107213. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Jolly, J.; Lawrence, D.A. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol. Appl. Pharmacol. 2017, 334, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Sharrocks, A.D.; Whitmarsh, A.J. MAP kinase signalling cascades and transcriptional regulation. Gene 2013, 513, 1–13. [Google Scholar] [CrossRef]
- Mostafalou, S.; Baeeri, M.; Bahadar, H.; Soltany-Rezaee-Rad, M.; Gholami, M.; Abdollahi, M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ. Toxicol. Pharmacol. 2015, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.B.; Hafida, S.; Stemmer, P.; Adhami, A.; Leff, T. Lead (Pb) exposure promotes diabetes in obese rodents. J. Trace Elem. Med. Biol. 2017, 39, 221–226. [Google Scholar] [CrossRef]
- Wan, H.; Wang, B.; Cui, Y.; Wang, Y.; Zhang, K.; Chen, C.; Xia, F.; Ye, L.; Wang, L.; Wang, N.; et al. Low-level lead exposure promotes hepatic gluconeogenesis and contributes to the elevation of fasting glucose level. Chemosphere 2021, 276, 130111. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Q.; Xu, X.; Huang, X.; Chen, J.; Huo, X. Associations of insulin sensitivity and immune inflammatory responses with child blood lead (Pb) and PM2.5 exposure at an e-waste recycling area during the COVID-19 lockdown. Environ. Geochem. Health 2024, 46, 296. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Xie, J.; Kang, L.-N.; Wang, L.; Xu, B. High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications. Mediat. Inflamm. 2016, 2016, 3896147. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamakuchi, M.; Biswas, K.K.; Aryal, B.; Yamada, S.; Hashiguchi, T.; Maruyama, I. HMGB1 is secreted by 3T3-L1 adipocytes through JNK signaling and the secretion is partially inhibited by adiponectin. Obesity 2016, 24, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-M.; Chiou, H.-Y.; Ho, I.-C.; Chen, C.-J.; Lee, T.-C. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environ. Health Perspect. 2003, 111, 1429–1438. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Liu, S.X.; Athar, M.; Lippai, I.; Waldren, C.; Hei, T.K. Induction of oxyradicals by arsenic: Implication for mechanism of genotoxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Wauson, E.M. Sodium Arsenite Inhibits and Reverses Expression of Adipogenic and Fat Cell-Specific Genes during in Vitro Adipogenesis. Toxicol. Sci. 2002, 65, 211–219. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Silbergeld, E.K.; Streeter, R.A.; Clark, J.M.; Burke, T.A.; Guallar, E. Arsenic Exposure and Type 2 Diabetes: A Systematic Review of the Experimental and Epidemiologic Evidence. Environ. Health Perspect. 2006, 114, 641–648. [Google Scholar] [CrossRef]
- Spaur, M.; Galvez-Fernandez, M.; Chen, Q.; Lombard, M.A.; Bostick, B.C.; Factor-Litvak, P.; Fretts, A.M.; Shea, S.J.; Navas-Acien, A.; Nigra, A.E. Association of Water Arsenic With Incident Diabetes in U.S. Adults: The Multi-Ethnic Study of Atherosclerosis and the Strong Heart Study. Diabetes Care 2024, 47, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Shokat, S.; Iqbal, R.; Riaz, S.; Yaqub, A. Association Between Arsenic Toxicity, AS3MT Gene Polymorphism and Onset of Type 2 Diabetes. Biol. Trace Elem. Res. 2024, 202, 1550–1558. [Google Scholar] [CrossRef]
- Olsson, I.-M.; Bensryd, I.; Lundh, T.; Ottosson, H.; Skerfving, S.; Oskarsson, A. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ. Health Perspect. 2002, 110, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.K.; Bolger, P.M.; Carrington, C.D. Update of US FDA’s Total Diet Study food list and diets. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 573–582. [Google Scholar] [CrossRef]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjørklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef]
- Salcedo-Bellido, I.; Gómez-Peña, C.; Pérez-Carrascosa, F.M.; Vrhovnik, P.; Mustieles, V.; Echeverría, R.; Fiket, Ž.; Pérez-Díaz, C.; Barrios-Rodríguez, R.; Jiménez-Moleón, J.J.; et al. Adipose tissue cadmium concentrations as a potential risk factor for insulin resistance and future type 2 diabetes mellitus in GraMo adult cohort. Sci. Total Environ. 2021, 780, 146359. [Google Scholar] [CrossRef]
- Abranches, M.V.; de Oliveira, F.C.E.; da Conceição, L.L.; Peluzio, M.d.C.G. Obesity and diabetes: The link between adipose tissue dysfunction and glucose homeostasis. Nutr. Res. Rev. 2015, 28, 121–132. [Google Scholar] [CrossRef]
- Echeverría, R.; Vrhovnik, P.; Salcedo-Bellido, I.; Iribarne-Durán, L.M.; Fiket, Ž.; Dolenec, M.; Martin-Olmedo, P.; Olea, N.; Arrebola, J.P. Levels and determinants of adipose tissue cadmium concentrations in an adult cohort from Southern Spain. Sci. Total Environ. 2019, 670, 1028–1036. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Odewumi, C.; Latinwo, L.M.; Sinclair, A.; Badisa, V.L.D.; Abdullah, A.; Badisa, R.B. Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells. Mol. Med. Rep. 2015, 12, 6422–6426. [Google Scholar] [CrossRef]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—Up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 681–692. [Google Scholar] [CrossRef]
- Papa, V.; Wannenes, F.; Crescioli, C.; Caporossi, D.; Lenzi, A.; Migliaccio, S.; Di Luigi, L. The environmental pollutant cadmium induces homeostasis alteration in muscle cells in vitro. J. Endocrinol. Investig. 2014, 37, 1073–1080. [Google Scholar] [CrossRef]
- Kukongviriyapan, U.; Apaijit, K.; Kukongviriyapan, V. Oxidative Stress and Cardiovascular Dysfunction Associated with Cadmium Exposure: Beneficial Effects of Curcumin and Tetrahydrocurcumin. Tohoku J. Exp. Med. 2016, 239, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Il’yasova, D.; Ivanova, A. Urinary Cadmium, Impaired Fasting Glucose, and Diabetes in the NHANES III. Diabetes Care 2003, 26, 468–470. [Google Scholar] [CrossRef]
- El Muayed, M.; Raja, M.R.; Zhang, X.; MacRenaris, K.W.; Bhatt, S.; Chen, X.; Urbanek, M.; O’Halloran, T.V.; Lowe, W.L., Jr. Accumulation of cadmium in insulin-producing β cells. Islets 2012, 4, 405–416. [Google Scholar] [CrossRef]
- Nie, X.; Wang, N.; Chen, Y.; Chen, C.; Han, B.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; Lu, M.; et al. Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ. Sci. Pollut. Res. 2016, 23, 18714–18723. [Google Scholar] [CrossRef]
- Jacquet, A.; Cottet-Rousselle, C.; Arnaud, J.; Julien Saint Amand, K.; Ben Messaoud, R.; Lénon, M.; Demeilliers, C.; Moulis, J.-M. Mitochondrial Morphology and Function of the Pancreatic β-Cells INS-1 Model upon Chronic Exposure to Sub-Lethal Cadmium Doses. Toxics 2018, 6, 20. [Google Scholar] [CrossRef]
- Huang, C.-C.; Kuo, C.-Y.; Yang, C.-Y.; Liu, J.-M.; Hsu, R.-J.; Lee, K.-I.; Su, C.-C.; Wu, C.-C.; Lin, C.-T.; Liu, S.-H.; et al. Cadmium exposure induces pancreatic β-cell death via a Ca2+-triggered JNK/CHOP-related apoptotic signaling pathway. Toxicology 2019, 425, 152252. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015, 6, 14. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Baig, J.A.; Sarfraz, R.A. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pract. 2008, 80, 280–288. [Google Scholar] [CrossRef]
- Little, B.B.; Reilly, R.; Walsh, B.; Vu, G.T. Cadmium Is Associated with Type 2 Diabetes in a Superfund Site Lead Smelter Community in Dallas, Texas. Int. J. Environ. Res. Public Health 2020, 17, 4558. [Google Scholar] [CrossRef]
- Barregard, L.; Bergström, G.; Fagerberg, B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: A cross-sectional and prospective study in women. Environ. Res. 2013, 121, 104–109. [Google Scholar] [CrossRef]
- Borné, Y.; Fagerberg, B.; Persson, M.; Sallsten, G.; Forsgard, N.; Hedblad, B.; Barregard, L.; Engström, G. Cadmium Exposure and Incidence of Diabetes Mellitus—Results from the Malmö Diet and Cancer Study. PLoS ONE 2014, 9, e112277. [Google Scholar] [CrossRef]
- Guo, F.-F.; Hu, Z.-Y.; Li, B.-Y.; Qin, L.-Q.; Fu, C.; Yu, H.; Zhang, Z.-L. Evaluation of the association between urinary cadmium levels below threshold limits and the risk of diabetes mellitus: A dose-response meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 19272–19281. [Google Scholar] [CrossRef]
- Roy, C.; Tremblay, P.-Y.; Ayotte, P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ. Res. 2017, 156, 747–760. [Google Scholar] [CrossRef]
- Eom, S.-Y.; Choi, S.-H.; Ahn, S.-J.; Kim, D.-K.; Kim, D.-W.; Lim, J.-A.; Choi, B.-S.; Shin, H.-J.; Yun, S.-W.; Yoon, H.-J.; et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int. Arch. Occup. Environ. Health 2014, 87, 501–513. [Google Scholar] [CrossRef]
- Maqbool, F.; Bahadar, H.; Niaz, K.; Baeeri, M.; Rahimifard, M.; Navaei-Nigjeh, M.; Ghasemi-Niri, S.F.; Abdollahi, M. Effects of methyl mercury on the activity and gene expression of mouse Langerhans islets and glucose metabolism. Food Chem. Toxicol. 2016, 93, 119–128. [Google Scholar] [CrossRef]
- Chen, Y.W.; Huang, C.F.; Tsai, K.S.; Yang, R.S.; Yen, C.C.; Yang, C.Y.; Lin-Shiau, S.Y.; Liu, S.H. The Role of Phosphoinositide 3-Kinase/Akt Signaling in Low-Dose Mercury–Induced Mouse Pancreatic β-Cell Dysfunction In Vitro and In Vivo. Diabetes 2006, 55, 1614–1624. [Google Scholar] [CrossRef]
- Chang, J.-W.; Chen, H.-L.; Su, H.-J.; Liao, P.-C.; Guo, H.-R.; Lee, C.-C. Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. J. Hazard. Mater. 2011, 185, 749–755. [Google Scholar] [CrossRef]
- Ali, N.D.A.; Ma, Y.; Reynolds, J.; Wise, J.P.; Inzucchi, S.E.; Katz, D.L. Chromium Effects on Glucose Tolerance and Insulin Sensitivity in Persons at Risk for Diabetes Mellitus. Endocr. Pract. 2011, 17, 16–25. [Google Scholar] [CrossRef]
- Althuis, M.D.; Jordan, N.E.; Ludington, E.A.; Wittes, J.T. Glucose and insulin responses to dietary chromium supplements: A meta-analysis. Am. J. Clin. Nutr. 2002, 76, 148–155. [Google Scholar] [CrossRef]
- Balk, E.M.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of Chromium Supplementation on Glucose Metabolism and Lipids. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef]
- Vajdi, M.; Khajeh, M.; Safaei, E.; Moeinolsadat, S.; Mousavi, S.; Seyedhosseini-Ghaheh, H.; Abbasalizad-Farhangi, M.; Askari, G. Effects of chromium supplementation on body composition in patients with type 2 diabetes: A dose-response systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2024, 81, 127338. [Google Scholar] [CrossRef]
- Son, Y.-O.; Hitron, J.A.; Wang, X.; Chang, Q.; Pan, J.; Zhang, Z.; Liu, J.; Wang, S.; Lee, J.-C.; Shi, X. Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 2010, 245, 226–235. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in Drinking Water: Sources, Metabolism, and Cancer Risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Bagchi, D. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu; Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks. Front. Cell. Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic migration and transformation pathways and exposure health risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef]
- Chartres, N.; Cooper, C.B.; Bland, G.; Pelch, K.E.; Gandhi, S.A.; BakenRa, A.; Woodruff, T.J. Effects of Microplastic Exposure on Human Digestive, Reproductive, and Respiratory Health: A Rapid Systematic Review. Environ. Sci. Technol. 2024, 58, 22843–22864. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Hantoro, I.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Widianarko, B.; Ragas, A.M.J. Microplastics in coastal areas and seafood: Implications for food safety. Food Addit. Contam. Part A 2019, 36, 674–711. [Google Scholar] [CrossRef]
- Cole, M.; Gomiero, A.; Jaén-Gil, A.; Haave, M.; Lusher, A. Microplastic and PTFE contamination of food from cookware. Sci. Total Environ. 2024, 929, 172577. [Google Scholar] [CrossRef]
- Snekkevik, V.K.; Cole, M.; Gomiero, A.; Haave, M.; Khan, F.R.; Lusher, A.L. Beyond the food on your plate: Investigating sources of microplastic contamination in home kitchens. Heliyon 2024, 10, e35022. [Google Scholar] [CrossRef]
- World Health Organization. Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health; World Health Organization: Geneva, Switzerland, 2022; Available online: https://iris.who.int/bitstream/handle/10665/362049/9789240054608-eng.pdf?sequence=1 (accessed on 28 August 2025).
- Blackburn, K.; Green, D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2022, 51, 518–530. [Google Scholar] [CrossRef]
- Roh, Y.; Kim, J.; Song, H.; Seol, A.; Kim, T.; Park, E.; Park, K.; Lim, S.; Wang, S.; Jung, Y.; et al. Impact of the Oral Administration of Polystyrene Microplastics on Hepatic Lipid, Glucose, and Amino Acid Metabolism in C57BL/6Korl and C57BL/6-Lepem1hwl/Korl Mice. Int. J. Mol. Sci. 2024, 25, 4964. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, Y.; Nie, P.; Wang, W.; Wu, H.; Wan, X.; Xu, H.; Fu, F. Polystyrene microplastics disturb maternal glucose homeostasis and induce adverse pregnancy outcomes. Ecotoxicol. Environ. Saf. 2024, 279, 116492. [Google Scholar] [CrossRef]
- Romano, N.; Renukdas, N.; Fischer, H.; Shrivastava, J.; Baruah, K.; Egnew, N.; Sinha, A.K. Differential modulation of oxidative stress, antioxidant defense, histomorphology, ion-regulation and growth marker gene expression in goldfish (Carassius auratus) following exposure to different dose of virgin microplastics. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 238, 108862. [Google Scholar] [CrossRef]
- Fraenkel, E.; Lazurova, I. IGF-1 and IGFBP3 as indirect markers of hepatic insulin resistance and their relation to metabolic syndrome parameters in liver steatosis patients. Endocr. Regul. 2023, 57, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, L.-B.; Wang, D.; Zhu, Q.-L.; Zheng, J.-L. Combined effects of polystyrene microplastics and cadmium on oxidative stress, apoptosis, and GH/IGF axis in zebrafish early life stages. Sci. Total Environ. 2022, 813, 152514. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Chen, X.; Peng, L.-B.; Wang, D.; Zhu, Q.-L.; Li, J.; Han, T. Particles rather than released Zn2+ from ZnO nanoparticles aggravate microplastics toxicity in early stages of exposed zebrafish and their unexposed offspring. J. Hazard. Mater. 2022, 424, 127589. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D. Intestinal mitochondrial unfolded protein response induced by nanoplastic particles in Caenorhabditis elegans. Chemosphere 2021, 267, 128917. [Google Scholar] [CrossRef]
- Tang, Y.; Suo, Y.; Sun, Z.; Wu, X.; Xing, Q.; Bai, Y. Microplastics induce insulin resistance by causing mitochondrial dysfunction associated with mROS in skeletal muscle in vitro. Ecotoxicol. Environ. Saf. 2025, 302, 118585. [Google Scholar] [CrossRef]
- Kim, J.E.; Sonar, N.S.; Thakuri, L.S.; Park, J.W.; Kim, K.-T.; Rhyu, D.Y. Mixtures of polystyrene micro and nanoplastics affects fat and glucose metabolism in 3T3-L1 adipocytes and zebrafish larvae. NanoImpact 2025, 37, 100549. [Google Scholar] [CrossRef]
- Fan, X.; Wei, X.; Hu, H.; Zhang, B.; Yang, D.; Du, H.; Zhu, R.; Sun, X.; Oh, Y.; Gu, N. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere 2022, 288, 132607. [Google Scholar] [CrossRef]
- Fan, X.; Li, X.; Li, J.; Zhang, Y.; Wei, X.; Hu, H.; Zhang, B.; Du, H.; Zhao, M.; Zhu, R.; et al. Polystyrene nanoplastics induce glycolipid metabolism disorder via NF-κB and MAPK signaling pathway in mice. J. Environ. Sci. 2024, 137, 553–566. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef]
- Zhao, J.; Gomes, D.; Jin, L.; Mathis, S.P.; Li, X.; Rouchka, E.C.; Bodduluri, H.; Conklin, D.J.; O’Toole, T.E. Polystyrene bead ingestion promotes adiposity and cardiometabolic disease in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113239. [Google Scholar] [CrossRef]
- Lee, A.G.; Kang, S.; Yoon, H.J.; Im, S.; Oh, S.J.; Pak, Y.K. Polystyrene Microplastics Exacerbate Systemic Inflammation in High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2023, 24, 12421. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Xu, K.; Wang, X.; Gao, X.; Han, Q.; Wang, S.; Chen, M. The effect and a mechanistic evaluation of polystyrene nanoplastics on a mouse model of type 2 diabetes. Food Chem. Toxicol. 2023, 173, 113642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, K.; Gao, X.; Wei, Z.; Han, Q.; Wang, S.; Du, W.; Chen, M. Polystyrene nanoplastics with different functional groups and charges have different impacts on type 2 diabetes. Part. Fibre Toxicol. 2024, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dong, H.; Gao, Y.; Liu, S.; Chen, L.; Ni, G.; Guo, X.; Wang, M.; Wang, C.; Chen, Y.; et al. Airborne Nanoplastics Exposure Inducing Irreversible Glucose Increase and Complete Hepatic Insulin Resistance. Environ. Sci. Technol. 2024, 58, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, P.; Xu, T.; Zhang, G.; Xu, Z.; Wang, X.; Zhao, L.; Yang, H. Combined exposure to lead and microplastics increased risk of glucose metabolism in mice via the Nrf2/NF-κB pathway. Environ. Toxicol. 2024, 39, 2502–2511. [Google Scholar] [CrossRef]
- Pani, B.S.U.L.; Chandrasekaran, N. Investigating the impact of nanoplastics in altering the efficacy of clarithromycin antibiotics through In vitro studies. Environ. Pollut. 2024, 363, 125144. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Gao, H. Potential mechanisms and modifications of dietary antioxidants on the associations between co-exposure to plastic additives and diabetes. Nutr. Diabetes 2024, 14, 72. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Zheng, Y.; Xu, M.; Zhou, J.; Wang, S.; Li, S.; Wang, M. Nanoplastics disrupt hepatic lipid metabolism via the inhibition of PPARγ: A study based on digestive system exposure. Toxicology 2025, 516, 154194. [Google Scholar] [CrossRef]
- Ryan, P.B.; Burke, T.A.; Hubal, E.A.C.; Cura, J.J.; McKone, T.E. Using Biomarkers to Inform Cumulative Risk Assessment. Environ. Health Perspect. 2007, 115, 833–840. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef]
- Niedzwiecki, M.M.; Walker, D.I.; Vermeulen, R.; Chadeau-Hyam, M.; Jones, D.P.; Miller, G.W. The Exposome: Molecules to Populations. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 107–127. [Google Scholar] [CrossRef]
- Sun, J.; Fang, R.; Wang, H.; Xu, D.-X.; Yang, J.; Huang, X.; Cozzolino, D.; Fang, M.; Huang, Y.A. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ. Int. 2022, 158, 106941. [Google Scholar] [CrossRef]
- Sarigiannis, D.; Karakitsios, S.; Anesti, O.; Stem, A.; Valvi, D.; Sumner, S.C.J.; Chatzi, L.; Snyder, M.P.; Thompson, D.C.; Va-siliou, V. Advancing translational exposomics: Bridging genome, exposome and personalized medicine. Hum. Genom. 2025, 19, 48. [Google Scholar] [CrossRef]
- Khraishah, H.; Chen, Z.; Rajagopalan, S. Understanding the Cardiovascular and Metabolic Health Effects of Air Pollution in the Context of Cumulative Exposomic Impacts. Circ. Res. 2024, 134, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Impact Assessment (HIA); WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/tools/health-impact-assessments (accessed on 18 August 2025).
- Xu, J.; Zhang, W.; Lu, Z.; Zhang, F.; Ding, W. Airborne PM2.5-Induced Hepatic Insulin Resistance by Nrf2/JNK-Mediated Signaling Pathway. Int. J. Environ. Res. Public Health 2017, 14, 787. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, W.; Liao, R.; Cao, W.; Huang, F.; Wang, X.; Li, M.; Li, Y. Subacute PM2.5 Exposure Induces Hepatic Insulin Resistance Through Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 812. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Protano, C.; Antonucci, A.; Vitali, M.; Sessa, R. Impact of Air Pollution on the Composition and Diversity of Human Gut Microbiota in General and Vulnerable Populations: A Systematic Review. Toxics 2022, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.; Talusan, A.; Vaidyanathan, S.; Veerappan, A.; Mikhail, M.; Ostrofsky, D.; Crowley, G.; Kim, J.; Kwon, S.; No-lan, A. Metabolic Syndrome and Air Pollution: A Narrative Review of Their Cardiopulmonary Effects. Toxics 2019, 7, 6. [Google Scholar] [CrossRef]
- Sîrbu, C.A.; Stefan, I.; Dumitru, R.; Mitrica, M.; Manole, A.M.; Vasile, T.M.; Stefani, C.; Ranetti, A.E. Air Pollution and Its Devastating Effects on the Central Nervous System. Healthcare 2022, 10, 1170. [Google Scholar] [CrossRef]
- Stojchevski, R.; Chandrasekaran, P.; Hadzi-Petrushev, N.; Mladenov, M.; Avtanski, D. Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences. Int. J. Mol. Sci. 2024, 25, 7849. [Google Scholar] [CrossRef]
| Pollutant | % Change in HOMA-IR (per 1 μg/m3) | 95% CI | I2 (%) | p-Value |
|---|---|---|---|---|
| PM2.5 | 0.40% | −0.03 to 0.84 | 67.4 | 0.009 |
| PM10 | 1.61% | 0.243, 2.968 | 49.1 | 0.001 |
| NO2 | 0.09% | −0.01, 0.19 | 83.2 | 0.002 |
| Pesticide | Type of Study | Molecular Effect of Exposure to Pesticides | Key References |
|---|---|---|---|
| Atrazine | In vivo | ↑ oxidative stress ↑ fatty acids ↓ triglycerides ↓ Akt | [85,86] |
| Arsenite and Methylarsonite | In vitro | ↑ GS ↑ GP ↓ Akt | [87] |
| Permethrin | In vivo | ↑ TNF-α ↓ GLUT4 ↓ Akt ↓ PDK1 ↓ GLUT4 | [88,89] |
| Imidacloprid | In vivo | ↑ CD36 ↑ SREBP1c ↑ TNF-α ↑ fatty acids ↑ PEPCK ↓ PPARα mRNA | [90] |
| Lindane | In vitro | ↑ SOD ↑ IκBα ↑ p38 MAPK ↑ JNK ↑ HSP25 ↑ HSP70 ↓ antioxidant ↓ IRS-1 tyrosine ↓ Akt serine | [91] |
| Chlorpyrifos | In vitro | ↑ p38MAPK ↑ IκBα ↓ IRS-1 tyrosine ↓ Akt serine | [92] |
| Plastic Type | Animal | Applied Dose/Concentration | Exposition | Results | Ref. |
|---|---|---|---|---|---|
| virgin polyvinyl chloride microplastics | goldfish | 0.5 mg/L | 4 days | ↓ expression of GHR and IGFBP-1 ↑ expression of CR | [176] |
| micropolystyrene | zebrafish embryos | 500 μg/L | 30 days | ↓ expression of GHR, IGF-1, IGFR-1, IGFBP-2 and IGFBP-6 | [178] |
| nanopolystyrene | C. elegans | 100 μg/L | 6.5 days | ↑ mitochondrial unfolded protein response in intestines | [180] |
| nanopolystyrene | mice | 15 mg/kg (in feeding chow) | 20 weeks | ↑ plasma glucose levels ↑ ROS production ↑ phosphorylation of IRS-1 impaired PI3K/Akt pathway signaling | [183] |
| nanopolystyrene | mice | 5 mg/kg (in feeding chow) | 20 weeks | ↑ ROS production ↑ activation of NRF2, NFκB and MAPK signaling pathways ↑ phosphorylation of IRS-1 ↓ Akt activity ↑ transcription of G6PC and PEPCK | [184] |
| micropolystyrene | mice | 55 μg/day (in feeding chow) | 2 weeks | ↓ I3A and phenylacetylglycine generation ↑ 4-guanidinobutyric acid and CDP-choline generation ↑ fasting glucose and insulin levels ↑ HOMA-IR index value | [174] |
| micropolysterene | mice | 80 mg/kg (in feeding chow) | 10 weeks | ↓ glucose tolerance ↑ HOMA-IR index value ↑ IL-1β, IL-6, TNF-α and CRP levels ↓ number of pancreatic islets ↑ vacuolization, nuclear pyknosis of hepatocytes ↓ microbiota richness ↑ Bacteroidetes presence in the intestines ↓ Firmicutes presence in the intestines | [185] |
| micropolystyrene | mice | 1 μg/mL (in drinking water) | 12 weeks | ↑ body weight ↑ fat content ↑ fasting glucose ↓ glucose tolerance ↑ HOMA-IR index value ↑ HDL (high-density lipoprotein) ↑ expression of IL-6 and MCP-1 in perivascular adipose tissue | [186] |
| micropolystyrene | mice | 0.125 µg/day (in feeding chow) | 6 weeks | ↑ blood glucose ↑ fasting insulin ↑ HOMA-IR index value | [187] |
| nanopolystyrene | mice | 30 mg/kg (in feeding chow) | 8 weeks | ↑ blood glucose ↓ glucose tolerance ↑ insulin resistance ↑ ROS levels ↑ lesion formation in liver and pancreas | [188] |
| nanopolystyrene modified with –COOH and –NH2 functional groups | mice | 5 mg/kg (in feeding chow) | 9 weeks | ↑ fasting blood glucose ↓ glucose tolerance ↑ HOMA-IR index value ↑ ROS levels in liver tissue ↑ glycogen accumulation in liver tissue ↑ cellular damage in liver and pancreas tissue ↓ p-Akt and FoxO1 levels in liver tissue All alterations were more severe in groups treated with nanopolystyrene modified with –NH2 functional groups | [189] |
| polyvinyl chloride and polyethylene microplastics combined with Pb | mice | 10 mg/L (in drinking water) | 6 weeks | ↓ glucose tolerance ↑ glycosylation of serum proteins ↓ SOD, GPX and CAT activity in liver tissue ↓ HO-1 levels in liver tissue ↓ Keap1 and NRF2 mRNA expression in liver tissue | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Grabarczyk, M.; Leszto, K.; Luba, G.; Motor, J.; Sosińska, A.; Rysz, J.; Franczyk, B. Environmental Insults to Glucose Metabolism: The Role of Pollutants in Insulin Resistance. Int. J. Mol. Sci. 2025, 26, 8979. https://doi.org/10.3390/ijms26188979
Młynarska E, Grabarczyk M, Leszto K, Luba G, Motor J, Sosińska A, Rysz J, Franczyk B. Environmental Insults to Glucose Metabolism: The Role of Pollutants in Insulin Resistance. International Journal of Molecular Sciences. 2025; 26(18):8979. https://doi.org/10.3390/ijms26188979
Chicago/Turabian StyleMłynarska, Ewelina, Mikołaj Grabarczyk, Klaudia Leszto, Gabriela Luba, Jakub Motor, Aleksandra Sosińska, Jacek Rysz, and Beata Franczyk. 2025. "Environmental Insults to Glucose Metabolism: The Role of Pollutants in Insulin Resistance" International Journal of Molecular Sciences 26, no. 18: 8979. https://doi.org/10.3390/ijms26188979
APA StyleMłynarska, E., Grabarczyk, M., Leszto, K., Luba, G., Motor, J., Sosińska, A., Rysz, J., & Franczyk, B. (2025). Environmental Insults to Glucose Metabolism: The Role of Pollutants in Insulin Resistance. International Journal of Molecular Sciences, 26(18), 8979. https://doi.org/10.3390/ijms26188979